Abstract
The effects of three resuscitation fluids, hydroxyethyl starch (HES), Haemaccel, and fresh autologous blood, on reticuloendothelial system phagocytic and catabolic functions and resistance to infection after 40% hemorrhages in BALB/c mice were studied. The mice, anesthetized with isoflurane, were bled over a 10-min period, left hypovolemic for 30 min, and then resuscitated with their shed blood or the same volume of asanguineous fluid. Normothermia was maintained throughout the experiments. The uptake and catabolism of intravenously injected double-labelled sheep erythrocytes (51Cr-125I-SRBC) in liver and spleen were determined at 1 and 48 h after hemorrhage. No significant changes in the uptake or catabolism of SRBC in liver or spleen were found at 1 h after hemorrhage and resuscitation with any of the fluids. However, at 48 h a significant increase in liver uptake of SRBC was seen in animals resuscitated with either Haemaccel or HES compared to that in animals resuscitated with shed blood or in animals subjected to a sham operation. The increase in liver uptake was accompanied by a small decrease in spleen uptake in animals resuscitated with Haemaccel but not with HES. No great changes in catabolic activity were seen at 48 h, although activity levels tended to be higher in animals resuscitated with Haemaccel. Separate groups of animals were challenged by an intraperitoneal injection with live Escherichia coli at 1 or 48 h after hemorrhage and resuscitation. Sixty-four percent of the animals resuscitated with shed blood survived the challenge with E. coli at 1 h after hemorrhage, whereas only 10 and 0% survival was seen for animals resuscitated with Haemaccel and HES, respectively. At 48 h survival was 80% for shed-blood-resuscitated animals and 60 and 70% for Haemaccel- and HES-resuscitated animals, respectively.
Patients sustaining severe traumatic shock (including hypovolemic shock) have an increased susceptibility to infection and sepsis. The basic treatment of hypovolemic shock involves hemostasis and fluid resuscitation, and the use of blood and blood products has played a central role over the last 5 decades for the treatment of the hypovolemic patient. However, the dangers of transfusion-transmitted diseases have recently reduced the use of blood (49). Indeed, the hematocrit values used as an indication for the administration of blood have been progressively reduced (11, 13), and the use of artificial fluids, such as colloids, has increased.
There are concerns that colloids might have deleterious effects on the reticuloendothelial system (RES). For example hydroxyethyl starch (HES) accumulates and persists in macrophages (51). Haemaccel has been reported to interact with fibronectin (10), a nonspecific opsonin which contributes to the phagocytosis of foreign antigens by macrophages (36). Such interactions between resuscitation fluids and the RES may well be detrimental to the injured patient, particularly as the RES is thought to play an important role in resistance to infection and sepsis (36).
In the present study the RES liver and spleen phagocytic and catabolic activities have been studied at 1 and 48 h after hemorrhage and resuscitation with either shed blood, HES, or Haemaccel. At the same times separate groups of animals were challenged with Escherichia coli, and their 96-h survival rates were recorded. These experiments tested the hypothesis that resuscitation with colloids modifies the macrophage function after hemorrhage and/or alters the susceptibility to infection.
MATERIALS AND METHODS
These studies were conducted in accordance with the Animals (Scientific Procedures) Act of 1986 which encompasses the National Institutes of Health guidelines for the care and use of experimental animals.
The asanguineous fluids used in these studies were HES (Laevosan; Laevosan-Gesellschaft MVH, Linz/Bonau, Austria), a 6% colloid solution in 0.9% saline with an weight average molecular mass of 250 kDa, and Haemaccel (Hoechst UK Ltd., Hounslow, United Kingdom), a 3.5% colloid solution with a calcium content of 6.26 mmol/liter and a potassium content of 5.1 mmol/liter. Haemaccel has a weight average molecular mass of 35 kDa. The solutions supplied were sterile and nonpyrogenic.
Preparation of 125I-51Cr-SRBC.
Sheep erythrocytes (SRBC) (TCS Microbiology, Buckingham, United Kingdom) were labelled with 125I by using the Bolton-Hunter reagent (Amersham; 5 mCi/ml in benzene containing 0.2% dimethylformadide) and with 51Cr (Amersham) by using a modified method described by Dzhandzhugazyan and Jorgensen (16). Briefly, 5 μl of 125I-Bolton Hunter reagent was transferred into a test tube and the solvent was evaporated under a stream of nitrogen. One milliliter of packed SRBC, washed previously in 0.1 M phosphate buffer (pH 9.0), was added to the test tube and the mixture was incubated for 15 min on ice with occasional shaking. The reaction was stopped by adding 0.2 ml of 0.2 M glycine in 0.1 M phosphate buffer. Then, 10 ml of phosphate buffer (diluted 1:1 with saline) was added, and the suspension was centrifuged for 10 min at 1,000 × g. The supernatant was removed, and the cells were washed twice in saline. The suspension was stored overnight in a refrigerator at 4°C. The following day the supernatant was removed and the cells were resuspended in saline and labelled with 51Cr according to the method described by Markus et al. (31). Both 125I and 51Cr remained associated with the SRBC when the mixture was incubated for 4 h in human plasma at 37°C. A 25% suspension in saline was used for injection.
Hemorrhage model.
Male BALB/c mice (23 to 27 g) were anesthetized with isoflurane (2.5%) in O2. A thermocouple was inserted 3 to 4 cm past the anus to measure core temperature, which was maintained at 36 to 38°C by external heating lamps. The left femoral artery was exposed through a small skin incision, dissected free of neighboring veins and nerves, and cannulated with PE10 tubing (Clay Adams, Becton Dickinson, Parsippany, N.J.). After surgery the concentration of isoflurane was reduced to 1.5 to 1.75%. Two units of heparin in 0.1 ml of saline was injected via the cannula. The animals were bled through the cannula at a rate of 0.1 ml min−1 until 40% of the animals’ blood volume (measured in a preliminary study as 9.3 ± 0.2 ml/100 g of body weight) had been removed (total bleeding time approximately 10 min). After a 30-min “shock” period the shed blood or an equal volume of HES or Haemaccel was infused at a rate of approximately 0.67 ml min−1. After resuscitation, the cannula was removed, the artery was ligated, and the groin incision was closed. Animals were allowed to recover and were housed individually. They were kept on a cycle of 12 h of light and 12 h of darkness at a constant temperature (21°C) with free access to food and water.
An additional group of animals underwent surgery but were not bled or resuscitated (sham-operation group).
Measurement of phagocytic and catabolic RES function.
Phagocytic uptake and catabolic activity can be determined with doubly labelled SRBC prepared as described above. The radioactivity associated with 51Cr remains largely within the reticuloendothelial cells once it has been phagocytosed, whereas that associated with 125I decreases much more rapidly, indicating catabolism of the injected particles rather than deiodination (6, 9, 25, 46).
At 1 h after the end of the hemorrhage (approximately 15 min after the end of resuscitation) animals were injected with 100 μl of 125I-51Cr-SRBC via the femoral cannula, which was then flushed with a small volume of saline. Preliminary experiments based on the studies of Schildt (43) showed that this dose exceeded the “critical dose” below which liver blood flow is the limiting factor in the clearance of SRBC. After cannula removal and closure of the incision, the animals were returned to their cages. At either 1 (T1) or 6 h (T2) after 125I-51Cr-SRBC injection, the animals were killed via cervical dislocation; a cardiac blood sample was collected by direct puncture after opening the thorax; and the liver, spleen, and lungs were removed, washed in saline, and weighed. The blood was centrifuged, and the radioactivity of the RBC and plasma, as well as that of the organs was measured by gamma scintillation with windows of 25 to 37 and 240 to 400 keV for 125I and 51Cr, respectively.
Those animals for which RES function was determined 48 h after hemorrhage were reanesthetized with isoflurane, and the femoral artery was recannulated to inject the 125I-51Cr-SRBC.
Preparation of bacterial suspension.
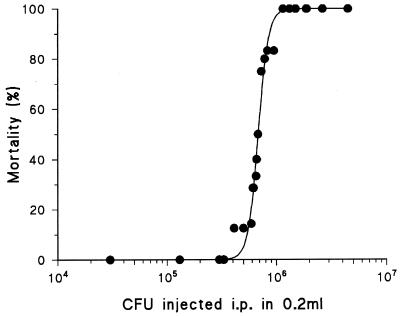
The inoculum for injection into mice was prepared by growing E. coli O18:K1 overnight in Luria broth at 37°C. Log-phase growth of the bacteria was initiated by adding 20 ml of Luria broth to 1 ml of culture, which was grown at 37°C in a shaking incubator for 2 h. The bacterial concentration was determined at 600 nm with a spectrophotometer. Preliminary experiments determined the linear relationship between optical density and bacterial concentration. The cell suspension was centrifuged for 15 min at 10,000 × g, and the supernatant was removed. The cell pellet was kept on ice and just before injection was resuspended in saline and further diluted to the required concentration. A sample aliquot of the actual challenge dose was serial diluted and cultured on Luria broth agar and incubated overnight at 37°C, and CFU were counted. In a preliminary series of experiments the 50% lethal dose (LD50) for hemorrhaged animals resuscitated with shed blood was calculated as 6.8 × 105 CFU (Fig. 1), and this was the “target” dose (range, 6.3 × 105 to 7.4 × 105 CFU) used in the present study. The LD50 was calculated by an adaptation of the method of Reed and Muench (33) whereby individual animals rather than small groups of animals were used at each dose (Table 1).
FIG. 1.
Mortality of hemorrhaged mice resuscitated with shed blood and injected with E. coli O18:K1 intraperitoneally (i.p.). LD50 was calculated by using allosteric Hill kinetics and an adaptation of the method of Reed and Muench (33) and was found to be 6.8 × 105 CFU.
TABLE 1.
Survival after hemorrhage and resuscitation with shed blood followed by intraperitoneal bacterial challenges of different severities
| CFU injected (106) | Outcome | Total no. that:
|
% Mortality | |
|---|---|---|---|---|
| Survived | Died | |||
| 2.63 | Died | 0 | 12 | 100.0 |
| 1.89 | Died | 0 | 11 | 100.0 |
| 1.87 | Died | 0 | 10 | 100.0 |
| 1.50 | Died | 0 | 9 | 100.0 |
| 1.49 | Died | 0 | 8 | 100.0 |
| 1.32 | Died | 0 | 7 | 100.0 |
| 1.15 | Died | 0 | 6 | 100.0 |
| 0.95 | Survived | 1 | 5 | 83.3 |
| 0.83 | Died | 1 | 5 | 83.3 |
| 0.78 | Died | 1 | 4 | 80.0 |
| 0.73 | Died | 1 | 3 | 75.0 |
| 0.68 | Survived | 2 | 2 | 50.0 |
| 0.66 | Survived | 3 | 2 | 40.0 |
| 0.65 | Survived | 4 | 2 | 33.3 |
| 0.62 | Survived | 5 | 2 | 28.6 |
| 0.61 | Died | 5 | 2 | 28.6 |
| 0.59 | Survived | 6 | 1 | 14.3 |
| 0.50 | Survived | 7 | 1 | 12.5 |
| 0.41 | Died | 7 | 1 | 12.5 |
| 0.33 | Survived | 8 | 0 | 0.0 |
| 0.30 | Survived | 9 | 0 | 0.0 |
| 0.13 | Survived | 10 | 0 | 0.0 |
| 0.03 | Survived | 11 | 0 | 0.0 |
Resistance to infection.
Animals received an intraperitoneal injection of 0.2 ml of the bacterial suspension immediately after the hemorrhage-and-resuscitation procedure or 48 h thereafter. Survival was monitored over the next 96 h.
Statistical methods.
Values are given as means ± standard errors of the means (SEM). Data were analyzed by factorial analysis of variance and the Fisher exact test. Those comparisons leading to P values that were <0.05 were interpreted as being significant.
RESULTS
The initial body weights of all the groups were the same, and there were no deaths which could be attributed to the hemorrhage-and-resuscitation procedure.
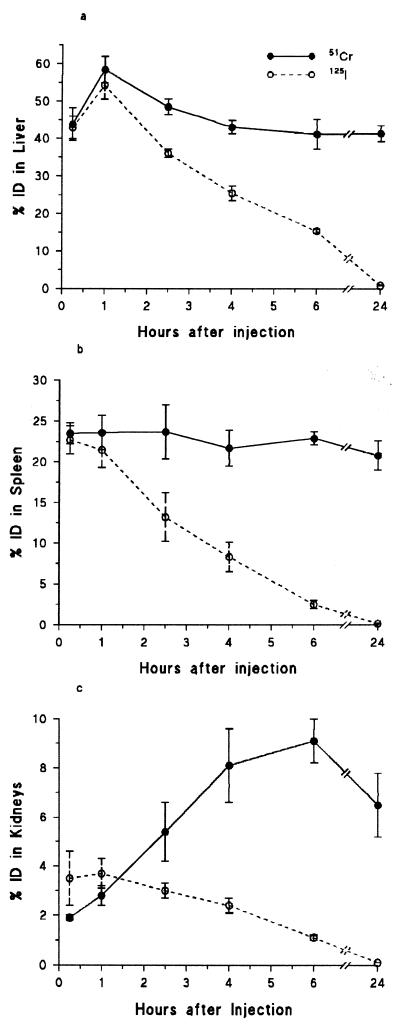
Levels of 51Cr and 125I in the organs of the RES were expressed as percentages of the total amount of 51Cr-125I-SRBC injected per total organ (% ID/TO). Preliminary experiments showed that the phagocytic uptake of the liver reached its maximum at 1 h, whereas spleen uptake was maximal at 15 min (Fig. 2). 51Cr levels remained constant for 24 h in the spleen but decreased in the liver by approximately 25% between 1 and 6 h, although between 6 and 24 h no further decrease was recorded. 125I levels were similar to 51Cr levels in both liver and spleen at 1 h after injection but thereafter decreased rapidly in both organs. At 6 h after injection, 50% of the 125I but only 10% of the 51Cr was found in the urine (data not shown). 51Cr levels in the kidneys increased to almost 9% and were still at 7% 18 h later. 125I levels in the kidneys were maximal at 1 h after injection (3.5%) and decreased thereafter such that at 24 h no 125I was detectable. At 1 and 6 h after injection almost no radioactivity was associated with the intravascular RBC (1 h, 3.4%; 6 h, 0.5%).
FIG. 2.
Changes in radioactivity, expressed as percentages of the total injected dose (ID), in liver (a), spleen (b), and kidney (c) following the intravenous injection of 51Cr-125I-labelled SRBC into control animals. Results are shown as means ± SEM. n = 3 at each time point.
Phagocytic uptake and catabolism of the SRBC did not change significantly over time, either for groups given a sham operation or for groups resuscitated with shed blood (Table 2). Significant increases in phagocytic liver uptake were found at 48 h in the animals resuscitated with either HES or Haemaccel (i.e., 51Cr levels in the liver at 48 h were significantly higher in HES- and Haemaccel-resuscitated animals than in the sham-operation group or the shed-blood-resuscitated group). The increase in phagocytic liver uptake was accompanied by a slight increase in catabolic activity (i.e., the decrease in 125I between T1 and T2 was significantly greater in animals resuscitated with Haemaccel than in sham-operation or shed-blood-resuscitated animals). Spleen uptake was normal in HES-resuscitated animals at 48 h, whereas the 51Cr levels in animals resuscitated with Haemaccel showed a small but significant decrease compared to the levels found when SRBC were injected at 1 h after hemorrhage and resuscitation with Haemaccel.
TABLE 2.
Effects of sham operation and hemorrhage plus resuscitation with either shed blood, HES, or Haemaccel on the uptake and catabolism of 51Cr-125I-SRBC in the liver and spleena
| Group | Mean % ID/TO ± SEM in:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver at:
|
Spleen at:
|
|||||||||||||||
| 1 h
|
48 h
|
1 h
|
48 h
|
|||||||||||||
|
51Cr
|
125I
|
51Cr
|
125I
|
51Cr
|
125I
|
51Cr
|
125I
|
|||||||||
| T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | |
| Sham operation | 57.2 ± 2.5 | 42.7 ± 2.4 | 49.5 ± 0.6 | 18.4 ± 0.8 | 61.7 ± 2.0 | 45.7 ± 3.0 | 57.1 ± 3.6 | 17.5 ± 2.2 | 24.9 ± 1.1 | 20.9 ± 0.9 | 22.6 ± 1.4 | 3.4 ± 0.3 | 20.8 ± 2.6 | 17.9 ± 1.4 | 19.7 ± 1.4 | 3.2 ± 0.4 |
| Shed blood | 62.3 ± 3.3 | 50.6 ± 4.0 | 56.3 ± 2.8 | 19.5 ± 2.0 | 62.3 ± 3.0 | 55.7 ± 4.1 | 54.0 ± 2.7 | 20.0 ± 1.6 | 20.7 ± 2.0 | 20.9 ± 1.1 | 19.9 ± 1.7 | 3.2 ± 0.7 | 19.0 ± 1.5 | 20.0 ± 1.7 | 18.1 ± 0.8 | 3.2 ± 0.5 |
| Haemaccel | 60.2 ± 2.6 | 49.0 ± 3.6 | 54.4 ± 2.3 | 21.2 ± 2.1 | 79.6 ± 1.5 | 70.2 ± 2.1b | 69.2 ± 1.9 | 19.6 ± 2.0d | 23.9 ± 1.6 | 21.8 ± 2.0 | 22.9 ± 2.0 | 3.3 ± 0.2 | 16.5 ± 1.1 | 16.9 ± 0.9c | 15.7 ± 1.7 | 2.2 ± 0.4 |
| HES | 53.0 ± 2.6 | 50.8 ± 2.8 | 49.5 ± 2.2 | 20.9 ± 1.8 | 74.8 ± 1.7 | 64.4 ± 4.4b | 60.5 ± 2.0 | 19.8 ± 1.2 | 24.7 ± 1.1 | 21.1 ± 1.6 | 22.7 ± 0.5 | 3.4 ± 0.7 | 21.4 ± 0.9 | 18.0 ± 1.9 | 18.1 ± 1.0 | 2.1 ± 0.3 |
% ID/TO is as defined in Results; T1 and T2 are as defined in Materials and Methods. n = 4 or 5 for each group.
Significantly greater than the corresponding value at 1 h and also significantly greater than the corresponding sham-operation and shed-blood group values at 48 h.
Significantly smaller than the corresponding value at 1 h and also significantly smaller than the corresponding values for other groups at 48 h.
The difference between this level and the T1 level is significantly greater than the difference between the 1-h T1 and T2 levels and is also significantly greater than the corresponding 48-h differences for the sham-operation and shed-blood groups.
In the mice resuscitated with asanguineous fluids there was a significant increase in spleen weight at 48 h compared to that at 1 h (Haemaccel: 110%; HES: 120%). No significant increase in spleen weight was seen in sham-operation or blood-resuscitated animals. Liver wet weight did not change over time or between groups.
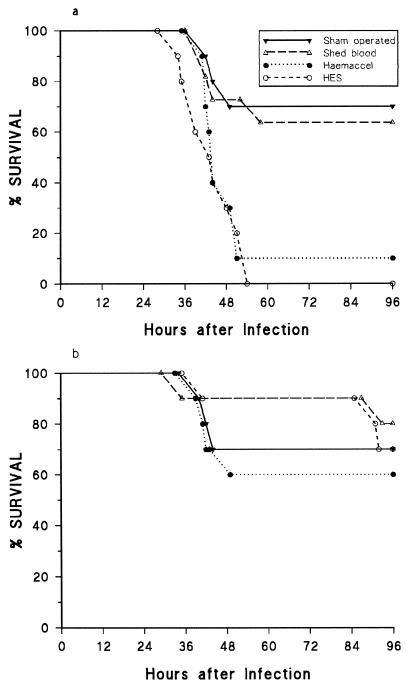
Survival rates after bacterial injection at both 1 and 48 h for the sham-operation and shed-blood-resuscitated groups were similar (64 to 80%) (Fig. 3). However, animals resuscitated with HES and Haemaccel showed significantly lower survival rates (0% and 10%, respectively) when challenged at 1 h after hemorrhage, a difference not seen if the bacterial challenge was delayed until 48 h after hemorrhage and resuscitation (Fig. 3).
FIG. 3.
Survival of mice after an intraperitoneal injection with E. coli O18:K1 at 1 (a) and 48 (b) h after hemorrhage and resuscitation. n = 10 or 11 in each group.
DISCUSSION
The present studies confirm that resuscitation with colloids after hemorrhage can increase the phagocytic activity of the RES (53) and show that these changes are accompanied by an increase in catabolic activity. However, the ability to withstand challenge with E. coli was significantly impaired in animals resuscitated with Haemaccel and HES at 1 h, but not at 48 h, after hemorrhage.
The most frequently used method for determining the in vivo activity of the RES is to monitor the disappearance from the bloodstream of an intravenously injected antigen. Studies by Biozzi et al. (6) showed that bacteria labelled with either 51Cr or 131I are quickly taken up by the cells of the RES; however, it was noted that 51Cr remained within the cells much longer than 131I. It has been shown that once iodinated antigens have been taken up they are broken down into low-molecular-weight metabolites (9), suggesting that the disappearance of radioactive iodine from the RES cells could be used as an index of catabolic activity (6, 25). The explanation for the initial release of 51Cr from the liver is unclear, although it might be related to the binding of up to 20% of the 51Cr to a small protein fraction in the RBC as well as to hemoglobin (1, 47) and selective digestion and excretion by the RES cells. In contrast to the situation in the liver the 51Cr levels in the spleen did not show an early decline, suggesting that the two organs handle injected antigens in different ways.
The RES plays an important role in the development after trauma of bacteraemia, sepsis, and multiple organ failure (36). Thus, it has been shown that depression of RES phagocytic function increases susceptibility to infection (37) whereas RES stimulation increases resistance to infection (54, 62).
The effects of trauma on in vivo RES function have been studied extensively in animal models. For example, it has been shown that insults as diverse as hemorrhage (2, 3, 63), Noble-Collip drum trauma (22), burns (44), limb ischemia (48), and surgery (38) decrease phagocytic clearance rate. Studies by Kaplan and Saba have shown that the phagocytic clearance rate is depressed soon after nonlethal trauma, returning to normal after 6 to 24 h, whereafter stimulated phagocytosis occurs (22, 36). The degree of initial phagocytic depression is dependent upon the severity of the injury (3). However, as trauma in the small laboratory animal is accompanied by an inhibition of thermoregulation and a fall in body temperature (50), it is important to exclude any such effects before attributing a change in RES function to trauma per se. Indeed, our earlier experiments using the model described in the present paper showed that there was no early depression in the phagocytic rate provided that normothermia was maintained (52); thus, in the current experiments care was taken to ensure that all animals were kept normothermic.
Phagocytic uptake and catabolic activity do not necessarily change in parallel. For example, Haglind et al. (20) showed that early after intestinal ischemia-reperfusion injuries in rats, hepatic phagocytic uptake was normal but hepatic catabolic activity was significantly decreased. Kondo et al. (26) showed that hepatic uptake was depressed immediately after hemorrhage followed by resuscitation with shed blood and remained depressed at 6 h but had normalized at 24 h. In contrast, although catabolic activity was also significantly decreased at 6 h, it was markedly stimulated at 24 h. Also, Schildt et al. (42) found that phagocytic activity in patients 2 to 4 days after injury was not significantly different from that in control subjects; however, there was a significant reduction in catabolic activity. In a subsequent study of patients undergoing cardiopulmonary bypass, both RES phagocytic and catabolic activities were slightly, but not significantly, decreased immediately and 6 days after surgery (40). Similarly, a study by Lahnborg et al. (27) showed no significant difference in either phagocytic or catabolic activity in patients before and shortly after surgery. A more recent study by Regel et al. (35) showed that the clearance rate was depressed on the first day after trauma, with a return to normal over the next 4 days. By contrast, a stimulation in clearance rate has been found at 24 to 48 h in patients after surgical trauma (61). Thus, there is no consensus about the effects of injury on either phagocytic or catabolic activity, and the situation is no less confused when the added influence of resuscitation fluids is taken into account.
Most studies on the effects of colloidal resuscitation fluids, such as HES and Haemaccel, on RES function have focused on phagocytic clearance rate in normovolemic animals and cannot, therefore, be directly compared to the present study. Schildt et al. (41) showed that the clearance rate of 51Cr-rabbit RBC was significantly depressed at 3 h after infusion of HES in mice (20 ml/kg of body weight) compared to that of control animals infused with plasma. For animals receiving the same volume of Haemaccel a depression at 1 h after infusion was noted. At 6 h the phagocytic clearance rate had returned to normal in both groups. When HES- or Haemaccel-infused animals were challenged with endotoxin at 1 or 3 h after colloid infusion, mortality was significantly increased compared to that of noninfused animals. Lemperle (29) found that the infusion of 0.5 ml of Haemaccel led to a transient reduction in the clearance rate of carbon particles, with a return to normal at 24 h. White et al. (60) showed that HES infusion (20 to 40 ml/kg) in mice had no effect on the clearance of SRBC over 7 days compared to saline infusion. Infusion of a larger dose of HES (80 ml/kg) depressed the clearance rate and liver uptake at 3 and 6 h after infusion. Recovery occurred at 24 h, and an increased liver uptake was found at 3 days. Oelschlager et al. (32) showed that the phagocytic clearance rate of carbon particles in rats at 2 h after hemorrhage and HES infusion (8 ml/kg) was not significantly different from that after hemorrhage and Ringer’s lactate infusion (16 ml/kg) or no infusion. However, the distribution of particles at 4 h showed lower levels in the livers and spleens of HES-infused animals than in those of animals receiving Ringer’s lactate or no infusion. Shatney and Chaudry (45) showed that neither the intravascular clearance rate nor the organ distribution of a gelatinized 131I-test lipid emulsion was affected at 24 or 48 h after infusion of HES (60 ml/kg) in rats. Similarly HES infusion 2 days prior to a septic challenge (cecal ligation and puncture) did not affect survival. Lawrence and Schell (28) infused HES (16 ml/kg) into mice together with live bacteria; no difference in survival between HES- and saline-treated animals was seen. In a clinical study Lenz et al. (30) infused HES (10 ml/kg) into volunteers and found that the phagocytic clearance rate of a gelatinized lipid emulsion was increased by 30 to 40% immediately after infusion and was still above normal 5 h later.
In a rat model of hemorrhage and resuscitation no significant depression in the phagocytic clearance rate of carbon particles was seen in HES-resuscitated animals compared to those receiving Ringer’s lactate or untreated controls (32). Hemorrhage and resuscitation with either HES or Ringer’s lactate increased mortality after cecal ligation and puncture by similar amounts. Croce et al. (12) used a similar model of hemorrhage and resuscitation and found no significant differences in bacterial numbers in blood, liver, or spleen cultures after an intravenous challenge with live bacteria.
We have recently studied the effects of resuscitation with either shed blood, HES, or Haemaccel on the clearance rate of intravenously injected 51Cr-SRBC in the mouse (53). Provided normothermia was maintained, no early depression was seen with any of the fluids, but an increased clearance rate and liver uptake were noticed in animals resuscitated with Haemaccel at 48 and 72 h after hemorrhage. The increase in liver uptake was accompanied by a reduction in spleen uptake (confirmed in the present study). This is likely to be related to competition for the SRBC between the liver and spleen (57). In the present study we labelled the 51Cr-SRBC with 125I to determine the effects of colloidal resuscitation fluids on catabolic activity as well as on phagocytic uptake. In addition we determined whether these resuscitation fluids affected the resistance to the intraperitoneal injection of live E. coli O18:K1 (18, 54). It could be argued that the E. coli should be given as an intravenous injection to challenge directly the main organs of the RES. However, such a challenge is not closely related to the clinical situation and so, on balance, it was decided that intraperitoneal challenge was more relevant (14, 15).
Hemorrhage plus resuscitation with any of the fluids had no effect on the catabolic activity of either the liver or the spleen at 1 h. Also sham-operation animals and those resuscitated with shed blood showed no change in catabolic activity at 48 h compared to the activity at 1 h. However, the catabolic activity of the liver at 48 h was significantly increased in animals resuscitated with Haemaccel compared to that in sham-operation or shed-blood-resuscitated animals. These findings contradict the fears that HES and Haemaccel compromise the RES system.
At 48 h an approximately 100% increase in spleen wet weight was seen in animals resuscitated with asanguineous fluids, probably reflecting the involvement of the spleen in erythropoiesis (8). Whether the erythropoietic response, regulated by erythropoietin, certain cytokines, and growth factors (19), plays a role in the increased hepatic uptake in this model of hemorrhagic shock remains to be determined.
Although hemorrhage and resuscitation did not impair either the uptake or catabolism of SRBCs, resuscitation with either HES or Haemacel did impair the ability to withstand an intraperitoneal challenge with E. coli at 1 h after hemorrhage. While macrophages of the liver and spleen did not show any depression in phagocytic activity, resuscitation with HES or Haemaccel might have left the peritoneal macrophages in a compromised state allowing the bacteria to spread and overwhelm host defences. It has further been reported that decreased migration by macrophages into the peritoneal cavity in response to inflammatory stimuli could increase susceptibility to infection (58, 59). It is not known whether HES or Haemaccel affects the ability of macrophages to migrate to the site of infection.
It has been shown in animal models, as well as in clinical studies, that infection leads to a stimulation of the RES (4, 5, 7, 56). The RES clearance function has been show to increase after intravenous bolus injection of E. coli endotoxin (34), but it has also been shown that intravenous and intraperitoneal injection of bacteria depresses the RES (23). It is not known whether asanguineous fluid resuscitation leads to a modification of the RES response to a bacterial challenge in our model; however, it is clear both from this and our previous studies (53) that the phagocytic rate and uptake by the main organs of the RES are not affected early after hemorrhage and resuscitation by asanguineous fluids.
Even when animals were resuscitated with HES plus RBC mortality remained high (data not shown), indicating that a low hematocrit was not responsible for the decreased resistance to infection. The dilution of plasma factors by the asanguineous fluids and/or an inadequate response by macrophages and leukocytes, i.e., inadequate production of various cytokines and prostaglandins, to a bacterial challenge after asanguineous resuscitation could also have led to the decreased resistance to infection.
At 48 h after hemorrhage the animals resuscitated with HES or Haemaccel had recovered from their increased susceptibility to infection. This recovery coincided with the increase in liver RES activity. Activation of the macrophage phagocytic activity by lipopolysaccharide or glucan has been shown to be protective in mice against a subsequent challenge with E. coli (55, 62). Previous studies of animals with stimulated RES function have shown that the number of Kupffer cells in the liver is increased due to proliferation and that the size of Kupffer cells is increased (17, 21, 24). A similar mechanism could have led to the increased phagocytic uptake in livers of asanguineously resuscitated animals.
These findings indicate that fluids such as HES and Haemaccel do not depress the phagocytic and catabolic activities of the liver and spleen but that they do affect the host defense mechanism early after trauma, leading to an increased susceptibility to sepsis. The exact mechanism by which HES and Haemaccel exert these deleterious effects requires further investigation.
ACKNOWLEDGMENTS
We are grateful to I. Chaudry for the advice and hospitality extended to one of us (E.v.R.) during the early phase of this study. We thank F. Biet for her expertise and help with the bacterial cultures.
The study was supported by the Ministry of Defence (United Kingdom).
REFERENCES
- 1.Aaseth J, Alexander J, Norseth T. Uptake of 51Cr-chromate by human erythrocytes. A role of glutathione. Acta Pharmacol Toxicol. 1982;50:310–315. doi: 10.1111/j.1600-0773.1982.tb00979.x. [DOI] [PubMed] [Google Scholar]
- 2.Altura B M. Hemorrhagic shock and reticuloendothelial system phagocytic function in pathogen-free animals. Circ Shock. 1974;1:295–300. [Google Scholar]
- 3.Altura B M, Hershey S G. Sequential changes in reticuloendothelial system function after acute hemorrhage. Proc Soc Exp Biol Med. 1972;139:935–939. doi: 10.3181/00379727-139-36270. [DOI] [PubMed] [Google Scholar]
- 4.Biozzi G, Stiffel C. The physiopathology of the reticuloendothelial cells of the liver and spleen. In: Popper H, Schaffner F, editors. Progress in liver disease. New York, N.Y: Grune and Stratton; 1965. pp. 166–187. [Google Scholar]
- 5.Biozzi G, Halpern B N, Benacerraf B, Stiffel C. Phagocytic activity of the reticulo-endothelial system in experimental infections. In: Halpern B N, editor. Physiopathology of the reticuloendothelial system. Oxford, United Kingdom: Blackwell; 1957. pp. 204–225. [Google Scholar]
- 6.Biozzi G, Howard J G, Halpern B N, Stiffel C, Mouton D. The kinetics of blood clearance of isotopically labelled Salmonella enteritidis by the reticuloendothelial system. Immunology. 1960;3:74–89. [PMC free article] [PubMed] [Google Scholar]
- 7.Bird D C, Sheagren J N. Evaluation of reticuloendothelial system phagocytic activity during systemic Candida albicans infection in mice. Proc Soc Exp Biol Med. 1970;133:34–37. doi: 10.3181/00379727-133-34401. [DOI] [PubMed] [Google Scholar]
- 8.Boggs D R, Geist A, Chervenick P A. Contribution of the mouse spleen to post-hemorrhagic erythropoiesis. Life Sci. 1969;8:587–599. doi: 10.1016/0024-3205(69)90020-4. [DOI] [PubMed] [Google Scholar]
- 9.Bouveng R, Schildt B, Sjoqvist J. Estimation of RES phagocytosis and catabolism in man by the use of 125I-labelled microaggregates of human serum albumin. J Reticuloendothel Soc. 1975;18:151–159. [PubMed] [Google Scholar]
- 10.Brodin B, Hesselvik F, Von Schenck H. Decrease of plasma fibronectin concentration following infusion of a gelatin-based plasma substitute in man. Scand J Lab Invest. 1984;44:529–533. doi: 10.1080/00365518409083606. [DOI] [PubMed] [Google Scholar]
- 11.Caroli G C, Borghi B, Pappalardo G, et al. Consensus conference: Risparmiare sangue: quali ancora i dubbi e i problemi. Minerva Anestesiol. 1994;60:285–293. [PubMed] [Google Scholar]
- 12.Croce M A, Fabian T C, Kudsk K A, Trenthem L L, Patterson C R. Delayed immune dysfunction following hemorrhagic shock and resuscitation. Am Surg. 1988;54:731–735. [PubMed] [Google Scholar]
- 13.Crosby E T. Perioperative haemotherapy: I. Indications for blood component transfusion. Can J Anaesth. 1992;39:695–707. doi: 10.1007/BF03008233. [DOI] [PubMed] [Google Scholar]
- 14.Cross A S, Opal S M, Sadoff J C, Gemski P. Choice of bacteria in animal models of sepsis. Infect Immun. 1993;61:2741–2747. doi: 10.1128/iai.61.7.2741-2747.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deitch E A. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Dzhandzhugazyan K N, Jorgensen P L. Asymmetric orientation of amino groups in the alpha-subunit and the beta-subunit of (Na+K)-ATPase in tight right-side-out vesicles of basolateral membranes from outer medulla. Biochim Biophys Acta. 1985;817:165–173. doi: 10.1016/0005-2736(85)90079-3. [DOI] [PubMed] [Google Scholar]
- 17.Filkins J P, Lubitz J M, Smith J J. The effect of zymosan and glucan on the reticuloendothelial system and on resistance to traumatic shock. Angiology. 1964;15:465–470. doi: 10.1177/000331976401501101. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths E, Cortes A, Gilbert N, Stevenson P, Macdonald S, Pepper D. Haemoglobin-based blood substitutes and sepsis. Lancet. 1995;345:158–160. doi: 10.1016/s0140-6736(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 19.Guilbert L J, Branch D R. Monocyte T-cell interaction and its mediators. Regulation of hematopoiesis by growth factors: proliferation of the murine macrophage as a model for stimulatory and inhibitory effects. In: Faist E, Ninneman J, Green D, editors. Immune consequences of trauma, shock and sepsis. Berlin, Germany: Springer-Verlag; 1989. pp. 35–44. [Google Scholar]
- 20.Haglind E, Wang D, Klein A S. Hepatic reticuloendothelial system dysfunction after intestinal ischemia-reperfusion. Shock. 1996;5:72–75. doi: 10.1097/00024382-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Ikejiri N, Tanikawa K. Effects of vitamin A and estrogen on the sinusoidal cells in the rat liver. In: Wisse E, Knook D L, editors. Kupffer cells and other liver sinusoidal cells. Amsterdam, The Netherlands: Elsevier; 1977. pp. 83–92. [Google Scholar]
- 22.Kaplan J E, Saba T M. Humoral deficiency and reticuloendothelial depression after traumatic shock. Am J Physiol. 1976;230:7–14. doi: 10.1152/ajplegacy.1976.230.1.7. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan J E, Scovill W A, Bernard H, Saba T M, Gray V. Reticuloendothelial phagocytic response to bacterial challenge after traumatic shock. Circ Shock. 1977;4:1–12. [PubMed] [Google Scholar]
- 24.Kelly L S, Dobson E L, Finney C R, Hirsch J D. Proliferation of the reticuloendothelial system in the liver. Am J Physiol. 1960;198:1134–1138. doi: 10.1152/ajplegacy.1960.198.5.1134. [DOI] [PubMed] [Google Scholar]
- 25.Klein A, Zhadkewich M, Margolick J, Winkelstein J, Bulkley G. Quantitative discrimination of hepatic reticuloendothelial clearance and phagocytic killing. J Leukocyte Biol. 1994;55:248–252. doi: 10.1002/jlb.55.2.248. [DOI] [PubMed] [Google Scholar]
- 26.Kondo S, Wang D, Mayumi T, Klein A S, Bulkley G B. Effect of hemorrhagic shock and resuscitation upon hepatic phagocytic clearance and killing of circulating microorganisms. Shock. 1996;5:106–111. doi: 10.1097/00024382-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Lahnborg G, Berghem L, Jarstrand C. Effect of dextran infusion on the phagocytic and metabolic functions of the reticuloendothelial system in man. Acta Chir Scand Suppl. 1979;489:271–277. [PubMed] [Google Scholar]
- 28.Lawrence D A, Schell R F. Influence of hydroxyethyl starch on humoral and cell-mediated immune responses in mice. Transfusion. 1985;25:223–229. doi: 10.1046/j.1537-2995.1985.25385219902.x. [DOI] [PubMed] [Google Scholar]
- 29.Lemperle G. Depression and stimulation of host defense mechanisms after severe burns. Plast Reconstr Surg. 1970;45:435–440. doi: 10.1097/00006534-197005000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Lenz G, Hempel V, Junger H, Werle H, Buckenmaier P. Auswirkungen von Hydroxyathylstarke, Oxypolygelatine und Humanalbumin auf die phagozytosefunction des Retikuloendothelialen Systems (RES) gesunder Probanden. Anaesthesist. 1986;35:423–428. [PubMed] [Google Scholar]
- 31.Markus R, Carmel N, Gross J, Stern K. Reticuloendothelial phagocytosis of Cr51-labelled sheep red cells in mice. Isr J Med Sci. 1972;8:1775–1782. [PubMed] [Google Scholar]
- 32.Oelschlager B K, Caragnano C, Carpenter J, Baker C C. Effect of resuscitation with hydroxyethyl starch and lactated Ringers on macrophage activity after hemorrhagic shock and sepsis. Shock. 1994;2:141–144. doi: 10.1097/00024382-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 34.Regel G, Nerlich M L, Dwenger A, Seidel J, Schmidt C, Sturm J A. Phagocytic function of polymorphonuclear leukocytes and the RES in endotoxemia. J Surg Res. 1987;42:74–84. doi: 10.1016/0022-4804(87)90068-0. [DOI] [PubMed] [Google Scholar]
- 35.Regel G, Dwenger A, Gratz K F, Nerlich M L, Sturm J A, Tscherne H. Humorale und zellulare veranderungen der unspezifischen immunabwehr nach schwerem trauma. Unfallchirurg. 1989;92:314–320. [PubMed] [Google Scholar]
- 36.Saba T M. Fibronectin: relevance to phagocytic host response to injury. Circ Shock. 1989;29:257–278. [PubMed] [Google Scholar]
- 37.Saba T M. Reticuloendothelial systemic host defense after surgery and traumatic shock. Circ Shock. 1975;2:91–108. [Google Scholar]
- 38.Saba T M, Di Luzio N R. Surgical stress and reticuloendothelial function. Surgery. 1969;65:803–807. [PubMed] [Google Scholar]
- 39.Salky N K, DiLuzio N R, P’Pool D B, Sutherland A J. Evaluation of RE function in man. JAMA. 1964;187:168–172. doi: 10.1001/jama.1964.03060230072019. [DOI] [PubMed] [Google Scholar]
- 40.Schildt B, Berghem L, Holm G, Jarstrand C, Lahnborg G, Palmblad J, Radegran K. Influence of cardiopulmonary bypass on some host defence functions in man. Scand J Thorac Cardiovasc Surg. 1980;14:207–211. doi: 10.3109/14017438009100999. [DOI] [PubMed] [Google Scholar]
- 41.Schildt B, Bouveng R, Sollenberg M. Plasma substitute induced impairment of the reticuloendothelial system function. Acta Chir Scand. 1975;141:7–13. [PubMed] [Google Scholar]
- 42.Schildt B, Gertz I, Wide L. Differentiated reticuloendothelial system (RES) function in some critical surgical conditions. Acta Chir Scand. 1974;140:611–617. [PubMed] [Google Scholar]
- 43.Schildt B E. Disappearance of i.v. injected 51Cr-RBC from circulation as a possible measure of RES function in mice. Acta Chir Scand. 1970;136:351–357. [PubMed] [Google Scholar]
- 44.Schildt B E. Function of the RES after thermal and mechanical trauma in mice. Acta Chir Scand. 1970;136:359–364. [PubMed] [Google Scholar]
- 45.Shatney C H, Chaudry I H. Hydroxyethyl starch administration does not depress reticuloendothelial function or increase mortality from sepsis. Circ Shock. 1984;13:21–26. [PubMed] [Google Scholar]
- 46.Shih L B, Thorpe S R, Griffiths G L, Diril H, Ong G L, Hansen H J, Goldenberg D M, Mattes M J. The processing and fate of antibodies and their radiolabels bound to the surface of tumor cells in vitro: a comparison of nine radiolabels. J Nucl Med. 1994;35:899–908. [PubMed] [Google Scholar]
- 47.Skrabut E M, Catsimpoolas N, Crowley J P, Valeri C R. (51Cr) sodium chromate incorporation into the soluble protein fraction of the human erythrocyte: binding not associated with the hemoglobin monomeric subunit. Biochem Biophys Res Commun. 1976;69:672–677. doi: 10.1016/0006-291x(76)90928-1. [DOI] [PubMed] [Google Scholar]
- 48.Stoner H B. Studies on the mechanism of shock: the activity of the reticuloendothelial system after limb ischaemia in the rat. Br J Exp Pathol. 1961;42:523–538. [PMC free article] [PubMed] [Google Scholar]
- 49.Surgenor D M, Wallace E L, Halt S G, Gilpatrick M W. Changing patterns of transfusion in four sets of United States hospitals, 1980 to 1985. Transfusion. 1988;28:513–518. doi: 10.1046/j.1537-2995.1988.28689059022.x. [DOI] [PubMed] [Google Scholar]
- 50.Tabor H, Rosenthal S M. Body temperature and oxygen consumption in traumatic shock and hemorrhage in mice. Am J Physiol. 1947;149:449–464. doi: 10.1152/ajplegacy.1947.149.2.449. [DOI] [PubMed] [Google Scholar]
- 51.Thompson W L, Fukushima T, Rutherford R B, Walton R P. Intravascular persistence, tissue storage, and excretion of hydoxyethyl starch. Surg Gynecol Obstet. 1970;131:965–972. [PubMed] [Google Scholar]
- 52.van Rijen E A M, Ward J J, Parry E, Little R A. Reticuloendothelial function after hemorrhage and hypothermia. Shock. 1997;7:300–303. doi: 10.1097/00024382-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 53.van Rijen E A M, Ward J J, Little R A. Phagocytic reticuloendothelial function after hemorrhage and resuscitation: modulation by different fluids. Shock. 1997;8:219–224. doi: 10.1097/00024382-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Vuopio-Varkila J, Makela P H. Killing of Escherichia coli in the peritoneal cavity of convalescent mice; role of specific and non-specific immune mechanisms. J Med Microbiol. 1988;25:205–211. doi: 10.1099/00222615-25-3-205. [DOI] [PubMed] [Google Scholar]
- 55.Vuopio-Varkila J, Nurminen M, Pyhala L, Makela P H. Lipopolysaccharide-induced non-specific resistance to systemic Escherichia coli infection in mice. J Med Microbiol. 1988;25:197–203. doi: 10.1099/00222615-25-3-197. [DOI] [PubMed] [Google Scholar]
- 56.Wagner H, Lio M, Hornick R. Studies of the reticuloendothelial system (RES). II. Changes in the phagocytic capacity of the RES in patients with certain infections. J Clin Invest. 1963;42:427–434. doi: 10.1172/JCI104730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warr G W, Sljivic V S. Studies on the organ uptake of 51Cr-labelled sheep erythrocytes in the evaluation of stimulation of RES phagocytic function in the mouse. J Reticuloendothel Soc. 1974;16:193–203. [PubMed] [Google Scholar]
- 58.Waymack J P, Robb J, Alexander J W. Effect of transfusion on immune function in a traumatized animal model. II. Effect on mortality rate following septic challenge in an animal model. Arch Surg. 1987;122:935–939. doi: 10.1001/archsurg.1987.01400200085016. [DOI] [PubMed] [Google Scholar]
- 59.Waymack J P, Warden G D, Alexander J W, Miskell P, Gonce S. Effect of blood transfusion or anaesthesia on resistance to bacterial peritonitis. J Surg Res. 1987;42:528–535. doi: 10.1016/0022-4804(87)90028-x. [DOI] [PubMed] [Google Scholar]
- 60.White K L, Krasula R W, Munson A E, Holsapple M P. Effects of hydroxyethyl starch (HespanR), a plasma expander, on the functional activity of the reticuloendothelial system. Comparison with human serum albumin and pyran copolymer. Drug Chem Toxicol. 1986;9:305–322. doi: 10.3109/01480548608998282. [DOI] [PubMed] [Google Scholar]
- 61.Wiklund-Hammarstrom B, Gheorghescu B, Liljedahl S O, Nylind B, Reizenstein P. Reticulo-endothelial activity and some metabolic changes related to trauma. Adv Exp Med Biol. 1971;15:333–346. [Google Scholar]
- 62.Williams D L, Browder W, Di Luzio N R. Immunotherapeutic modification of Escherichia coli-induced experimental peritonitis and bacteremia by glucan. Surgery. 1983;93:448–454. [PubMed] [Google Scholar]
- 63.Zweifach B W, Benacerraf B. Effect of hemorrhagic shock on the phagocytic function of Kupffer cells. Circ Res. 1958;6:83–87. doi: 10.1161/01.res.6.1.83. [DOI] [PubMed] [Google Scholar]