Abstract
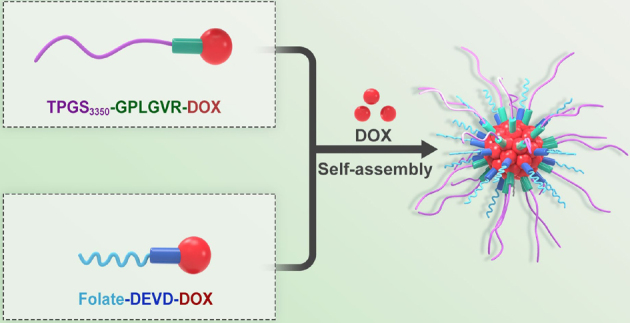
Recently, the incidence of cancer keeps increasing, seriously endangers human health, and has evolved into the main culprit of human death. Conventional chemotherapeutic drugs, such as paclitaxel and doxorubicin (DOX), have some disadvantages, including low therapeutic effect, poor water solubility, high toxic side effects, short blood circulation time in the body, and so on. To improve the anti-tumor effect of the drug in vivo and reduce its side effects on the body, researchers have designed and developed a variety of responsive nanocarriers. In this work, we synthesized D-α-tocopherol polyethylene glycol 3350 succinate (TPGS3350)-Gly-Pro-Leu-Gly-Val-Arg (GPLGVR)-DOX (TPD) prodrugs in response to extracellular enzymes of matrix metalloproteinase (MMP-9) in the tumor microenvironment and FA-Asp-Glu-Val-Asp (DEVD)-DOX (FPD) prodrugs responsive to intracellular enzymes of caspase-3. Then, intracellular and extracellular enzyme-responsive TPD&FPD micelles with DOX (TPD&FPD&D) were successfully prepared through dialysis method. The outer layer of TPGS3350 can prolong the blood circulation time of micelles in vivo, followed by accumulation of micelles at tumor tissue through enhanced permeability and retention (EPR) effect. The peptide of GPLGVR can be cleaved by MMP-9 enzymes to remove the outer layer of TPGS3350, exposing the targeting molecule of folate, and then the micelles are engulfed by tumor cells through folate receptor-mediated endocytosis. After entering the tumor cells, the free DOX loaded in the micelles is released, which induces tumor cell apoptosis to activate caspase-3 in the cells, cutting the peptide DEVD to accelerate the intracellular release of the DOX, which further enhances cytotoxicity to improve antitumor effect.
Electronic Supplementary Material
Supplementary material () is available in the online version of this article at 10.1007/s12274-022-4967-1.
Keywords: cancer, micelle, peptide, enzyme-responsive, folate
Electronic Supplementary Material
Intracellular and extracellular enzymatic responsive micelle for intelligent therapy of cancer
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 22078246, 22178270, and 82102204). The authors also appreciate the support by the Postdoctoral Research Foundation of China (No. 2020M680478).
Contributor Information
Fangzhou Li, Email: lifz2020@nanoctr.cn.
Yi Liu, Email: yiliuchem@whu.edu.cn.
Jie Pan, Email: panjie@tiangong.edu.cn.
References
- [1].Sung H, Ferlay J, Siegel R L, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R L, Miller K D, Fuchs H E, Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- [3].Sengupta S, Kulkarni A. Design principles for clinical efficacy of cancer nanomedicine: A look into the basics. ACS Nano. 2013;7:2878–2882. doi: 10.1021/nn4015399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wolfram J, Ferrari M. Clinical cancer nanomedicine. Nano Today. 2019;25:85–98. doi: 10.1016/j.nantod.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rosenblum D, Joshi N, Tao W, Karp J M, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018;9:1410. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Poon W, Kingston B R, Ouyang B, Ngo W, Chan W C W. A framework for designing delivery systems. Nat. Nanotechnol. 2020;15:819–829. doi: 10.1038/s41565-020-0759-5. [DOI] [PubMed] [Google Scholar]
- [7].Manzari M T, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller D A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021;6:351–370. doi: 10.1038/s41578-020-00269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhou J, Rao L, Yu G C, Cook T R, Chen X Y, Huang F H. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021;50:2839–2891. doi: 10.1039/D0CS00011F. [DOI] [PubMed] [Google Scholar]
- [9].Wan D, Xi Y J, Li S F, Pan J. Progress on nanocarriers in responsive to tumor microenvironment. Chem. Ind. Eng. 2021;38:80–87. [Google Scholar]
- [10].Li Y X, Sun J, Li J J, Liu K, Zhang H J. Engineered protein nanodrug as an emerging therapeutic tool. Nano Res. 2022;15:5161–5172. doi: 10.1007/s12274-022-4103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao D C, Yang N L, Xu L K, Du J, Yang Y, Wang D. Hollow structures as drug carriers: Recognition, response, and release. Nano Res. 2022;15:739–757. doi: 10.1007/s12274-021-3595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Blanco E, Shen H F, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mitchell M J, Billingsley M M, Haley R M, Wechsler M E, Peppas N A, Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barenholz Y. Doxil®-the first FDA-approved nano-drug: Lessons learned. J. Control. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- [15].Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- [16].Attia M F, Anton N, Wallyn J, Omran Z, Vandamme T F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019;71:1185–1198. doi: 10.1111/jphp.13098. [DOI] [PubMed] [Google Scholar]
- [17].Han X J, Alu A, Liu H M, Shi Y, Wei X W, Cai L L, Wei Y Q. Biomaterial-assisted biotherapy: A brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact. Mater. 2022;17:29–48. doi: 10.1016/j.bioactmat.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jin Q, Deng Y Y, Chen X H, Ji J. Rational design of cancer nanomedicine for simultaneous stealth surface and enhanced cellular uptake. ACS Nano. 2019;13:954–977. doi: 10.1021/acsnano.8b07746. [DOI] [PubMed] [Google Scholar]
- [19].Wang Y Q, Li S M, Wang X H, Chen Q, He Z G, Luo C, Sun J. Smart transformable nanomedicines for cancer therapy. Biomaterials. 2021;271:120737. doi: 10.1016/j.biomaterials.2021.120737. [DOI] [PubMed] [Google Scholar]
- [20].Chong G W, Zang J, Han Y, Su R P, Weeranoppanant N, Dong H Q, Li Y Y. Bioengineering of nano metal-organic frameworks for cancer immunotherapy. Nano Res. 2021;14:1244–1259. doi: 10.1007/s12274-020-3179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fan, M. L.; Liu, W.; Fan, C. Y.; Zheng, X. Y.; Hui, J. F.; Hu, C. Q.; Fan, D. D. Ce and Se co-doped MBG/SA/HLC microgel bone powder for repairing tumor bone defects. Nano Res., in press, 10.1007/s12274-022-4630-x.
- [22].El-Sawy H S, Al-Abd A M, Ahmed T A, El-Say K M, Torchilin V P. Stimuli-responsive nano-architecture drug-delivery systems to solid tumor micromilieu: Past, present, and future perspectives. ACS Nano. 2018;12:10636–10664. doi: 10.1021/acsnano.8b06104. [DOI] [PubMed] [Google Scholar]
- [23].Grzelczak M, Liz-Marzan L M, Klajn R. Stimuli-responsive self-assembly of nanoparticles. Chem. Soc. Rev. 2019;48:1342–1361. doi: 10.1039/C8CS00787J. [DOI] [PubMed] [Google Scholar]
- [24].Van Der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder W J M, Lammers T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019;14:1007–1017. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li Z M, Yang Y, Wei H X, Shan X T, Wang X Z, Ou M T, Liu Q Y, Gao N S, Chen H Z, Mei L, et al. Charge-reversal biodegradable MSNs for tumor synergetic chemo/photothermal and visualized therapy. J. Control. Release. 2021;338:719–730. doi: 10.1016/j.jconrel.2021.09.005. [DOI] [PubMed] [Google Scholar]
- [26].Zhong Q, Zhang J H, Guo R W. Facile preparation of hollow mesoporous silica nanoparticles coated with pH-sensitive polymer. Chem. Ind. Eng. 2017;34:50–54. [Google Scholar]
- [27].Zhu X H, Li C, Lu Y, Liu Y J, Wan D, Zhu D W, Pan J, Ma G L. Tumor microenvironment-activated therapeutic peptide-conjugated prodrug nanoparticles for enhanced tumor penetration and local T cell activation in the tumor microenvironment. Acta Biomater. 2021;119:337–348. doi: 10.1016/j.actbio.2020.11.008. [DOI] [PubMed] [Google Scholar]
- [28].Liu Y J, Lu Y, Zhu X H, Li C, Yan M M, Pan J, Ma G L. Tumor microenvironment-responsive prodrug nanoplatform via co-self-assembly of photothermal agent and IDO inhibitor for enhanced tumor penetration and cancer immunotherapy. Biomaterials. 2020;242:119933. doi: 10.1016/j.biomaterials.2020.119933. [DOI] [PubMed] [Google Scholar]
- [29].Xue, X. D.; Qu, H. J.; Li, Y. P. Stimuli-responsive crosslinked nanomedicine for cancer treatment. Exploration, in press, 10.1002/EXP.20210134. [DOI] [PMC free article] [PubMed]
- [30].Shahriari M, Zahiri M, Abnous K, Taghdisi S M, Ramezani M, Alibolandi M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release. 2019;308:172–189. doi: 10.1016/j.jconrel.2019.07.004. [DOI] [PubMed] [Google Scholar]
- [31].Li L, Yang Z, Chen X Y. Recent advances in stimuli-responsive platforms for cancer immunotherapy. Acc. Chem. Res. 2020;53:2044–2054. doi: 10.1021/acs.accounts.0c00334. [DOI] [PubMed] [Google Scholar]
- [32].Liu H H, Yang F W, Chen W J, Gong T, Zhou Y, Dai X Y, Leung W, Xu C S. Enzyme-responsive materials as carriers for improving photodynamic therapy. Front. Chem. 2021;9:763057. doi: 10.3389/fchem.2021.763057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Braun A C, Gutmann M, Ebert R, Jakob F, Gieseler H, Lühmann T, Meinel L. Matrix metalloproteinase responsive delivery of myostatin inhibitors. Pharm. Res. 2017;34:58–72. doi: 10.1007/s11095-016-2038-6. [DOI] [PubMed] [Google Scholar]
- [34].Han H J, Valdepérez D, Jin Q, Yang B, Li Z H, Wu Y L, Pelaz B, Parak W J, Ji J. Dual enzymatic reaction-assisted gemcitabine delivery systems for programmed pancreatic cancer therapy. ACS Nano. 2017;11:1281–1291. doi: 10.1021/acsnano.6b05541. [DOI] [PubMed] [Google Scholar]
- [35].Han M, Huang-Fu M Y, Guo W W, Guo N N, Chen J J, Liu H N, Xie Z Q, Lin M T, Wei Q C, Gao J Q. MMP-2-sensitive HA end-conjugated poly(amidoamine) dendrimers via click reaction to enhance drug penetration into solid tumor. ACS Appl. Mater. Interfaces. 2017;9:42459–42470. doi: 10.1021/acsami.7b10098. [DOI] [PubMed] [Google Scholar]
- [36].Ke W D, Zha Z S, Mukerabigwi J F, Chen W J, Wang Y H, He C X, Ge Z S. Matrix metalloproteinase-responsive multifunctional peptide-linked amphiphilic block copolymers for intelligent systemic anticancer drug delivery. Bioconjugate Chem. 2017;28:2190–2198. doi: 10.1021/acs.bioconjchem.7b00330. [DOI] [PubMed] [Google Scholar]
- [37].Lv Y Q, Xu C R, Zhao X M, Lin C S, Yang X, Xin X F, Zhang L, Qin C, Han X P, Yang L, et al. Nanoplatform assembled from a CD44-targeted prodrug and smart liposomes for dual targeting of tumor microenvironment and cancer cells. ACS Nano. 2018;12:1519–1536. doi: 10.1021/acsnano.7b08051. [DOI] [PubMed] [Google Scholar]
- [38].Yao Q, Kou L F, Tu Y, Zhu L. MMP-responsive “smart” drug delivery and tumor targeting. Trends Pharmacol. Sci. 2018;39:766–781. doi: 10.1016/j.tips.2018.06.003. [DOI] [PubMed] [Google Scholar]
- [39].Pan J, Li P J, Wang Y, Chang L, Wan D, Wang H. Active targeted drug delivery of MMP-2 sensitive polymeric nanoparticles. Chem. Commun. 2018;54:11092–11095. doi: 10.1039/C8CC05504A. [DOI] [PubMed] [Google Scholar]
- [40].Porter A G, Jänicke R U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- [41].Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shi Y G. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell. 2002;9:459–470. doi: 10.1016/S1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- [43].Riedl S J, Salvesen G S. The apoptosome: Signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- [44].Riedl S J, Shi Y G. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- [45].Yuan Y Y, Kwok R T K, Tang B Z, Liu B. Targeted theranostic platinum(IV) prodrug with a built-in aggregation-induced emission light-up apoptosis sensor for noninvasive early evaluation of its therapeutic responses in situ. J. Am. Chem. Soc. 2014;136:2546–2554. doi: 10.1021/ja411811w. [DOI] [PubMed] [Google Scholar]
- [46].Pan J, Wan D, Bian Y X, Guo Y G, Jin F M, Wang T, Gong J L. Reduction of nonspecific binding for cellular imaging using quantum dots conjugated with vitamin E. AIChE J. 2014;60:1591–1597. doi: 10.1002/aic.14382. [DOI] [Google Scholar]
- [47].Yu M, Liu K, Mao Z B, Luo J Y, Gu W, Zhao W H. USP11 is a negative regulator to γH2AX ubiquitylation by RNF8/RNF168. J. Biol. Chem. 2016;291:959–967. doi: 10.1074/jbc.M114.624478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wan D, Li S F, Zhang J X, Ma G L, Pan J. Intelligent self-assembly prodrug micelles loading doxorubicin in response to tumor microenvironment for targeted tumors therapy. Chin. J. Chem. Eng. 2021;39:219–227. doi: 10.1016/j.cjche.2021.06.023. [DOI] [Google Scholar]
- [49].Chen H B, Gu Z J, An H W, Chen C Y, Chen J, Cui R, Chen S Q, Chen W H, Chen X S, Chen X Y, et al. Precise nanomedicine for intelligent therapy of cancer. Sci. China Chem. 2018;61:1503–1552. doi: 10.1007/s11426-018-9397-5. [DOI] [Google Scholar]
- [50].Chandrashekar D S, Bashel B, Balasubramanya S A H, Creighton C J, Ponce-Rodriguez I, Chakravarthi B V S K, Varambally S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sun T M, Zhang Y S, Pang B, Hyun D C, Yang M X, Xia Y N. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem., Int. Ed. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intracellular and extracellular enzymatic responsive micelle for intelligent therapy of cancer


