Abstract
Astragalus membranaceus Bunge, known as Huangqi, has been used to treat various diseases for a long time. Astragaloside IV (AS-IV) is one of the primary active ingredients of the aqueous Huangqi extract. Many experimental models have shown that AS-IV exerts broad beneficial effects on cardiovascular disease, nervous system diseases, lung disease, diabetes, organ injury, kidney disease, and gynaecological diseases. This review demonstrates and summarizes the structure, solubility, pharmacokinetics, toxicity, pharmacological effects, and autophagic mechanism of AS-IV. The autophagic effects are associated with multiple signalling pathways in experimental models, including the PI3KI/Akt/mTOR, PI3K III/Beclin-1/Bcl-2, PI3K/Akt, AMPK/mTOR, PI3K/Akt/mTOR, SIRT1–NF-κB, PI3K/AKT/AS160, and TGF-β/Smad signalling pathways. Based on this evidence, AS-IV could be used as a replacement therapy for treating the multiple diseases referenced above.
Keywords: Astragaloside IV, Pharmacological effect, Autophagy, Inflammation
Core Tip: Astragaloside IV (AS-IV) is one of the main active ingredients of the aqueous extract Huangqi. Many experimental models have shown that AS-IV has broad beneficial effects on various diseases. This review demonstrates and summarizes the pharmacological effects and autophagic mechanism of AS-IV. The autophagic effects are associated with inflammation or not and multiple signalling pathways in experimental models. Based on this evidence, AS-IV will be used as replacement therapy for treating the above various diseases.
INTRODUCTION
Traditional Chinese medicine (TCM) has been used to treat various diseases with great effects for a long time[1]. Radix Astragali (Huangqi) is a prevalent TCM. Compared to western medicine, TCM has significantly fewer side effects[2]. In addition to polysaccharides, flavones and amino acids, the primary components are astragalosides[3]. Astragaloside IV (AS-IV) is the primary active astragaloside, whose pharmacological effects have been reported in various diseases in vitro and in vivo. A search strategy was performed of published studies from 2001 to present using keyword searches “(astragaloside IV) and (inflamm)” in PubMed. Then we included the studies related to autophagy and excluded the studies not related to autophagy. In this review, we summarize the protective effects and autophagic mechanism of AS-IV.
PHARMACOKINETICS AND TOXICITY OF AS- IV
The Chinese Pharmacopoeia lists AS-IV as a quality test for Astragalus membranaceus[4,5]. Figure 1 shows the structural formula of AS-IV. As a cycloartane triterpene saponin (CAS number 84687-43-4), the molecular formula and molecular weight of AS-IV are C14H68O14 (Figure 1) and 784.97, respectively[4,6]. AS-IV is easily soluble in ethanol, methanol or acetone[7]. The methods of reflux, ultrafiltration, high-speed centrifugation, water extraction, ultrasonic extraction, and alcohol precipitation are combined to extract and isolate AS-IV[8].
Figure 1.
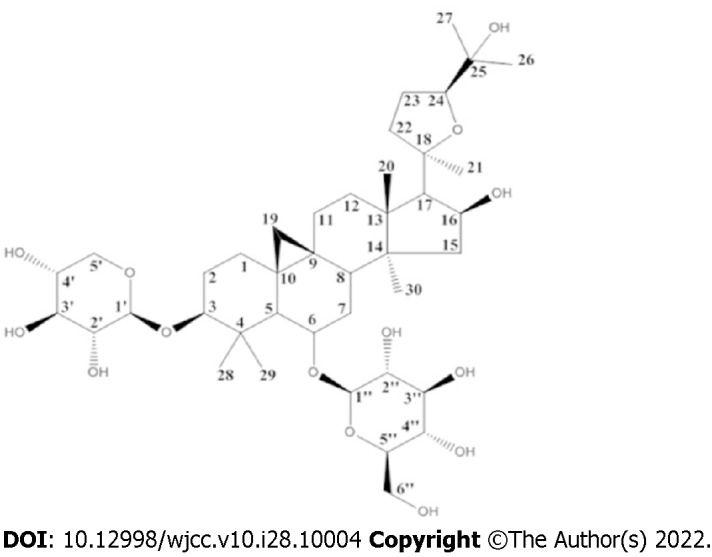
Chemical structure of astragaloside IV.
Tissues, including kidney, lung, spleen, liver, skin, adipose tissue, heart, muscle, duodenum, brain, stomach and ovary, in rats after intravenous AS-IV, can detect AS-IV[9]. The highest concentration of AS-IV is in the liver and kidney, followed by the lung, heart and spleen[10], with limited distribution in the brain. Plasma proteins combined with AS-IV, and in urine and faeces, 50% AS-IV was recovered[9]. The linear pharmacokinetic features of AS-IV have been characterized in rats[9]. AS-IV lacks first-pass elimination and is little metabolised in the liver. The absolute bioavailability of oral administration AS-IV is 3.66% in rats and only 7.4% in beagle dogs[11], which restricts its oral administration. AS-IV causes no obvious adverse reactions or preclinical toxicity. Liver and renal function were not affected after oral administration[3,12]. In rats, no maternal toxicity appeared at doses from 0.25 to 1.0 mg/kg, but caution should be taken when using AS-IV to treat cardiovascular disease (CVD) in pregnant women at the dose of 1.0 mg/kg/d[13,14]. AS-IV exhibits maternal toxicity (intravenously guttae 1.0 mg/kg) and foetal toxicity (≥ 0.5 mg/kg) in rats, but there are no teratogenic effects in rats or rabbits[13]. The reproductive toxicity of AS-IV includes a delay in fur development, the cliff parry reflex of pups, and eye-opening under 1.0 mg/kg AS-IV for 4 wk[14]. Hence, AS-IV should be used with caution in pregnant women.
PHARMACOLOGICAL EFFECTS OF AS-IV ON VARIOUS DISEASES
AS-IV protects against various diseases, including CVD, nervous system diseases, lung disease, diabetes, organ injury, kidney disease, and gynaecological diseases. The specific diseases in each category and their related experimental models are shown in Tables 1–7.
Table 1.
Protective effect of astragaloside IV on cardiovascular disease
|
Disease categories
|
Study object/model
|
Effect induced by autophagy
|
Mechanism (targets or pathways)
|
Ref.
|
| Myocardial I/R injury | H2O2 in cardiomyocytes; LAD in mice | (-) Myocardial I/R injury via (-) I/R-caused autophagosome accumulation | (+) SOD2, (-) O2 | Huang et al[21] |
| Myocardial injury | Doxorubicin in rats | (-) The heart damage of rats via (-) autophagy | (+) PI3K/Akt pathway | Luo et al[24] |
| Cardiac dysfunction | LPS in rats | (-) Cardiac dysfunction, reduce heart injury, (-) autophagy | (+) Calcium- and mitochondrial energy metabolism-related proteins | Wang et al[19] |
| Myocardial hypertrophy | The abdominal aorta narrow in rats; mechanically stretching cardiomyocytes | (+) Cardiac function, cardiomyocyte morphology; (+) Autophagy | (+) LC3 II expression, (-) p62 levels | Zhang et al[20] |
| Myocardial infarction | H/R injured H9C2 cells | (-) The H/R injury induced apoptosis and autophagy | (-) Autophagy related genes (Beclin 1 and LC3 II); the interactions between Bcl-2 and Beclin-1 enhanced by GATA | Yang et al[22] |
| Acute ischaemic heart disease | High glucose in rat cardiomyocytes H9C2 | (-) Cardiomyocyte injury, (-) HG-induced oxidative stress and autophagy | Pathways [miR-34a/Bcl2/(LC3 II/LC3 I) and pAKT/Bcl2/(LC3 II/LC3 I)] | Zhu et al[23] |
| Atherosclerosis | High-fat diet in ApoE-/-mice; β-glycerophosphate in human VSMCs | (-) Autophagy and mineralization of VSMCs in atherosclerosis | (-) DUSP5 and autophagy-related proteins; (+) H19, p-ERK1/2 and p-mTOR | Song et al[59] |
| Mitochondrial dysfunction | Ang II in rat aortic VSMCs | (-) Ang II-induced mitochondrial dysfunction in rat VSMCs via (+) mitochondrial autophagy | (-) OCRs, ATP and mtDNA, the disruption of mitochondrial structural integrity | Lu et al[58] |
LAD: Left anterior descending; LPS: Lipopolysaccharide; LC: Lung cancer; H/R: Hypoxia/reoxygenation; HG: High glucose; H9C2: A subclone of the original clonal cell line which exhibits many of the properties of skeletal muscle; VSMC: Vascular smooth muscle cell; OCR: Oxygen consumption rate.
Protective effect of AS-IV on CVD
CVDs are pathologies related to the heart and blood vessels[15]. As the leading cause of death, CVDs have major impacts on human health and overburden the global economy[1,16]. According to current trends, the annual death toll due to CVDs will reach 22.2 million by 2030[15]. Although research on the pathophysiology and pharmacological mechanisms of CVD has progressed significantly, morbidity and mortality remain high[17]. However, many people fail to recognize the risks of CVD. High blood pressure, weight problems, and changes in glucose or cholesterol levels are all signs of CVD risk. To prevent and treat these disorders, drugs with increased therapeutic efficacy and fewer side effects are urgently needed. According to the literature, Astragalus membranaceus preparations are widely used to treat CVDs[18]. Consistently, through an autophagic mechanism, AS-IV exerts a protective effect on CVDs, including heart dysfunction, myocardial hypertrophy, and cardiomyocyte injury caused by ischaemia, hypoxic high glucose, and doxorubicin[19-24] (Table 1). AS-IV improved lipopolysaccharide (LPS)-induced cardiac dysfunction by inhibiting autophagy and regulating the expression of mitochondrial energy metabolism-related proteins[19]. By activating autophagy and decreasing inflammation, AS-IV inhibits cardiac hypertrophy caused by mechanical stress[20]. Decreased inflammation was confirmed by decreased expression of NLR family, pyrin domain containing 3 (NLRP3) and interleukin (IL)-1β[20]. Activated autophagy is demonstrated by increased microtubule-associated protein light chain 3 (LC3II) expression and decreased p62 levels. AS-IV alleviated myocardial ischaemia–reperfusion (I/R) injury by attenuating I/R-induced autophagosome accumulation, which is regulated by reactive oxygen species (ROS). AS-IV upregulated SOD2 and downregulated O2 in myocardial I/R injury in vivo and H9C2 (a subclone of the original clonal cell line which exhibits many of the properties of skeletal muscle) cardiomyocyte injury in vitro[21]. AS-IV ameliorated autophagosome numbers in H9C2 cells caused by I/R injury and decreased autophagy-related genes (Beclin-1 and LC3 II)[22]. AS-IV improves cardiomyocyte injury by inhibiting high glucose-induced oxidative stress and autophagy via the miR-34a/Bcl2/(LC3II/LC3I) and pAKT/Bcl2/(LC3II/LC3I) pathways[23]. AS-IV protected against heart damage in rats induced by doxorubicin through autophagy regulation and PI3K/Akt pathway activation[24].
Protective effect of AS-IV on the brain and nervous system
Neurological disorders include stroke, dementia, cerebral ischaemia, epilepsy, Alzheimer’s disease and Parkinson’s disease[25]. Among them, stroke is the leading cause of death worldwide[26]. Ischaemic stroke causes I/R, leading to brain injury, in which autophagy plays an important role. Huangqi has been used to treat stroke, especially in China, where it has been used for thousands of years[22]. AS-IV can cross the blood–brain barrier[26] and alleviate brain injury due to I/R through autophagy, which has been reported in several studies. AS-IV decreased cerebral I/R injury in middle cerebral artery occlusion rats. AS-IV increased autophagy in HT22 cells in vitro after oxygen and glucose deprivation/reoxygenation (OGD/R). Expression levels of the autophagy marker p62 were reduced, and the ratio of LC3II/LC3I was augmented[27]. However, Xu et al[28] found that AS-IV decreased the increased autophagy markers LC3 II, Beclin-1, and autophagy-related gene ATG12. Moreover, AS-IV (intraperitoneal injection 10 mg/kg/d) may promote functional recovery after spinal cord injury and stimulate autophagy in neuronal cells in vitro[29]. AS-IV reduced autophagic injury in PC12 cells, demonstrating that cell survival was increased and lactate dehydrogenase leakage was decreased by OGD/R. The protective mechanism is related to decreased autophagy, indicating reduced autophagosomes, reduced LC3 II/LC3 I ratio, and p62 protein upregulation. The autophagy-related mechanisms may be associated with PI3K III/Beclin-1/Bcl-2 and the PI3KI/Akt/mTOR signalling pathways[30]. AS-IV suppressed astrocyte senescence of LPS/MPP+ induced premature senescence in vitro and loss of dopamine neurons in a murine model of Parkinson’s disease. The mechanism included promotion of mitophagy, which reduced generation of mitochondrial ROS and accumulation of damaged mitochondria[31]. Table 2 lists the protective effect of AS-IV on the brain and nervous system.
Table 2.
Protective effect of astragaloside IV on the brain and nervous system
|
Disease categories
|
Study object/model
|
Effect induced by autophagy
|
Mechanism (targets or pathways)
|
Ref.
|
| Ischemic stroke | MCAO in SD rats; OGD/R in HT22 cells | A neuroprotective role (-) apoptosis (+) autophagy | (+) cell viability, balanced Bcl-2 and Bax expression, (-) the rate of apoptosis, (-) p62, (+) LC3 II/LC3 I | Zhang et al[27] |
| Acute ischaemic stroke | Acute ischaemic stroke mice | (-) The abnormal intestinal microbial; (-) ROS, homocysteine and FFA, NOX2/4, and autophagy marker | (-) Autophagy-related gene (Beclin 1, LC3 II, Atg 12 | Xu et al[28] |
| Ischemic stroke | OGD/R in PC12 cells | (-) Excessive autophagy and damage in PC12 cells | The PI3K I/Akt/mTOR and PI3K III/Becline-1/Bcl-2 signalling pathways | Huang et al[30] |
| Spinal cord injury | Vascular clip to clamp the spinal cord in SD rats | (+) Functional recovery in the spinal cord; (-) apoptosis via (+) autophagy in neuronal cells | (-) mTORC1 (+) lysosomal biogenesis through TFEB | Lin et al[29] |
| Parkinson’s disease | MPTP-induced PD mouse model | (-) The loss of dopamine neurons and behavioural deficits; (+) mitophagy | (-) Damaged mitochondria accumulation, (-) mitochondrial ROS generation | Xia et al[31] |
MCAO: Middle cerebral artery occlusion; OGD/R: Oxygen and glucose deprivation/reoxygenation; PC12: A neuron cell line; LC: Lung cancer; ROS: Reactive oxygen species; FFA: Free fatty acids; NOX: NADPH oxidases; Atg: Autophagy; MPTP: 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD: Parkinson’s disease.
Protective effect of AS-IV on lung disease
As-IV has been reported to exert protective effects in various diseases, including non-small cell lung cancer (NSCLC), lung toxicity caused by fine particulate matter (PM2.5, < 2.5 μm), and acute respiratory distress syndrome (ARDS), which are related to autophagic mechanisms (Table 3). Lung cancer is one of the most serious diseases worldwide[32]. AS-IV not only suppresses lung cancer in vivo by enhancing immune responses[33], but also increases the chemosensitivity of NSCLC[34]. For NSCLC cells treated with cisplatin, AS-IV inhibited the increased autophagy of proteins Beclin1 and LC3 I/II[35]. Serious lung toxicity is caused by prolonged exposure to PM2.5, and no effective prevention or treatment measures currently exist[36,37]. PM2.5-induced lung toxicity in a rat model in vivo was established using PM2.5 dust suspension through intratracheal instillation. AS-IV increased autophagic flux and inflammation by activating the AMPK/mTOR pathway in rat models[36]. An ARDS model in vitro was established in MLE-12 cells induced by LPS[34], and AS-IV downregulated autophagy levels, resulting in decreased expression levels of LC3II, Beclin-1 and ATG5[34] .
Table 3.
Protective effect of astragaloside IV on lung disease
|
Disease categories
|
Study object/model
|
Effect induced by autophagy
|
Mechanism (targets or pathways)
|
Ref.
|
| Lung injury | PM2.5-induced lung toxicity in rats | (-) PM2.5-induced lung toxicity; (+) autophagic flux | (+) AMPK/mTOR pathway | Wang et al[36] |
| Lung injury | PM2.5 in rats and rat alveolar macrophages | (-) Severe inflammation and oxidative stress, (+) autophagic flux mainly via autophagosome degradation | (-) The PI3K/Akt/mTOR pathway to (+) autophagy and (-) inflammation | Pei et al[37] |
| Lung injury | LPS in pulmonary epithelial cell | (-) Apoptosis in cell model, (-) autophagy initiation | (-) The oxidative stress and inflammatory response | Liu et al[34] |
| Lung adenocarcinoma | Bevacizumab in A549 cells | (-) Proliferation inhibition and apoptosis promotion (-) inhibiting autophagy pathway | Autophagy-related proteins (p62, LC3 II/LC3 I), p-AKT and p-Mtor | Li et al[57] |
| NSCLC | Cisplatin-resistant the NSCLC cell lines | (-) Chemoresistance to cisplatin in NSCLC cells via (-) inhibition of ER stress or autophagy | Autophagy-related proteins (Beclin1, LC3 II/I) | Lai et al[35] |
AMPK: AMP-activated protein kinase; LPS: Lipopolysaccharide; LC: Lung cancer; NSCLC: Non-small cell lung cancer; ER: Endoplasmic reticulum
Protective effect of AS-IV on diabetes
As a metabolic disorder, diabetes is marked by high levels of glucose, which can damage the kidneys, heart, eyes, gastric mucosa, and even cause coma and death[11]. AS-IV can lower blood glucose levels and can alleviate diabetic complications in diabetic mice[11]. One of the chronic diabetes complications is diabetic peripheral neuropathy (DPN), whose prevention and treatment have become a hot research topic[38]. Several studies have shown that AS-IV protects against DPN through an autophagic mechanism (Table 4). AS-IV treatment improved renal function and renal fibrosis in KK-Ay mice with spontaneous diabetes. AS-IV alleviated the overactivation of mitophagy and maintained mitochondrial function in Schwann cells, significantly downregulating expression levels of LC3 in diabetic KK-Ay mice[38]. Moreover, AS-IV prevented the progression of DPN partly through AMPKα-promoted autophagy induction[39] and exerted renoprotective effects on podocyte epithelial–mesenchymal transition through autophagy activation and modulation of the SIRT1–NF-κB pathway[40]. AS-IV also exerted neuroprotection by alleviating peripheral nerve myelin sheath injury by inhibiting autophagy and upregulating PI3K/Akt/mTOR signalling pathways[41]. A diabetic liver injury model in type 2 diabetes mellitus (T2DM) rats was induced by highfat diets/streptozotocin. ASIV treatment improved liver injury and suppressed liver autophagy in T2DM rats, which was correlated with upregulation of AMPK/mTORmediated autophagy[42] (Table 4).
Table 4.
Protective effect of astragaloside IV on diabetes
|
Disease categories
|
Study object/model
|
Effect induced by autophagy
|
Mechanism (targets or pathways)
|
Ref.
|
| Diabetic peripheral neuropathy | A high-glucose medium in Schwann cells | Antioxidant activity via (-) the autophagy overactivation of Schwann cells | (-) Reactive oxygen species and (-) autophagy-related proteins (LC3, PINK and Parkin); protective effect (mitochondrial morphology and membrane potential) | Wei et al[38] |
| Diabetic peripheral neuropathy | High-fat diet in rats; high glucose in Schwann RSC96 cells | (-) The myelin sheath injury by the apoptosis of Schwann cells via (+) autophagy | (-) The activation of the PI3K/Akt/mTOR signalling pathway by (+) miR-155 expression | Yin et al[41] |
| DN | KK-Ay diabetic mice; immortalized mouse podocytes | (-) Glucose-induced podocyte EMT and (+) enhanced autophagy | The SIRT1–NF-κB pathway | Wang et al[40] |
| DN | STZ diabetic mice; high glucose in podocytes | (-) The progression of DN via (+) autophagy induction | AMPKα-promoted autophagy induction | Guo et al[39] |
| Liver injury in diabetics | Highfat diets + lowdose STZ in diabetic liver injury rats | (+) Autophagy in the liver of T2DM rats; (-) IR, dyslipidaemia, oxidative stress and inflammation | The promotion of AMPK/mTORmediated autophagy | Zhu et al[42] |
LC: Lung cancer; PINK: PTEN-induced putative kinase 1; RSC: Rat Schwann cells; KK-Ay: Spontaneous diabetes; EMT: Epithelial-mesenchymal transition; STZ: Streptozotocin; DN: Diabetic nephropathy; IR: Immunoreactive; T2DM: Type 2 diabetes mellitus.
Protective effect of AS-IV on organ injury
Many vital roles of the liver, such as drug metabolism and detoxification, cause drug-induced liver injury. Recent studies have shown that AS-IV protects the liver from injury[11] (Table 5). LO2 cells were treated with an overdose of iron dextran, which was selected as a liver injury in the cell model. Excessive autophagy of hepatocytes was induced in this model, primarily causing hepatocyte damage. AS-IV reduced the growing number of autophagosomes, which was induced by iron dextran. LC3II/I was also significantly downregulated, and p62 was increased in the cell model. Hence, AS-IV reversed this damage by inhibiting excess autophagy[43]. Liver and kidney injuries were induced by cisplatin in rats. AS-IV plays a protective role by inducing autophagy and limiting the expression of proinflammatory mediators[44]. We found that AS-IV alleviated liver injury in acute liver failure induced by D-galactosamine/lipopolysaccharide by reducing the autophagy levels of monocytes/macrophages (data not shown).
Table 5.
Protective effect of astragaloside IV on organ injury
|
Disease categories
|
Study object/model
|
Effect induced by autophagy
|
Mechanism (targets or pathways)
|
Ref.
|
| Liver injury | Iron overload (iron dextran) in LO2 cells | (-) Damage to hepatocytes, excessive autophagy, autophagosomes and apoptosis of hepatocytes by the iron overload | (-) LC3 II/I, (+) p62 | Xie et al[43] |
| Liver and kidney injury | Cisplatin in rats | Protected against cisplatin-induced injury by (+) autophagy | (-) Autophagy-mediated NLRP3 | Qu et al[44] |
LO2: Human normal embryonic hepatocytes; LC: Lung cancer; NLRP3: NLR family, pyrin domain containing 3.
Protective effect of AS-IV on kidney disease
Kidney diseases for which AS-IV has been tested include chronic glomerulonephritis (CGN), and glomerular diseases, which are associated with autophagy treated by AS-IV (Table 6). As an immune-mediated disease, CGN is the most common glomerular disease[45]. AS-IV protects against CGN through autophagy activation via the PI3K/AKT/AS160 pathway, which is demonstrated by improved kidney function and ameliorated kidney lesions[46]. AS-IV not only alleviated fibrosis but also improved renal function and morphology in diabetic KK-Ay mice[47] (Table 6). Many glomerular diseases related to renal fibrosis are associated with mesangial cell activation. AS-IV inhibited mesangial cell activation and enhanced autophagy via the SIRT1/NF-κB p65 pathway, and these effects were eliminated by an autophagy inhibitor[47].
Table 6.
Protective effect of astragaloside IV on kidney disease
|
Disease categories
|
Study object/model
|
Effect induced by autophagy
|
Mechanism (targets or pathways)
|
Ref.
|
| Chronic glomerular nephritis | Cationic bovine serum in rats | (+) Kidney function, (-) kidney lesion, (-) inflammatory, (+) autophagy | (-) The activation of PI3K/AKT/AS160 pathway | Lu et al[46] |
| Diabetic kidney disease | A high-fat diet in the diabetic KK-Ay mice | (+) Renal function and morphology by (+) autophagy | (-) MC activation through the SIRT1-NF-κB pathway | Wang et al[47] |
KK-Ay: Spontaneous diabetes; MC: Mesangial cell.
Protective effect of AS-IV on gynaecological diseases
The gynaecological diseases treated by AS-IV through an autophagic mechanism include triple-negative breast cancer, cervical cancer, and vulvar squamous cell carcinoma (VSCC) (Table 7). Triple-negative breast cancer seriously threatens women’s health worldwide. AS-IV is one of the four active ingredients of SANT (a novel Chinese herbal monomer combination) treatment. The efficacy and safety of SANT as an antitumor agent were evaluated in mouse models. SANT administration exerted significant antitumor efficacy, which enhanced autophagic flux and increased gene expression levels of ATG16L1, ATG9B and ATG4D[48]. The survival rate of advanced cervical cancer remains low. AS-IV decreased the tumour growth curves and suppressed cell invasion through autophagy induction. The autophagy regulatory proteins mRNA-decapping enzyme 1A (DCP1A) and thymosin beta-4 (TMSB4X) were increased in cervical cancer cells in response to AS-IV. Hence, by inducing autophagy, AS-IV inhibits cervical cancer invasion[49]. AS-IV also inhibited the proliferation of VSCC (SW962 cells) by increasing autophagic activity, as evidenced by increased Beclin-1 and LC3 II and decreased p62. AS-IV decreased apoptosis after autophagy inhibition by 3-methyladenine[50].
Table 7.
Protective effect of astragaloside IV on gynaecological diseases
|
Disease categories
|
Study object/model
|
Effect induced by autophagy
|
Mechanism (targets or pathways)
|
Ref.
|
| Triple-negative breast cancer | The MDA-MB-231 orthotopic mammary tumour in BALB/c nude mice | (-) Cancer cells' proliferation and migration, (+) autophagy flux | (+) The ATG16L1, ATG9B, ATG4D via SANT; (-) TMEM74 and TNF gene expressions | Li et al[48] |
| Cervical cancer | A SiHa cell in the nude mice | (-) Cervical cancer invasion, (+) autophagy | (+) Atg12 and (+) cancer cell autophagy via DCP1A and TMSB4X | Xia et al[49] |
| Vulvar squamous cell carcinoma | The human VSCC cell line SW962 | (-) Cell proliferation, (+) apoptosis and autophagy | The TGF-β/Smad signalling pathway; (+) Beclin 1 and LC3 II, (-) p62 | Zhao et al[50] |
LC: Lung cancer; MDA-MB-231: Human breast cancer cell line; BALB/c: The white mutant laboratory mouse; Atg: Autophagy; VSCC: Vulvar squamous cell carcinoma; SW962: Human vulva phosphorous cancer cell line; SANT: A novel Chinese herbal monomer combination; DCP1A: mRNA-decapping enzyme 1A; TMSB4X: Thymosin beta-4; TNF: Tumour necrosis factor.
Other diseases
AS-IV also has beneficial effects on gastric mucosa, flap survival, Graves’ orbitopathy (GO), and so on. AS-IV protected the gastric mucosa in a rat model and decreased expression levels of the autophagic proteins Beclin1, p62, ATG5 and ATG12[51]. AS-IV may have beneficial functions for flap survival. AS-IV increased the flap survival area and reduced tissue oedema. AS-IV promoted survival of skin flaps decreased tissue oedema by activating autophagy in a rat model[52]. AS-IV significantly decreased IL-1β secretion in influenza A (H1N1) infection by activating autophagy[53]. AS-IV decreased inflammatory responses and reduced excessive autophagy induced by heat in vitro and in vivo[54]. AS-IV or rapamycin played an antiapoptotic role by increasing autophagic activity in IL-1β-treated chondrocytes[55]. GO is a disease affecting the cornea of the eye. AS-IV protects against GO orbital inflammation by suppressing autophagy induced by IL-1β[56].
THE AUTOPHAGY PROMOTING OR INHIBITORY EFFECTS OF AS-IV
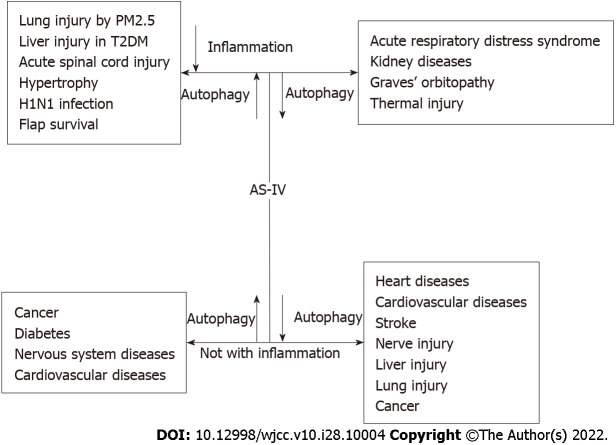
AS-IV promotes or inhibits autophagy to exert beneficial effects on various diseases. The related signalling pathways of the autophagic mechanism are shown in Table 8. AS-IV promotes or inhibits autophagy to alleviate inflammation and disease severity (Figure 2). AS-IV also promotes or inhibits autophagy to alleviate disease severity not associated with inflammation (Figure 2).
Table 8.
The autophagy promotion or inhibition effects of astragaloside IV
|
Autophagy effects (+, -) and inflammation (R, NR)
|
Diseases or study model
|
Effect induced by autophagy
|
Mechanism (targets or pathways)
|
Ref.
|
| "+; R" | Lung injury rats induced by PM2.5 | (-) GM-CSF, ICAM-1, IFN-γ, TNF-α, IL-6, IL-18 and CRP | The AMPK/mTOR; PI3K/Akt/mTOR pathway | Wang et al[36], Pei et al[37] |
| "+; R" | Liver injury in T2DM rats | (-) TNFα and IL6 | The AMPK/mTOR signalling pathway | Zhu et al[42] |
| "+; R" | Liver and kidney injury in rats induced by cisplatin | (-) The NLRP3 inflammasome | (+) LC3 II/I and (-) p62 | Qu et al[44] |
| "+; R" | Acute spinal cord injury | (-) neuroinflammation; (-) iNOS, COX-2 and TNF-α | Polarize towards an M2 phenotype in microglial cells | Lin et al[29] |
| "+; R" | Myocardial hypertrophy by mechanical stress | (-) NLRP3 and IL-1β in cardiomyocytes | (+) LC3 II/I and (-) p62 | Zhang et al[20] |
| "+; R" | H1N1 infection | (-) IL-1β | (+) Autophagosome formation, (+) autolysosomes, (+) the fusion of autophagosome and lysosome | Zhang et al[53] |
| "+; R" | The rat "McFarlane flap" model | Skin flap survival; (-) TNF-α, IL-1β and IL6 and (-) leukocyte infiltration | (+) Autophagosome formation related protein, Beclin 1 and LC3 II/I | Lin et al[52] |
| "+; NR" | Lung cancer | Favourable in lung cancer | The p53/AMPK/mTOR signalling pathway | Yang et al[32] |
| "+; NR" | Lung adenocarcinoma cells | (-) The viability and promote the apoptosis of A549 cells | The AKT and mTOR pathways | Li et al[57] |
| "+; NR" | Vulvar squamous cell carcinoma | (-) Cell proliferation | The TGF-β/Smad pathway | Zhao et al[50] |
| "+; NR" | The gastric mucosa | A beneficial effect on gastric mucosa in vivo | (+) Beclin1, p62, ATG5, and ATG12 | Cai et al[51] |
| "+; NR" | Diabetic KK-Ay mice | Improve renal fibrosis and function | The SIRT1–NF-κB pathway; (-) mesangial cell activation through the SIRT1-NF-κB pathway | Wang et al[40,47] |
| "+; NR" | DPN induced by Schwann cell apoptosis | (-) Myelin sheath injury | (-) The PI3K/Akt/mTOR signalling pathway | Yin et al[41] |
| "+; NR" | Diabetic rats | (-) Liver injury and insulin resistance | The AMPK/mTOR pathway | Zhu et al[42] |
| "+; NR" | Nervous system diseases | (-) Parkinson's disease | (-) Astrocyte senescence | Xia et al[31] |
| "+; NR" | Nervous system diseases | (-) Brain injury caused by ischaemic stroke | Further (+) LC3II/LC3 I | Zhang et al[27] |
| "+; NR" | Cardiovascular diseases; rat VSMCs induced by Ang II | Favourable effects on mitochondrial dysfunction | Drp1 and parkin are vital to mitochondrial autophagy | Lu et al[58] |
| "-; R" | Acute respiratory distress syndrome; the pulmonary endothelial ARDS cell model stimulated by LPS | (-) Inflammation and apoptosis | (-) Autophagy proteins | Liu et al[34] |
| "-; R" | Kidney disease; CGN rats | (-) Kidney injury and (-) inflammation | (-) The PI3K/AKT/AS160 pathway | Lu et al[46] |
| "-; R" | Graves' orbitopathy | Protect against Graves' orbitopathy; (-) IL-6, IL-8, TNF-α, and MCP-1 | (-) Beclin 1, Atg 5 and LC3 II/LC3 I | Li et al[56] |
| "-; R" | Thermal injury in vitro and in vivo | (-) Inflammatory responses | The PERK-eIF2α pathway | Dong et al[54] |
| "-; NR" | Heart diseases | (-) The cardiotoxicity of rats; (-) H/R-injured H9C2 cells | PI3K/Akt pathway activation | Huang et al[21] |
| "-; NR" | Heart diseases | Improve heart dysfunction induced by LPS | (-) Calcium-mediated apoptosis and autophagy by targeting miR-1 | Wang et al[19] |
| "-; NR" | Atherosclerosis; VSMCs in thoracic aorta of mice and in vitro VSMCs model | (-) Mineralization in vitro and in vivo models | (-) DUSP5 and autophagy-related proteins and (+) H19, p-ERK1/2 and p-mTOR | Song et al[59] |
| "-; NR" | Nerve injury; PC12 cells in response to OGD/R | (-) Excessive autophagy injury | (-) The number of autophagosomes; (-) LC3 II/LC3 I, (+) p62; PI3K I/Akt/mTOR pathway | Huang et al[30] |
| "-; NR" | Nerve injury; Schwann cells induced by high glucose | (-) Mitophagy and excessive autophagy | (-) Autophagy markers Beclin-1, Atg12, and LC3 II | Wei et al[38] |
| "-; NR" | Liver injury; L02 hepatocytes induced by iron overload | (-) The damage to L02 hepatocytes | (-) Autophagosome formation; (+) p62, (-) LC3II/LC3 I | Xie et al[43] |
| "-; NR" | Lung injury caused by PM2.5 in vivo and in vitro | (-) Lung injury | Degraded autophagosomes | Pei et al[37] |
| "-; NR" | Cancer; NSCLC cells treated with cisplatin | Counteract chemoresistance | (-) Autophagy (Beclin 1) and ER stress (GPR78) | Lai et al[35] |
| "-; NR" | Cancer | (-) Invasion of cervical cancer | (-) Atg7/Atg12, (-) DCP1A and TMSB4X | Li et al[48] |
Autophagy effects (+, -): “+” Indicates autophagy promotion, “-” indicates autophagy inhibition. Inflammation (R, NR): “R” indicates “related”, “NR” indicates “not related”. LC: Lung cancer; GM-CSF: Granulocyte-macrophage colony-stimulating factor; ICAM: Intercellular adhesion molecule; IFN: Inborn errors of interferon; TNF: Tumour necrosis factor; IL: Interleukin; Atg: Autophagy; NLRP3: NLR family, pyrin domain containing 3; H1N1: Influenza A; CRP: C-reactive protein; T2DM: Type 2 diabetes mellitus; iNOS: Inducible nitric oxide synthases; COX-2: Cyclooxygenase-2; KK-Ay: Spontaneous diabetes; DPN: Diabetic peripheral neuropathy; VSMC: Vascular smooth muscle cell; ARDS: Acute respiratory distress syndrome; LPS: Lipopolysaccharide; CGN: Chronic glomerular nephritis; MCP-1: Monocyte chemotactic protein-1; H9C2: A subclone of the original clonal cell line which exhibits many of the properties of skeletal muscle; PC12: A neuron cell line; H/R: Hypoxia/reoxygenation; OGD/R: Oxygen and glucose deprivation/reoxygenation; NSCLC: Non-small cell lung cancer; DCP1A: mRNA-decapping enzyme 1A; TMSB4X: Thymosin beta-4.
Figure 2.
Autopahgy promotion or inhibition to alleviate diseases and inflammation. AS-IV: Astragaloside IV; T2DM: Type 2 diabetes mellitus.
The autophagy-promoting effects of AS-IV are associated with inflammation
AS-IV has beneficial effects on various diseases by promoting autophagy to alleviate inflammation. These diseases include lung injury induced by PM2.5, liver injury in type 2 diabetic rats, acute spinal cord injury, myocardial hypertrophy, H1N1 infection, and flap survival.
AS-IV protected against PM2.5-induced lung toxicity in rats and in vitro through increasing autophagic flux and inhibiting severe inflammation[36,37]. The inflammatory mediators granulocyte-macrophage colony-stimulating factor, intercellular adhesion molecule 1, tumour necrosis factor (TNF) α, IL 6, IL-18 and C-reactive protein were inhibited by AS-IV[36,37]. The associated mechanism includes the AMPK/mTOR or PI3K/Akt/mTOR pathway[36,37]. AS-IV induced autophagic flux depending on AMPK activation and inhibiting phosphorylation of mTOR[36]. AS-IV primarily restored autophagic flux through autophagosome degradation and increased autophagosome-lysosome fusion[37]. AS-IV significantly inhibited the protein expression of phosphorylated phosphatidylinositol 3-kinase (p-PI3K), p-Akt and phosphorylated mechanistic target of rapamycin (p-mTOR)[37].
ASIV promoted autophagy through the AMPK/mTOR signalling pathway to further inhibit expression of TNFα and IL6 and protect against liver injury in T2DM rats[42]. ASIV reversed the suppression of the AMPK/mTOR pathway, elevated the pAMPK/AMPK ratio and reduced the pmTOR/mTOR ratio[42]. AS-IV induced autophagy to protect against liver and kidney injury in rats induced by cisplatin, inhibiting the expression of inflammatory mediators, such as the NLRP3 inflammasome[44]. AS-IV may promote functional recovery in vivo after spinal cord injury through a mechanism related to autophagy promotion and inflammatory inhibition, such as the downregulated expression of inducible NO synthase, cyclo-oxygenase-2 and TNF-α. AS-IV may promote autophagy in neuronal cells to inhibit apoptosis and polarize towards an M2 phenotype in microglial cells to attenuate neuroinflammation[29]. AS-IV activated autophagy and reduced the inflammatory mediators NLRP3 and IL-1β in cardiomyocytes, preventing hypertrophy and improving cardiac function induced by mechanical stress[20]. AS-IV activated autophagy triggered by H1N1 infection to reduce secretion of the inflammatory mediator IL-1β[53]. AS-IV promoted skin flap survival via autophagic activation in the rat McFarlane flap model[52]. The related mechanism also involved inflammatory inhibition, which was evidenced by decreased expression levels of TNF-α, IL-1β and IL6 and inhibited leukocyte infiltration[52].
The autophagy-promoting effects of AS-IV are not associated with inflammation
Diseases that benefit from the autophagic mechanism of AS-IV are not related to inflammation and include cancer, diabetes, nervous system diseases, and CVDs.
AS-IV exerted anticancer effects on lung cancer, lung adenocarcinoma cells, VSCC, and the gastric mucosa. AS-IV promoted autophagy mediated by the p53/AMPK/mTOR signalling pathway and was favourable in lung cancer[32]. AS-IV enhanced autophagy levels associated with the AKT and mTOR pathways, which was inhibited by bevacizumab in lung adenocarcinoma cells[57]. AS-IV induced autophagy and inhibited cell proliferation in VSCC, which was associated with the TGF-β/Smad pathway[50]. AS-IV promoted the expression level of the autophagy proteins Beclin1, p62, ATG5, and ATG12 to have a beneficial effect on gastric mucosa in vivo[51].
AS-IV exerted beneficial effects on diabetic complications, such as DPN and liver injury. AS-IV activated autophagy to improve renal fibrosis and function in diabetic KK-Ay mice, while an autophagy inhibitor abrogated the effect of AS-IV[40,47]. AS-IV induced autophagy promoted by AMPKα to inhibit DPN progression[39]. AS-IV enhanced autophagy to alleviate myelin sheath injury in DPN induced by Schwann cell apoptosis. Enhanced autophagy was associated with inhibition of the PI3K/Akt/mTOR signalling pathway[41]. ASIV promoted autophagy mediated by the AMPK/mTOR pathway to alleviate liver injury and insulin resistance in diabetic rats[42] .
AS-IV exerts a protective role against senescent astrocytes, ischaemic stroke, CVD and arthritis. AS-IV induced mitophagy to reduce astrocyte senescence, which is involved in PD[31]. I/R caused by ischaemic stroke leads to brain injury, and autophagy reduction plays a role in its pathology, which can be reversed by AS-IV[27]. AS-IV-induced mitochondrial autophagy has favourable effects on mitochondrial dysfunction in rat vascular smooth muscle cells induced by Ang II[58]. Autophagy was promoted by AS-IV or rapamycin to reduce chondrocyte apoptosis caused by IL-1β[55] .
The autophagy inhibition effects of AS-IV are associated with inflammation
AS-IV has beneficial effects on various diseases through autophagy inhibition to alleviate inflammation. These diseases include acute respiratory distress syndrome, kidney disease, GO, and thermal injury. For the pulmonary endothelial ARDS cell model stimulated by LPS, AS-IV inhibited inflammation and apoptosis to limit autophagy initiation, which was evidenced by the downregulated expression of autophagy proteins[34]. AS-IV-activated autophagy is associated with inhibition of the PI3K/ AKT/AS160 pathway to reduce kidney injury and inhibit inflammation in CGN rats[45]. AS-IV suppresses autophagy to reduce the expression of inflammatory mediators such as IL-6, IL-8, TNF-α, and monocyte chemoattractant protein-1, thus protecting against GO[56]. AS-IV alleviated excessive autophagy and inflammatory responses induced by heat injury in vitro and in vivo. Autophagy induced by heat stress can activate and cross talk with the PERK/eIF2α pathway[54].
The autophagy inhibition effects of AS-IV are not associated with inflammation
AS-IV exerts beneficial effects on various diseases through autophagy inhibition, which is not associated with inflammation. These diseases include heart diseases, CVD, nerve injury, liver injury, lung injury, and cancer.
AS-IV can reduce ROS-mediated autophagosome accumulation and myocardial injury caused by I/R[21]. AS-IV inhibits the cardiotoxicity of rats via autophagy inhibition and PI3K/Akt pathway activation[21]. AS-IV decreased apoptosis and autophagosome number, which were increased in hypoxia/ reoxygenation-injured H9C2 cells[21]. AS-IV inhibited autophagy and improved heart dysfunction induced by LPS[19]. AS-IV inhibited HG-induced autophagy and improved cardiac dysfunction[23]. AS-IV inhibited autophagy and decreased mineralization in in vitro and in vivo models of atherosclerosis[59]. AS-IV reversed the upregulation of autophagic proteins, such as Beclin-1, LC3II and ATG12, caused by the intestinal microbiota[28]. AS-IV blocked excessive autophagy injury in PC12 cells in response to OGD/R[30]. AS-IV inhibited mitophagy and excessive autophagy in Schwann cells induced by high glucose[38]. AS-IV reduced the number of autophagosomes and excessive autophagy, alleviating the damage to L02 hepatocytes induced by iron overload[43]. AS-IV degraded autophagosomes to alleviate lung injury caused by PM2.5 in vivo and in vitro[37]. AS-IV inhibited autophagy to counteract chemoresistance in NSCLC cells treated with cisplatin[35]. AS-IV induces autophagy to inhibit the invasion of cervical cancer[49].
CONCLUSION
AS-IV, the primary active astragaloside from Radix Astragali, has been reported to have pharmacological effects on various diseases. The pharmacokinetics characterization revealed that AS-IV was detected in 12 tissues, including the liver and kidney. AS-IV not only promotes but also inhibits autophagic activity through a variety of signalling pathways to improve various diseases. These pathways include the PI3K I/Akt/mTOR, PI3K III/Beclin-1/Bcl-2, PI3K/Akt, AMPK/mTOR, PI3K/Akt/mTOR, SIRT1–NF-κB, PI3K/AKT/AS160, and TGF-β/Smad signalling pathways. The suggested pathway in this literature review is that the autophagic proteins Atg7/Atg12 are mediated by DCP1A and TMSB4X. When the level of Atg7/Atg12 is reduced, the level of DCP1A and TMSB4X is also decreased. The autophagy-related proteins include Beclin-1, LC3II, p62, ATG16L1, ATG9B and ATG4D. AS-IV is distributed widely in various tissues and the autophagic mechanism of AS-IV is a basic biological mechanism. Hence, AS-IV is an effective therapeutic drug for various diseases. According to existing research, AS-IV possesses the potential for broad application in many diseases, and the autophagy mechanism deserves further investigation.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: April 17, 2022
First decision: May 11, 2022
Article in press: August 25, 2022
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Novita BD, Indonesia; Ortiz-Masia D, Spain; Xu G, China S-Editor: Wang DM L-Editor: Kerr C P-Editor: Wang DM
Contributor Information
Ying Yang, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Meng Hong, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Wen-Wen Lian, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Zhi Chen, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China. zjuchenzhi@zju.edu.cn.
References
- 1.Buttar HS, Li T, Ravi N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp Clin Cardiol. 2005;10:229–249. [PMC free article] [PubMed] [Google Scholar]
- 2.Fu S, Zhang J, Gao X, Xia Y, Ferrelli R, Fauci A, Guerra R, Hu L. Clinical practice of traditional Chinese medicines for chronic heart failure. Heart Asia. 2010;2:24–27. doi: 10.1136/ha.2009.001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zang Y, Wan J, Zhang Z, Huang S, Liu X, Zhang W. An updated role of astragaloside IV in heart failure. Biomed Pharmacother. 2020;126:110012. doi: 10.1016/j.biopha.2020.110012. [DOI] [PubMed] [Google Scholar]
- 4.Chinese Pharmacopoeia Commission Pharmacopoeia of the People’s Republic of China. Beijing: China medical science and technology press, 2015: 302. [Google Scholar]
- 5.Sun GX, Zhao YY, Miao PP, Yang XY, Miao Q, Li J, Xue BJ, Su J, Zhang YJ. [Stability study in biological samples and metabolites analysis of astragaloside IV in rat intestinal bacteria in vitro] Zhongguo Zhong Yao Za Zhi. 2014;39:4258–4264. [PubMed] [Google Scholar]
- 6.Monschein M, Ardjomand-Woelkart K, Rieder J, Wolf I, Heydel B, Kunert O, Heuberger H, Bauer R. Accelerated sample preparation and formation of astragaloside IV in Astragali Radix. Pharm Biol. 2013 doi: 10.3109/13880209.2013.839712. [DOI] [PubMed] [Google Scholar]
- 7.Tan YQ, Chen HW, Li J. Astragaloside IV: An Effective Drug for the Treatment of Cardiovascular Diseases. Drug Des Devel Ther. 2020;14:3731–3746. doi: 10.2147/DDDT.S272355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J ZY, Zhang C, Han J, Sun S, Wang R. Pharmacokinetics and absolute bioavailability of Astragaloside IV inclusion compound. Chin Pharm J. 2011;46:615–618. [Google Scholar]
- 9.Zhang WD, Zhang C, Liu RH, Li HL, Zhang JT, Mao C, Moran S, Chen CL. Preclinical pharmacokinetics and tissue distribution of a natural cardioprotective agent astragaloside IV in rats and dogs. Life Sci. 2006;79:808–815. doi: 10.1016/j.lfs.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Chang YX, Sun YG, Li J, Zhang QH, Guo XR, Zhang BL, Jin H, Gao XM. The experimental study of Astragalus membranaceus on meridian tropsim: the distribution study of astragaloside IV in rat tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;911:71–75. doi: 10.1016/j.jchromb.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Wu C, Gao L, Du G, Qin X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv Pharmacol. 2020;87:89–112. doi: 10.1016/bs.apha.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Gui D, Guo Y, Wang F, Liu W, Chen J, Chen Y, Huang J, Wang N. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS One. 2012;7:e39824. doi: 10.1371/journal.pone.0039824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiangbo Z, Xuying W, Yuping Z, Xili M, Yiwen Z, Tianbao Z. Effect of astragaloside IV on the embryo-fetal development of Sprague-Dawley rats and New Zealand White rabbits. J Appl Toxicol. 2009;29:381–385. doi: 10.1002/jat.1422. [DOI] [PubMed] [Google Scholar]
- 14.Xuying W, Jiangbo Z, Yuping Z, Xili M, Yiwen Z, Tianbao Z, Weidong Z. Effect of astragaloside IV on the general and peripartum reproductive toxicity in Sprague-Dawley rats. Int J Toxicol. 2010;29:505–516. doi: 10.1177/1091581810376840. [DOI] [PubMed] [Google Scholar]
- 15.Tran BX, Nghiem S, Afoakwah C, Ha GH, Doan LP, Nguyen TP, Le TT, Latkin CA, Ho CSH, Ho RCM. Global mapping of interventions to improve the quality of life of patients with cardiovascular diseases during 1990-2018. Health Qual Life Outcomes. 2020;18:254. doi: 10.1186/s12955-020-01507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moghaddam AS, Afshari JT, Esmaeili SA, Saburi E, Joneidi Z, Momtazi-Borojeni AA. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis. 2019;285:1–9. doi: 10.1016/j.atherosclerosis.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 17.WHO library cataloguing-in-publication data. Hearts: technical package for cardiovascular disease management in primary health care. September 3, 2020. [cited 17 April, 2022] Available from: https://wwwwhoint/cardiovascular_diseases/hearts/Hearts_package.pdf .
- 18.Li S, Nong Y, Gao Q, Liu J, Li Y, Cui X, Wan J, Lu J, Sun M, Wu Q, Shi X, Cui H, Liu W, Zhou M, Li L, Lin Q. Astragalus Granule Prevents Ca2+ Current Remodeling in Heart Failure by the Downregulation of CaMKII. Evid Based Complement Alternat Med. 2017;2017:7517358. doi: 10.1155/2017/7517358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Yang X, Song Y, Sun X, Li W, Zhang L, Hu X, Wang H, Zhao N, Zhuang R, Xie X, Tang F. Astragaloside IV-targeting miRNA-1 attenuates lipopolysaccharide-induced cardiac dysfunction in rats through inhibition of apoptosis and autophagy. Life Sci. 2021;275:119414. doi: 10.1016/j.lfs.2021.119414. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Wang H, Lu M, Zhao K, Yin J, Liu Y, Sun Y. Astragaloside IV prevents myocardial hypertrophy induced by mechanical stress by activating autophagy and reducing inflammation. Am J Transl Res. 2020;12:5332–5342. [PMC free article] [PubMed] [Google Scholar]
- 21.Huang KY, Yu YW, Liu S, Zhou YY, Wang JS, Peng YP, Ji KT, Xue YJ. A Single, Acute Astragaloside IV Therapy Protects Cardiomyocyte Through Attenuating Superoxide Anion-Mediated Accumulation of Autophagosomes in Myocardial Ischemia-Reperfusion Injury. Front Pharmacol. 2021;12:642925. doi: 10.3389/fphar.2021.642925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang JJ, Zhang XH, Ma XH, Duan WJ, Xu NG, Chen YJ, Liang L. Astragaloside IV enhances GATA-4 mediated myocardial protection effect in hypoxia/reoxygenation injured H9c2 cells. Nutr Metab Cardiovasc Dis. 2020;30:829–842. doi: 10.1016/j.numecd.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Qian X, Li J, Lin X, Luo J, Huang J, Jin Z. Astragaloside-IV protects H9C2(2-1) cardiomyocytes from high glucose-induced injury via miR-34a-mediated autophagy pathway. Artif Cells Nanomed Biotechnol. 2019;47:4172–4181. doi: 10.1080/21691401.2019.1687492. [DOI] [PubMed] [Google Scholar]
- 24.Luo LF, Qin LY, Wang JX, Guan P, Wang N, Ji ES. Astragaloside IV Attenuates the Myocardial Injury Caused by Adriamycin by Inhibiting Autophagy. Front Pharmacol. 2021;12:669782. doi: 10.3389/fphar.2021.669782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang HL, Zhou QH, Xu MB, Zhou XL, Zheng GQ. Astragaloside IV for Experimental Focal Cerebral Ischemia: Preclinical Evidence and Possible Mechanisms. Oxid Med Cell Longev. 2017;2017:8424326. doi: 10.1155/2017/8424326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang X, Su S, Hong W, Geng W, Tang H. Research Progress on the Ability of Astragaloside IV to Protect the Brain Against Ischemia-Reperfusion Injury. Front Neurosci. 2021;15:755902. doi: 10.3389/fnins.2021.755902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhang Y, Jin XF, Zhou XH, Dong XH, Yu WT, Gao WJ. The Role of Astragaloside IV against Cerebral Ischemia/Reperfusion Injury: Suppression of Apoptosis via Promotion of P62-LC3-Autophagy. Molecules. 2019;24 doi: 10.3390/molecules24091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu N, Kan P, Yao X, Yang P, Wang J, Xiang L, Zhu Y. Astragaloside IV reversed the autophagy and oxidative stress induced by the intestinal microbiota of AIS in mice. J Microbiol. 2018;56:838–846. doi: 10.1007/s12275-018-8327-5. [DOI] [PubMed] [Google Scholar]
- 29.Lin J, Pan X, Huang C, Gu M, Chen X, Zheng X, Shao Z, Hu S, Wang B, Lin H, Wu Y, Tian N, Gao W, Zhou Y, Zhang X, Wang X. Dual regulation of microglia and neurons by Astragaloside IV-mediated mTORC1 suppression promotes functional recovery after acute spinal cord injury. J Cell Mol Med. 2020;24:671–685. doi: 10.1111/jcmm.14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang XP, Ding H, Yang XQ, Li JX, Tang B, Liu XD, Tang YH, Deng CQ. Synergism and mechanism of Astragaloside IV combined with Ginsenoside Rg1 against autophagic injury of PC12 cells induced by oxygen glucose deprivation/reoxygenation. Biomed Pharmacother. 2017;89:124–134. doi: 10.1016/j.biopha.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Xia ML, Xie XH, Ding JH, Du RH, Hu G. Astragaloside IV inhibits astrocyte senescence: implication in Parkinson's disease. J Neuroinflammation. 2020;17:105. doi: 10.1186/s12974-020-01791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B, Yang N, Chen Y, Zhu M, Lian Y, Xiong Z, Wang B, Feng L, Jia X. An Integrated Strategy for Effective-Component Discovery of Astragali Radix in the Treatment of Lung Cancer. Front Pharmacol. 2020;11:580978. doi: 10.3389/fphar.2020.580978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang A, Zheng Y, Que Z, Zhang L, Lin S, Le V, Liu J, Tian J. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J Cancer Res Clin Oncol. 2014;140:1883–1890. doi: 10.1007/s00432-014-1744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu B, Zhao H, Wang Y, Zhang H, Ma Y. Astragaloside IV Attenuates Lipopolysaccharides-Induced Pulmonary Epithelial Cell Injury through Inhibiting Autophagy. Pharmacology. 2020;105:90–101. doi: 10.1159/000502865. [DOI] [PubMed] [Google Scholar]
- 35.Lai ST, Wang Y, Peng F. Astragaloside IV sensitizes non-small cell lung cancer cells to cisplatin by suppressing endoplasmic reticulum stress and autophagy. J Thorac Dis. 2020;12:3715–3724. doi: 10.21037/jtd-20-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Wu Y, Pei C, Wang M, Wang X, Shi S, Huang D, Wang Y, Li S, Xiao W, He Y, Wang F. Astragaloside IV pre-treatment attenuates PM2.5-induced lung injury in rats: Impact on autophagy, apoptosis and inflammation. Phytomedicine. 2022;96:153912. doi: 10.1016/j.phymed.2021.153912. [DOI] [PubMed] [Google Scholar]
- 37.Pei C, Wang F, Huang D, Shi S, Wang X, Wang Y, Li S, Wu Y, Wang Z. Astragaloside IV Protects from PM2.5-Induced Lung Injury by Regulating Autophagy via Inhibition of PI3K/Akt/mTOR Signaling in vivo and in vitro. J Inflamm Res. 2021;14:4707–4721. doi: 10.2147/JIR.S312167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei X, Zheng Y, Ai Y, Li B. Regulatory Effects of Astragaloside IV on Hyperglycemia-Induced Mitophagy in Schwann Cells. Evid Based Complement Alternat Med. 2022;2022:7864308. doi: 10.1155/2022/7864308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo H, Wang Y, Zhang X, Zang Y, Zhang Y, Wang L, Wang H, Cao A, Peng W. Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKα-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci Rep. 2017;7:6852. doi: 10.1038/s41598-017-07061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Gao Y, Tian N, Wang T, Shi Y, Xu J, Wu B. Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT-NF-κB p65 axis. Sci Rep. 2019;9:323. doi: 10.1038/s41598-018-36911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin Y, Qu H, Yang Q, Fang Z, Gao R. Astragaloside IV alleviates Schwann cell injury in diabetic peripheral neuropathy by regulating microRNA-155-mediated autophagy. Phytomedicine. 2021;92:153749. doi: 10.1016/j.phymed.2021.153749. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Y, Su Y, Zhang J, Zhang Y, Li Y, Han Y, Dong X, Li W. Astragaloside IV alleviates liver injury in type 2 diabetes due to promotion of AMPK/mTORmediated autophagy. Mol Med Rep. 2021;23 doi: 10.3892/mmr.2021.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie D, Zhou P, Liu L, Jiang W, Xie H, Zhang L, Xie D. Protective Effect of Astragaloside IV on Hepatic Injury Induced by Iron Overload. Biomed Res Int. 2019;2019:3103946. doi: 10.1155/2019/3103946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qu X, Gao H, Tao L, Zhang Y, Zhai J, Sun J, Song Y, Zhang S. Astragaloside IV protects against cisplatin-induced liver and kidney injury via autophagy-mediated inhibition of NLRP3 in rats. J Toxicol Sci. 2019;44:167–175. doi: 10.2131/jts.44.167. [DOI] [PubMed] [Google Scholar]
- 45.Floege J, Amann K. Primary glomerulonephritides. Lancet. 2016;387:2036–2048. doi: 10.1016/S0140-6736(16)00272-5. [DOI] [PubMed] [Google Scholar]
- 46.Lu R, Chen J, Liu B, Lin H, Bai L, Zhang P, Chen D, Li H, Li J, Pang Y, Zhou Y, Zhou J, Wu J. Protective role of Astragaloside IV in chronic glomerulonephritis by activating autophagy through PI3K/AKT/AS160 pathway. Phytother Res. 2020;34:3236–3248. doi: 10.1002/ptr.6772. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Gao Y, Tian N, Zhu Z, Wang T, Xu J, Wu B, Zhang N. Astragaloside IV represses high glucose-induced mesangial cells activation by enhancing autophagy via SIRT1 deacetylation of NF-κB p65 subunit. Drug Des Devel Ther. 2018;12:2971–2980. doi: 10.2147/DDDT.S174058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li QW, Zhang GL, Hao CX, Ma YF, Sun X, Zhang Y, Cao KX, Li BX, Yang GW, Wang XM. SANT, a novel Chinese herbal monomer combination, decreasing tumor growth and angiogenesis via modulating autophagy in heparanase overexpressed triple-negative breast cancer. J Ethnopharmacol. 2021;266:113430. doi: 10.1016/j.jep.2020.113430. [DOI] [PubMed] [Google Scholar]
- 49.Xia C, He Z, Cai Y. Quantitative proteomics analysis of differentially expressed proteins induced by astragaloside IV in cervical cancer cell invasion. Cell Mol Biol Lett. 2020;25:25. doi: 10.1186/s11658-020-00218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Wang L, Wang Y, Dong S, Yang S, Guan Y, Wu X. Astragaloside IV inhibits cell proliferation in vulvar squamous cell carcinoma through the TGF-β/Smad signaling pathway. Dermatol Ther. 2019;32:e12802. doi: 10.1111/dth.12802. [DOI] [PubMed] [Google Scholar]
- 51.Cai T, Zhang C, Zhao Z, Li S, Cai H, Chen X, Cai D, Liu W, Yan Y, Xie K, Pan H, Zeng X. The gastric mucosal protective effects of astragaloside IV in mnng-induced GPL rats. Biomed Pharmacother. 2018;104:291–299. doi: 10.1016/j.biopha.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 52.Lin R, Chen H, Callow D, Li S, Wang L, Chen L, Ding J, Gao W, Xu H, Kong J, Zhou K. Multifaceted effects of astragaloside IV on promotion of random pattern skin flap survival in rats. Am J Transl Res. 2017;9:4161–4172. [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J, Zhang W, Ren L, He Y, Mei Z, Feng J, Shi T, Zhang H, Song Z, Jie Z. Astragaloside IV attenuates IL-1β secretion by enhancing autophagy in H1N1 infection. FEMS Microbiol Lett. 2020;367 doi: 10.1093/femsle/fnaa007. [DOI] [PubMed] [Google Scholar]
- 54.Dong Z, Zhou J, Zhang Y, Chen Y, Yang Z, Huang G, Yuan Z, Peng Y, Cao T. Astragaloside-IV Alleviates Heat-Induced Inflammation by Inhibiting Endoplasmic Reticulum Stress and Autophagy. Cell Physiol Biochem. 2017;42:824–837. doi: 10.1159/000478626. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Meng Q, Jing H, Zhou S. Astragaloside IV protects against apoptosis in human degenerative chondrocytes through autophagy activation. Mol Med Rep. 2017;16:3269–3275. doi: 10.3892/mmr.2017.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Zhang Y, Min J, Gao L, Zhang R, Yang Y. Astragaloside IV attenuates orbital inflammation in Graves' orbitopathy through suppression of autophagy. Inflamm Res. 2018;67:117–127. doi: 10.1007/s00011-017-1100-0. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Li G, Chen M, Cai R. Astragaloside IV enhances the sensibility of lung adenocarcinoma cells to bevacizumab by inhibiting autophagy. Drug Dev Res. 2022;83:461–469. doi: 10.1002/ddr.21878. [DOI] [PubMed] [Google Scholar]
- 58.Lu Y, Li S, Wu H, Bian Z, Xu J, Gu C, Chen X, Yang D. Beneficial effects of astragaloside IV against angiotensin II-induced mitochondrial dysfunction in rat vascular smooth muscle cells. Int J Mol Med. 2015;36:1223–1232. doi: 10.3892/ijmm.2015.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song Z, Wei D, Chen Y, Chen L, Bian Y, Shen Y, Chen J, Pan Y. Association of astragaloside IV-inhibited autophagy and mineralization in vascular smooth muscle cells with lncRNA H19 and DUSP5-mediated ERK signaling. Toxicol Appl Pharmacol. 2019;364:45–54. doi: 10.1016/j.taap.2018.12.002. [DOI] [PubMed] [Google Scholar]