Abstract
Pesticide toxicity, both acute and chronic, is a global public health concern. Pesticides are involved in abnormal inflammatory responses by interfering with the normal physiology and metabolic status of cells. In this regard, inflammatory indices aggregate index of systemic inflammation (AISI), monocyte-to-high-density lipoprotein ratio, monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte platelet ratio (NLPR), neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune inflammation index, and systemic inflammation response index (SIRI) have been used as predictive markers of inflammatory status in several diseases and also in acute poisoning events. This study aimed to determine systemic inflammation indices and their relationship with pesticide exposure from urban sprayers in 302 individuals categorized into three groups (reference group and moderate and high exposure groups). The data suggest that the AISI, MLR, NLPR, and SIRI indices were significantly higher in the exposed groups compared with the reference group. In conclusion, this study proposes that inflammation indices warrant further attention in order to assess their value as early biomarkers of acute and chronic pesticide intoxication.
Keywords: Pesticide, Occupational exposure, Inflammation index, Systemic inflammation, Urban sprayers, Inflammatory response
Introduction
Exposure to pesticide mixtures has resulted in serious problems for human health and the environment. Several studies have confirmed the link between exposure to pesticides and the incidence of a large number of adverse outcomes, such as reproductive effects, endocrine disorders, birth defects, and neurological, hepatic, respiratory, hematopoietic, and immunological effects, as well as the development of various types of cancer, including multiple myeloma, leukemia, and non-Hodgkin’s lymphoma (Piccoli et al. 2019; Cestonaro et al. 2020; Mu et al. 2021). In addition, the incidence of diabetes, obesity, and cardiovascular diseases has also been associated with pesticide exposure (Dayton et al. 2010; Everett and Matheson 2010; Legler et al. 2011). The common pathogenic mechanisms of these diseases are an increase in oxidative stress and a general inflammatory state (Madani et al. 2016).
Pesticides may trigger an abnormal inflammatory response via interference with normal physiology and the metabolic state of immune cells (Block and Hong 2005; Kumar et al. 2014). In consideration of these, several inflammation and immune-based prognostic scores have been used for the recurrence of inflammatory processes and the prognosis of acute toxicity, such as neutrophil-to-lymphocyte ratio (NLR) (Dundar et al. 2014; Zhou et al. 2016; Cao et al. 2019; Jeong and Sun 2020; Lionte et al. 2021; Mu et al. 2021), monocyte-to-lymphocyte ratio (MLR) (Lionte et al. 2021), platelet-to-lymphocyte ratio (PLR) (Elhosary and Abdelbar 2018; Wang et al. 2019; Lionte et al. 2021; Ortiz-López et al. 2022), monocyte-to-high-density lipoprotein ratio (MHR), and neutrophil-to-lymphocyte platelet ratio (NLPR) (Kanbay et al. 2014; Fois et al. 2020).
In addition, numerous novel inflammatory markers that include three or more indicators have been developed to reflect the balance of host inflammatory and immune status, including the systemic immune inflammation index (SII) based on the number of lymphocytes, neutrophils, and platelets, is an indicator associated with inflammation and that can reflect the immune and inflammatory state (Hu et al. 2014; Meng et al. 2018). Also, some studies have associated the SII with the prognosis of diseases (Miyamoto et al. 2019; Li et al. 2020), and this parameter can properly represent the inflammatory and immune status in COVID-19 patients (Fois et al. 2020; Hamad et al. 2022). The systemic inflammation response index (SIRI, the quotient of neutrophils and monocytes, divided by lymphocyte count) has been used to predict that survival can fully evaluate the balance between host immune and inflammatory conditions in cancer (Chen et al. 2020).
On the other hand, the aggregate index of systemic inflammation (AISI) has been proposed as a predictive tool that includes lymphocytes, neutrophils, platelets, and monocytes, which might be superior to simpler indexes as it better reflects the inflammatory status in the context of specific disease states (Zinellu et al. 2021).
Few studies have explored the relationship between pesticide exposure and hematological indices as indicators of inflammation. In most of these studies, only NLR, PLR, MLR, and SII indices were found to be determinate in acute intoxication (Dundar et al. 2014; Elhosary and Abdelbar 2018; Wang et al. 2019; Nejatifar et al. 2022). To the best of our knowledge, this is the first study in which eight inflammatory indices (AISI, MHR, MLR, NLPR, NLR, PLR, SII, and SIRI) are evaluated in a population chronically exposed to pesticides. This study aimed to determine the systemic inflammation indices and their relationship with pesticide exposure.
Methodology
Study population
A cross-sectional study was carried out on a population of 302 individuals, of whom 181 were engaged in occupational pesticide spraying and the remaining 121 did not experience occupational exposure to pesticides. The inclusion criteria taken into account in the present study were individuals from Nayarit, Mexico, ≥ 18 years old, who decided to participate voluntarily in the present study, providing a signed written-informed consent letter. On the other hand, exclusion criteria were found: people who have constant exposure to x-rays or who taking immunosuppressants or corticosteroids. Each study participant donated a blood sample and completed a structured questionnaire designed to collect anthropometric and socioeconomic characteristics, age, harmful habits (smoking, consumption of drugs, and alcohol), medical history, as well as information on pesticide use and management, exposure time, and other variables. The study was approved by the Bioethics Commission of the State of Nayarit, Mexico (CEBN/0112017).
Peripheral blood samples were collected in EDTA tubes. Blood was drawn from the participants after fasting for 8–12 h. A certified laboratory carried out the blood work: a complete blood count and biochemical profile, including hemoglobin, hematocrit, platelets, glucose, urea, BUN creatinine, uric acid, cholesterol, liver function tests, lipid profile, and absolute count of leukocytes, neutrophils, eosinophils, basophils, lymphocytes, and monocytes. Inflammation-related indices were calculated from the blood profile data, as presented in Table 1.
Table 1.
Formulas to determine inflammation indices
| Inflammation index | Formula |
|---|---|
| AISI | |
| MHR | |
| MLR | |
| NLPR | |
| NLR | |
| PLR | |
| SII | |
| SIRI |
AISI, aggregate index of systemic inflammation; MHR, monocyte-to-high-density lipoprotein ratio; MLR, monocyte‐to-lymphocyte ratio; NLPR, neutrophil-to-lymphocyte platelet ratio; NLR, neutrophil‐to-lymphocyte ratio; PLR, platelet‐to-lymphocyte ratio; SII, systemic immune inflammation index; SIRI, systemic inflammation response index; HDLC, high-density lipoprotein cholesterol (Kanbay et al. 2014 and Fois et al. 2020)
Statistical analysis
The geometric means (GM) with a 95% confidence interval (95% CI) in nonparametric variables and arithmetic means ± standard deviation (± SD) in parametric variables were calculated, and the proportions were analyzed via the Chi-square test. ANOVA with post hoc Bonferroni test and Kruskal–Wallis with post hoc Dunn’s test were applied to the parametric and nonparametric data, respectively. Statistical significance was considered at p values of < 0.05. Statistical analysis was performed with Stata version 14 (Stata Statistical Software, Stata Corporation, College Station, Texas, USA) and GraphPad Prism version 6.02 (Graph Pad software, San Diego, California, USA).
Results
Characteristics of the study population
The participants were categorized into three groups: (a) reference group, i.e., individuals without activities related to the use of pesticides, either permanent or temporary (office workers and others); (b) moderate exposure group, i.e., individuals who carried out spraying activities occasionally; and (c) high exposure group, i.e., individuals who participated in permanent fumigation activities. The GM of exposure to pesticides in the moderate exposure group (b) was 6.3 years (95% CI: 4.71–7.82), while in the high exposure group (c) was 6.7 years (95% CI: 5.02–8.45). Previously, our working group reported differences in some pesticide biomarkers of exposure among the study groups (Zepeda-Arce et al. 2017; Herrera-Moreno et al. 2021).
The population consisted of 36.75% women and 63.25% men, and the GM of the participants’ age was 34.80 years (95% CI: 33.64–35.99). With respect to body mass index (BMI), 35.10% of the participants were overweight, according to the World Health Organization (WHO) classification (2022), and 39.74% were obese. Table 2 presents the general characteristics of the study population for the exposition group (n = 302). A significant difference was observed in age, BMI, and educational level between the study groups. Regarding the BMI observed in the study population, overweight and obesity are mainly linked to cardiovascular diseases (WHO 2021), which could have negative implications for the health of the participants, in addition to the damage caused by occupational exposure to pesticides.
Table 2.
General characteristics of the study population
| Group | ||||
|---|---|---|---|---|
| Characteristics | Reference | Moderate exposure | High exposure | p value |
| Total [n(%)] | 121 (40.07) | 121 (40.07) | 60 (19.87) | |
| Sex | 0.10 | |||
| Male [n(%)] | 73 (60.33) | 73 (60.33) | 45 (75.00) | |
| Female [n(%)] | 48 (39.67) | 48 (39.67) | 15 (25.00) | |
| Age [years (95% CI)] | 32.63 (30.81–34.56) | 37.17 (35.32–39.12) | 34.68 (32.48–37.04) | < 0.01 |
| BMI (kg/m2) | < 0.001 | |||
| Underweight [n(%)] | 1 (100) | – | – | |
| Normal weight [n(%)] | 43 (57.33) | 17 (22.67) | 15 (20.00) | |
| Overweight [n(%)] | 43 (40.57) | 42 (39.62) | 21 (19.81) | |
| Obesity [n(%)] | 34 (28.33) | 62 (51.67) | 24 (20.00) | |
| Education* [years (95% CI)] | 16.04 (15.47–16.64) | 11.52 (11.01–12.06) | 11.50 (10.89–12.14) | < 0.001 |
*Values are presented as geometric means
95% CI, 95% confidence interval
Body mass index (BMI) according to the World Health Organization (WHO 2022): underweight (≤ 18.5 kg/m2), normal weight (> 18.5 kg/m2, ≤ 25 kg/m2), overweight (> 25 kg/m2, ≤ 30 kg/m2), and obesity (> 30 kg/m2)
As shown in Fig. 1, organophosphates (OP), pyrethroids (PYR), and carbamates (CB) were the most used pesticides (by frequency) by the moderate and high exposure groups. In addition, temephos was the most used pesticide in the OP group, followed by chlorpyrifos and malathion. In the PYR group, the most used pesticides (by frequency) were deltamethrin, lambda-cyhalothrin, and permethrin. Bendiocarb is the most widely used pesticide in the case of CB.
Fig. 1.
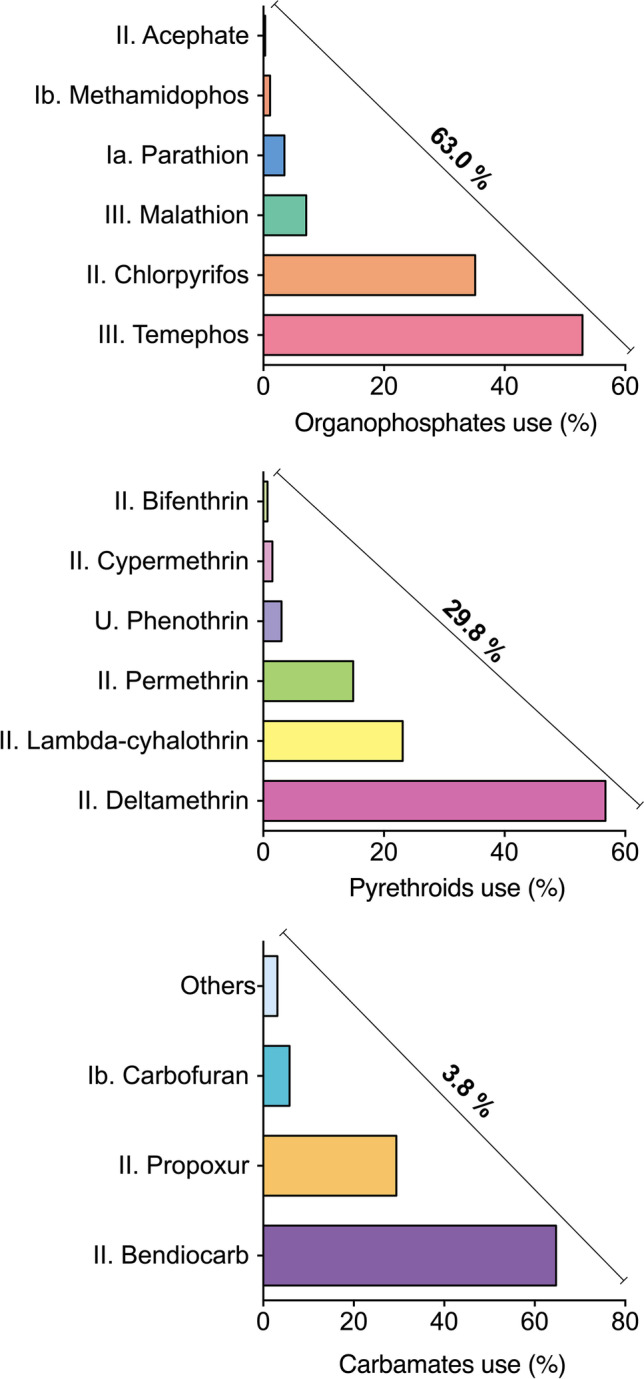
Pesticides used by the study population and WHO classification. Ia (extremely hazardous), Ib (very hazardous), II (moderately hazardous), III (slightly hazardous), and U (unlikely to present an acute hazard) (WHO 2019). The remaining 3.4% belongs to other groups
The main symptomatology reported by the study population exposed to pesticides included headache (68.51%), skin discomforts such as burning or itching (53.89%), weakness (50.83%), constant thirst (49.16%), burning in the eyes (48.07%), and abnormal tiredness (46.61%).
Clinical parameters
Table 3 shows data on the clinical parameters of the study population. Significant differences were observed between the groups in the following parameters: urea, uric acid, cholesterol, GOT, GPT, GGT, globulin, and hematocrit. A significant increase in the values of leukocytes, neutrophils, basophils, and lymphocytes was observed in the high exposure group.
Table 3.
Clinical parameters of the study population
| Group | ||||
|---|---|---|---|---|
| Parameter | Reference | Moderate exposure | High exposure | p value |
| Glucose [mg/dL (95% CI)] | 84.16 (82.43–85.94) | 95.59 (85.47–95.23) | 84.50 (79.63–89.65) | 0.22a |
| Urea [mg/dL (95% CI)] | 23.99 (22.92–25.11) | 22.25 (21.02–23.54) | 22.29 (20.76–23.94) | 0.03a |
| BUN [mg/dL (95% CI)] | 11.21 (10.71–11.73) | 10.39 (9.82–11.00) | 10.81 (9.83–11.88) | 0.05a |
| Creatinine [mg/dL (95% CI)] | 0.76 (0.73–0.79) | 0.73 (0.70–0.77) | 0.76 (0.71–0.80) | 0.09a |
| Uric acid [mg/dL (± SD)] | 5.32 (1.31) | 5.80 (1.66) | 5.97 (1.77) | 0.02b |
| Cholesterol [mg/dL (± SD)] | 182.62 (39.25) | 200.23 (37.64) | 182.59 (35.68) | < 0.001b |
| GOT [UI/L (± SD)] | 24.42 (20.96) | 30.99 (32.25) | 23.54 (12.11) | < 0.01b |
| GPT [UI/L (95% CI)] | 21.09 (18.96–23.47) | 30.17 (26.42–34.44) | 25.11 (21.58–29.22) | < 0.001a |
| GGT [UI/L (95% CI)] | 26.04 (23.17–29.25) | 40.11 (34.85–46.16) | 32.73 (26.90–39.82) | < 0.001a |
| Albumin [g/dL (95% CI)] | 4.70 (4.65–4.75) | 4.75 (4.69–4.81) | 4.77 (4.69–4.85) | 0.06a |
| Globulin [g/dL(± SD)] | 2.72 (0.33) | 2.83 (0.34) | 2.83 (0.43) | 0.04b |
| Hemoglobin [g/dL (± SD)] | 15.06 (1.41) | 14.79 (1.48) | 14.80 (1.40) | 0.27b |
| Hematocrit [%(± SD)] | 44.50 (3.83) | 44.65 (3.91) | 44.83 (3.36) | 0.84b |
| Leukocytes [/mm3 (± SD)] | 6702.31 (1592.33) | 7769.01 (1775.91) | 8187.33 (2219.47) | < 0.001b |
| Neutrophils [/mm3 (95% CI)] | 3471.52 (3241.85–3717.46) | 4097.15 (3868.08–4339.78) | 4284.46 (3815.55–4810.98) | < 0.001a |
| Eosinophils [/mm3 (95% CI)] | 258.00 (218.52–304.61) | 309.71 (265.18–361.73) | 293.72 (235.54–366.27) | 0.17a |
| Basophils [/mm3 (95% CI)] | 67.59 (61.08–74.81) | 73.53 (63.49–85.17) | 99.94 (80.30–124.39) | < 0.01a |
| Lymphocytes [/mm3 (95% CI)] | 2216.34 (2115.63–2321.85) | 2470.69 (2333.91–2615.48) | 2393.40 (2192.15–2613.13) | < 0.01a |
| Monocytes [/mm3 (± SD)] | 326.12 (173.78) | 468.49 (186.97) | 224.34 (244.34) | < 0.001b |
| Platelets [/mm3 (± SD)] | 264,644.6 (61,745.16) | 258,289.3 (63,096.15) | 254,916.7 (65,862.8) | 0.56b |
BUN, blood urea nitrogen; GOT, glutamic oxalacetic transaminase; GPT, glutamic pyruvic transaminase; GGT, gamma-glutamyl transferase
ap values were obtained by Kruskal − Wallis and post hoc Dunn’s test
bp values were obtained by ANOVA, with post hoc Bonferroni test
95% CI, 95% confidence interval; ± SD, ± standard deviation
Inflammation parameters
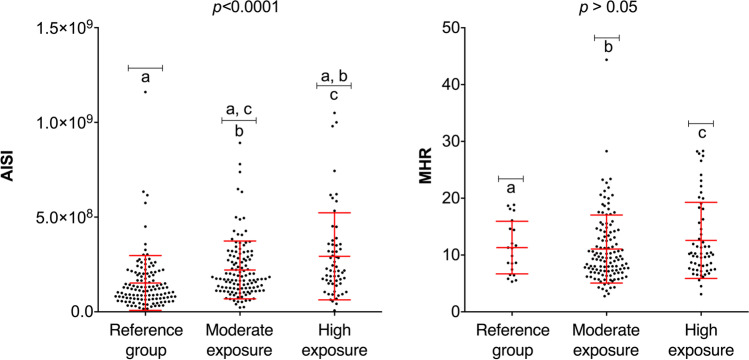
The AISI and MHR indices were analyzed with respect to the exposure groups (Fig. 2). The results show that AISI was significantly higher in both the moderate and high exposure groups compared with the reference group. In contrast, no significant difference was found in the values of the MHR index among the different groups.
Fig. 2.
AISI and MHR index values of the three exposure groups. AISI, aggregate index of systemic inflammation; MHR, monocyte/high-density lipoprotein cholesterol ratio. (a) Reference group individuals; (b) moderate exposure group individuals; (c) high exposure group individuals. Statistical analysis included ANOVA, Bonferroni test (ASI), and Kruskal–Wallis and Dunn’s test (MHR). Significant differences are indicated with the letters a, b, and c (p < 0.05)
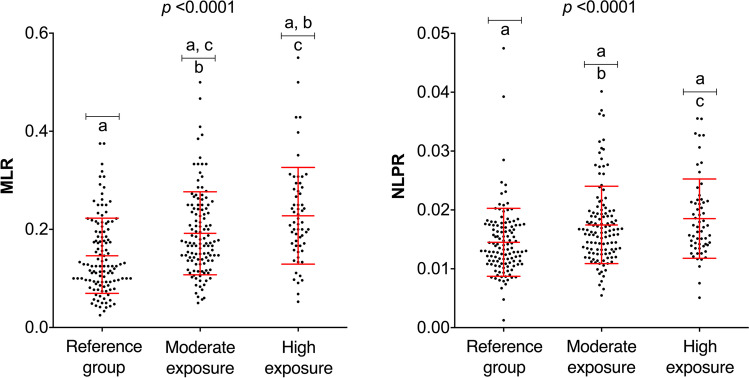
As shown in Fig. 3, the values of the indices MLR and NLPR are plotted by exposure group. The results show an increasing trend of MLR values with respect to occupational exposure to pesticides. A significant difference in NLPR values is evident in the moderate and high exposure groups compared with the reference group.
Fig. 3.
MLR and NLPR index values of the three exposure groups. MLR, monocyte-to-lymphocyte ratio; NLPR, neutrophil-to-lymphocyte platelet ratio. (a) Reference group individuals; (b) moderate exposure group individuals; (c) high exposure group individuals. Statistical analysis included ANOVA, Bonferroni test (MLR), and Kruskal–Wallis and Dunn’s test (NLPR). Significant differences are indicated with the letters a, b, and c (p < 0.05)
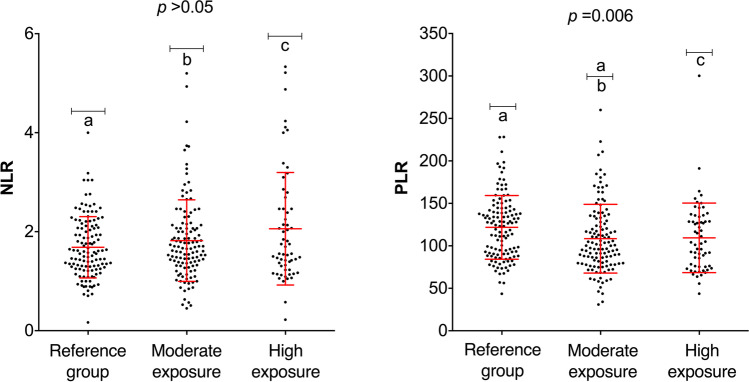
In the case of NLR values, no significant difference was found between the exposure groups (Fig. 4). However, PLR values were significantly different in the moderate exposure group compared with the reference group. Interestingly, the individuals with the highest exposure did not show statistically significant differences in PLR values compared with the reference group.
Fig. 4.
NLR and PLR index values of the three exposure groups. NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio. (a) Reference group individuals; (b) moderate exposure group individuals; (c) high exposure group individuals. Statistical analysis included Kruskal–Wallis and Dunn’s test (NLR and PLR). Significant differences are indicated with the letters a, b, and c (p < 0.05)
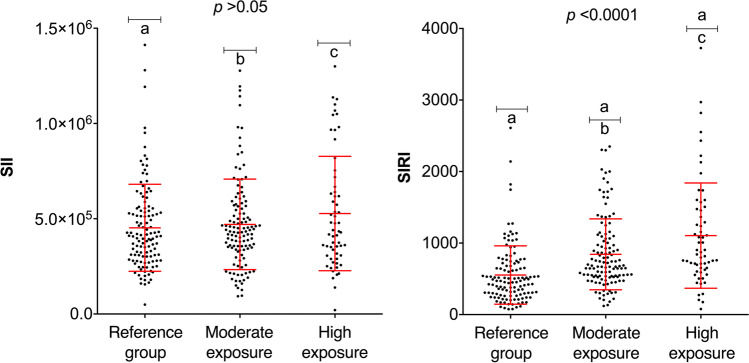
Figure 5 shows the SII and SIRI values with respect to the exposure groups. The SII values were not influenced by the study population’s exposure to pesticides; however, differences were observed in the SIRI values in the moderate and high exposure groups when compared with the reference group.
Fig. 5.
SII and SIRI index values of the three exposure groups. SII systemic inflammatory index; SIRI, systemic inflammatory response index. (a) Reference group individuals; (b) moderate exposure group individuals; (c) high exposure group individuals. Statistical analysis was performed with the Kruskal–Wallis and Dunn’s test (SII and SIRI). Significant differences are indicated with the letters a, b, and c (p < 0.05)
Discussion
The threat of disease-transmitting vectors has led to the use of pesticides as the main strategy for pest control in many Latin American countries, including Mexico (Gómez-Arroyo et al. 2013). Personnel who apply pesticides or who handle different formulations in closed environments are at risk, and the severity of risk depends upon (among other factors) the duration of handling or application; in many cases, this is nearly constant throughout the year (Benitez-Trinidad et al. 2018).
Inflammation is essential for effective immunity, including tissue repair and the return to homeostasis (Afonina et al. 2017; Eming et al. 2017; Gong et al. 2020). However, in unregulated conditions, inflammation is regarded as an unwanted response, particularly as it can lead to serious consequences, such as immune dysfunction, further tissue damage, sepsis, organ failure, or even death (Iqbal et al. 2017). The complexity of the process is reflected in the large number of cells involved in the molecular patterns associated with damage, mainly monocytes/macrophages, dendritic cells, neutrophils, mast cells, natural killer cells, and eosinophils (Gong et al. 2020). Accordingly, there is keen interest among researchers in characterizing diverse parameters of inflammation that can serve as prognostic tools in a broad array of diseases (Li et al. 2018; Aydin et al. 2022; Kara et al. 2022). Indices based on cell counts have been used to detect inflammatory events that are triggered by exposure to pesticides, and NLR is one of the most widely used indices (Dundar et al. 2014; Nejatifar et al. 2022). In the present study, we found significant differences in hematological parameters between the different exposure groups (moderate and high pesticide exposure compared to the reference group). These results are similar to those published recently by Nejatifar et al. (2022), who observed that NLR and other hematologic parameters, such as platelet count, red blood cell distribution, and the number of white blood cells, neutrophils, basophils, and eosinophils, in a group exposed to pesticides were significantly higher than in the unexposed group.
Moreover, the prognostic value of NLR, PLR, and other hematologic parameters measured in patients intoxicated with pesticides (within the first 24 h of admission to the emergency department) shows that the value of NLR and PLR was significantly higher in patients suffering from severe poisoning (Dundar et al. 2014). However, our data suggest that individuals subjected to high (occupational) exposure did not show significant differences in these index values compared with the control subjects. Therefore, in cases of pesticide intoxication, it is important to evaluate whether the degree of intoxication—based on the NLR and PLR indices—could be masked in subjects with chronic exposure. In addition, in the clinical studies mentioned above, patients requiring mechanical ventilation were found to have significantly higher leukocyte and neutrophil counts, as well as higher values of NLR and PLR and lower lymphocyte counts compared to non-ventilated patients (Dundar et al. 2014). Wang et al. (2019) determined that PLR is not a predictor for patients with acute paraquat poisoning; however, our data indicate that there is a decrease in PLR values compared to the reference group, similar to the results of a study in Egypt of patients with acute pesticide poisoning, in which it was shown that NLR and PLR were statistically elevated in the group of individuals who died compared to patients who experienced either complete recovery or complicated recovery (Elhosary and Abdelbar 2018).
To the best of our knowledge, the rest of the parameters evaluated in our study (AISI, MHR, NLPR, and SIRI) have not been evaluated in individuals exposed to pesticides, possibly because these biomarkers can be influenced by factors other than exposure to pesticides. Complete hematological indices would yield a more detailed picture of the relationship among variables in inflammatory diseases induced by pesticide exposure.
Regardless of the route and/or duration of exposure to one or more pesticides, it is likely that inflammation events will be constantly generated due to the physiological impacts of intoxication, both acute and chronic (Le Couteur et al. 1999; Kirby et al. 2001; Barlow et al. 2005; Hirschfield et al. 2010; Astiz et al. 2012; Cave et al. 2012; MacFarlane et al. 2013; Wong et al. 2017, 2021; Petrescu et al. 2018; Chen et al. 2019; Vilas-Boas et al. 2019). The main groups of pesticides (OP, CB, and PYR) to which urban sprayers are exposed can generate considerable alterations in many cellular structures, culminating in tissue-specific or system-wide damage (Le Couteur et al. 1999; Kirby et al. 2001; Barlow et al. 2005; Hirschfield et al. 2010; Astiz et al. 2012; Cave et al. 2012; MacFarlane et al. 2013; Wong et al. 2017, 2021; Petrescu et al. 2018; Chen et al. 2019; Vilas-Boas et al. 2019). Among these deleterious effects is the stimulation of the cholinergic system, which is not limited to nervous system cells. On the contrary, the cholinergic system is widely distributed in the body and is of great importance in the function of many cell types, including immune system cells (Fujii et al. 2017; Koureas et al. 2017; Andrew and Lein 2021; Lu and Wu 2021).
Similarly, neuroinflammation has been proposed as a key mechanism in abnormalities in ducks (Anatidae) related to exposure to pesticides. Consequently, the imbalances generated over long periods would culminate in neurodegenerative disorders (Kirby et al. 2001; Baltazar et al. 2014; Li et al. 2011; Andrew and Lein 2021). Most of these disorders are closely related to the production of proinflammatory mediators (di Penta et al. 2013; Gargouri et al. 2018). During these inflammatory processes, a redox imbalance is generated, resulting in the reduction of glutathione concentrations, an increase in inducible nitric oxide synthase production, and generation of reactive oxygen and nitrogen species derived from hydrophilic and hydrophobic components (Astiz et al. 2012; di Penta et al. 2013; Gargouri et al. 2018). Microglia and/or astrocytes are the main cells involved in these inflammatory processes. These cells are prevalent in the nervous system and present macrophage-like characteristics, behaving like cells of the innate immune system (Duffield 2003; Banks and Lein 2012; Gargouri et al. 2018). Microglia promote inflammation as part of the process of repairing injured nerve tissue; however, when the stimulus is uncontrolled, it is usually associated with diseases such as Parkinson’s and/or Alzheimer’s (Koziorowski et al. 2012; Spangenberg and Green 2017).
In this way, pesticides would generate an inflammatory response by interacting with components of the cholinergic and other systems and generating pathophysiological alterations related to inflammation (Banks and Lein 2012; Andrew and Lein 2021).
Figure 6 illustrates the mechanism of pesticide uptake, the inflammatory environment, and their relationship. Pesticides induce oxidative stress through the generation of reactive oxygen species and reactive nitrogen species (RNS) by the activation of NADPH oxidases (NOX). This could induce the oxidation of lipids, proteins, and DNA damage. Continuous stress triggers inflammation and apoptosis. Additionally, these stressors lead to the activation of TNFR1/TNF-α, MAPK, NF-κB, and mitochondrial apoptosis pathways. Moreover, increased levels of nitric oxide (NO) and increased Ca2+ uptake stimulate the production of RNS. Intracellular Ca2+ induces an inflammatory stage through NF-κB activation, promoting the increase in the levels of proinflammatory cytokines (TNF-α and IL-6). Lastly, the inflammatory environment is associated with several chronic diseases, such as endocrine disorders, cancers, and deleterious effects on the neurological, immunological, and respiratory systems; all of these effects have been linked with pesticide exposure.
Fig. 6.
Indices of inflammation, mechanisms of the pesticide-induced inflammatory process, and chronic toxicity related to inflammatory. B cell CLL/lymphoma-2 (BCL-2), pro-apoptotic factors (BAX and BAD), reduced NADP (NADPH), nuclear factor-kB (NF-κB), nitric oxide (NO), inducible nitric oxide synthase (iNOS), neural nitric oxide synthase (nNOS), superoxide anion (O2•−), and tumor necrosis factor alpha (TNF-α). Adapted from Mostafalou and Abdollahi (2013), Jabłońska-Trypuć (2017), and Sule et al. (2022)
The main limitation of this study was not evaluating diseases that could interfere with hematological indices; however, this was in all the study groups. Our recommendation is that in future studies, cytokines as well as C-reactive protein could complement the inflammatory status.
In conclusion, we propose that inflammation indices warrant more attention in order to evaluate their value as early biomarkers of both acute and chronic pesticide poisoning and to explore the link between pesticide exposure and the development of inflammatory-related diseases.
Acknowledgements
We are grateful to all the workers who participated in the study.
Author contribution
Ruíz-Arias Miguel Alfonso: conceptualization, formal analysis, writing – original draft preparation, writing – reviewing and editing. Medina-Díaz Irma Martha: writing – original draft preparation, writing – reviewing and editing. Bernal-Hernández Yael Yvette: writing – original draft preparation, writing – reviewing and editing. Agraz-Cibrian Juan Manuel: writing – original draft preparation, writing – reviewing and editing. González-Arias Cyndia Azucena: writing – original draft preparation, writing – reviewing and editing. Barrón-Vivanco Briscia Socorro: writing – original draft preparation, writing – reviewing and editing. Herrera-Moreno José Francisco: writing – original draft preparation, writing – reviewing and editing. Verdín-Betancourt Francisco Alberto: writing – original draft preparation, writing – reviewing and editing. Zambrano-Zaragoza José Francisco: writing – original draft preparation, writing – reviewing and editing. Rojas-García Aurora Elizabeth: conceptualization, writing – original draft preparation, formal analysis, writing – reviewing and editing, project administration.
Funding
This work was supported by CONACyT (Grant #233803 and 314829).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
The study was approved by the Bioethics Commission of the State of Nayarit, Mexico (CEBN/0112017).
Consent to participate
All individuals voluntarily participated in the study and gave their informed written consent.
Consent for publication
Written informed consent was obtained from all participants.
Competing interests
The authors declare no competing interests.
Footnotes
Highlights
• Significant differences were observed in clinical parameters in pesticide sprayers.
• AISI, MLR, NLPR, and SIRI indices were significantly higher in the exposed groups compared with the reference group.
• Significant higher inflammation indicators were observed in pesticide sprayers.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afonina IS, Zhong Z, Karin M, et al. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat Immunol. 2017;18(8):861–869. doi: 10.1038/ni.3772. [DOI] [PubMed] [Google Scholar]
- Andrew PM, Lein PJ. Neuroinflammation as a therapeutic target for mitigating the long-term consequences of acute organophosphate intoxication. Front Pharmacol. 2021;12:674325. doi: 10.3389/fphar.2021.674325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astiz M, de Alaniz MJ, Marra CA. The oxidative damage and inflammation caused by pesticides are reverted by lipoic acid in rat brain. Neurochem Int. 2012;61(7):1231–1241. doi: 10.1016/j.neuint.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Aydin C, Alpsoy Ş, Yildirim İ. Predictive values of inflammation indexes in predicting mortality in patients with COVID 19 hospitalized in general intensive care unit. Türk Sağlık Bilimleri Dergisi. 2022;7(1):32–39. doi: 10.26453/otjhs.984345. [DOI] [Google Scholar]
- Baltazar MT, Dinis-Oliveira RJ, de Lourdes BM, et al. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases – a mechanistic approach. Toxicol Lett. 2014;230(2):85–103. doi: 10.1016/j.toxlet.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Banks CN, Lein PJ. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology. 2012;33(3):575–584. doi: 10.1016/j.neuro.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow BK, Lee DW, Cory-Slechta DA, et al. Modulation of antioxidant defense systems by the environmental pesticide maneb in dopaminergic cells. Neurotoxicology. 2005;26(1):63–75. doi: 10.1016/j.neuro.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Benitez-Trinidad AB, Medina-Díaz IM, Bernal-Hernández YY, et al. Relationship between LINE-1 methylation pattern and pesticide exposure in urban sprayers. Food Chem Toxicol. 2018;113:125–133. doi: 10.1016/j.fct.2018.01.035. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Cao ZX, Song YQ, Bai WJ, et al. Neutrophil-lymphocyte ratio as an early predictor for patients with acute paraquat poisoning: a retrospective analysis. Medicine. 2019;98(37):e17199. doi: 10.1097/MD.0000000000017199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave M, Falkner KC, McClain C. Chapter 27 Occupational and environmental hepatotoxicity. In: Boyer TD, Manns MP, Sanyal AJ, editors. Zakim and Boyer's Hepatology. China: Elsevier Inc; 2012. pp. 476–492. [Google Scholar]
- Cestonaro LV, Garcia SC, Nascimento S, et al. Biochemical, hematological and immunological parameters and relationship with occupational exposure to pesticides and metals. Environ Sci Pollut Res Int. 2020;27(23):29291–29302. doi: 10.1007/s11356-020-09203-3. [DOI] [PubMed] [Google Scholar]
- Chen Z, Xu Y, Li N, et al. A national-scale cumulative exposure assessment of organophosphorus pesticides through dietary vegetable consumption in China. Food Control. 2019;104:34–41. doi: 10.1016/j.foodcont.2019.04.015. [DOI] [Google Scholar]
- Chen L, Kong X, Wang Z, et al. Pretreatment systemic infammation response index in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Cancer Manag Res. 2020;12:1543. doi: 10.2147/CMAR.S235519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton S, Sandler D, Blair A, et al. Pesticide use and myocardial infarction incidence among farm women in the agricultural health study. J Occup Environ Med. 2010;52(7):693–697. doi: 10.1097/JOM.0b013e3181e66d25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Penta A, Moreno B, Reix S, et al. Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS ONE. 2013;8(2):e54722. doi: 10.1371/journal.pone.0054722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci. 2003;104(1):27–38. doi: 10.1042/CS20020240. [DOI] [PubMed] [Google Scholar]
- Dundar ZD, Ergin M, Koylu R, et al. Neutrophil-lymphocyte ratio in patients with pesticide poisoning. J Emerg Med. 2014;47(3):286–293. doi: 10.1016/j.jemermed.2014.01.034. [DOI] [PubMed] [Google Scholar]
- Elhosary NM, Abdelbar E. RED CELL distribution width, neutrophil lymphocyte and platelet lymphocyte ratios as prognostic markers in acutely pesticides-poisoned patients. EJFSAT. 2018;18(4):29–40. doi: 10.21608/EJFSAT.2018.5005.1022. [DOI] [Google Scholar]
- Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- Everett C, Matheson E. Biomarkers of pesticide exposure and diabetes in the 1999–2004 national health and nutrition examination survey. Environ Int. 2010;36(4):398–401. doi: 10.1016/j.envint.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Fois AG, Paliogiannis P, Scano V, et al. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules. 2020;25(23):5725. doi: 10.3390/molecules25235725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Mashimo M, Moriwaki Y, et al. Expression and function of the cholinergic system in immune cells. Front Immunol. 2017;8:1085. doi: 10.3389/fimmu.2017.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargouri B, Yousif NM, Bouchard M, et al. Inflammatory and cytotoxic effects of bifenthrin in primary microglia and organotypic hippocampal slice cultures. J Neuroinflammation. 2018;15(1):159. doi: 10.1186/s12974-018-1198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Arroyo S, Martínez-Valenzuela C, Carbajal-López Y, et al. Riesgo genotóxico por la exposición ocupacional a plaguicidas en América Latina. Rev Int De Contam Ambient. 2013;29:159–180. [Google Scholar]
- Gong T, Liu L, Jiang W, et al. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- Hamad DA, Aly MM, Abdelhameid MA, et al. Combined blood indexes of systemic inflammation as a mirror to admission to intensive care unit in COVID-19 patients: a multicentric study. J Epidemiol Glob Health. 2022;12(1):64–73. doi: 10.1007/s44197-021-00021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Moreno JF, Medina-Díaz IM, Bernal-Hernández YY, et al. Organophosphorus pesticide exposure biomarkers in a Mexican population. Environ Sci Pollut Res. 2021;28(36):50825–50834. doi: 10.1007/s11356-021-14270-1. [DOI] [PubMed] [Google Scholar]
- Hirschfield GM, Heathcote EJ, Gershwin ME. Reviews in basic and clinical gastroenterology and hepatology. Gastroenterology. 2010;139:1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Hu B, Yang X-R, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- Iqbal AJ, Fisher EA, Greaves DR. Chapter 18 inflammation—a critical appreciation of the role of myeloid cells. In: Gordon S, editor. Myeloid Cells in Health and Disease: A Synthesis. Washington: ASM Press; 2017. pp. 325–342. [Google Scholar]
- Jabłońska-Trypuć A. Pesticides as inducers of oxidative stress. React Oxyg Species. 2017;3(8):96–110. [Google Scholar]
- Jeong JH, Sun KH. Role of neutrophil/lymphocyte ratio as a predictor of mortality in organophosphate poisoning. J Korea Acad-Industr Coop Soc. 2020;21(5):384–390. doi: 10.5762/KAIS.2020.21.5.384. [DOI] [Google Scholar]
- Kanbay M, Solak Y, Unal HU, et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol. 2014;46(8):1619–1625. doi: 10.1007/s11255-014-0730-1. [DOI] [PubMed] [Google Scholar]
- Kara SP, Altunan B, Unal A. Investigation of the peripheral inflammation (neutrophil–lymphocyte ratio) in two neurodegenerative diseases of the central nervous system. Neurol Sci. 2022;43(3):1799–1807. doi: 10.1007/s10072-021-05507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML, Barlow RL, Bloomquist JR. Neurotoxicity of the organochlorine insecticide heptachlor to murine striatal dopaminergic pathways. Toxicol Sci. 2001;61(1):100–106. doi: 10.1093/toxsci/61.1.100. [DOI] [PubMed] [Google Scholar]
- Koureas M, Rachiotis G, Tsakalof A, et al. Increased frequency of rheumatoid arthritis and allergic rhinitis among pesticide sprayers and associations with pesticide use. Int J Environ Res Public Health. 2017;14(8):865. doi: 10.3390/ijerph14080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziorowski D, Tomasiuk R, Szlufik S, et al. Inflammatory cytokines and NT-proCNP in Parkinson's disease patients. Cytokine. 2012;60(3):762–766. doi: 10.1016/j.cyto.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Kumar J, Lind PM, Salihovic S, et al. Persistent organic pollutants and inflammatory markers in a cross-sectional study of elderly Swedish people: the PIVUS cohort. Environ Health Perspect. 2014;122(9):977–983. doi: 10.1289/ehp.1307613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, McLean AJ, Taylor MC, et al. Pesticides and Parkinson’s disease. Biomed Pharmacother. 1999;53(3):122–130. doi: 10.1016/S0753-3322(99)80077-8. [DOI] [PubMed] [Google Scholar]
- Legler J, Hamers T, van Eck van der Sluijs-van de Bor M, , et al. The OBELIX project: early life exposure to endocrine disruptors and obesity. Am J Clin Nutr. 2011;94(6 Suppl):1933–1938. doi: 10.3945/ajcn.110.001669. [DOI] [PubMed] [Google Scholar]
- Li Y, Lein PJ, Liu C, et al. Spatiotemporal pattern of neuronal injury induced by DFP in rats: a model for delayed neuronal cell death following acute OP intoxication. Toxicol Appl Pharmacol. 2011;253(3):261–269. doi: 10.1016/j.taap.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Tian W, Zhao F, et al. Systemic immune-inflammation index, SII, for prognosis of elderly patients with newly diagnosed tumors. Oncotarget. 2018;9(82):35293. doi: 10.18632/oncotarget.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Huang J, Pan W et al (2020) Systemic immune-inflammatory index predicts prognosis of patients with COVID-19: a retrospective study. Research Square 1–30. 10.21203/rs.3.rs-30701/v1
- Lionte C, Bologa C, Sorodoc V, et al. Biomarkers of inflammation and inflammation-related indexes upon emergency department admission are predictive for the risk of intensive care unit hospitalization and mortality in acute poisoning: a 6-year prospective observational study. Dis Markers. 2021;2021:4696156. doi: 10.1155/2021/4696156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wu W. Cholinergic modulation of the immune system – a novel therapeutic target for myocardial inflammation. Int Immunopharmacol. 2021;93:107391. doi: 10.1016/j.intimp.2021.107391. [DOI] [PubMed] [Google Scholar]
- Macfarlane E, Carey R, Keegel T, et al. Dermal exposure associated with occupational end use of pesticides and the role of protective measures. Saf Health Work. 2013;4(3):136–141. doi: 10.1016/j.shaw.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani FZ, Hafida M, Merzouk SA, et al. Hemostatic, inflammatory, and oxidative markers in pesticide user farmers. Biomarkers. 2016;21(2):138–145. doi: 10.3109/1354750X.2015.1118545. [DOI] [PubMed] [Google Scholar]
- Meng X, Chang Q, Liu Y, et al. Determinant roles of gender and age on SII, PLR, NLR, LMR and MLR and their reference intervals defining in Henan, China: a posteriori and big-data-based. J Clin Lab Anal. 2018;32(2):e22228. doi: 10.1002/jcla.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Hiyoshi Y, Daitoku N, et al. Naples prognostic score is a useful prognostic marker in patients with metastatic colorectal cancer. Dis Colon Rectum. 2019;62(12):1485–1493. doi: 10.1097/dcr.0000000000001484. [DOI] [PubMed] [Google Scholar]
- Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013;268(2):157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Mu Y, Hu B, Gao N, et al. Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning. Open Life Sci. 2021;16(1):703–710. doi: 10.1515/biol-2021-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejatifar F, Abdollahi M, Attarchi M, et al. Evaluation of hematological indices among insecticides factory workers. Heliyon. 2022;8(3):e09040. doi: 10.1016/j.heliyon.2022.e09040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-López D, Acosta-Mérida MA, Casimiro-Pérez JA et al (2022) Valor del Ratio Neutrófilo-Linfocito, Ratio Plaqueta-Linfocito y Proteína C Reactiva del primer día como predictores de complicaciones postoperatorias tras cirugía oncológica gástrica. Revista de Gastroenterología de México 87(2):142–148. 10.1016/j.rgmx.2020.10.003 [DOI] [PubMed]
- Petrescu AD, Grant S, Frampton G, et al. Gulf war illness-related chemicals increase CD11b/c+ monocyte infiltration into the liver and aggravate hepatic cholestasis in a rodent model. Sci Rep. 2018;8(1):13147. doi: 10.1038/s41598-018-31599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli C, Cremonese C, Koifman R, et al. Occupational exposure to pesticides and hematological alterations: a survey of farm residents in the South of Brazil. Cien Saude Colet. 2019;24(6):2325–2340. doi: 10.1590/1413-81232018246.13142017. [DOI] [PubMed] [Google Scholar]
- Spangenberg EE, Green KN. Inflammation in Alzheimer’s disease: lessons learned from microglia-depletion models. Brain Behav Immun. 2017;61:1–11. doi: 10.1016/j.bbi.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sule RO, Condon L, Gomes AV. A common feature of pesticides: oxidative stress—the role of oxidative stress in pesticide-induced toxicity. Oxid Med Cell Longev. 2022;2022:1–31. doi: 10.1155/2022/5563759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilas-Boas V, Gijbels E, Cooreman A, et al. Industrial, biocide, and cosmetic chemical inducers of cholestasis. Chem Res Toxicol. 2019;32(7):1327–1334. doi: 10.1021/acs.chemrestox.9b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WJ, Cao ZX, Feng SY, et al. Platelet-lymphocyte ratio is not a prognostic predictor for acute paraquat-intoxicated patients: a retrospective analysis. Medicine. 2019;98(20):e15702 . doi: 10.1097/MD.0000000000015702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. The WHO recommended classification of pesticides by hazard and guidelines to classification, (2019) edition Available online: https://www.who.int/publications/i/item/9789240005662 (accessed on 30 March 2022).
- WHO. Obesity and overweight (2021) Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 8 August 2022).
- WHO. Body mass index (BMI) (2022) available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index (accessed on 30 March 2022).
- Wong HL, Garthwaite DG, Ramwell CT, et al. How does exposure to pesticides vary in space and time for residents living near to treated orchards? Environ Sci Pollut Res Int. 2017;24(34):26444–26461. doi: 10.1007/s11356-017-0064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LS, Yen YT, Lee CH. The implications of Pruritogens in the pathogenesis of atopic dermatitis. Int J Mol Sci. 2021;22(13):7227. doi: 10.3390/ijms22137227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepeda-Arce R, Rojas-García AE, Benitez-Trinidad A, et al. Oxidative stress and genetic damage among workers exposed primarily to organophosphate and pyrethroid pesticides. Environ Toxicol. 2017;32(6):1754–1764. doi: 10.1002/tox.22398. [DOI] [PubMed] [Google Scholar]
- Zhou DC, Zhang H, Luo ZM, et al. Prognostic value of hematological parameters in patients with paraquat poisoning. Sci Rep. 2016;6(1):1–9. doi: 10.1038/srep36235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinellu A, Collu C, Nasser M, et al. The aggregate index of systemic inflammation (AISI): a novel prognostic biomarker in idiopathic pulmonary fibrosis. J Clin Med. 2021;10(18):4134. doi: 10.3390/jcm10184134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.