Abstract
BACKGROUND
The incidence and mortality rate of breast cancer in China rank 120th and 163rd, worldwide, respectively. The incidence of breast cancer is on the rise; the risk increases with age but is slightly reduced after menopause. Early screening, diagnosis, and timely determination of the best treatment plan can ensure clinical efficacy and prognosis.
AIM
To evaluate the clinical value of magnetic resonance imaging (MRI) combined with digital breast tomosynthesis (DBT) in diagnosing early breast cancer and the effect of breast-conserving surgery by arc incision.
METHODS
This study was divided into two parts. Firstly, 110 patients with early breast cancer confirmed by pathological examination and 110 with benign breast diseases diagnosed simultaneously in Changzhi People’s Hospital of Shanxi Province and Shanxi Dayi Hospital from May 2019 to September 2020 were included in the breast cancer group and the benign group, respectively. Both groups underwent DBT and MRI examination, and the pathological results were used as the gold standard to evaluate the effectiveness of the combined application of DBT and MRI in the diagnosis of early breast cancer. Secondly, according to the operation method, 110 patients with breast cancer were divided into either a breast-conserving group (69 patients) or a modified radical mastectomy group (41 patients). The surgical effect, cosmetic effect, and quality of life of the two groups were compared.
RESULTS
Among the 110 cases of breast cancer, 66 were of invasive ductal carcinoma (60.00%), and 22 were of ductal carcinoma in situ (20.00%). Among the 110 cases of benign breast tumors, 55 were of breast fibromas (50.00%), and 27 were of breast adenosis (24.55%). The sensitivity, specificity, and area under the curve (AUC) of DBT in the differential diagnosis of benign and malignant breast tumors were 73.64%, 84.55%, and 0.791, respectively. The sensitivity, specificity, and AUC of MRI in the differential diagnosis of benign and malignant breast tumors were 84.55%, 85.45%, and 0.850, respectively. The sensitivity, specificity, and AUC of DBT combined with MRI in the differential diagnosis of benign and malignant breast tumors were 97.27%, 93.64%, and 0.955, respectively. The blood loss, operation time and hospitalization time of the breast-conserving group were significantly lower than those of the modified radical treatment group, and the difference was statistically significant (P < 0.05). After 3 mo of observation, the breast cosmetic effect of the breast-conserving group was better than that of the modified radical group, and the difference was statistically significant (P < 0.05). Before surgery, the quality-of-life scores of the breast-conserving and modified radical mastectomy groups did not differ (P > 0.05). Three months after surgery, the quality-of-life scores in both groups were higher than those before surgery (P < 0.05), and the quality-of-life score of the breast-conserving group was higher than that of the modified radical group (P < 0.05). In the observation of tumor recurrence rate two years after the operation, four patients in the breast-conserving group and one in the modified radical treatment group had a postoperative recurrence. There was no significant difference in the recurrence rate between the two groups (χ2 = 0.668, P = 0.414 > 0.05).
CONCLUSION
MRI combined with DBT in diagnosing early breast cancer can significantly improve the diagnostic efficacy compared with the two alone. Breast-conserving surgery leads to better cosmetic breast effects and reduces the impact of surgery on postoperative quality of life.
Keywords: Breast cancer, Magnetic resonance, Digital mammography, Clinical value, Arc incision, Breast-conserving surgery, Digital breast tomosynthesis
Core Tip: This study analyzed the diagnostic method, compared the efficacy of magnetic resonance imaging combined with digital breast tomosynthesis in diagnosing breast cancer in the Shanxi Province, China, and analyzed the advantages of breast-conserving surgery over modified radical mastectomy. The purpose was to select the best diagnostic method and surgical plan for breast cancer to improve the diagnostic accuracy and patients’ quality of life.
INTRODUCTION
Breast X-ray examination, such as digital breast tomosynthesis (DBT), is a commonly used method in clinical practice. It is easy to operate, safe and non-invasive, and has advantages in detecting microcalcifications. However, it is prone to failure due to the overlapping effects of gland and pathological tissue[1]. Breast magnetic resonance imaging (MRI) has a high soft-tissue resolution, does not expose patients to radiation, and has a high sensitivity to breast cancer. However, it has low specificity for breast cancer and a high false-positive rate. Presently, there are few reports on the combined application of these two methods in clinical practice[2]. Surgical treatment is the preferred method for the clinical treatment of breast cancer. Traditional modified radical mastectomy causes great trauma to patients. Breast-conserving surgery can narrow the scope of surgery and reduce physical trauma, especially if the postoperative cosmetic effect is better. However, the clinical application of arc incision breast-conserving surgery is relatively rare[3,4]. This study analyzed the clinical value of MRI combined with DBT in diagnosing early breast cancer and observed the effect of breast-conserving surgery with a small incision to provide a basis for clinical practice.
MATERIALS AND METHODS
Inclusion and exclusion criteria
Participants for this study were selected after presenting at the Changzhi Peoples' Hospital of Shanxi Province or Shanxi Dayi Hospital, China, from May 2019 to September 2020. The inclusion criteria were as follows: (1) Patients aged 18-55 years; (2) Patients with breast cancer or with benign breast tumors, as confirmed by pathological examination; (3) Tumor pathological stage I or II; (4) Patients at the first examination without a history of radiotherapy and chemotherapy; and (5) The interval between DBT and MRI should not have exceeded one week. The exclusion criteria were as follows: (1) Lack of data; (2) Patients with no normal understanding and communication skills; (3) Patients with severe systemic infectious diseases; and (4) Patients with related surgical contraindications.
Research tenders and related materials shall be implemented after submission to the Medical Ethics Committee for research approval [House (Lun) lot 16].
DBT and MRI examinations
DBT was performed using German Siemens MAMMOMAT Inspiration Digital Mammography. The automatic exposure mode was selected. Full-Field Digital Mammography imaging was performed first, and then DBT imaging was performed on the affected side. The X-ray tube was rotated around the breast at a scanning angle of 50° and a range of +25° to -25°. Each rotation was by 2°, and low-dose exposure was performed once, with a total of 25 exposures. A low-dose 2D image was obtained. The original image was reconstructed with a thickness of 1 mm in the direction parallel to the detector to obtain the 3D breast tomography image. The inspection positions included the bilateral craniocaudal and mediolateral oblique. Physicians performing diagnostic imaging followed the recommendations for breast imaging, taking into consideration the patient history and palpation findings.
MRI examinations were conducted using a Siemens Avanto 1.5T superconducting MRI instrument, and a breast phased array coil was used.
The conventional MRI scanning conditions are provided below. Transverse T2_tirm sequences were as follows: TR/TE 4600 ms/59 ms, field of view (FOV) 34 cm × 34 cm, slice thickness/layer spacing 4 mm/0.8 mm, matrix 224 × 320; the transverse T1_fl3d sequence was as follows: TR/TE 8.6 ms/4.7 ms, FOV 34 cm × 34 cm, slice thickness/slice spacing 1.2 mm/0.24 mm, matrix 276 × 384; the transverse DWI ep2d_diff sequence was as follows: TR/TE 9500 ms/133 ms, FOV 34 cm × 34 cm, slice thickness/layer spacing 4 mm/0.8 mm, matrix 132 × 148, b value 0 and 800 s/mm2.
The T1_fl3d axial dynamic enhanced scans were performed according to the following parameters: TR/TE 4.6 ms/1.7 ms, FOV 36 cm × 36 cm, slice thickness 2 mm, matrix 326 × 38. The scans were run once before injection of contrast agent and repeated seven times after injection of contrast agent after 30 s. The contrast agent was Gd-DTPA, the injection dose was 0.2 mL/kg, and the speed of injection was 2 mL/s.
Surgical methods
Small incision breast-conserving group: The use of breast and axillary double incision, according to the location of the tumor, and breast size, to focus on the choice of arc or radiation small incision (about 5 cm length) into the glandular layer after touching the mass, along with the mass and its surrounding visible under the naked eye 1-2 cm normal tissue block resection. The mammary glands around the residual cavity were dissociated from the surface of the pectoralis major muscle to fill the residual cavity or reduce the residual cavity to reshape the breast shape. The sutures in different directions of the specimen were marked, and the internal, external, upper, lower, basal, and surface edges were cut from the inner wall of the residual cavity. A rapid pathological examination of frozen tissue was performed to ensure that all the cut edges were negative. Extended resection was done if cut edges were positive, though it was not carried out more than twice. Modified radical resection was performed if the dissected edge was still positive after two cycles.
Modified radical mastectomy group: Auchincloss modified radical mastectomy with preservation of the pectoralis major and minor muscles was adopted. Sentinel lymph node biopsy or axillary lymph node dissection was performed according to the preoperative imaging examination or pathological results of axillary lymph node punctures.
RESULTS
Comparability analysis of breast cancer group and benign group
Of 110 patients in the breast cancer group were aged from 27 to 55 years old, with an average age of 37.8 ± 7.3 years old; body mass index (BMI) ranged from 21.6 to 25.7 kg/m2, with an average BMI of 23.6 ± 1.9 kg/m2. Distribution of affected side: Left 59 cases, right 51 cases. Of 110 patients in the benign group were aged from 24 to 55 years old, with an average age of 39.0 ± 8.6 years old; BMI ranged from 22.0 to 25.9 kg/m2 with an average of 23.9 ± 2.3 kg/m2. Distribution of affected side: 52 cases on the left and 58 cases on the right. The age, BMI and ipsilateral distribution were compared between the two groups, and the difference was not statistically significant (P > 0.05).
Pathological results of breast cancer group and benign group
Among the 110 patients with breast cancer, 66 cases (60.00%) were invasive ductal carcinoma and 22 cases (20.00%) were ductal carcinoma in situ. Among the 110 patients with benign breast tumors, 55 cases (50.00%) were breast fibroma and 27 cases (24.55% were breast adenosis (Table 1).
Table 1.
Pathological results of breast cancer group and benign group
|
Pathological results
|
No. of cases
|
Composition ratio (%)
|
| Breast cancer patient | 110 | 100.00 |
| Invasive ductal carcinoma | 66 | 60.00 |
| Ductal carcinoma in situ | 22 | 20.00 |
| Lobular carcinoma in situ | 13 | 11.82 |
| Other types | 9 | 8.18 |
| Patients with benign breast tumors | 110 | 100.00 |
| Breast fibroma | 55 | 50.00 |
| Breast disease | 27 | 24.55 |
| Cystic hyperplasia of breast | 14 | 12.73 |
| Granulomatous lobular mastitis | 8 | 7.27 |
| Other types | 6 | 5.45 |
The value of MRI and DBT in differential diagnosis of breast benign and malignant diseases
With pathological results as the gold standard, four tables were drawn respectively, and the sensitivity, specificity and the area under the curve (AUC) of DBT in the differential diagnosis of benign and malignant breast tumors were 73.64%, 84.55% and 0.791, respectively. The sensitivity, specificity and AUC of MRI in the differential diagnosis of benign and malignant breast tumors were 84.55%, 85.45% and 0.850, respectively. The sensitivity, specificity and AUC of DBT combined with MRI in differential diagnosis of benign and malignant breast tumors were 97.27%, 93.64% and 0.955, respectively (Tables 2 and 3, Figures 1 and 2).
Table 2.
Matches four tables
|
DBT
|
Malignant
|
Benign
|
Total
|
|
| Pathology | Malignant | 81 | 29 | 110 |
| Benign | 17 | 93 | 110 | |
| Total | 98 | 122 | 220 | |
| MRI | ||||
| Pathology | Malignant | 93 | 17 | 110 |
| 16 | 94 | 110 | ||
| Benign | ||||
| Total | 109 | 111 | 220 | |
| MRI + DBT | ||||
| Pathology | Malignant | 107 | 3 | 110 |
| Benign | 7 | 103 | 110 | |
| Total | 114 | 106 | 220 | |
MRI: Magnetic resonance imaging; DBT: Digital breast tomosynthesis.
Table 3.
The value of magnetic resonance imaging and digital breast tomosynthesis in differential diagnosis of breast benign and malignant diseases
|
Inspection method
|
Sensitivity
|
Specificity
|
Missed diagnosis rate
|
Misdiagnosis rate
|
AUC
|
| DBT | 73.64% | 84.55% | 26.36% | 15.45% | 0.791 |
| MRI | 84.55% | 85.45% | 15.45% | 14.55% | 0.850 |
| DBT + MRI | 97.27% | 93.64% | 2.73% | 6.36% | 0.955 |
MRI: Magnetic resonance imaging; DBT: Digital breast tomosynthesis; AUC: Area under the curve.
Figure 1.
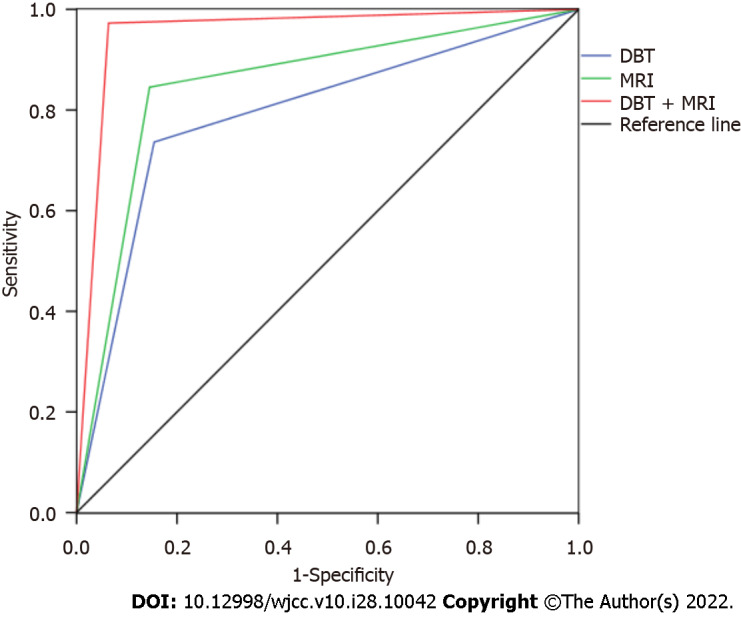
Receiver operating characteristic curve of magnetic resonance imaging and digital breast tomosynthesis differential diagnosis of breast benign and malignant diseases. MRI: Magnetic resonance imaging; DBT: Digital breast tomosynthesis.
Figure 2.
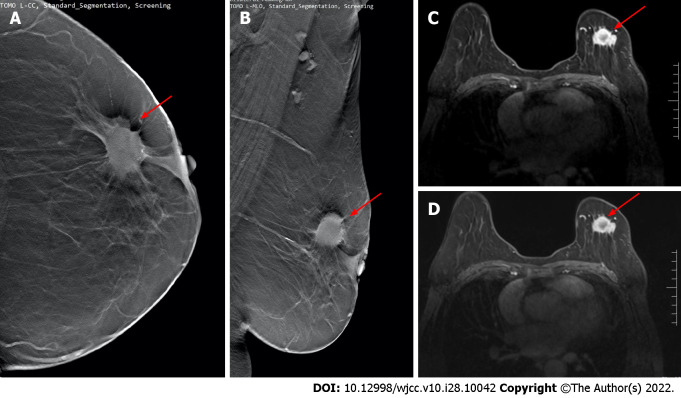
Digital breast tomosynthesis and magnetic resonance imaging examination. A and B: Images of digital breast tomosynthesis examination. Irregular lumps in the upper quadrant of the left breast and burr signs can be seen on the periphery (red arrows); C and D: Magnetic resonance imaging examination images of the same patient. The irregular lumps on the left breast show obvious postoperative disease (red arrows). It was confirmed by physiology as stage II invasive ductal carcinoma.
Comparison of general data between the breast-conserving group and the modified radical treatment group
The age, BMI, ipsilateral distribution, lesion diameter and pathological type between the breast-conserving group and the modified radical treatment group, and the difference was not statistically significant (P > 0.05) (Table 4).
Table 4.
Comparison of general data between the breast-conserving group and the modified radical treatment group
|
Group
|
Breast-conserving group
|
Modified radical cure group
|
t
/χ2
|
P
value
|
|
| n | 69 | 41 | |||
| Age (yr) | 37.1 ± 7.1 | 39.2 ± 6.8 | -1.523 | 0.131 | |
| BMI (kg/m2) | 23.9 ± 1.9 | 23.2 ± 1.7 | 1.941 | 0.055 | |
| Affected side distribution, n (%) | Left side | 34 (49.28) | 25 (60.98) | 1.416 | 0.243 |
| Right side | 35 (50.72) | 16 (39.02) | |||
| Lesion diameter (cm) | 2.18 ± 0.68 | 2.34 ± 0.70 | -1.18 | 0.24 | |
| Pathology type | Invasive ductal carcinoma | 43 (62.32) | 23 (56.1) | 2.171 | 0.538 |
| Ductal carcinoma in situ | 15 (21.74) | 7 (17.07) | |||
| Lobular carcinoma in situ | 6 (8.70) | 7 (17.07) | |||
| Other types | 5 (7.25) | 4 (9.76) | |||
BMI: Body mass index.
Comparison of perioperative indexes between the breast-conserving group and the modified radical treatment group
The amount of bleeding, operation time and hospitalization time in the breast conserving group were significantly lower than those in the modified radical group, and the difference was not statistically significant (P < 0.05) (Table 5).
Table 5.
Comparison of perioperative indexes between the breast-conserving group and the modified radical treatment group (mean ± SD)
|
Group
|
n
|
Surgical bleeding (mL)
|
Operation time (min)
|
Hospital stay (d)
|
| Breast-conserving group | 69 | 66.2 ± 15.8 | 143.8 ± 24.1 | 9.5 ± 2.2 |
| Modified radical cure group | 41 | 106.7 ± 19.6 | 185.5 ± 28.0 | 13.7 ± 2.8 |
| t | -11.869 | -8.256 | -8.731 | |
| P value | 0.000 | 0.000 | 0.000 |
Comparison of postoperative cosmetic effects between breast-conserving group and modified radical treatment group
Three months after operation, the cosmetic effect of breast-conserving group was better than that of modified radical mastectomy group, and the difference was not statistically significant (P < 0.05) (Table 6 and Figure 3).
Table 6.
Comparison of postoperative cosmetic effects between breast-conserving group and modified radical treatment group, n (%)
|
Group
|
n
|
Excellent
|
Good
|
Error
|
| Breast-conserving group | 69 | 58 (84.06) | 11 (15.94) | 0 (0.00) |
| Modified radical cure group | 41 | 8 (19.51) | 22 (53.66) | 11 (26.83) |
| Z | -6.921 | |||
| P value | 0.000 | |||
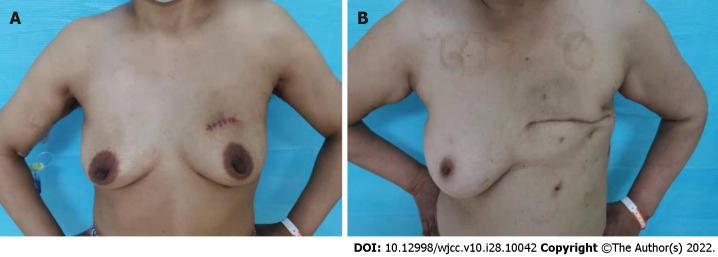
Figure 3.
The postoperative appearance of patients undergoing small incision breast-conserving surgery and modified radical surgery. A: The postoperative appearance of patients undergoing small incision breast-conserving surgery; B: The postoperative appearance of modified radical surgery.
Comparison of quality of life between the breast-conserving group and modified radical treatment group
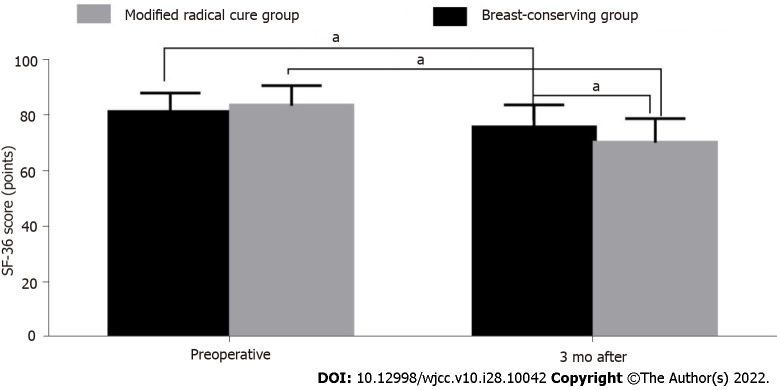
Before operation, the quality of life scores of the breast-conserving group and the modified radical treatment group were compared, and the difference was not statistically significant (P > 0.05). Three months after the operation, the quality of life scores in both groups were significantly higher than before the operation (P < 0.05). The quality of life score of the breast-conserving group was higher than that of the modified radical group (P < 0.05) (Table 7 and Figure 4). In the observation of tumor recurrence rate two years after the operation, four patients in the breast-conserving group and one in the modified radical treatment group had a postoperative recurrence. There was no significant difference in the recurrence rate between the two groups (χ2 = 0.668, P = 0.414 > 0.05).
Table 7.
Comparison of quality of life between breast-conserving group and modified radical treatment group (mean ± SD)
|
Group
|
n
|
Preoperative
|
3 mo after surgery
|
t
|
P
value
|
| Breast-conserving group | 69 | 81.03 ± 6.82 | 75.46 ± 8.14 | 3.850 | 0.000 |
| Modified radical cure group | 41 | 83.26 ± 7.24 | 70.04 ± 8.65 | 8.604 | 0.000 |
| t | -1.621 | 3.299 | |||
| P value | 0.108 | 0.001 |
Figure 4.
Comparison of the quality of life scores between the breast-conserving group and the modified radical treatment group. a P < 0.05.
DISCUSSION
The incidence of breast cancer has increased annually in recent years. Presently, there are 1.67 million newly diagnosed breast cancer patients worldwide yearly. Although breast cancer treatment tends to be comprehensive, including surgery, radiotherapy, chemotherapy, targeting, and endocrine therapy, surgical treatment is still the most important treatment method[3,4]. In recent years, studies have found that the clinical symptoms of breast cancer are not obvious, and many cancers are highly concealed, and are prone to spreading and metastasis. Therefore, early detection and diagnosis of lesions through imaging examination technology are key to reducing mortality and improving prognosis and recovery[5,6].
Imaging examinations play a critical role in the early detection and diagnosis of breast cancer. The commonly used methods in clinical practice include breast radiography (which can be on film, by one-dimensional digital imaging or DBT) and MRI. DBT reduces or eliminates the influence of the fibrous breast gland and pathological tissue that overlap during the imaging process by obtaining three-dimensional tomographic images and enhancing the visibility of the lesion[7]. In patients with breast cancer, DBT can be performed by analyzing the degree of irregularity of the tumor and its lobulation. The burr sign can be seen on tumor edges, calcification is most common, and the breast is limited and has a dense asymmetric shadow[8]. MRI has a high soft-tissue resolution and is obtained by multi-angle, multi-plane, multi-sequence, and multi-parameter imaging. It is less affected by the density of the breast gland; thus, it plays an important role in the differential diagnosis of breast cancer[9].
It was found that the specificity and accuracy of the diagnosis of benign and malignant breast lesions are not high when the conventional plain scan sequence of breast MRI is used alone in the clinic, and MRI is prone to missed diagnoses and misdiagnoses[10]. Traditional breast X-ray photography generates two-dimensional images, which mask burrs and lobulations and other diagnostic signs or display false images of malignant lesions, thus increasing false negative and false positive rates[11-13]. In our study, pathological results were used as the gold standard. The sensitivity and specificity of DBT combined with MRI in the differential diagnosis of benign and malignant breast tumors were 97.27% and 93.64%, respectively, which were higher than those of the single examination methods, suggesting that DBT combined with MRI in the differential diagnosis of benign and malignant breast tumors has higher clinical diagnostic value. DBT has been found to be of great significance for breast BI-RADS classification, improving the consistency between BI-RADS classification results and pathological diagnosis results of diagnostic doctors in the Department of Radiology. However, the combination of the two imaging methods, due to overlapping signs of benign and malignant breast lesions or different sensitivity to calcification, can further improve the clinical diagnostic efficiency, which is consistent with the results of our study[14-16].
With the growing aesthetic requirements of the population, the current principle of breast cancer surgery is to take into account the postoperative patients’ breast cosmetic effects while guaranteeing complete tumor resection. Traditional radical mastectomy for breast cancer causes serious bodily trauma, affecting the beauty of women’s breasts. At the same time, the lack of female characteristics after surgery is also a blow to the patient’s spirit, affecting their quality of life, and physical and mental health[17-19]. In our study, breast-conserving surgery with a small incision was performed. The amount of bleeding, operation and hospitalization time in the breast-conserving group were significantly lower than those in the modified radical mastectomy group. Small incision breast-conserving surgery leads to a better appearance, less trauma, and shorter operation time[20,21].
This study also found that the breast cosmetic effect of the breast-conserving surgery group was better than that of the modified radical mastectomy group 3 mo after the operation. There was no difference in tumor recurrence rate between the two groups 2 years after the operation, suggesting that breast-conserving surgery has a better cosmetic effect in the treatment of patients with breast cancer and does not increase the recurrence rate. Moreover, breast-conserving surgery effectively filled the defects of breast tissue and closed the residual cavity of surgery through the application of gland flap transfer technology or autologous tissue replacement technology in breast-conserving surgery, which improved the cosmetic effect[22]. This study also found that, three months after surgery, the quality-of-life scores of the two groups of patients were significantly higher than those before surgery. Moreover, the quality-of-life score of the breast-conserving group was higher than that of the modified radical group. Through the implementation of breast-conserving surgery, the breast can be retained as a symbol of physical beauty, which reduces the patient's physical and psychological trauma and sense of embarrassment. Consequently, it improves postoperative work and social conditions, among others[23,24].
This study showed that DBT combined with MRI had better diagnostic efficacy in the differential diagnosis of benign and malignant breast tumors, providing a better diagnostic method for early clinical detection, early diagnosis, and timely and appropriate treatment of breast cancer. The combined use of the two diagnostic methods reduces missed clinical diagnoses and misdiagnoses and has greater development potential. Additionally, the advantages of breast-conserving surgery were experimentally confirmed, especially regarding the improvement of women's postoperative quality of life and cosmetic effect, which is more in line with the aesthetic requirements of modern women, while also providing a basis for clinical summaries of breast-conserving surgery. However, our study has certain limitations. The sample size in our study was not large, and the determinants of postoperative breast cosmetic effects included many aspects. Likewise, it was impossible to evaluate the long-term effects of surgery on patients in this study, and further prospective, multi-center, large-scale research and demonstration analysis are needed.
CONCLUSION
In summary, MRI combined with DBT can significantly improve the diagnostic efficiency of early breast cancer compared with the two alone; patients with early breast cancer with breast-conserving surgery can better achieve breast cosmetic effects and reduce the impact of surgery on postoperative quality of life.
ARTICLE HIGHLIGHTS
Research background
The incidence of breast cancer has increased annually in recent years. Presently, there are 1.67 million newly diagnosed breast cancer patients worldwide yearly. Although breast cancer treatment tends to be comprehensive, including surgery, radiotherapy, chemotherapy, targeting, and endocrine therapy, surgical treatment is still the most important treatment method.
Research motivation
In recent years, studies have found that the clinical symptoms of breast cancer are not obvious, and many cancers are highly concealed, and are prone to spreading and metastasis. Therefore, early detection and diagnosis of lesions through imaging examination technology are key to reducing mortality and improving prognosis and recovery.
Research objectives
The purpose was to select the best diagnostic method and surgical plan for breast cancer to improve the diagnostic accuracy and patients’ quality of life.
Research methods
We analyzed the value of digital breast tomosynthesis (DBT) combined with magnetic resonance imaging (MRI) in the differential diagnosis of benign and malignant breast tumors, and compared the blood loss, operation time, hospitalization time, breast cosmetic effect, and quality of life between small incision breast-conserving surgery and modified radical mastectomy.
Research results
The combined use of the two diagnostic methods has advantages of reducing clinical missed diagnosis and misdiagnosis, and have more clinical value and greater development potential. By this study, we have confirmed the advantages of breast-conserving surgery, which had lower blood loss, lower operation time, lower hospital stay time, higher quality of life scores and better breast cosmetic effects.
Research conclusions
In early breast cancer, MRI combined with DBT can significantly improve the diagnostic performance compared with the two alone. Compared with modified radical surgery, breast-conserving surgery in patients with early breast cancer can achieve better breast cosmetic effect and postoperative quality of life.
Research perspectives
We believe that the clinical value of early breast cancer using MRI combined with DBT is higher, and breast-conserving surgery with small incision is more effective.
Footnotes
Institutional review board statement: This study was approved by the Changzhi People's Hospital Affiliated to Shanxi Medical University Medical Ethics Committee.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All the authors have nothing to disclose.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: May 26, 2022
First decision: June 15, 2022
Article in press: August 22, 2022
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Reviewer: de Azambuja E, Belgium; Lamghari M, Portugal S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
Contributor Information
Yun Ren, Department of Breast Surgery, Changzhi People's Hospital Affiliated to Shanxi Medical University, Changzhi 046000, Shanxi Province, China.
Jiao Zhang, Department of Diagnostic Radiology, Changzhi People's Hospital Affiliated to Shanxi Medical University, Changzhi 046000, Shanxi Province, China.
Jin-Dan Zhang, Department of Breast Surgery, Changzhi People's Hospital Affiliated to Shanxi Medical University, Changzhi 046000, Shanxi Province, China.
Jian-Zhong Xu, Department of Breast Surgery, Changzhi People's Hospital Affiliated to Shanxi Medical University, Changzhi 046000, Shanxi Province, China. xjzzhuren@163.com.
Data sharing statement
Not available.
References
- 1.Kim JY, Jung EJ, Kim JM, Lee HS, Kwag SJ, Park JH, Park T, Jeong SH, Jeong CY, Ju YT. Dynamic changes of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predicts breast cancer prognosis. BMC Cancer. 2020;20:1206. doi: 10.1186/s12885-020-07700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trusler O, Goodwin J, Laslett AL. BRCA1 and BRCA2 associated breast cancer and the roles of current modelling systems in drug discovery. Biochim Biophys Acta Rev Cancer. 2021;1875:188459. doi: 10.1016/j.bbcan.2020.188459. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez BD, Eisel SL, Qin B, Llanos AAM, Savard J, Hoogland AI, Jim H, Lin Y, Demissie K, Hong CC, Bandera EV. Prevalence, risk factors, and trajectories of sleep disturbance in a cohort of African-American breast cancer survivors. Support Care Cancer. 2021;29:2761–2770. doi: 10.1007/s00520-020-05786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen-Sträuli BD, Vorburger D, Frauchiger-Heuer H, Bringolf L, Maggi N, Talimi-Schnabel J, Dedes KJ. Prepectoral implant-based breast reconstruction with TiLOOP® Bra Pocket - a single-centre retrospective study. J Plast Reconstr Aesthet Surg. 2022;75:104–111. doi: 10.1016/j.bjps.2021.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Sato K, Fuchikami H, Takeda N, Shimo T, Kato M, Okawa T. Moving incision for covert breast-conserving surgery may prevent early wound complications in brachytherapy-based partial-breast irradiation. Brachytherapy. 2019;18:645–650. doi: 10.1016/j.brachy.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy KM, Zilkens R, Allen WM, Foo KY, Fang Q, Chin L, Sanderson RW, Anstie J, Wijesinghe P, Curatolo A, Tan HEI, Morin N, Kunjuraman B, Yeomans C, Chin SL, DeJong H, Giles K, Dessauvagie BF, Latham B, Saunders CM, Kennedy BF. Diagnostic Accuracy of Quantitative Micro-Elastography for Margin Assessment in Breast-Conserving Surgery. Cancer Res. 2020;80:1773–1783. doi: 10.1158/0008-5472.CAN-19-1240. [DOI] [PubMed] [Google Scholar]
- 7.Bassez A, Vos H, Van Dyck L, Floris G, Arijs I, Desmedt C, Boeckx B, Vanden Bempt M, Nevelsteen I, Lambein K, Punie K, Neven P, Garg AD, Wildiers H, Qian J, Smeets A, Lambrechts D. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat Med. 2021;27:820–832. doi: 10.1038/s41591-021-01323-8. [DOI] [PubMed] [Google Scholar]
- 8.Tamanuki T, Namura M, Aoyagi T, Shimizu S, Suwa T, Matsuzaki H. Effect of Intraoperative Imprint Cytology Followed by Frozen Section on Margin Assessment in Breast-Conserving Surgery. Ann Surg Oncol. 2021;28:1338–1346. doi: 10.1245/s10434-020-08955-z. [DOI] [PubMed] [Google Scholar]
- 9.Keelan S, Heeney A, Downey E, Hegarty A, Roche T, Power C, Mhuircheartaigh NN, Duke D, Kerr J, Hambly N, Hill A. Breast cancer patients with a negative axillary ultrasound may have clinically significant nodal metastasis. Breast Cancer Res Treat. 2021;187:303–310. doi: 10.1007/s10549-021-06194-8. [DOI] [PubMed] [Google Scholar]
- 10.Killander F, Wieslander E, Karlsson P, Holmberg E, Lundstedt D, Holmberg L, Werner L, Koul S, Haghanegi M, Kjellen E, Nilsson P, Malmström P. No Increased Cardiac Mortality or Morbidity of Radiation Therapy in Breast Cancer Patients After Breast-Conserving Surgery: 20-Year Follow-up of the Randomized SweBCGRT Trial. Int J Radiat Oncol Biol Phys. 2020;107:701–709. doi: 10.1016/j.ijrobp.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Hermann N, Haas I, Malinger P, Kaufman Z. Margin assessment before intraoperative radiotherapy during breast conserving surgery-Does the addition of MarginProbe decrease the need for addition of fractionated whole breast radiation? Breast J. 2020;26:1343–1346. doi: 10.1111/tbj.13865. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen CT, Thanh La M, Ann J, Nam G, Park HJ, Min Park J, Kim YJ, Young Kim J, Hong Seo J, Lee J. Discovery of a simplified deguelin analog as an HSP90 C-terminal inhibitor for HER2-positive breast cancer. Bioorg Med Chem Lett. 2021;45:128134. doi: 10.1016/j.bmcl.2021.128134. [DOI] [PubMed] [Google Scholar]
- 13.Tom MC, Sittenfeld SMC, Shah C, Bauer-Nilsen K, Tendulkar R, Cherian S, Al-Hilli Z, Arthur D, Recht A, Vicini F. Use of a Radiation Tumor Bed Boost After Breast-Conserving Surgery and Whole-Breast Irradiation: Time Trends and Correlates. Int J Radiat Oncol Biol Phys. 2021;109:273–280. doi: 10.1016/j.ijrobp.2020.07.2624. [DOI] [PubMed] [Google Scholar]
- 14.Beebeejaun Y, Athithan A, Copeland TP, Kamath MS, Sarris I, Sunkara SK. Risk of breast cancer in women treated with ovarian stimulation drugs for infertility: a systematic review and meta-analysis. Fertil Steril. 2021;116:198–207. doi: 10.1016/j.fertnstert.2021.01.044. [DOI] [PubMed] [Google Scholar]
- 15.Patel P, Umapathy D, Manivannan S, Nadar VM, Venkatesan R, Joseph Arokiyam VA, Pappu S, Ponnuchamy K. A doxorubicin-platinum conjugate system: impacts on PI3K/AKT actuation and apoptosis in breast cancer cells. RSC Adv. 2021;11:4818–4828. doi: 10.1039/d0ra06708c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klement RJ, Koebrunner PS, Krage K, Weigel MM, Sweeney RA. Short-term effects of a Paleolithic lifestyle intervention in breast cancer patients undergoing radiotherapy: a pilot and feasibility study. Med Oncol. 2020;38:1. doi: 10.1007/s12032-020-01443-0. [DOI] [PubMed] [Google Scholar]
- 17.Ouldamer L, Goupille C, Vildé A, Arbion F, Guimaraes C, Jourdan ML, Bougnoux P, Body G, Chevalier S. Total long-chain polyunsaturated n-3 fatty acids level is an independent predictive factor of breast cancer multifocality in women with positive hormone-receptors tumors. Surg Oncol. 2021;38:101597. doi: 10.1016/j.suronc.2021.101597. [DOI] [PubMed] [Google Scholar]
- 18.Lopez P, Galvão DA, Taaffe DR, Newton RU, Souza G, Trajano GS, Pinto RS. Resistance training in breast cancer patients undergoing primary treatment: a systematic review and meta-regression of exercise dosage. Breast Cancer. 2021;28:16–24. doi: 10.1007/s12282-020-01147-3. [DOI] [PubMed] [Google Scholar]
- 19.de Faria Castro Fleury E, Jasmin Huanca Bernal K, Lucena Miranda Madeiro A, Luis Cervera Ocana W, Carlos Vendramini Fleury J, Caobianco L. Side effects in breast implants related to radiotherapy in breast cancer reconstructive surgery. Tech Innov Patient Support Radiat Oncol. 2021;18:8–11. doi: 10.1016/j.tipsro.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh CY, Patmore S, Smolenski A, Howard J, Evans S, O'Sullivan J, McCann A. The role of von Willebrand factor in breast cancer metastasis. Transl Oncol. 2021;14:101033. doi: 10.1016/j.tranon.2021.101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai HW, Mok CW, Chang YT, Chen DR, Kuo SJ, Chen ST. Endoscopic assisted breast conserving surgery for breast cancer: Clinical outcome, learning curve, and patient reported aesthetic results from preliminary 100 procedures. Eur J Surg Oncol. 2020;46:1446–1455. doi: 10.1016/j.ejso.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed RS, Mohammed RS. Assessment of uranium concentration in blood of Iraqi females diagnosed with breast cancer. Radiat Environ Biophys. 2021;60:193–201. doi: 10.1007/s00411-020-00881-8. [DOI] [PubMed] [Google Scholar]
- 23.Ugras SK, Layeequr Rahman R. Hormone replacement therapy after breast cancer: Yes, No or maybe? Mol Cell Endocrinol. 2021;525:111180. doi: 10.1016/j.mce.2021.111180. [DOI] [PubMed] [Google Scholar]
- 24.Poulin R, Poirier D, Merand Y, Thériault C, Bélanger A, Labrie F. Extensive esterification of adrenal C19-delta 5-sex steroids to long-chain fatty acids in the ZR-75-1 human breast cancer cell line. J Biol Chem. 1989;264:9335–9343. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not available.