Abstract
Purpose:
The addition of cytotoxic chemotherapy to immune-checkpoint inhibitor (ICI) may enhance antitumor effects. We conducted an open-label randomized phase II/III study to evaluate nivolumab + docetaxel combination therapy in comparison with nivolumab monotherapy for previously treated ICI-naïve non–small cell lung cancer (NSCLC).
Patients and Methods:
The primary endpoint of the phase III study was overall survival (OS), and the secondary endpoints included progression-free survival (PFS), overall response rate (ORR), and toxicity. As ICI and platinum-doublet combination chemotherapy was approved in the first-line setting during this study, patient accrual was discontinued.
Results:
One hundred twenty-eight patients (each arm, n = 64) were included in the full analysis set. The median OS in nivolumab (arm A) and nivolumab + docetaxel (arm B) was 14.7 months (95% CI, 11.4–18.7) and 23.1 months (95% CI, 16.7–NR), respectively. The HR for OS was 0.63 (90% CI, 0.42–0.95; P = 0.0310). The median PFS in arms A and arm B was 3.1 months (95% CI, 2.0–3.9) and 6.7 months (95% CI, 3.8–9.4), respectively. The HR for progression was 0.58 (95% CI, 0.39–0.88; P = 0.0095). The ORR was 14.0% (95% CI, 6.3–25.8) in arm A and 41.8% (95% CI, 28.7–55.9) in arm B. Hematotoxicity and gastrointestinal adverse events were more common in arm B than in arm A. Two treatment-related deaths were observed, including one patient in arm A who died of pneumonitis and one in arm B who died of myocarditis.
Conclusions:
Despite a slightly elevated toxicity, the addition of docetaxel to nivolumab has significantly prolonged the OS and PFS of patients with previously treated ICI-naïve NSCLC.
Translational Relevance.
Multiple prospective studies compared chemotherapy with and without immune-checkpoint inhibitors (ICI) and proved that the combination therapy prolongs survival. However, no prospective studies comparing ICI with and without chemotherapy have been announced. We herein report the result of an open-label randomized phase II/III study that evaluated nivolumab + docetaxel combination therapy in comparison with nivolumab monotherapy for previously treated ICI-naïve non–small cell lung cancer. The protocol was amended to reduce the total sample size and to shorten the follow-up period, due to the first-line approval of chemotherapy + ICI regimens during this study. The overall survival, progression-free survival, and overall response rate were significantly better in combination with nivolumab + docetaxel than nivolumab monotherapy. Although the toxicity profile, especially hematotoxicity, was more significant in the nivolumab + docetaxel arm, most of them were manageable. The survival benefit of the chemotherapy + ICI combination compared with ICI monotherapy proved by our study is quite innovative, although these results have to be viewed with caution due to the early termination.
Introduction
Lung cancer is the world's leading cause of cancer death, and non–small cell lung cancer (NSCLC) accounts for >80% of all lung cancer cases. Despite the development of whole-genome sequencing techniques, and efforts to create new targeted therapies, lung cancer still carries a dismal prognosis. Nevertheless, the introduction of immune-checkpoint inhibitors (ICI) has drastically changed the course of treatment for various malignancies, including lung cancer, giving hope for a prolonged response that would not have been expected with any other chemotherapies.
Cancer cells generally take over checkpoints that control the immune system and secure an escape route from the cytotoxic T-cell (CTL) attack. Nivolumab, an anti–PD-1 inhibitor, blocks immune-checkpoint molecules on the T-cell surface and reverses their suppression. Cytotoxic chemotherapy helps release tumor antigens and activate CTLs (1). As reported in animal models, chemotherapy also makes tumor cells vulnerable to lymphocyte-mediated tag cells (2). Consequently, the combination of cytotoxic chemotherapy and ICI could enhance antitumor benefits. In fact, multiple global clinical trials have demonstrated the efficacy of combined chemo-ICI in previously untreated NSCLC patients. Each study showed a promising survival benefit without intolerable toxicities (3, 4, 5).
Second-line nivolumab treatment was well evaluated in Checkmate017 and Checkmate057 (6, 7). Both studies compared docetaxel and nivolumab in second-line treatment, with Checkmate017 targeting squamous cell carcinoma and Checkmate057 treating nonsquamous cell carcinoma. Since the superiority of nivolumab was proven in both studies, it successively replaced the previously established docetaxel therapy for refractory or recurrent NSCLC after first-line platinum-doublet therapy. The addition of a cytotoxic agent to an ICI (as demonstrated in first-line combination therapy) may enhance the clinical advantage over nivolumab alone. In addition, we hypothesized that disease progression at an early stage after ICI initiation, which is often encountered in clinical practice, can be avoided by administering ICI plus chemotherapy together.
Therefore, we conducted a randomized phase II/III study comparing nivolumab monotherapy with nivolumab + docetaxel combination therapy. This is the first study to compare chemo-ICI and ICI alone for previously treated or recurrent ICI-naïve advanced NSCLC. Our main objective is to prove the superiority of docetaxel and nivolumab therapy over nivolumab alone in this population.
Patients and Methods
Patient selection
Patients with histologically or cytologically proven NSCLC, stage IIIB/ IIIC/IV (UICC-TNM classification, 8th edition) or postoperative recurrence, who received one or two previous chemotherapy regimens, excluding EGFR or ALK tyrosine kinase inhibitors (TKI), were eligible for inclusion. The switch maintenance therapy was considered as two regimens, whereas the continuous maintenance therapy was counted as one. The adjuvant chemotherapy was counted as one regimen when administered within 1 year in cases with postoperative recurrence. Cases involving NSCLC with known driver mutations (e.g., EGFR mutation or EML4–ALK translocation) required pretreatment with TKIs, as instructed in the NCCN guidelines. The other eligibility criteria included age ≥20 years, performance status (PS) 0–1, neutrophil count ≥1,500 /mm3, hemoglobin ≥9.0 g/dL, platelet count ≥100,000 /mm3, aspartate aminotransferase (AST) ≤100 IU/L, alanine aminotransferase (ALT) ≤100 IU/L, creatinine ≤1.5 mg/dL, and SpO2 ≥92% or PaO2 ≥60 torr in room air.
Major exclusion criteria included the previous administration of docetaxel or ICI (e.g., anti–PD-1/anti–PD-L1/CTLA-4 inhibitors), usage of immunosuppressive medication or steroid therapy equivalent to ≥5 mg/day of prednisolone, interstitial pneumonia on CT, symptomatic brain metastasis, active concomitant malignancy, major operations within 28 days, and palliative radiotherapy within 2 weeks, or curative radiotherapy within 6 weeks prior to the study enrollment. Tumor PD-L1 expression (measured with 28-8 kit) was evaluated as a biomarker when available (patients were enrolled irrespective of the expression).
Each patient gave their written informed consent before enrollment. This study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol fulfilled the criteria for a specified clinical trial based on the Clinical Trials Act (Act No. 16, 2017) and for advanced medical care B (approved by the Minister of Health, Labour and Welfare with the aim of insurance coverage in the future) in Japan, and was approved by the institutional review board of each study site. This study was registered in the Japan Registry of Clinical Trials (protocol no. jRCTs031180331).
Study design and treatment
This multi-institutional, open-label, phase II/III clinical research was initiated in November 2017. Enrolled patients were randomly assigned to arm A or B with stratification factors of PS (0 vs. 1), histologic type (squamous cell carcinoma vs. nonsquamous cell carcinoma), sex (male vs. female), and driver oncogene status (EGFR mutation and ALK translocation).
Nivolumab (240 mg per body, every 2 weeks) was administered in both arms, and docetaxel (60 mg/m2) was administered every 4 weeks. Triweekly docetaxel 60 mg/m2 is the recommended dose and schedule in Japan for advanced NSCLC based on the result of two phase II trials (8, 9). The schedule was modified in order to administer both nivolumab and docetaxel at the same timing. Both treatments were continued until the development of progressive disease (PD), as defined by the Response Evaluation Criteria in Solid Tumors (RECIST v1.1) system, or the occurrence of intolerable adverse events.
The patients must meet certain criteria in order to receive the next cycle of treatment, including PS 0–2, afebrile, neutrophil count ≥1,500 /mm3 (≥1,000/mm3 for day 15), hemoglobin ≥8.0 g/dL, platelet count ≥100,000 /mm3, AST ≤100 IU/L, ALT ≤100 IU/L, creatinine ≤1.5 mg/dL, albumin ≥2.0 g/dL, total bilirubin ≤2.0 mg/dL, no pneumonitis, and nonhematologic toxicities of grade 2 or less. The protocol treatment was withdrawn unless the next cycle was started within 42 days. The dose modification criteria for docetaxel included grade 4 neutropenia and thrombocytopenia, and grade 3 or worse nonhematologic adverse events. Dose of docetaxel was reduced to 50 mg/m2 and to 40 mg/m2 every time the modification criteria were noted. Monotherapy with either nivolumab or docetaxel was not allowed in arm B within the protocol. Post-study treatment had no restrictions.
Evaluation
Imaging studies were repeated every 8 ± 1 weeks for the first 6 months, and then every 12 ± 1 weeks thereafter. The overall response rate (ORR) was defined as the proportion of patients with a complete or partial response to therapy; a 6-week term was required to establish stable disease. Progression-free survival (PFS) was assessed from the date of enrollment to disease progression (according to RECIST v1.1) or death from any cause. Overall survival (OS) was defined as the time from enrollment until death.
Adverse events were graded using the NCI–Common Terminology Criteria for Adverse Events v4.0 (NCI-CTCAE v4.0).
Statistical analysis
Since data on combined ICI and cytotoxic chemotherapy were limited in 2017, when this study was initiated, the safety profile required assessment in a phase II study before proceeding to phase III. The phase II population was also included in the phase III analysis. The primary endpoints for the phase II noncomparative trial were the 6-month PFS rate and the occurrence of grade ≥3 pneumonitis within 12 weeks. In the phase II part, according to the previous Checkmate017/057 and Keynote-010 studies (6, 7, 10), the threshold and expected 6-month PFS rates for arm B were set 20% and 35%, respectively. Forty-six subjects would be required with a one-sided α of 0.1 and 85% power. Thus, phase II study registration was held off once 50 patients were assigned to arm B. Once the 6-month PFS rate was evaluated for the null hypothesis of arm B to be rejected, and the lower limit of the 80% confidence interval of grade ≥3 pneumonitis incidents was confirmed to be <7%, the phase III study was initiated.
The primary endpoint for the phase III comparative part was OS; the secondary endpoints were PFS, ORR, and safety. We assumed a median OS (mOS) of 10.5 months in patients receiving second-line nivolumab therapy based on Checkmate017 and Checkmate057 (6, 7). An absolute improvement of 3.5 months was considered to indicate a potential benefit from the addition of docetaxel, because the mOS of docetaxel versus best supportive care in previously treated NSCLC was 7.5 versus 4.6 months, respectively (11). We estimated the mOS of arms A and B as 10.5 and 14.0 months, respectively, which resulted in a hazard ratio of 0.75. We required 169 subjects in each arm to achieve 80% power with a one-sided α of 0.05 and a 2-year follow-up period. With the assumption of and accommodations for an anticipated 10% loss to follow-up, the planned initial sample size was 350 patients.
OS, PFS, and ORR were analyzed in the full analysis set (all patients who received the protocol treatment at least once and who met the eligibility criteria). OS and PFS were compared using a stratified log-rank test based on stratification variables of PS, histology, sex, and driver mutation status. Hazard ratios were calculated using a stratified Cox proportional hazards model. Survival curves were estimated using the Kaplan–Meier method. The ORR was compared between the two arms using Fisher exact test. One-sided P < 0.05 for the primary endpoint and two-sided P < 0.05 for the secondary endpoint were considered statistically significant.
In Japan, ICIs were approved as part of first-line therapy either as a monotherapy or combination therapy with platinum-doublet in late 2018, which drastically slowed patient enrollment. On June 30, 2020, according to the recommendations of an Independent Data Monitoring Committee, the protocol was amended to reduce the total sample size to 131. A total of 128 patients were thus subjected to endpoint analysis, and (1 −β) was calculated to be 0.46, with a one-sided α of 0.05.
Given that the pooled median survival time for both arms was 16.7 months, it was considered virtually impossible for the power to exceed 50% when calculating with the hazard ratio of 0.75 at the point of 6 months after enrollment discontinuation. In addition, because approximately 80% of the subjects were enrolled by June 2019, an Independent Data Monitoring Committee recommended that the follow-up period be shortened from 2 to 1 year from accrual cessation (June 2021).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author (Y.T.) upon reasonable request.
Results
Phase II trial
As the 50th patient in the phase II part was assigned to arm B on June 27, 2019, accrual was temporarily closed to assess phase II results. The 6-month PFS rate for arm B was 64.4% (80% CI, 53.4–73.5), in which the lower limit of 80% CI successfully exceeded the threshold 6-month PFS rate (20%). No cases of grade ≥3 pneumonitis occurred at the data cutoff point. Accordingly, an Independent Data Monitoring Committee recommended proceeding to the phase III study.
Phase III trial
Patient characteristics
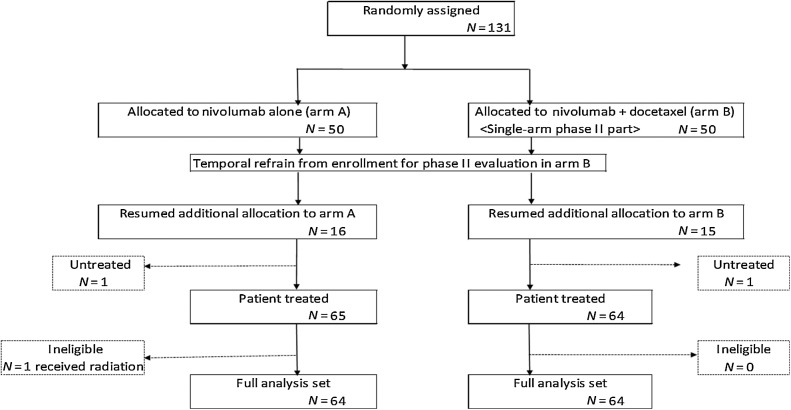
Out of 131 enrolled patients, 66 and 65 patients were assigned to arms A and B, respectively. One patient from each arm did not receive the protocol treatment because of patient refusal, and one patient of arm A was found to be ineligible after randomization. A total of 128 patients (each arm, n = 64) were included in the full analysis set; 129 patients (arm A, n = 65; arm B, n = 64) were included in the safety set (Fig. 1). Patient characteristics were well balanced between each arm (Table 1). PD-L1 (28–8) was examined in 43.8% (28/64) and 34.4% (22/64) of the patients in arms A and B, respectively, and was not significantly different. EGFR mutation was detected in 14 (21.9%) and 13 (20.3%) patients in arms A and B, respectively. ALK translocation was not detected in any patients.
Figure 1.
Summary of patient enrollment and disposition.
Table 1.
Patient characteristics.
| Group, N (%) | ||
|---|---|---|
| Arm A (N = 64) | Arm B (N = 64) | |
| Sex | ||
| Male | 44 (68.8) | 45 (70.3) |
| Female | 20 (31.3) | 19 (29.7) |
| Age | ||
| Median (range) | 69.5 (35–84) | 69 (45–83) |
| <65 | 22 (34.4) | 18 (28.1) |
| ≥65 | 42 (65.6) | 46 (71.9) |
| PS | ||
| 0 | 22 (34.4) | 21 (32.8) |
| 1 | 42 (65.6) | 43 (67.2) |
| Histology | ||
| Squamous cell carcinoma | 14 (21.9) | 12 (18.8) |
| Nonsquamous cell carcinoma | 50 (78.1) | 52 (81.3) |
| Staging | ||
| IIIB–IIIC | 5 (7.8) | 2 (3.1) |
| IVA | 29 (45.3) | 29 (45.3) |
| IVB | 30 (46.9) | 33 (51.6) |
| Recurrence | 20 (31.3) | 15 (23.4) |
| PD-L1 (28-8) | ||
| ≥50 | 3 (4.6) | 2 (3.1) |
| 1–49 | 13 (20.3) | 10 (15.6) |
| 0 | 12 (18.8) | 10 (15.6) |
| Unknown | 36 (56.3) | 42 (65.6) |
| Smoking history | ||
| Never | 15 (23.4) | 12 (18.8) |
| Ever | 49 (76.6) | 52 (81.3) |
| No. of previous systemic regimens | ||
| 1 | 57 (89.1) | 58 (90.6) |
| 2 | 7 (10.9) | 6 (9.4) |
| Oncotarget mutation | ||
| Positive EGFR mutation status | 14 (21.9) | 13 (20.3) |
| Positive EML4-ALK translocation status | 0 | 0 |
| CNS metastasis | 9 (14.1) | 14 (21.9) |
Abbreviation: CNS, central nervous system.
Treatment delivery
As of June 2021 (data cutoff), the median follow-up time was 12.5 months (range, 0.2–31.8) and 18.9 months (range, 0.6–39.1) in arms A and B, respectively. Four patients in arm A and two patients in arm B were still on the protocol treatment at the data cutoff. The median number of treatment cycles was 2 (range, 1–28) and 4 (range, 1–31) in arms A and B, respectively. Forty-six (71.9%) in arm A and 33 (51.6%) in arm B discontinued the treatment due to disease progression, while 6 (9.4%) patients in arm A and 25 (39.1%) in arm B stopped treatment due to adverse events (Table 2). Out of 28 patients who discontinued treatment due to adverse events or patients’ wishes in arm B, 5 continued nivolumab monotherapy as off-protocol treatment. Docetaxel was reduced to 50 mg/m2 in 37.5% (24/64) and to 40 mg/m2 in 18.8% (12/64). The median RDI (relative dose intensity) for docetaxel was 90.0% (range, 65.7–100). The interval between treatment cycles was extended in 42.2% (27/64) and 84.4% (54/64) of patients in arms A and B, respectively.
Table 2.
Treatment cycles and the reasons for discontinuation.
| Arm A (N = 64) | Arm B (N = 64) | |
|---|---|---|
| Treatment courses, median (range) | 2 (1–28) | 4 (1–31) |
| Reasons for discontinuation | ||
| Disease progression, n (%) | 46 (71.9) | 33 (51.6) |
| Adverse events, n (%) | 6 (9.4) | 25 (39.1) |
| Participants' wish, n (%) | 7 (10.9) | 3 (4.7) |
| Death, n (%) | 0 | 1 (1.6) |
| Others, n (%) | 1 (1.6) | 0 |
Efficacy (OS, PFS, and ORR)
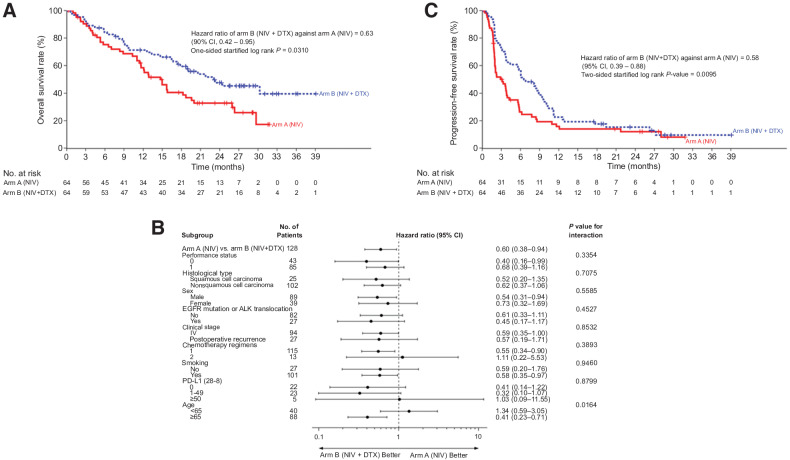
OS curves according to the assigned treatment are shown in Fig. 2A. Overall, with 75 events (arm A, n = 42; arm B, n = 33), the mOS was 14.7 months (95% CI, 11.4–18.7) and 23.1 months (95% CI, 16.7–NR) in arms A and B, respectively. The HR of OS was 0.63 (90% CI, 0.42–0.95; P = 0.0310). An OS subgroup analysis was conducted according to patient characteristics, and the HRs consistently favored arm B across all prespecified and post hoc patient subgroups, except patients who received 2 previous chemotherapy lines, patients with >50% PD-L1 expression, and patients of <65 years of age (Fig. 2B). The mOS of arm B was still better than that of arm A who received docetaxel-containing regimens as a subsequent therapy (15.0 months in cross-over arm A; 95% CI, 11.0–18.7; vs. 23.1 months in arm B; 95% CI, 16.7–NR; HR 0.53; 95% CI, 0.31–0.90).
Figure 2.
Kaplan–Meier curves for (A) overall survival, (B) subgroup analysis for overall survival, and (C) progression-free survival. CI, confidence interval; HR, hazard ratio.
With 109 events (arm A, n = 54; arm B, n = 55), the median PFS in arms A and B was 3.1 months (95% CI, 2.0–3.9) and 6.7 months (95% CI, 3.8–9.4), respectively (Fig. 2C). The corresponding HR for PFS was 0.58 (95% CI, 0.39–0.88; P = 0.0095), and the 6-month and 12-month PFS rates were 26.4% (95% CI, 16.0–37.9) and 15.8% (95% CI, 43.3–67.4), respectively, in arm A, and 56.3% (95% CI, 7.9–26.3) and 22.6% (95% CI, 13.2–33.5) in arm B. PFS subgroup analysis is shown in Supplementary Fig. S1; there was a consistent tendency to favor arm B across all patient subgroups, except for patients receiving third-line or later chemotherapy. The survival data of each subgroup are shown in Supplementary Table S1.
The ORR was 14.0% (95% CI, 6.3–25.8) in arm A and 41.8% (95% CI, 28.7–55.9) in arm B (P = 0.0014; Table 3). A waterfall plot showing the best response in target lesions from baseline in each treatment arm is shown in Supplementary Fig. S2.
Table 3.
Overall response rate.
| Arm A | Arm B | ||||
|---|---|---|---|---|---|
| (N = 57) | (N = 55) | ||||
| n (%) | [95% CI] | n (%) | [95% CI] | P | |
| Best overall response | |||||
| CR | 0 | — | 1 (1.8) | — | |
| PR | 8 (14.0) | — | 22 (40.0) | — | |
| SD | 20 (35.1) | — | 21 (38.2) | — | |
| PD | 29 (50.9) | — | 11 (20.0) | — | |
| Response rate | 8 (14.0) | [6.3–25.8] | 23 (41.8) | [28.7–55.9] | 0.0014 |
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
As for the EGFR-mutant subgroup, mOS (11.0 months in arm A; 95% CI, 3.5–14.0; vs. 20.6 months in arm B; 95% CI, 5.8–NR; HR 0.45; 95% CI, 0.17–1.17) and mPFS (1.8 months in arm A; 95% CI, 1.4–2.0; vs. 3.7 months in arm B; 95% CI, 1.9–6.0; HR 0.26; 95% CI, 0.10–0.65) both tended to be better in arm B compared with arm A. The ORR in this population was 7.1% in arm A and 30.8% in arm B.
Toxicities
Toxicities are shown in Table 4. Febrile neutropenia occurred in 0% (n = 0) and 20.3% (n = 13) of the patients in arms A and B, respectively. In arms A and B, 2 (3.1%) and 8 (12.5%) patients developed grade 3–4 anemia, respectively. Although all-grade gastrointestinal disorders were more commonly seen in arm B (n = 34, 53.1%) than in arm A (n = 21, 32.3%), the incidence of grade 3–4 was not significantly different. Three patients (arm A, n = 1; arm B, n = 2) developed grade 3 pneumonitis. Grade 5 pneumonitis occurred in one case in arm A, and grade 5 myocarditis occurred in one case in arm B. A case of grade 4 myasthenia gravis was reported in arm B.
Table 4.
Toxicities.
| Group, N (%) | ||||||
|---|---|---|---|---|---|---|
| Arm A (N = 65) | Arm B (N = 64) | |||||
| Any grade | Grade 3/4 | Grade 5 | Any grade | Grade 3/4 | Grade 5 | |
| Neutrophil count decreased | 7 (10.8) | 1 (1.5) | 0 | 60 (93.8) | 57 (89.1) | 0 |
| Platelet count decreased | 7 (10.8) | 0 | 0 | 26 (40.6) | 1 (1.6) | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 13 (20.3) | 13 (20.3) | 0 |
| Anemia | 17 (26.2) | 2 (3.1) | 0 | 46 (71.9) | 8 (12.5) | 0 |
| Nausea | 5 (7.7) | 0 | 0 | 12 (18.8) | 1 (1.6) | 0 |
| Diarrhea | 12 (18.5) | 1 (1.5) | 0 | 10 (15.6) | 0 | 0 |
| Mucositis oral | 3 (4.6) | 0 | 0 | 8 (12.5) | 1 (1.6) | 0 |
| Constipation | 8 (12.3) | 0 | 0 | 15 (23.4) | 0 | 0 |
| Vomiting | 2 (3.1) | 0 | 0 | 7 (10.9) | 0 | 0 |
| Fatigue | 20 (30.8) | 0 | 0 | 37 (57.8) | 0 | 0 |
| Malaise | 8 (12.3) | 2 (3.1) | 0 | 13 (20.3) | 3 (4.7) | 0 |
| Sepsis | 1 (1.5) | 1 (1.5) | 0 | 0 | 0 | 0 |
| Lung infection | 3 (4.6) | 0 | 0 | 6 (9.4) | 2 (3.1) | 0 |
| Creatine phosphokinase increased | 1 (1.5) | 1 (1.5) | 0 | 1 (1.6) | 1 (1.6) | 0 |
| ALT increased | 10 (15.4) | 1 (1.5) | 0 | 20 (31.3) | 2 (3.1) | 0 |
| AST increased | 16 (24.6) | 2 (3.1) | 0 | 23 (35.9) | 5 (7.8) | 0 |
| Creatinine increased | 17 (26.2) | 1 (1.5) | 0 | 14 (21.9) | 1 (1.6) | 0 |
| Hyponatremia | 14 (21.5) | 1 (1.5) | 0 | 25 (39.1) | 5 (7.8) | 0 |
| Myocarditis | 0 | 0 | 0 | 1 (1.6) | 0 (0) | 1 (1.6) |
| Sclerosing cholangitis | 1 (1.5) | 1 (1.5) | 0 | 0 | 0 | 0 |
| Myositis | 0 | 0 | 0 | 1 (1.6) | 1 (1.6) | 0 |
| Myasthenia gravis | 0 | 0 | 0 | 1 (1.6) | 1 (1.6) | 0 |
| Peripheral sensory neuropathy | 2 (3.1) | 0 | 0 | 7 (10.9) | 0 | 0 |
| Pneumonitis | 9 (13.8) | 1 (1.5) | 1 (1.5) | 8 (12.5) | 2 (3.1) | 0 |
| Alopecia | 0 | 0 | 0 | 28 (43.8) | 0 | 0 |
| Dermatologic disorders | 6 (9.2) | 0 | 0 | 37 (57.8) | 1 (1.6) | 0 |
The detail of the adverse events leading to discontinuation is listed in Supplementary Table S2, and most of which in arm B was docetaxel-related hematotoxicity. There are 3 and 6 discontinuations following pneumonitis events in arms A and B, respectively. Two of 3 in arm A and 2 of 6 in arm B were graded 3 or more.
Postprotocol treatment
In arms A and B, 85.2% (46/54) and 71.2% (37/52) of patients received subsequent chemotherapy after disease progression, respectively. Out of 46 patients who received successive therapy in arm A, 32 (69.6%) received docetaxel-containing regimens (15 with docetaxel alone, 17 with docetaxel plus ramucirumab). ICIs, including nivolumab, were administered to 15.2% and 54.1% of the patients in arms A and B, respectively (Supplementary Table S3).
Discussion
To our knowledge, this is the first randomized clinical trial suggesting that the addition of cytotoxic chemotherapy to ICI significantly improved survival benefit over ICI monotherapy in any treatment line for any cancer type. According to our study, regardless of sex, PS, histologic type, the expression of PD-L1, or driver mutation status, the addition of docetaxel to nivolumab provides a survival benefit in second-line or subsequent treatments for NSCLC. OS and PFS in patients who received nivolumab + docetaxel dual therapy were significantly prolonged in comparison with those who received standard nivolumab monotherapy. Moreover, the ORR was significantly better in arm B. The OS subgroup analysis showed that OS was consistently better in arm B across all baseline clinical and demographic characteristics, except for patients who received the protocol treatment as third-line chemotherapy, patients with high PD-L1 expression, and patients <65 years of age.
In our study, arms A and B both appeared to have comparable tail plateaus at approximately 20 months on the Kaplan–Meier curve for PFS. However, the slight gap between the OS plateaus of each arm is worth mentioning, considering that 69.6% (32/46) of patients in arm A who encountered PD received subsequent therapy containing docetaxel. The addition of chemotherapy to ICI at an early stage shrinks the tumor size so significantly that a longer time is required before the patient reaches a critical status, even after progression. Also, ICI rechallenge may have contributed to the survival benefit, given that ICIs were readministered after PD to 54.1% of arm B, while only 15.2% in arm A received ICI as subsequent therapy. Those who ceased the protocol treatment due to adverse events or the patient's wish could have another chance to derive benefit from ICI in the following course of treatment.
The OS data in our study were better than our initial expectation. In fact, mOS in arm A was 14.7 months and was 4.2 months longer than our original assumption of 10.5 months. A recently published pooled analysis that combined two phase II studies conducted in Japan, mOS of second-line nivolumab was as long as 16.3 months (12.4–25.2) and 17.1 months (13.3–23.0) in squamous and nonsquamous cell carcinoma patients, respectively. The difference in OS results could simply be attributed to racial disparity (12).
The toxicity profile, especially hematotoxicity, was more significant in arm B, which was predictable given that both chemotherapy-related and ICI-related adverse events could occur together. Most cytotoxic chemotherapy-related adverse effects and immune-related adverse events (irAE) were manageable, with the exception of one case of grade 5 pneumonitis in arm A and one case of grade 5 myocarditis in arm B. The incidence of all-grade pneumonitis in our study (arm A, 13.8%; arm B, 12.5%) was higher in comparison with Checkmate017 and Checkmate057 (Checkmate017, 5%; Checkmate057, 1%); however, the rate of irAE interstitial lung disease (ILD) in Japanese populations has been demonstrated to be higher in comparison with non-Japanese populations (13). Moreover, grade ≥3 ILD was rare in both arms; thus, additional chemotherapy does not seem to increase this toxicity.
A relevant clinical question could be whether concomitant docetaxel and nivolumab is better than sequential. The mOS of arm B was still better than that of arm A, which received docetaxel-containing regimens as a subsequent therapy (15.0 months in cross-over arm A; 95% CI, 11.0–18.7; vs. 23.1 months in arm B; 95% CI, 16.7–NR; HR, 0.53; 95% CI, 0.31–0.90), although 17 of 32 (53.1%) received additional ramucirumab on top of docetaxel. Additional toxicity might be a trade-off, given the synergetic efficacy of concomitant docetaxel and nivolumab therapy rather than sequential.
Interestingly, the OS subgroup analysis suggested the possible benefit of the addition of docetaxel to nivolumab monotherapy, even for EGFR-mutant NSCLC. In fact, a better tendency toward arm B than toward A was seen in all PFS, OS, and ORR in the EGFR-mutant subgroup in our study. The toxicities were similar to the original data with no pneumonitis seen in either arm. Previously reported systemic review and meta-analysis comparing ICI to docetaxel in pretreated NSCLC (14) proved that ICIs were not associated with prolonged OS in the EGFR-mutant subgroup, unlike the EGFR wild-type subgroup. Although ICI is generally less effective in EGFR-mutant patients, the OS HR (0.45) in arm B versus A of this study was unexpectedly good, which may suggest that not only the added docetaxel itself but also a synergetic effect of docetaxel + ICI given together worked effectively. Also, the exploratory analysis of IMPOWER150 revealed atezolizumab plus chemotherapy exceeded chemotherapy in OS for those with sensitizing EGFR mutations (15). Our study implies adding docetaxel to nivolumab therapy could be promising for EGFR-mutant patients even in a second-line setting. Further studies are warranted.
Docetaxel is a candidate inducer of immunogenic cell death (ICD), which promotes synergetic effects when combined with ICI therapy. When the ICD prediction score was applied, which assesses a cytotoxicant's physicochemical characteristics and surrogate markers (e.g., calreticulin, ATP, and HGMB1 release), docetaxel, cisplatin, and carboplatin are the most promising ICD inducers (16).
Admittedly, the implication of this regimen is minimal for clinical practice, given the first-line approval of ICI and chemo-ICI regimens, which essentially preclude access to the relevant population. Moreover, it is not clear that these data could be extended to those with poor PS, because this study limited enrollment to PS 0–1 only. However, a potential use of this regimen is for ICI rechallenge, where the first-line combination ICI therapy failed owing to either adverse events or disease progression. irAEs are considered positive clinical biomarkers that predict ICI efficacy (17, 18), and some reports suggest that the ICI readministration is relatively safe and could be effective for patients who cease the ICI treatment due to irAEs (19). Moreover, the efficacy of the second ICI treatment following PD was mentioned in some reports, one of which demonstrated that the efficacy was associated with the duration of PFS with the first ICI therapy (20, 21).
Multiple prospective studies compared chemotherapy with and without ICI in various cancer types, including head and neck cancer (22), triple-negative breast cancer (23, 24), gastric/gastroesophageal junction/esophageal cancers (25, 26), cervical cancer (27), and lung cancer (3, 4, 5). However, the survival time in the standard chemotherapy arm may have been underestimated in those trials depending on the percentage of patients who received ICI as subsequent therapy, because ICI obviously shows long-lasting responses. Our study design, which clearly compares ICI with and without chemotherapy is quite innovative.
This study has some limitations. The small study population reduced the statistical power to <50%, and the lack of statistical power is truly regrettable. Although this study is originally intended as a phase III study, it is possible to consider this as having the same impact as a phase II study, due to the reduced sample size. Furthermore, patient backgrounds (e.g., postoperative recurrence and driver oncogene mutation) were heterogeneous, which may have influenced the OS. Also, the biomarker analysis beyond the assessment of EGFR/ALK and PD-L1 was not pursued in this study. Plus, more than half of the patients had unknown PD-L1 status, because all NSCLC patients could receive second-line nivolumab regardless of PD-L1 status at the time of enrollment in Japan. Most of all, whether to incorporate immunotherapy, either alone or in combination with chemotherapy, is a first-line decision. Due to changes in practice patterns, the patient population of this study may not be clinically relevant to routine clinical care.
These results have to be viewed with caution due to the above limitations and the early termination. Also, the use of ICIs in oncogene-driven NSCLC has not yet been shown to be appropriate for routine clinical use despite the results seen in the EGFR-mutant cohort in this study.
In conclusion, the addition of nivolumab to docetaxel produced a significant improvement in OS/PFS/ORR in comparison with nivolumab alone for previously treated ICI-naïve NSCLC. Despite slightly increased toxicity, nivolumab + docetaxel may be a second-line treatment option.
Supplementary Material
Acknowledgments
This study was funded by Ono Pharmaceutical Co., Ltd and Bristol Myers Squibb. UMIN Clinical Trials Registry (UMIN-CTR): 000021813.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
T. Shimokawa reports other support from MSD, Taiho, Chugai, and BMS outside the submitted work. Y. Takiguchi reports grants and personal fees from Ono Pharmaceutical Co. and Bristol Meyers Squib during the conduct of the study. Y. Takiguchi also reports grants and personal fees from Taiho Pharmaceutical Co., Chugai Pharmaceutical Co., AstraZeneca, Pfizer, MSD, Novartis, Daiichi Sankyo Pharmaceutical Co., Eli Lilly, Boehringer Ingelheim, and Kyowa Kirin Pharmaceutical Co.; grants from Eisai; and personal fees from Takeda Pharmaceutical Co. outside the submitted work. Y. Kawashima reports personal fees from Taiho Pharmaceutical, Eli Lilly, AstraZeneca, and Chugai Pharmaceutical outside the submitted work. N. Furuya reports personal fees from Eli Lilly Japan, Chugai, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim Japan, Taiho, Pfizer Japan, and Novartis outside the submitted work. Y. Shiraishi reports grants and personal fees from Chugai Pharma, as well as personal fees from Eli Lilly, Ono Pharmaceutical, AstraZeneca, Taiho Pharmaceutical, and Bristol Myers Squibb outside the submitted work. H. Tanaka reports personal fees from AstraZeneca, Chugai Pharmaceutical Co., Boehringer Ingelheim Japan Inc, Pfizer Japan Inc, Ono Pharmaceutical Co. Ltd, and Bristol Myers Squibb outside the submitted work. S. Miura reports personal fees from Chugai Pharmaceutical, Taiho Pharmaceutical, Ono Pharmaceutical, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Boehringer Ingelheim Japan, Pfizer, Novartis, MSD, Kyowa Hakko Kirin, Daiichi Sankyo, AbbVie, Nippon Kayaku, Amgen, Merck Biopharma, and Takeda Pharmaceutical outside the submitted work. Y. Nakahara reports grants from JSPS Kakenhi; grants and personal fees from Takeda Pharmaceutical Company and Bristol Myers Squibb; and personal fees from Ono Pharmaceutical Co. Ltd, AstraZeneca plc, Chugai Pharmaceutical Co. Ltd, Eli Lilly Japan K.K., Merck KGaA, and Boehringer Ingelheim GmbH outside the submitted work. T. Tokito reports personal fees from Chugai Pharmaceutical, AstraZeneca, MSD, Novartis, and Bristol Myers Squibb outside the submitted work. K. Naoki reports grants from Ono Pharmaceutical and Bristol Myers Squibb during the conduct of the study, as well as grants from AstraZeneca, Chugai Pharmaceutical, Nippon Boehringer Ingelheim, Taiho Pharmaceutical, and Parexel International Inc outside the submitted work. A. Bessho reports grants and personal fees from Ono Pharmaceutical, as well as personal fees from Bristol Myers Squibb during the conduct of the study; A. Bessho also reports grants and personal fees from Chugai Pharmaceutical, MSD, and AstraZeneca outside the submitted work. M. Seike reports personal fees from AstraZeneca, Takeda Pharmaceutical, Bristol Myers Squibb, Pfizer, Nihon Kayaku, Kyowa Kirin, Ono Pharmaceutical, and MSD, as well as grants and personal fees from Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly, and Boehringer Ingelheim during the conduct of the study. H. Okamoto reports grants from MSD, Taiho, Chugai, and Bristol Myers Squibb outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
Y. Taniguchi: Conceptualization, investigation, writing–original draft, writing–review and editing. T. Shimokawa: Conceptualization, supervision, investigation, methodology. Y. Takiguchi: Conceptualization, supervision, investigation, methodology. T. Misumi: Data curation, formal analysis, methodology. Y. Nakamura: Conceptualization, supervision, investigation, methodology. Y. Kawashima: Resources, supervision. N. Furuya: Resources, supervision. Y. Shiraishi: Resources, supervision. T. Harada: Resources, supervision. H. Tanaka: Resources, supervision. S. Miura: Resources, supervision. A. Uchiyama: Resources, supervision. Y. Nakahara: Resources, supervision. T. Tokito: Resources, supervision. K. Naoki: Resources, supervision. A. Bessho: Resources, supervision. Y. Goto: Resources, supervision. M. Seike: Resources, supervision. H. Okamoto: Conceptualization, supervision, investigation, methodology.
References
- 1. Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res 2008;68:4026–30. [DOI] [PubMed] [Google Scholar]
- 2. Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho H-I, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest 2010;120:1111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis FV, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. J Engl J Med 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 4. Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA) international, randomized, open-label, phase 3 trial. Lancet Oncol 2021;22:198–211. [DOI] [PubMed] [Google Scholar]
- 5. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–301. [DOI] [PubMed] [Google Scholar]
- 6. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kunitoh H, Watanabe K, Onoshi T, Furuse K, Niitani H, Taguchi T. Phase II trial of docetaxel in previously untreated advanced non-small-cell lung cancer: a Japanese cooperative study. J Clin Oncol 1996;14:1649–55. [DOI] [PubMed] [Google Scholar]
- 9. Kudo S, Hino M, Fujita A, Igarashi T, Arita K, Niitani H, et al. Late phase II clinical study of RP56976 (docetaxel) in patients with non-small cell lung cancer. Gan To Kagaku Ryoho 1994;21:2617–23 [PubMed] [Google Scholar]
- 10. Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell cancer (KEYNOTE-010): a randomized controlled trial. Lancet 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
- 11. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095–103. [DOI] [PubMed] [Google Scholar]
- 12. Saka H, Nishio M, Hida T, Nakagawa K, Sakai H, Nogami N, et al. Five-year follow-up results from phase II studies of nivolumab in Japanese patients with previously treated advanced non-small cell lung cancer: pooled analysis of the ONO-4538-05 and ONO-4538-06 studies. Jpn J Clin Oncol 2021;51:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto N, Nakanishi Y, Gemma A, Nakagawa K, Sakamoto T, Akamatsu A, et al. Real-world safety of nivolumab in patients with non-small-cell lung cancer in Japan: postmarketing surveillance. Cancer Sci 2021;112:4692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systemic review and meta-analysis. JAMA Oncol 2018;4:210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nogami N, Barlesi F, Socinski MA, Reck M, Thomas CA, Cappuzzo F, et al. Impower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol 2022;17:309–23. [DOI] [PubMed] [Google Scholar]
- 16. Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol 2020;17:725–41. [DOI] [PubMed] [Google Scholar]
- 17. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018;4:374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 2019;145:479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 2018;6:1093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nomura M, Otsuka A, Kondo T, Nagai H, Nonomura Y, Kaku Y, et al. Efficacy and safety of retreatment with nivolumab in metastatic melanoma patients previously treated with nivolumab. Cancer Chemother Pharmacol 2017;80:999–1004. [DOI] [PubMed] [Google Scholar]
- 21. Reckamp KL, Redman MW, Dragnev KH, Minichiello K, Villaruz LC, Faller B, et al. Phase II randomized study of ramcirmab and pembrolizumab versus standard of care in advanced non-small-cell lung cancer previously treated with immunotherapy-lung-MAP S1800A. J Clin Oncol 2022:JCO2200912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomized, open-label, phase 3 study. Lancet 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- 23. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. [DOI] [PubMed] [Google Scholar]
- 24. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im S-A, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomized, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020;396:1817–28. [DOI] [PubMed] [Google Scholar]
- 25. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang YK, Chen LT, Ryu MH, Oh D-Y, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomized, multicenter, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:234–47. [DOI] [PubMed] [Google Scholar]
- 27. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med 2021;385:1856–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Y.T.) upon reasonable request.