Despite the advancement of treatments, adults with relapsed/refractory (R/R) B-lineage acute lymphoblastic leukemia (B-ALL) have poor prognosis, with an expected five-year overall survival (OS) rate of 10%‒20% (Nguyen et al., 2008; Oriol et al., 2010). Extramedullary relapse of B-ALL is regarded as a high-risk factor generally associated with poor survival, occurring in about 15% to 20% of all relapsed patients (Ding et al., 2017; Sun et al., 2018). The central nervous system (CNS) and the testes are the most common sites of extramedullary relapse of B-ALL. In addition, extramedullary leukemia can appear in the skin, eyes, breasts, bones, muscles, and abdominal organs. The prognosis of relapsed extramedullary B-ALL after allogeneic hematopoietic stem cell transplantation (allo-HSCT) is extremely poor (Spyridonidis et al., 2012; Dahlberg et al., 2019). Conventional chemotherapy or radiation is often ineffective in such patients. At present, there are no optimal treatment strategies for treating extramedullary leukemia after allo-HSCT.
In recent years, chimeric antigen receptor-T (CAR-T) targeting cluster of differentiation 19 (CD19) has shown remarkable therapeutic efficacy for R/R B-cell malignancies (Huang et al., 2020), with a minimal residual disease (MRD)-negative complete remission (CR) of 60%‒90% (Maude et al., 2014, 2018; Lee et al., 2015; Park et al., 2018). Yet 30%‒50% of patients failed to exhibit durable remission and relapsed after CAR-T therapy (Maude et al., 2018). The efficacy of second CAR-T immunotherapy against the same target is often limited. For relapsed patients after HSCT, donor-derived CAR-T cell therapy has been considered as a possible approach to reinduce remission. However, experience using second CAR-T cells derived from donors against extramedullary leukemia has been limited. This is partly due to the exclusion of such patients from pivotal clinical studies. Herein, we report the application of second donor-derived CD19 CAR-T immunotherapy to treat an older patient with multi-site extramedullary relapses after the first auto-CAR-T therapy, followed by haploidentical HSCT (haplo-HSCT).
A 62-year-old woman was referred to the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China, due to fever and abdominal discomfort for one week on July 21, 2020. She had a history of hepatitis B. Bone marrow (BM) examination indicated an increase in blasts up to 88%. The detection of MRD by flow cytometry revealed that 82.69% of blasts were positive for CD19, CD22, CD200, cytoplasmic CD79a (cyCD79a), nuclear terminal deoxyribonucleotidyl transferase (nTdT), and CD10. The karyotype analysis yielded 46, XX, t(1, 19). A positive result for E2A-PBX1 fusion gene was identified by reverse transcription-polymerase chain reaction (RT-PCR). Next-generation sequence analysis of BM revealed SET domain-containing 2 (SETD2), suppressor of Zeste 12 (SUZ12), and Runt-related transcription factor 1 (RUNX1) mutations. Based on these findings, the patient was diagnosed with B-ALL. Induction chemotherapy with venetoclax and prednisone was initiated but this failed to achieve any response. The patient then received reinduction therapy of hyper-CVAD-A chemotherapy regimen consisting of cyclophosphamide, vindesine, epirubicin, and dexamethasone. The morphology of BM after the chemotherapy indicated CR with an MRD value of 0.011%. Following the second course of hyper-CVAD-A, repeated BM assessment demonstrated ALL relapse with 16% blast cells and MRD of 2.38%.
Considering the poor response to conventional chemotherapy, the patient was enrolled in CD19 CAR-T trial at our center. She was infused with autologous CAR-T targeting CD19 at a dose of 2.99×106 kg-1 (transduction efficiency, 51.5%) following a FC regimen (fludarabine 30 mg/m2 from Days -4 to -2, cyclophosphamide 500 mg/m2 from Days -3 to -2). On Day +7 post-CAR-T cells, the patient developed grade 2 cytokine release syndrome (CRS), which was treated with single tocilizumab (400 mg). On Day +30, the patient achieved CR with negative MRD. Three months later, she underwent human leukocyte antigen (HLA)-haploidentical HSCT with peripheral blood stem cells taken from her daughter after reduced-intensity conditioning (RIC) with fludarabin/cyclophosphamide/anti-thymocyte globulin (Flu/Cy/ATG) in February 2021. The counts of mononuclear cells (MNCs) and CD34+ were 12.25×108 kg-1 and 14.98×106 kg-1, respectively. Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporin A, methotrexate, and low-dose mycophenolate mofetil. Neutrophil and platelet engraftments were observed on Days +18 and +20. One month after HSCT, complete donor chimerism (99.9%) was achieved, and the BM assessment indicated CR.
In April 2021, two months after allo-HSCT, the patient presented progressive blurred vision and pain in the left eye for weeks. The intraocular pressure of the left eye was 35 mmHg (1 mmHg=133.322 Pa). The clinical examination result of the right eye was normal. The BM evaluation showed no evidence of leukemia with MRD negativity. Subsequently, left eye enucleation was performed at our hospital, and the postoperative pathological result demonstrated extramedullary B-ALL.
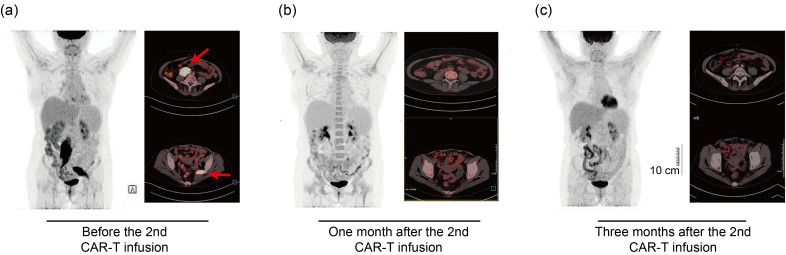
At six months after HSCT, the patient was admitted to the gastroenterology department of our hospital for a gradually increased level of carcino-embryonic antigen (CEA; peak of 18.1 ng/mL within one month). She complained of muscle pain in the left leg. The histopathological and immunohistochemical examination of biopsies sampled during gastroscopy revealed the recurrence of extramedullary B-ALL with positive staining for TdT, B cell-specific activation protein (BSAP or PAX-5), CD19, and CD22. The positron emission tomography/computed tomography (PET/CT) scanning detected irregular soft tissue density masses in the right retroperitoneal cavities (size of 3.5 cm×4.7 cm×12.6 cm) and left pelvic wall (size of 2.5 cm×4.3 cm×8.8 cm), with increased 18F-fluorodeoxyglucose (FDG) metabolism (Fig. 1a). No leukemic cells were detectable in the BM with MRD negativity. The lumbar puncture was also negative. These findings confirmed isolated extramedullary ALL relapses after allo-HSCT. Due to multiple relapses, chemorefractory, and HSCT, donor-derived CD19 CAR-T therapy was recommended. Before the second CAR-T cell infusion, good functional immune reconstitution post-HSCT was observed in the patient. Meanwhile, she developed skin rash over her entire body, suggestive of chronic GVHD and treatment with prednisone.
Fig. 1. Positron emission tomography/computed tomography (PET/CT) scanning at different time points. (a) PET/CT before the second donor-derived chimeric antigen receptor-T (CAR-T) cell infusion. The red arrows indicate abnormal uptake involving the right retroperitoneal cavities and the left pelvic wall. (b) The PET/CT scans show significantly diminished leukemia lesions one month after the second donor-derived CAR-T cell infusion. (c) The PET/CT scans prove complete remission three months after the second donor-derived CAR-T cell infusion. Scale bar=10 cm.
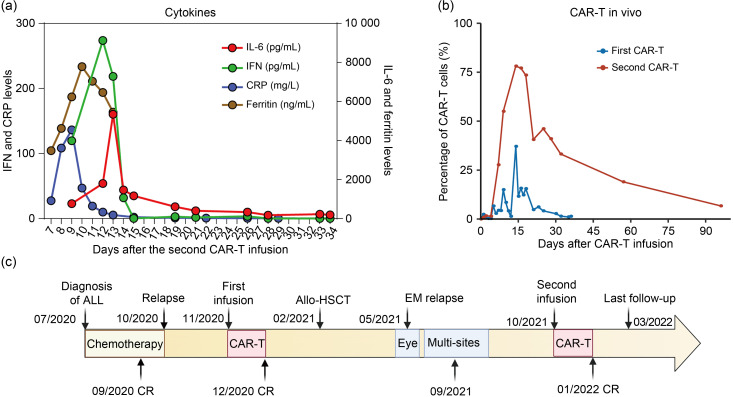
On Sept. 29, 2021, the patient received a lymphodepletion regimen with fludarabine and cyclophosphamide, followed by the second infusion of donor-derived CD19 CAR-T with a total dose of 2.01×106 kg-1 (transduction efficiency, 56.2%). On Day +1 after the infusion, she presented persistent fever with a maximum temperature of 39 ℃, accompanied by the increased level of interleukin-6 (IL-6), IL-10, and interferon-γ (IFN-γ), suggestive of grade 2 CRS (Fig. 2a). Single tocilizumab (480 mg) was administrated on Day +8 and the body temperature returned normal on Day +12. The allogeneic CAR-T cells reached the highest level in the peripheral blood on Day +14 and then declined gradually (Fig. 2b). One month after the second infusion, the patient was re-examined and the morphology of BM showed CR with negative MRD. PET/CT performed one month after the second CAR-T infusion identified lesion reduction in the right retroperitoneal cavities and a regression of lesions in the left pelvic wall (Fig. 1b). BM analysis also suggested CR. The third follow-up PET/CT was conducted three months after the second CAR-T infusion, and it indicated the full resolution of all extramedullary leukemia lesions (Fig. 1c). Additionally, 6.7% of CAR-T cells were still detected in the peripheral blood. However, the level of platelets remained consistently low after the second CAR-T therapy, and she was treated with eltrombopag of 50 mg once daily. At the last follow-up of Mar. 6, 2022, the patient was in good clinical condition without evidence of disease. No signs of acute GVHD (aGVHD) were observed during the entire treatment. The latest blood routine examination showed a white blood cell count of 3.3×109 L-1, a hemoglobin level of 130 g/L, and a platelet count of 6.5×1010 L-1. The therapeutic process is presented by Fig. 2c.
Fig. 2. Clinical data of the patient. (a) Levels of cytokine and ferritin during the second CAR-T infusion. (b) CAR-T percentages of the first and second CAR-T therapies assessed by flow cytometry. (c) Therapeutic process. CAR-T: chimeric antigen receptor-T; IL-6: interleukin-6; IFN: interferon; CRP: C-reactive protein; ALL: acute lymphoblastic leukemia; Allo-HSCT: allogeneic hematopoietic cell transplantation; CR: complete remission; EM: extramedullary.
Despite the era of new drugs, extramedullary relapse of ALL after HSCT remains intractable. Extramedullary tissue presents a haven for tumor cells’ escape from the immune system, and conventional chemotherapy treatments have weak effects. Meanwhile, the role of CAR-T therapy in R/R extramedullary disease remains to be determined. Talekar et al. (2017) reported that 6 in 10 patients with extramedullary relapse of ALL after HSCT achieved durable remission after CAR-T therapy. Another study of 55 pediatric R/R B-ALL patients with extramedullary involvement (both CNS and non-CNS) observed an 87.5% (35/40) CR rate in patients with CNS and 66.7% (10/15) with non-CNS after CAR-T treatment (Fabrizio et al., 2022). These results suggested that CAR-T therapy is a reasonable option to eradicate extramedullary lesions in B-ALL.
Compared to the first CAR-T therapy, the efficacy of the second CAR-T infusion against the same target is usually unsatisfactory. In a retrospective analysis of 44 B-ALL patients relapsed or refractory to the first CAR-T infusion, Gauthier et al. (2021) reported low CR rates of 22% in chronic lymphocytic leukemia (CLL), 19% in non-Hodgkin lymphoma (NHL), and 21% in ALL patients after the second autologous CAR-T infusion, even for relatively high infused dose. The failure mechanisms of second CAR T-cell therapy are not clearly understood, and the possible relevant factors include poor T function, an immunosuppressive tumor microenvironment, the loss of expression of the targeted antigen, and anti-CAR immune responses primed in reaction to the first CAR-T (Turtle et al., 2016; Gauthier et al., 2021). Compared with autologous CAR-T therapy, donor-derived CAR-T as the second infusion seems to be a better strategy to improve the outcome of patients relapsed after allo-HSCT. Therapy with donor-derived CAR-T cells is free from the influence of patient’s T cell dysfunction and such cells are easier to harvest. Some clinical trials have reported successful treatments with allogeneic CAR-T cells for patients relapsed after allo-HSCT (Cruz et al., 2013; Kochenderfer et al., 2013; Maude et al., 2014; Brudno et al., 2016). The safety of allogeneic CAR-T cells, particularly that regarding severe GVHD and CRS, is a major concern. Thus far, no cases of severe aGVHD following donor-derived CAR-T cells treatment have been reported among patients relapsed after allo-HSCT, and only few patients developed mild chronic GVHD. These patients also exhibited good tolerance to donor-derived CAR-T cells with mild-to-moderate CRS (Cruz et al., 2013; Kochenderfer et al., 2013; Maude et al., 2014; Brudno et al., 2016). Altogether, donor-derived CAR-T therapy can exert graft-versus-leukemic effects mediated by alloreactive effector T cells but is incapable of causing severe GVHD.
The case of this study demonstrated a good efficacy of allogenic CAR-T for treating extramedullary lesions. Obviously, during the first infusion, autologous CAR-T cells did not expand well in vivo and were not detectable at Day +40 after infusion. Thus, although the patient subsequently bridged to allo-HSCT, multiple-site EM leukemia emerged rapidly. Conversely, during the second infusion, we observed that the second donor-derived CAR-T cells had significantly higher peak expansion up to 78.1%, compared with the first CAR-T therapy at 37.1%. The allogeneic CAR-T cells could still be detected until +3 months after infusion. On the other hand, in the first and second CAR-T therapies, the patient only presented mild CRS and the related symptoms were controlled after the administration of tocilizumab. No signs of GVHD after all-HSCT were observed. Consequently, the expansion and persistence of allogenic CAR-T cells would not be affected by the first CAR-T infusion, showing no increased risk of potential complications.
In conclusion, although the follow-up period was relatively short, our case report indicated that the second donor-derived CAR-T can be feasible and safe in patients relapsed after the first CAR-T therapy and those with extramedullary involvement. The second donor-derived CAR-T may serve as a bridge to transplantation or other immunotherapies, such as sequential CD22 CAR-T therapy, which further consolidates its efficacy and prevents leukemia relapse. Long-term follow-up and prospective clinical studies are required to fully elucidate the role of second donor-derived CAR-T in the treatment of extramedullary relapse.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81730008, 82130003, and 81870153).
Author contributions
Delin KONG contributed to reviewing the patient and writing the manuscript. Tingting YANG wrote part of the manuscript and reviewed the literature. Jia GENG supported the imaging materials of the patient and provided her comments on this manuscript. Ruirui JING, Qiqi ZHANG, and Guoqing WEI revised, edited, and checked the final version. He HUANG and Yongxian HU were mainly in charge of the patient, and critically reviewed the patient and the manuscript. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Delin KONG, Tingting YANG, Jia GENG, Ruirui JING, Qiqi ZHANG, Guoqing Wei, He HUANG, and Yongxian HU declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (the Ethics Review Committee of the First Affiliated Hospital of Zhejiang University (No. NCT04532268)) and with the Helsinki Declaration of 1975, as revised in 2008(5). Informed consent was obtained from the patient for being included in the report.
References
- Brudno JN, Somerville RPT, Shi V, et al. , 2016. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol, 34(10): 1112-1121. 10.1200/JCO.2015.64.5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz CRY, Micklethwaite KP, Savoldo B, et al. , 2013. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood, 122(17): 2965-2973. 10.1182/blood-2013-06-506741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A, Leisenring W, Bleakley M, et al. , 2019. Prognosis of relapse after hematopoietic cell transplant (HCT) for treatment of leukemia or myelodysplastic syndrome (MDS) in children. Bone Marrow Transplant, 54(8): 1337-1345. 10.1038/s41409-019-0438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding LW, Sun QY, Mayakonda A, et al. , 2017. Mutational profiling of acute lymphoblastic leukemia with testicular relapse. J Hematol Oncol, 10: 65. 10.1186/s13045-017-0434-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio VA, Phillips CL, Lane A, et al. , 2022. Tisagenlecleucel outcomes in relapsed/refractory extramedullary ALL: a pediatric real world CAR consortium report. Blood Adv, 6(2): 600-610. 10.1182/bloodadvances.2021005564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier J, Bezerra ED, Hirayama AV, et al. , 2021. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood, 137(3): 323-335. 10.1182/blood.2020006770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Wu HW, Hu YX, 2020. Current advances in chimeric antigen receptor T-cell therapy for refractory/relapsed multiple myeloma. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 21(1): 29-41. 10.1631/jzus.B1900351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Carpenter RO, et al. , 2013. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood, 122(25): 4129-4139. 10.1182/blood-2013-08-519413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. , 2015. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet, 385(9967): 517-528. 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, et al. , 2014. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med, 371(16): 1507-1517. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Laetsch TW, Buechner J, et al. , 2018. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med, 378(5): 439-448. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K, Devidas M, Cheng SC, et al. , 2008. Factors influencing survival after relapse from acute lymphoblastic leukemia: a children’s oncology group study. Leukemia, 22(12): 2142-2150. 10.1038/leu.2008.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriol A, Vives S, Hernández-Rivas JM, et al. , 2010. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA study group. Haematologica, 95(4): 589-596. 10.3324/haematol.2009.014274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Riviere I, Gonen M, et al. , 2018. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med, 378(5): 449-459. 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyridonidis A, Labopin M, Schmid C, et al. , 2012. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the acute leukemia working party of EBMT. Leukemia, 26(6): 1211-1217. 10.1038/leu.2011.351 [DOI] [PubMed] [Google Scholar]
- Sun WL, Malvar J, Sposto R, et al. , 2018. Outcome of children with multiply relapsed B-cell acute lymphoblastic leukemia: a therapeutic advances in childhood leukemia & lymphoma study. Leukemia, 32(11): 2316-2325. 10.1038/s41375-018-0094-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talekar MK, Maude SL, Hucks GE, et al. , 2017. Effect of chimeric antigen receptor-modified T (CAR-T) cells on responses in children with non-CNS extramedullary relapse of CD19+ acute lymphoblastic leukemia (ALL). J Clin Oncol, 35(15 Suppl): 10507-10507. 10.1200/JCO.2017.35.15_suppl.10507 [DOI] [Google Scholar]
- Turtle CJ, Hanafi LA, Berger C, et al. , 2016. CD19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest, 126(6): 2123-2138. 10.1172/JCI85309 [DOI] [PMC free article] [PubMed] [Google Scholar]