Abstract
Background
Neonatal maternal separation (NMS) is a major early life stress that can induce visceral pain and mental disorders. We have shown that an enriched environment (EE) can alleviate NMS-induced negative effects, but the time window over which EE works is unclear. Therefore, this study aimed to investigate the time window through which EE alleviates NMS-induced visceral pain, anxiousness, and depressive behaviors.
Methods
In this study, we used male C57BL/6J mice. The mice were randomly divided into five groups: control group, NMS group, prepubertal EE group (EE1 group), pubertal EE group (EE2 group), and adult EE group (EE3 group). The visceral pain threshold test (PTT), open field test (OFT), elevated plus maze (EPM), forced swimming test (FST), and sucrose preference test (SPT) were performed in all five groups to assay visceral pain, anxiety-, and depression-like behaviors in mice, respectively. Enzyme-linked immunosorbent assay (ELISA) for corticosterone was performed in all five groups to assess the function of the hypothalamic-pituitary-adrenal (HPA) axis.
Results
There was no significant change in weight between groups. It was shown that NMS induced visceral pain, anxiety, and depression, and EE1 and EE2 reversed these negative effects, but EE3 had no significant effect. Likewise, EE1 and EE2 reversed the NMS-induced increase of corticosterone, but EE3 did not.
Conclusions
Adverse life experiences in early life can lead to visceral pain, anxiety, and depression in adulthood, which can be effectively prevented by EE interventions in prepuberty and puberty.
Keywords: Neonatal maternal separation (NMS), enriched environment (EE), visceral pain, anxiety- and depression-like behaviors
Introduction
Chronic pain is a very common disease, often accompanied by mental disorders such as anxiety and depression, which imposes a heavy burden on patients. However, the pathogenesis of such comorbidities is not clear, which leads to an unsatisfactory treatment effect in many patients (1). Studies have shown that early life stresses can affect the development of the central nervous system (CNS) and predispose individuals to chronic pain and mental disorders (2,3). For example, early postpartum neonatal maternal separation (NMS) alters normal mother-child interaction in rodents, prolongs stress-induced hypothalamic-pituitary-adrenal (HPA) axis responses, and negatively affects synaptic plasticity, brain anatomy, and function in mice (4). In addition, exposure to NMS increases vulnerability to chronic pain and psychiatric disorders (5,6).
In contrast to the negative effects of early life stress, an enriched environment (EE) can promote CNS development and improve chronic pain and mental disorders, including visceral pain, anxiety, and depression (7). An EE is an environmental intervention frequently used in animal studies, defined as a combination of “complex inanimate objects and social stimuli”, aiming to increase sensory, physical, and social stimuli (8). It is well known that harmful stress causes abnormal HPA axis function, which is associated with visceral pain and psychiatric symptoms. Several studies have shown that EE can reduce anxiety or depressive behaviors and increase inquisitive behaviors in rodents (9,10). In addition, EE can reduce the expression level of corticosterone (11,12). We, and others, have previously shown that EE alleviates NMS-induced visceral pain, anxiety- and depression-like behaviors (7). However, these studies have not systematically explored the optimal timing of EE intervention.
The development of the CNS is a long process that continues into early adulthood. During certain critical periods, the brain seems to be particularly sensitive to environmental stimuli, triggering structural changes that can have lasting physical and mental effects (13). Research has shown that the brain is particularly sensitive to early adolescent stress, which can lead to extensive pruning of synapses in the frontal cortex. In addition, most mental illnesses, including depression and anxiety, begin to develop around age 14 (14). Therefore, we wondered whether there might be an optimal time for EE intervention, perhaps before the onset of overt abnormal behaviors, that might prevent or ameliorate visceral pain, anxiety, and depressive behaviors caused by early life stress.
In this study, we compared the effects of different periods of EE (prepuberty, puberty, and early adulthood) on visceral pain, anxiety, and depression symptoms in NMS-underwent mice. We also assessed HPA axis reactivity by measuring plasma corticosterone levels to better understand the underlying mechanisms of EE. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-475/rc).
Methods
Animals
Pregnant female mice were purchased from the Experimental Animal Center of Xuzhou Medical University (Xuzhou, China). Mice were placed in a constant temperature and humidity environment with a 12-hour light/dark cycle with free access to water and food. Mice were killed immediately if they developed disease. After behavioral tests, the mice were euthanized. All experimental procedures were approved by the Animal Care and Use Committee of Xuzhou Medical University (No. 202010A132), in compliance with the national guidelines for the care and use of animals. The study protocol was prepared before the study but was not registered.
NMS
From postnatal day 1 (P1) to P21, neonatal mice were kept away from their mother for 6 hours every day. After weaning at P21, male mice were used for subsequent experiments.
EE
Cages were furnished with various running wheels, climbing platforms, toys, and pipes. Interior items and colors are rearranged and updated weekly.
Experimental design
According to the random number table method (Figure 1), 40 male mice were blindly and randomly divided into five groups (n=8 mice per group). A previous study showed that pubertal timing in mice was around P28 (4 weeks) and the period between 60 and 90 days after birth was called adulthood (15). The NMS group: from P1 to P21, neonatal mice were separated from their mothers for 6 hours every day until weaning at P21; Control group: neonatal mice were kept with their mothers until weaning at P21; prepubertal EE group (EE1 group): from P1 to P21, neonatal mice were separated from their mothers for 6 hours per day until weaning at P21, and placed in EE from P1 to 4 weeks (prepubertal period); pubertal EE group (EE2 group): from P1 to P21, neonatal mice were separated from their mothers for 6 hours per day until weaning at P21, and placed in EE from weeks 5 to 8 (pubertal period); and adult EE group (EE3 group): neonatal mice were separated from their mothers for 6 hours daily from P1 to P21 until weaning at P21 and placed in EE from weeks 9 to 12 (adulthood). At week 13, mice were subjected to behavioral testing and then sacrificed for corticosterone determination. All researchers who performed the animal experiments and analyzed the data were blinded to the treatment conditions.
Figure 1.
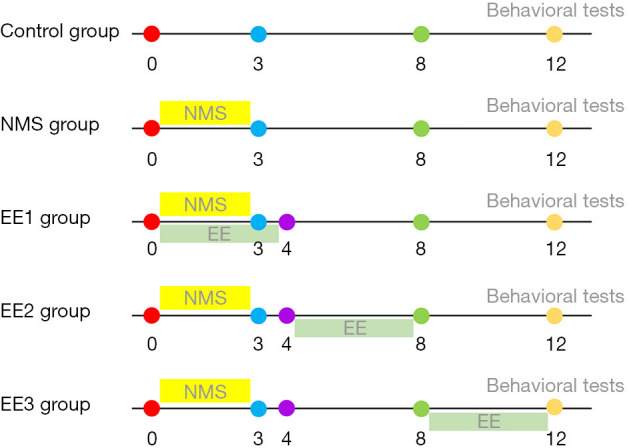
Timeline of experiment. Mice in NMS group were removed from their mothers for 6 hours daily from P1 to P21 (3 weeks). In addition of NMS, EE1, EE2, and EE3 groups, mice also received EE interventions at 0 to 4 weeks, 5 to 8 weeks, and 9 to 12 weeks, respectively. NMS, neonatal maternal separation; EE, enriched environment; EE1 group, prepubertal EE group; EE2 group, pubertal EE group; EE3 group, adult EE group; P1, postnatal day 1; P21, postnatal day 21.
Visceral pain threshold test (PTT)
After the mice were anesthetized, an uninflated balloon was placed in the colorectum. After a moment of adaptation, the balloon was inflated to give pressure to the intestinal wall, and the pressure value when the abdominal wall muscle left the table was the visceral pain threshold.
Open field test (OFT)
The device was divided into 9 squares equally. The area in the middle was called the central area. The mouse was placed gently in the central area and their behavior was recorded for 5 minutes. The observation indicators included the following: total distance, center time, and center distance.
Elevated plus maze (EPM)
The EPM has a pair of open arms and a pair of closed arms. At the beginning of the experiment, the mice were placed in the central grid and their activity was recorded for 5 minutes. The observation indicators included the open arm time and closed arm time.
Sucrose preference test (SPT)
Mice were housed in single cages and trained on sucrose drinking water for 48 hours. Two bottles of 1–2% sucrose water were given in the first 24 hours. In the second 24 hours, 1 bottle contained 1–2% sucrose water, and the other bottle contained pure water (the positions of the 2 bottles were exchanged halfway). After the mice had fasted for 14–23 hours, the sucrose water preference index was measured: the amount of water (g) that the mice drank from 2 bottles of water within 1 hour was determined. Sucrose preference was used as the evaluation index, sucrose preference (%) = sucrose water consumption/(sucrose water consumption + drinking water consumption) × 100%.
Forced swimming test (FST)
The mice were placed in glass tanks (diameter: 11 cm, height: 25 cm), which were filled with tap water at 21–24 ℃. The mice were forced to swim for 6 minutes and the time of immobility during the last 4 minutes was measured as an indicator of depression-like behavior.
Enzyme-linked immunosorbent assay (ELISA)
The levels of corticosterone were quantified with ELISA kits according to the manufacturers’ instructions (Bluegene, E03C0006, Shanghai, China). Mice were sacrificed after prior anesthesia and then blood samples were collected and centrifuged (3,500 rpm) at 4 ℃ for 15 minutes. The concentrations of corticosterone in serum were assessed using ELISA kits.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). Comparisons between multiple groups were performed by one-way analysis of variance (ANOVA) or two-way ANOVA followed by post hoc Bonferroni. A P value less than 0.05 was considered statistically significant.
Results
Body weight
There was no significant difference in body weight among mice subject to different housing conditions in each period (Figure 2).
Figure 2.
The weight of mice in each group. There was no significant difference in weight between groups. All data were expressed as mean ± SD. NMS, neonatal maternal separation; EE, enriched environment; EE1 group, prepubertal EE group; EE2 group, pubertal EE group; EE3 group, adult EE group; SD, standard deviation.
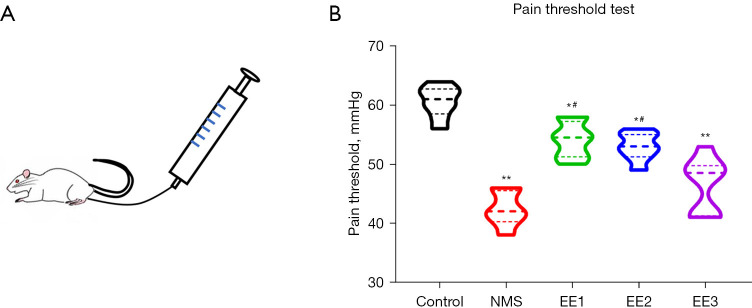
EE1 and EE2 groups alleviated NMS-induced adolescent visceral pain
Compared to the Control group, the visceral pain threshold decreased in the other groups; compared with the NMS group, the visceral pain threshold was significantly increased in the EE1 and EE2 groups, but not in the EE3 group (Figure 3).
Figure 3.
The visceral pain threshold of mice in each group. (A) Schematic of PTT. (B) The visceral pain threshold of each group. All data were expressed as mean ± SD. *, P<0.05, **, P<0.01 vs. Control group; #, P<0.05 vs. NMS group. NMS, neonatal maternal separation; EE, enriched environment; EE1 group, prepubertal EE group; EE2 group, pubertal EE group; EE3 group, adult EE group; PTT, pain threshold test; SD, standard deviation.
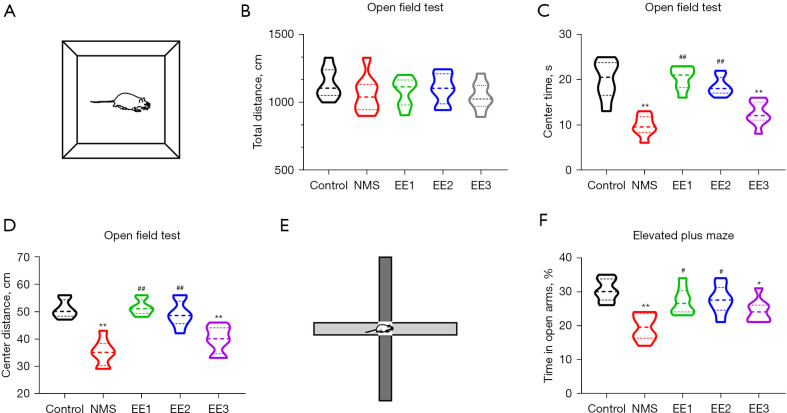
EE1 and EE2 groups alleviated NMS-induced anxiety-like behaviors
To assess anxiety-like behaviors, the OFT and EPM were performed (9). There was no significant difference in total distance between groups (Figure 4A,4B). Compared with the Control group, the NMS and EE3 groups decreased the center time and center distance, exhibiting anxiety-like behavior, and compared with the NMS group, the EE1 and EE2 groups greatly increased the center time and center distance in the OFT (Figure 4C,4D). Compared with the Control group, the NMS and EE3 groups decreased the time in open arms in the EPM, exhibiting anxiety-like behavior, and compared with the NMS group, the EE1 and EE2 groups greatly increased the time in open arms of NMS mice (Figure 4E,4F).
Figure 4.
EE1 and EE2 groups alleviated NMS-induced anxiety-like behaviors. (A) Schematic of OFT. (B) The total distance, (C,D) center time and center distance of NMS group mice in the OFT. (E) Schematic of EPM. (F) The time in open arms of NMS group mice in the EPM. All data were expressed as mean ± SD. *, P<0.05, **, P<0.01 vs. Control group; #, P<0.05, ##, P<0.01 vs. NMS group. NMS, neonatal maternal separation; EE, enriched environment; EE1 group, prepubertal EE group; EE2 group, pubertal EE group; EE3 group, adult EE group; OFT, open field test; EPM, elevated plus maze; SD, standard deviation.
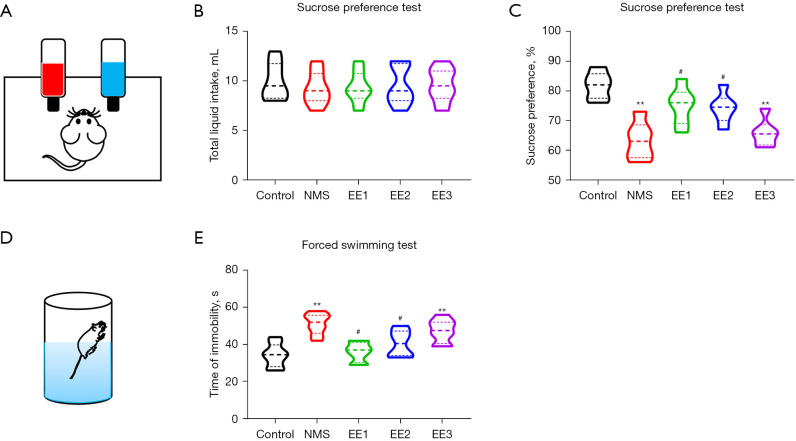
EE1 and EE2 groups alleviated NMS-induced depression-like behaviors
To assess depression-like behaviors, the SPT and FST were performed (9). There was no significant difference in total liquid intake between groups (Figure 5A,5B). Compared to the Control group, the NMS and EE3 groups mice had decreased sucrose intake percentages, exhibiting depression-like behavior, and compared to the NMS group, the EE1 and EE2 groups mice had increased sucrose intake percentages in the SPT (Figure 5C). Compared to the Control group, the NMS and EE3 groups increased the time of immobility, exhibiting depression-like behavior, and compared to the NMS group, the EE1 and EE2 groups mice had decreased times of immobility in the FST (Figure 5D,5E).
Figure 5.
EE1 and EE2 groups alleviated NMS-induced depression-like behaviors. (A) Schematic of SPT. (B) Total liquid intake and (C) sucrose preference rate in SPT. (D) Schematic of FST. (E) Time of immobility in FST. All data were expressed as mean ± SD. **, P<0.01 vs. Control group; #, P<0.05 vs. NMS group. NMS, neonatal maternal separation; EE, enriched environment; EE1 group, prepubertal EE group; EE2 group, pubertal EE group; EE3 group, adult EE group; SPT, sucrose preference test; FST, forced swimming test; SD, standard deviation.
EE1 and EE2 groups decreased NMS-induced corticosterone level
Since the HPA axis is a major target of stress and is associated with visceral pain, anxiety, and depression, we examined corticosterone level. Compared to the Control group, NMS and EE3 groups increased corticosterone level, and compared to the NMS group, EE1 and EE2 groups decreased corticosterone level (Figure 6).
Figure 6.
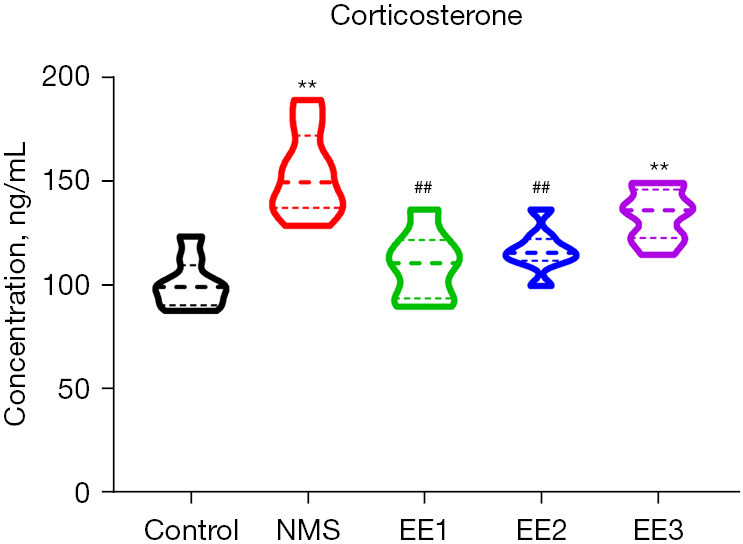
EE1 and EE2 groups decreased NMS-induced corticosterone level. NMS and EE3 groups increased corticosterone level compared to the control group, and EE1 and EE2 groups decreased corticosterone level compared to the NMS group. All data were expressed as mean ± SD. **, P<0.01 vs. Control group; ##, P<0.01 vs. NMS group. NMS, neonatal maternal separation; EE, enriched environment; EE1 group, prepubertal EE group; EE2 group, pubertal EE group; EE3 group, adult EE group; SD, standard deviation.
Discussion
In this study, we used several behavioral tests, including visceral pain, anxiety, and depressive behaviors, and measured plasma corticosterone levels using ELISA to explore the optimal time window for EE intervention to improve NMS-induced visceral pain, anxiety, and depressive behaviors. The results showed that NMS resulted in a lower visceral pain threshold, decreased the center time and center distance in the OFT, decreased the time in open arms in the EPM, decreased sucrose intake percentage in the SPT, increased the time of immobility in the FST, increased the plasma corticosterone levels, exhibiting visceral pain, anxiety, and depressive behaviors, and EE1 and EE2 groups intervention successfully reversed the NMS-induced symptoms, but EE3 group did not achieve the same effect. Our study showed that EE intervention during prepuberty and puberty effectively prevented/alleviated NMS-induced visceral pain, anxiety, and depressive behaviors and reversed the increase in plasma corticosterone, yet EE intervention in early adulthood had no significant effect.
Clinical and basic studies have shown that early life stress may negatively affect brain development, neuroplasticity, and behavior, and increase individual vulnerability to chronic pain and psychiatric disorders, including chronic visceral pain, depression, and anxiety (2,5-7,16). In this study, we observed that exposure to NMS resulted in a decreased pain threshold and disturbed emotional response in mice. The visceral PTT was used to evaluate visceral pain sensitivity, and our results showed that the visceral pain threshold was decreased, indicating that NMS mice were in a state of visceral hypersensitivity. We used SPT and FST to assess depressive behaviors. The present results show that NMS reduces adult sucrose consumption, a central symptom of anhedonia (17), and at the same time NMS significantly increases the duration of immobility in FST, suggesting that NMS causes behavioral despair. These results suggest that NMS leads to depression-like behaviors. We used OFT and EPM to assess anxiety-like behavior and showed that NMS also reduced OFT central time to central distance, while reducing EPM open-arm time, indicating increased anxiety levels in adult mice. These results are consistent with previous findings (18,19).
In contrast to the negative effects of early life stress, EE had a positive effect on brain development and behavior. We have previously shown that EE from P1 to P40 can alleviate visceral pain and mental disorders in adolescence (7). Since EE appears to ameliorate stress-induced negative responses in early life, is there an optimal time window for intervention? Given that brain development continues into early adulthood, we investigated the efficacy of EE interventions in puberty, prepuberty, and early adulthood. According to our results, both prepubertal and pubertal EE intervention increased sucrose preference rate in the SPT and decreased the time of immobility in the FST, suggesting that preadult EE interventions attenuated NMS-induced depression in mice. Similarly, prepubertal and pubertal EE intervention also reduced anxiety in NMS mice in EPM and OFT. However, EE in early adulthood showed no significant positive effect. This is consistent with the brain being most sensitive to stress during adolescence. These results suggest that EE interventions should be implemented as early as possible to deal with the damage caused by stress in early life, preferably in pre-adult adolescence rather than later in life, when the effects are likely to be weak. Although we found no significant effect of EE intervention in early adulthood on NMS mice, which is inconsistent with some reports, we found a certain improvement trend in the experiment, which may be caused by the short duration of EE intervention, and extending the duration of EE intervention may also achieve positive effects. In addition, previously studies shown that elevating levels of corticosterone increases anxiety-and depression-like behaviors, induces visceral hypersensitivity, and decreases somatic pain thresholds (20-24). Consistent with this, preadult EE tended to normalize plasma corticosterone levels in NMS mice, whereas adult EE had no positive effect on plasma corticosterone reduction, suggesting that corticosterone plays an important role of EE in the improvement of visceral pain, anxiety, and depressive behaviors induced by NMS.
In conclusion, we believe that EE intervention before adulthood (prepuberty, puberty) is the best time window, and only a relatively short intervention time is needed to improve the negative effects caused by NMS. Although EE intervention after adulthood may miss the best intervention time, it should not be given up, and longer intervention may also have a positive effect. Therefore, our study suggests that EE intervention before adulthood may have important guiding significance in the clinical treatment visceral pain, anxiety, and depressive behaviors induced by early life stress.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the Talent Construction and Research Start-Up Fund of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (to Ming Xia).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All experimental procedures were approved by the Animal Care and Use Committee of Xuzhou Medical University (No. 202010A132), in compliance with the national guidelines for the care and use of animals.
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-475/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-475/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-475/coif). The authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Gaynes B. Assessing the risk factors for difficult-to-treat depression and treatment-resistant depression. J Clin Psychiatry 2016;77 Suppl 1:4-8. 10.4088/JCP.14077su1c.01 [DOI] [PubMed] [Google Scholar]
- 2.Bick J, Nelson CA. Early experience and brain development. Wiley Interdiscip Rev Cogn Sci 2017. doi: . 10.1002/wcs.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolb B, Mychasiuk R, Gibb R. Brain development, experience, and behavior. Pediatr Blood Cancer 2014;61:1720-3. 10.1002/pbc.24908 [DOI] [PubMed] [Google Scholar]
- 4.Tractenberg SG, Levandowski ML, de Azeredo LA, et al. An overview of maternal separation effects on behavioural outcomes in mice: Evidence from a four-stage methodological systematic review. Neurosci Biobehav Rev 2016;68:489-503. 10.1016/j.neubiorev.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 5.Huang ST, Song ZJ, Liu Y, et al. BNSTAV GABA-PVNCRF Circuit Regulates Visceral Hypersensitivity Induced by Maternal Separation in Vgat-Cre Mice. Front Pharmacol 2021;12:615202. 10.3389/fphar.2021.615202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin X, Liu XX, Wang Y, et al. Early life stress induces anxiety-like behavior during adulthood through dysregulation of neuronal plasticity in the basolateral amygdala. Life Sci 2021;285:119959. 10.1016/j.lfs.2021.119959 [DOI] [PubMed] [Google Scholar]
- 7.Ji NN, Xia M. Enriched environment alleviates adolescent visceral pain, anxiety- and depression-like behaviors induced by neonatal maternal separation. Transl Pediatr 2022;11:1398-407. 10.21037/tp-22-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res 1996;78:57-65. 10.1016/0166-4328(95)00216-2 [DOI] [PubMed] [Google Scholar]
- 9.Gong X, Chen Y, Chang J, et al. Environmental enrichment reduces adolescent anxiety- and depression-like behaviors of rats subjected to infant nerve injury. J Neuroinflammation 2018;15:262. 10.1186/s12974-018-1301-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Gu JY, Han JH, et al. Enriched environment combined with fluoxetine ameliorates depression-like behaviors and hippocampal SYP expression in a rat CUS model. Brain Res Bull 2017;135:33-9. 10.1016/j.brainresbull.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 11.Ilin Y, Richter-Levin G. Enriched environment experience overcomes learning deficits and depressive-like behavior induced by juvenile stress. PLoS One 2009;4:e4329. 10.1371/journal.pone.0004329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morley-Fletcher S, Rea M, Maccari S, et al. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci 2003;18:3367-74. 10.1111/j.1460-9568.2003.03070.x [DOI] [PubMed] [Google Scholar]
- 13.Hübener M, Bonhoeffer T. Neuronal plasticity: beyond the critical period. Cell 2014;159:727-37. 10.1016/j.cell.2014.10.035 [DOI] [PubMed] [Google Scholar]
- 14.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 2008;9:947-57. 10.1038/nrn2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naulé L, Robert V, Parmentier C, et al. Delayed pubertal onset and prepubertal Kiss1 expression in female mice lacking central oestrogen receptor beta. Hum Mol Genet 2015;24:7326-38. 10.1093/hmg/ddv430 [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Baram TZ. Toward Understanding How Early-Life Stress Reprograms Cognitive and Emotional Brain Networks. Neuropsychopharmacology 2016;41:197-206. 10.1038/npp.2015.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu MY, Yin CY, Zhu LJ, et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 2018;13:1686-98. 10.1038/s41596-018-0011-z [DOI] [PubMed] [Google Scholar]
- 18.Cui Y, Cao K, Lin H, et al. Early-Life Stress Induces Depression-Like Behavior and Synaptic-Plasticity Changes in a Maternal Separation Rat Model: Gender Difference and Metabolomics Study. Front Pharmacol 2020;11:102. 10.3389/fphar.2020.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houwing DJ, Ramsteijn AS, Riemersma IW, et al. Maternal separation induces anhedonia in female heterozygous serotonin transporter knockout rats. Behav Brain Res 2019;356:204-7. 10.1016/j.bbr.2018.08.031 [DOI] [PubMed] [Google Scholar]
- 20.Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol 2008;583:115-27. 10.1016/j.ejphar.2008.01.014 [DOI] [PubMed] [Google Scholar]
- 21.Dubey VK, Ansari F, Vohora D, et al. Possible involvement of corticosterone and serotonin in antidepressant and antianxiety effects of chromium picolinate in chronic unpredictable mild stress induced depression and anxiety in rats. J Trace Elem Med Biol 2015;29:222-6. 10.1016/j.jtemb.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 22.Spinieli RL, Cazuza RA, Sales AJ, et al. Persistent inflammatory pain is linked with anxiety-like behaviors, increased blood corticosterone, and reduced global DNA methylation in the rat amygdala. Mol Pain 2022;18:17448069221121307. 10.1177/17448069221121307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benedetti M, Merino R, Kusuda R, et al. Plasma corticosterone levels in mouse models of pain. Eur J Pain 2012;16:803-15. 10.1002/j.1532-2149.2011.00066.x [DOI] [PubMed] [Google Scholar]
- 24.Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behav Brain Res 2010;214:465-9. 10.1016/j.bbr.2010.05.049 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as