Abstract
Immune checkpoint inhibitors (ICI) targeting cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) proteins transformed the management of advanced cancers. Many tumor-intrinsic factors modulate immunological and clinical responses to such therapies, but ample evidence also implicates the gut microbiome in responses. The gut microbiome, comprising the bacteria, archaea, fungi, and viruses that live in the human digestive tract, is an established determinant of host immunity, but its impact on response to ICI therapy in mice and humans with cancer has only recently been appreciated. Therapeutic interventions to optimize microbiota composition to improve immunotherapy outcomes show promise in mice and humans with cancer. In this review, we discuss the rationale for gut microbiome–based cancer therapies, the results from early-phase clinical trials, and possible future developments.
Introduction
Tumor antigen-specific cytotoxic T cells are present in advanced cancers but often fail to induce tumor rejection (1–3) because cancer cells utilize many tumor-intrinsic mechanisms to escape immune-mediated destruction. Among these, the programmed death 1/programmed death ligand 1 (PD-1/PD-L1) axis is a key player in the inhibition of cytotoxic CD8+ T cells, and immune checkpoint inhibitors (ICI) targeting PD-L1 promote effective and often durable responses in patients with a variety of cancer types, including melanoma, non–small cell lung cancer (NSCLC), and renal cell carcinoma (RCC; refs. 4–6). Biomarkers of response to PD-L1 blockade include CD8+ tumor-infiltrating lymphocytes (TIL; refs. 7, 8), IFNγ gene expression (9, 10), high tumor mutation burden (TMB; refs. 10–12), or PD-L1 expression on tumor or T cells (5, 13). Strikingly, multiple studies support the role of the gut microbiome in regulating clinical responses to ICI in various preclinical models and in patients with cancer. In this review, we (i) summarize preclinical and clinical data supporting the role of the gut microbiome in determining response to cancer immunotherapy; (ii) describe the spectrum of therapeutic strategies to target the microbiome in cancer and their present status; and (iii) present key unanswered questions to address in ongoing and future research.
The Gut Microbiome in Cancer Immunotherapy
Preclinical data implicating gut microbiome composition in cancer immunotherapy
Evidence supports the role of gut commensals in mediating the efficacy of anticancer therapies, including chemotherapy, radiotherapy, and immunotherapies, in mouse tumor models. For example, cyclophosphamide induces gut transmucosal translocation of Enterococcus hirae to secondary lymphoid organs with consequent stimulation of Th17 responses and IFN-producing CD8+ T-effector cells in sarcoma models and lung adenocarcinoma models (14, 15). In addition, commensal bacteria mediate oxaliplatin-induced immunogenic cell death by modulating reactive oxygen species (ROS; ref. 16) and the adjuvanticity of ileal bacteria (Erysipelotrichaceae spp., Bacteroides fragilis) that dictate the balance between antitumor T-follicular helper (Tfh) cells and deleterious Th17 responses in colon cancer (17). The gut microbiome also mediates the effects of hypofractionated radiotherapy on irradiated and nonradiated tumors in an IFNγ and cytotoxic T-cell–dependent manner in melanoma and human papillomavirus (HPV)-driven cancer models (18). Notably, the efficacy of adoptive cellular therapy (ACT) following total body irradiation (TBI) depends on lipopolysaccharide (LPS) and host Toll-like receptor 4 (TLR4) signaling mediated by bacterial translocation (19, 20). Systemically administered Bifidobacterium spp. can accumulate in tumors and improves anti-CD47 immunotherapy in a stimulator of IFN genes (STING) and IFN-dependent fashion (21). In addition, anticytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) efficacy depends on distinct Bacteroides spp., and broad-spectrum antibiotic treatment or housing in germ-free facilities attentuates the efficacy of CTLA-4 blockade in mice bearing MCA205 fibrosarcoma. In such mice, anti-CTL4 efficacy is partially restored after treatment with oral vancomycin, oral gavage with B. fragilis, immunization with B. fragilis polysaccharides, or adoptive transfer of B. fragilis–specific Th1 cells (22). Further, anti–CTLA-4 therapy appears to directly perturb the gut microbiome, specifically causing reductions in Bacteroidales and Burkholderiales and increases in Clostridiales spp. (22). Similarly, the efficacy of PD-1 blockade depends on Bifidobacterium spp. that act upon host dendritic cells (DC) to augment T-cell function in preclinical models of melanoma (23). Interestingly, E. faecium, but not E. faecalis, contains peptidoglycan hydrolase secreted antigen A (SagA) that generates nucleotide-binding oligomerization domain 2 (NOD2)-active muropeptides able to modulate anti–PD-1 treatment efficacy in vivo (24).
Finally, emerging evidence indicates that carcinogenesis induces regenerating islet-derived protein 3 γ (Reg3-γ)-mediated β-adrenergic receptor-dependent epithelial permeability and, consequently, Clostridium spp.-dominant dysbiosis. This finding implicates the gut microbiota in carcinogenesis and cancer progression (25). Collectively, these data provide compelling preclinical evidence that gut microbiota mediate the efficacy of a range of anticancer therapies, including chemotherapy, radiotherapy, and immunotherapy.
Human data implicating gut microbiome composition in cancer immunotherapy
Multiple observations underscore the role of gut microbiota in determining response in ICI-treated patients. Firstly, in line with preclinical studies that showed that broad-spectrum antibiotics promoted unfavorable clinical outcomes to treatment with ICI targeting CTLA-4 (22) or PD-1/PD-L1 (26–28), patients with cancer who received broad-spectrum antibiotics shortly before or during ICI therapy had poorer progression-free survival (PFS) and overall survival compared with those who did not receive antibiotics (29, 30). These observations suggest that a diverse gut microbiota plays a critical role in promoting antitumor immunity (29, 31–34). Second, studies of the gut microbiota composition of ICI-treated patients with melanoma (26, 27, 35–37), NSCLC (28, 38–40), RCC (28, 41), and gastrointestinal (GI) cancers (42) reveal higher levels of distinct bacterial taxa in ICI-responders (R) compared with non-responders (NR; Table 1). These studies identified specific members of each of the major bacterial phyla colonizing humans—Firmicutes, Actinobacteria, Proteobacteria, Bacteroidetes, and Verrucomicrobia—in patients who derived durable benefit from PD-1, CTLA-4, or dual PD-1/CTLA-4 blockade (Table 1). Conversely, the gut microbiota of NRs exhibited increased abundance of genera Bacteroides (phylum Bacteroidetes) and Ruminococcus and Roseburia (both Firmicutes; Table 1). While individually significant, these studies had a striking feature; each largely identified different sets of microbes. Notably, while increased alpha diversity was correlated with improved outcome in early studies (26), evidence for an association with ICI benefit is lacking.
Table 1.
Published studies evaluating gut microbiome composition in patients with advanced cancer treated with ICI.
| Study reference | Histology | ICI | Geographic location of samples | Sample size | Collection timepoint | Responder definition and timepoint | Sequencing method; taxonomic assignment | Bacterial taxa associated with improved benefit (study-specific; top 10) | Association between alpha diversity metrics and outcome |
|---|---|---|---|---|---|---|---|---|---|
| Chaput and colleagues (35) | Melanoma | Anti–CTLA-4 (ipilimumab) | Villejuif, France | 26 (9 R, 17 NR) | Pre-, and on- treatment (all paired) | Long-term clinical benefit (tumor shrinkage ≥50% relative to baseline; or tumor <50% shrinkage relative to baseline but <25% increase relative to nadir); 6 months | 16S; QIIME | Faecalibacterium spp.; Gemmiger spp. | Diversity metrics were not associated with outcome. Decreased diversity (lower Shannon index) was associated with colitis. |
| Frankel and colleagues (36) | Melanoma | Anti–PD-1 (nivolumab or pembrolizumab), anti–CTLA-4 (ipilimumab), and ipilimumab/nivolumab | Dallas, TX | 39 (24 R, 15 NR) | Pre- | Radiographic response per RECIST v1.1; 2–3 months | MSS; MetaPhlAn | Bacteroides caccae; Streptococcus parasanguinis; PD-1 patients: Dorea formicigenerans, PD-1/CTLA-4 patients: Faecalibacterium prausnitzii, Holdemania filiformis | Diversity metrics were not associated with outcome. |
| Gopalakrishnan and colleagues (26) | Melanoma | Anti–PD-1 (nivolumab or pembrolizumab) | Houston, TX | 43 (30 R, 13 NR) | Pre- (41), and on- (2) treatment | Radiographic response per RECIST v1.1; 6 months | 16S; BLAST and mothur | Firmicutes spp., Clostridia spp., Ruminococcaceae spp., F. prausnitzii, R. bromii, Porphyromonas pasteri, Veilonellaceae spp., C. hungatei, Phascolarctobacterium spp., P. faecium | Increased diversity (higher inverse Simpson) was associated with improved outcome. |
| Matson and colleagues (27) | Melanoma | Anti–PD-1 (nivolumab or pembrolizumab), anti–CTLA-4 (ipilimumab) | Chicago, IL | 42 (16R, 26 NR) | Pre- | Radiographic response per RECIST v1.1; not stated | 16S; QIIME MSS; MetaPhlAn2 | Bifidobacteriaceae spp., Coriobacteriaceae spp., Lachospiraceae spp., Lactobacillaceae spp., Enterococcaceae spp., Enterobacteriaceae spp., Porphyromonadaceae spp. | Diversity metrics were not associated with outcome. |
| Peters and colleagues (37) | Melanoma | Anti–PD-1 (nivolumab or pembrolizumab), anti–CTLA-4 (ipilimumab), and ipilimumab/nivolumab | New York, NY | 27 | Pre- | PFS | 16S; QIIME MSS; MetaPhlAn2 | Faecalibacterium prausnitzii, Coprococcus eutactus, Prevotella stercorea, Streptococcus sanguinis, Streptococcus anginosus, and Lachnospiraceae bacterium 3 1 46FAA | Increased diversity (higher Shannon) was associated with improved outcome in 16S data but not in MSS data. |
| Routy and colleagues (28) | RCC, NSCLC | Anti–PD-1 (nivolumab or pembrolizumab) | Villejuif, France | 153 (86 R, 67 NR) Discovery: NSCLC 60 (31 R, 29 NR); RCC (25R, 15NR) Validation: NSCLC 27 (10 R, 17 NR); RCC 26 (20 R, 6 NR) | Pre-, and on- treatment (all paired) | Best radiographic response per RECIST v1.1 (including SD); 3-month PFS | MSS; METEOR and MetaOMineR | Radiographic response: A. muciniphila, Firmicutes spp., Eubacterium spp., Lachnospiraceae spp., Erysipelotrichaceae spp., Cloacibacillus porcorum, E. faecium, Intestinimonas spp., Clostridiales spp., Alistipes spp. Improved PFS: Firmicutes spp., Eubacterium spp., Alistipes spp., A. muciniphila, Intestinimonas spp., B. nordii, Bacteroides xylanisolvens, Blautia spp., Lachnospiraceae spp., Clostridiales spp. | Diversity metrics were not associated with outcome. |
| Jin and colleagues (38) | NSCLC | Anti–PD-1 nivolumab | Shanghai, China | 37 (23 R, 14 NR) | Pre- | Best radiographic response per RECIST v1.1 (including SD ≥6 months) | 16S; QIIME | Ruminococcus spp., A. putredinis, P. copri, B. longum, Clostridiales spp., Parabacteroides spp., Shigella spp. | Increased diversity (higher Shannon and inverse Simpson) was associated with improved outcome. |
| Hakozaki and colleagues (39) | NSCLC | Anti–PD-(L)1 (nivolumab, pembrolizumab, or atezolizumab) | Tokyo, Japan | 70 (24 Ra, 27 NR) | Pre- | Best radiographic response per RECIST v1.1 (including SD ≥6 months); PFS | 16S; SILVA | Ruminococcaceae UCG 13 and Agathobacter spp. | Diversity metrics were not associated with outcome. Antibiotic use was associated with reduced diversity metrics (Shannon and inverse Simpson). |
| Derosa and colleagues (40) | NSCLC | Anti–PD-(L)1 (nivolumab, pembrolizumab or atezolizumab) singly or in combination with chemotherapy in either first- or second-line NSCLC | Villejuif, France | 338 patients (R - 28% Akk+ and 18% Akk−; NR - 44% Akk+ and 50% Akk−) | Pre- (V1), and early on-treatment (V2) | Best radiographic response per RECIST v1.1 | MSS; MetaOMineR and MetaPhlAn3 | Akkermansia muciniphila | Increased diversity (higher Shannon) was associated with improved outcome. |
| Derosa and colleagues (41) | RCC | PD-1 (nivolumab) treated in NIVOREN GETUG-AFU 26 phase II study | Villejuif, France | 58 (30 R, 28 NR) after exclusion of 11 patients who received antibiotics | Pre- | Best radiographic response per RECIST v1.1 (including SD ≥12 months); PFS | MSS; MetaOMineR and MetaPhlAn2 | A. muciniphila, Eubacterium spp., Firmicutes bacterium CAG_124, Methanobrevibacter smithii, Firmicutes bacterium CAG_103, Dialister succinatiphilus YIT_11850, Eubacterium siraeum CAG_80, Bacteroides cellulosilyticus, Acinetobacter spp. CAG_196, Faecalibacterium spp. CAG_74 | Diversity metrics were not associated with outcome. Antibiotic use was associated with reduced beta diversity (ANOSIM). |
| Peng and colleagues (42) | GI (including esophageal, gastric, colorectal, and others) | Anti–PD-(L)1 (nivolumab, pembrolizumab, or atezolizumab) or anti–PD-1/anti–CTLA-4 combination (ipilimumab with nivolumab) | Beijing, China | 74 (45 R, 29 NR) | Pre-, and on- treatment | Best radiographic response per RECIST v1.1 (including SD ≥3 months); PFS | 16S; SILVA and HUMAnN2 | All radiographic responders: Ruminococcaceae OTU185 spp., CAG-352 OTU217 spp., Prevotella OTU264 spp., Dialister OTU18 spp., Lachnospiraceae OTU77 spp., Ruminiclostridium OTU216 spp., Lachnospira OTU159 spp., Ruminococcus OTU222 spp., Ruminiclostridium OTU196 spp., Parabacteroides OTU295 spp. Esophageal carcinoma (N = 14): Prevotella _9 OTU261 spp., Dialister OTU18 spp., Lachnospiraceae OTU97 spp., Ruminococcus_2 OTU222 spp., Bacteroides OTU266 spp., Parasutterella OTU207 spp., Phascolarctobacterium OTU1 spp., Bacteroides OTU277 spp., Lachnospiraceae OTU105 spp., Lachnospiraceae OTU128 spp. Gastric cancer (N = 23): Prevotella_9 OTU263 spp., Bifidobacterium OTU202 spp., Prevotella _2 OTU264 spp., Lachnospira OTU156 spp., Bacteroides OTU274 spp., Ruminococcaceae OTU190 spp., Agathobacter OTU79 spp., Bacteroides OTU275 spp. Colorectal carcinoma (N = 19): Lachnoclostridium OTU104 spp., Parabacteroides OTU294 spp., Parabacteroides OTU293 spp., Lachnospira OTU155 spp., Ruminococcaceae OTU187 spp., Flavonifractor OTU197 spp., Dialister OTU18 spp., Ruminococcaceae OTU187 spp., Lachnospiraceae OTU77 spp., Lachnospiraceae OTU103 spp. | Diversity metrics were not associated with outcome. |
Abbreviation: MSS, microsatellite stable.
aIn Hakozaki and colleagues, while stable disease (SD) was observed in 17 patients and patients with SD ≥ 6 months were counted as R, this number was not reported.
The startling lack of consensus regarding the microbial signals associated with clinical response raises many questions about the significance of these findings. Multiple factors may impede the interpretation of the data linking ICI and gut microbiome composition, including the small sizes of individual cohorts, variable definitions of clinical response, distinct bioinformatic analytic pipelines, and the many confounding factors influencing gut microbiome composition (diet, treatment, geography, ethnicity, etc.) (highlighted in Table 1). To determine the effect of analytic pipelines upon observed taxa disparity, two studies utilized a consistent bioinformatic approach for taxonomic assignment with appropriate statistical corrections and analyzed raw sequencing data from multiple published datasets (43, 44). Gharaibeh and colleagues determined that the Gopalakrishnan and colleagues, Matson and colleagues, and Routy and colleagues datasets each had a unique microbial 16S rDNA signal and that there were shared orthologues in pairwise comparisons. However, there was a lack of common orthologues in all three datasets, suggesting that differences in analytic pipelines could not explain the signal differences across studies and that models based on microbial gene composition (function) rather than community composition (form) may have better predictive power (43). Separately, Shaikh and colleagues developed biomarkers of clinical outcome to ICIs (R index and NR index, respectively) based on gut microbial signatures in Rs and NRs, and concluded that the NR-index outperformed the R-index and literature-based indices in predicting outcome to ICI therapy in both a random effects model and a sensitivity/specificity analysis (44). Population-specific differences exert important effects in microbiome studies (45, 46) that may be overcome by using large datasets comprising multiple cohorts and performing intra-cohort and cross-cohort comparisons to yield better combined-cohort prediction. Also, ICI therapy, in particular, can yield novel patterns of response, meaning that time-to-event analyses evaluating a landmark timepoint [1 year PFS rather than objective response rate (ORR)] may better define the magnitude of benefit and the impact of a predictive biomarker (47, 48). To test whether a large dataset could overcome population-specific differences, Lee and colleagues used source-level metadata and a machine learning framework to evaluate normalized baseline metagenomic data from five new observational cohorts (n = 165) and four previously described cohorts (n = 147) of patients with ICI-naïve melanoma, and determined how combined taxonomic and functional microbiome signatures performed against radiographic assessment of response (49). The authors found cohort-specific microbial signatures. However, neither taxonomic nor functional microbiome biomarkers identified consistent signals across cohorts, suggesting that even unbiased machine learning methods are not sufficient to overcome between-cohort and microbiota differences. This fact is particularly apt when cohorts are small and/or between-cohort differences are significant (49). To determine the role of time-to-event analyses in predicting how microbiome-based signatures affect ICI outcome, McCulloch and colleagues evaluated a melanoma cohort treated with PD-1 monotherapy, along with four published datasets, and utilized PFS—rather than radiographic response—to infer PD-1 benefit (50). They found that baseline microbiota composition was optimally associated with clinical outcome at approximately 1 year after initiating treatment. Meta-analysis and other bioinformatic analyses of the combined data across cohorts revealed that bacteria associated with specific microbiota signatures correlated with favorable (Lachnospiraceae and Ruminococcaceae families of Firmicutes phylum and Actinobacteria phylum) or unfavorable (members of Bacteroidetes and Proteobacteria phyla) outcomes to PD-1 ICI therapy (50). These findings provide a scientific rationale for selecting the optimal endpoint (time-to-event rather than ORR) for inferring how baseline gut microbiome composition affects ICI response. Together with the ability of fecal microbiome transplantation (FMT) to overcome primary PD-1 resistance (51), they suggest that the early response is dominated by host-intrinsic and tumor-intrinsic factors but early nonresponse may be determined by the gut microbiota composition. This effect wanes in significance late in treatment when adaptive resistance mechanisms dominate.
Evidence suggests that human gut microbiota comprise many discrete ecologically balanced communities—“enterotypes” or “enteric microbiotypes”—that tend to be temporally resilient but still modifiable by diet, drugs, and lifestyle (52–57). Despite considerable effort to establish the link between the composition of individual taxa to PD-1 ICI response, the relationship between distinct enteric microbiotypes and this response remains poorly understood. McCulloch and colleagues mapped patient-level metagenomic data onto a microbiome map derived from an American Gut Project database of more than 10,000 fecal samples from across the United States and found that the distinct enteric microbiotypes of different geographical areas accounted for most of the between-cohort differences. This finding suggests that enteric microbiotypes could be accurately identified in large datasets despite being not entirely discrete and varying tremendously by taxonomic, functional, and ecologic properties (58). These data remain to be validated in larger series but still underscore the utility of such classifiers in developing microbiota-based diagnostics and/or therapeutic interventions. This work further helps to illuminate how strain-level population structure and genetic diversity contribute to immunotherapy outcome (58–60).
Collectively, these observations underscore the need to perform studies with large and deeply annotated datasets, clinically meaningful endpoints, geographically distinct populations, and stool samples collected and processed in a uniform fashion. Whether the findings observed in PD-1–treated melanoma apply to other cancers treated with other ICIs and/or ICI combinations will need to be evaluated. Developing novel analytic methods to better explore the individual-specific microbial diversity will aid these endeavors (58–60).
Potential mechanism(s) to explain the impact of gut microbiota on cancer immunotherapy
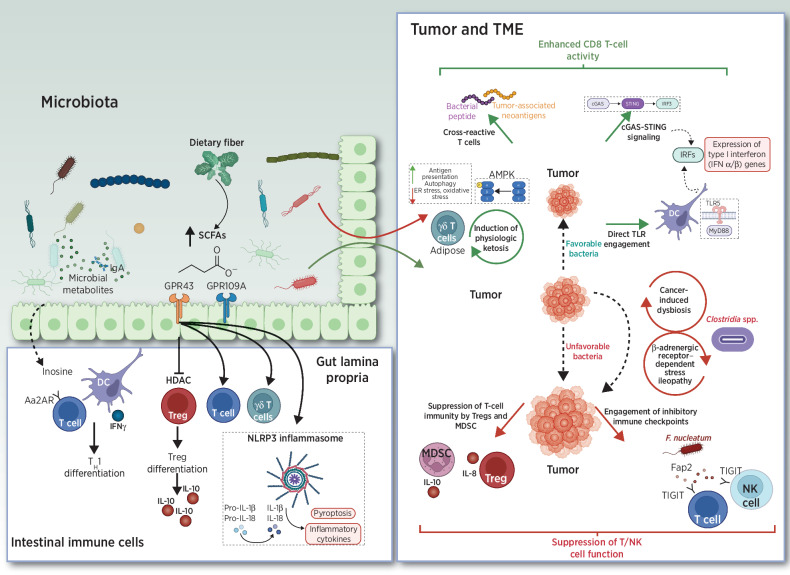
The precise mechanisms underlying how gut microbiota influence cancer immunotherapy are poorly understood, but research has converged upon three themes (see below; and Fig. 1): bacteria or bacterial components that directly stimulate antitumor T-cell responses, molecular mimicry between shared bacterial and tumoral epitopes, and bacterial metabolites that shape antitumor immunity.
Figure 1.
Mechanisms for the effects of bacteria or bacterial metabolites on antitumor immune responses. MDSC, myeloid-derived suppressor cell; TME, tumor microenvironment.
Some gut bacteria can elicit defined antigen-specific T-cell responses, including Helicobacter spp. [RORγt+ FOXP3+ inducible T-regulatory cells (iTreg)] and A. muciniphila (IgG1 antibodies and Tfh cells; refs. 61–64). Others exert immunostimulatory properties either directly or after being sensed by DCs in gut-associated lymphoid tissue (GALT), spleen, and/or tumor draining lymph nodes. These include: B. thetaiotaomicron or B. fragilis, which enhance the efficacy of CTLA-4 treatment (22), and E. hirae and B. intestihominis, which directly enhance the intratumoral CD8/Treg ratio and IFNγ-producing γδ T cells, respectively, following cyclophosphamide treatment (14). Others include B. rodentium, which stimulates antitumor responses in Rnf5−/− mice in a My8DD/TLR-mediated fashion (65), and Bifidobacterium spp., which sensitizes mice to anti-CD47 immunotherapy in a STING-and IFN-dependent fashion (21). Separately, bacterial flagellin directly interacts with TLR5; in cancer, bacterial flagellin derived from E. gallinarum and S. typhimurium demonstrate immunostimulatory potential (66, 67).
Molecular mimicry between pathogens and tumor antigens can also elicit cross-reactive T cells via antigenic mimicry (Fig. 1). Preclinically, both Bifidobacterium breve and the E. hirae bacteriophage elicited commensal-specific T cells that cross-reacted with candidate neoantigens (19, 68). In humans, long-term survival in pancreatic cancer was associated with the development of highly immunogenic neoantigens with predicted cross-reactivity to microbial epitopes (69). Fusobacterium nucleatum is associated with colorectal cancer and promotes colonic tumor formation in preclinical models; this bacterium interacts with the inhibitory T-cell receptor TIGIT through FAP2 and can directly suppress antitumor immunity and inhibit tumor killing by natural killer (NK) cells (70, 71). Collectively, these data suggest that certain commensals may influence adaptive and/or innate responses to cancer by modulating inhibitory checkpoint pathways. Finally, human melanoma expresses a repertoire of human leukocyte antigen class I (HLA-I) and class II (HLA-II) restricted peptides derived from intratumoral bacteria that can be presented by antigen-presenting cells and elicit T-cell responses (72).
Metabolites synthesized de novo by gut microbiota or produced by the host and biochemically modified by gut bacteria can exert immunomodulatory effects (Fig. 1). Endogenous nucleosides adenosine and inosine exert a wide range of anti-inflammatory and immunomodulatory effects in vivo through adenosine receptors (AR). Pharmacologic inhibition of adenosine signaling—either through direct A2AR inhibition or inhibition of CD38, CD39, or CD73—enhances antitumor immunity in multiple preclinical models and is a focus of drug development (73, 74). Surprisingly, supplementation of inosine, which is formed by adenosine catabolism, was recently reported to enhance efficacy of anti–PD-1 therapy and cell therapy but only in a low-glucose environment (75). In multiple mouse tumor models, key gut microbiota—specifically A. muciniphila and B. pseudolongum—produce inosine and enhance the effects of anti–CTLA-4 therapy (76).
Major classes of microbial-derived molecules such as short-chain fatty acids (SCFA) and secondary bile acids (BA) are immunomodulatory (Fig 1). Commensal bacteria convert primary to secondary BAs, which promotes hepatic CXCR6+ NKT cells that inhibit liver-selective metastases in preclinical models of primary and metastatic liver cancer (77). SCFAs such as acetate, propionate, and butyrate are the byproduct of bacterial fermentation of dietary fiber in the colon (78). These products bind to specific SCFA-sensing G-protein–coupled receptors (GPCR; GPR41, GPR43, GPCR109A) and regulate histone acetyltransferase (HAT) and histone deacetylase (HDAC) activity. SCFAs modulate intestinal immune homeostasis, affecting Tregs, effector T cells, and γδ T cells (79–85). Preclinically, the effects of SCFA supplementation in the context of cancer immunotherapy is controversial. Both favorable or unfavorable effects may occur (86–88). In two separate studies of anti–PD-1–treated patients with cancer, higher levels of SCFAs were associated with favorable response (89, 90). However, given the relatively small number of patients evaluated in both studies and the context-dependent cues governing SCFA production in humans that were not independently evaluated, the role of SCFA supplementation upon ICI efficacy remains unclear.
Ketone bodies are a vital alternative metabolic fuel source for all domains of life. Ketogenic diets (KD) alter the composition of gut microbiota in mice (91, 92). Further, systemic ketosis increases the abundance of bile-tolerant members of the Bacteroidetes phylum (Alistipes spp., Bilophila spp., and Bacteroides spp.) and decreases the levels of butyrate-producing members of the Firmicutes phylum (Roseburia spp., Eubacterium rectale, and Ruminococcus bromii) in humans (93, 94). KDs improve tumor antigen-specific innate and adaptive immune responses in multiple preclinical models (95, 96), possibly related to 3-hydroxybutyrate-mediated increases in Eisenbergiella massiliensis (97) or direct AMP-activated protein kinase (AMPK) activation and consequent enhancement of antigen presentation (97). KDs also appear to expand protective adipose-resident γδ T cells (98). However, it is unclear whether the observed immunological effects are secondary to gut microbiome-induced changes in the intestinal immune environment or due to circulating ketone bodies that may directly affect immune-cell function (98, 99).
In McCulloch and colleagues, noninvasive exfoliated transcriptome (exfoliome) analysis implicated LPS-producing Gram-negative bacteria enriched for key bacterial genes including α-l-fucosidase, α-galactosidase, and glycosyltransferases in mediating nonresponse to PD-1 (50). These genes are linked with mucus degradation and LPS synthesis, respectively, suggesting potential mechanisms of action used by the human gut microbiota to modulate host immunity and outcome upon PD-1 ICI therapy (100–102).
Preclinical and human data implicating gut microbiome composition in the development of adverse events to anticancer agents
In addition to their putative antitumor effects, commensal organisms are strongly implicated in mediating toxicity to a spectrum of anticancer agents, including ICIs. Commensal microbiota mediate methotrexate-induced gut toxicity controlled by ABCB1/MDR1-encoded p-glycoprotein drug efflux pumps in CD11b+ intestinal myeloid cells in a TLR2-dependent fashion (103). Irinotecan (CPT-11)-related diarrhea is mediated by metabolite SN-38, in turn mediated by microbial β-glucuronidases (GUS). Inhibitors of GUS enhance irinotecan efficacy by blocking the irinotecan-induced bloom of Enterobacteriaceae spp. (104). Streptomycin reduces the severity of irinotecan-mediated delayed-onset diarrhea by reducing ileum and colonic tissue exposure to irinotecan and related metabolites (105). Gemcitabine (dFdC) is less efficacious in the presence of certain bacteria, e.g., Gammaproteobacteria spp., that have increased expression of a long isoform of bacterial cytidine deaminase (CDDL). This CDDL expression increases metabolic degradation of gemcitabine into difluorodeoxyuridine (dFdU; ref. 106). Thus, antibiotic use is associated with increased gemcitabine efficacy in preclinical models of pancreatic cancer (106) and improved survival of humans with pancreatic cancer (107).Tyrosine kinase inhibitor (TKI) therapy-related diarrhea is associated with higher levels of Bacteroides spp. and lower levels of Prevotella spp. in patients with RCC (108).
During PD-1 and/or CTLA-4 ICI treatments, species belonging to Bacteroidetes, Clostridia, and Proteobacteria phyla have been linked to increased incidence and severity of immune-related adverse events (irAE; refs. 35, 109, 110). Of these events, ICI-related colitis has been most closely linked with commensal microbiota, specifically members of the Firmicutes phyla (F. prausnitzi, G. formicilis, Ruminococcaceae spp.), during anti–CTLA-4 ICI therapy (35, 109). In addition, B. intestinalis (phylum Bacteroidetes) was linked to colitis in patients treated with anti–PD-1 and anti–CTLA-4 combination therapy (110).
McCulloch and colleagues identified two microbial signatures associated with specific irAE profiles and divergent clinical outcomes (58). One signature exhibited increased abundance of members of the Lachnospiraceae family and was associated with favorable response to anti–PD-1. The other was dominated by Streptococcus spp. and associated with shorter PFS and a high frequency of distinct irAEs, particularly arthritis (58). Increased abundance of Streptococcus was associated with proton pump inhibitor (PPI) use. Thus, it is tempting to speculate that PPIs increase the survival rate of oral bacteria during gastric passage (i.e., oralization), inducing a shift in gut microbiome composition. Altogether, these findings reconcile the discordant published results linking irAEs and clinical response to anti–PD-1 (58).
Single-cell analyses of intestinal luminal samples from ICI-treated patients who developed colitis identified transcriptionally-distinct T cells (characterized by IFNG, GBP5, HLA-DR, and CD74 expression) and myeloid cells (characterized by TNF, IL1B, and OSM expression) that drove the emergence of CD8+ T-effector cells (111). Similarly, increased numbers of IFN-expressing CD8+ effector T cells and IL1β-expressing monocytes have been related to ICI-related pneumonitis (112). Thus, it is unsurprising that immunosuppressive therapies, including corticosteroids and TNFα inhibitors, are effective in treating ICI-related colitis and pneumonitis. More recently, healthy donor-derived FMT was found to efficiently treat biologic (TNFα and/or integrin α₄β₇ inhibitor) refractory ICI colitis, supporting that pathobionts can mediate the development of this irAE (113, 114). These observations suggest pathobionts influence the development of irAEs in epithelial organs with a local microbiota and large populations of tissue-resident T cells via a TNF/CXCL8/IL1β and NLRP3 inflammasome-mediated program. Whether this holds true for other irAEs in epithelial organs besides the colon, e.g., the skin and/or lung, remains unknown.
Microbiome-based therapy of cancer
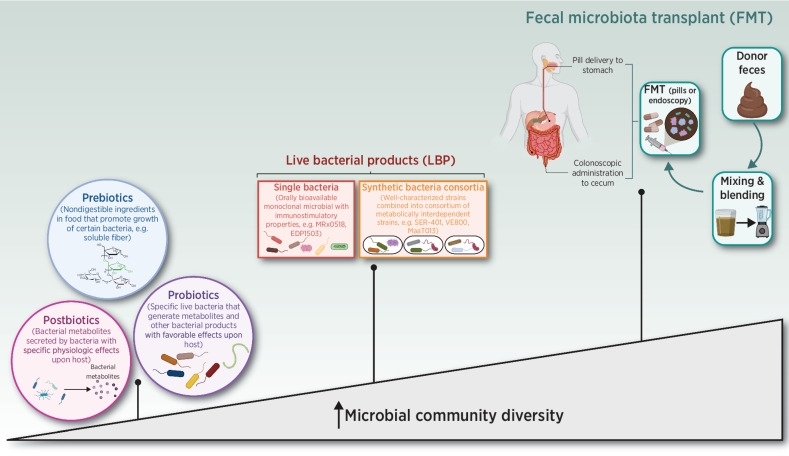
Most therapeutic interventions aimed at targeting the microbiome in cancer have focused on the gut (rather than the skin or the pulmonary) microbiome; these approaches are summarized in Fig. 2 and Table 2. Patients with Lugano stage I or II of Helicobacter pylori (H. pylori)-associated gastric marginal zone lymphoma (MZL) are typically treated with H. pylori–directed triple or quadruple antibiotic therapy (115). Outside of this, there is little therapeutic use of antibiotics in cancer. Preclinical studies suggest that antibiotic administration improves outcomes in models of primary/metastatic lung, colon, and pancreatic cancer by facilitating a more immunogenic tumor microenvironment (116–119), but accumulating evidence from patients with solid organ cancer tumors treated with ICI therapy indicates that systemic antibiotic therapy associates with reduced bacterial diversity, diminished ICI efficacy, and poorer survival in multiple cancers (29, 31–34). Similarly, the use of antibiotics is associated with leukemic progression in genetically predisposed hosts (120, 121). Therapeutic and/or prophylactic use of broad-spectrum antibiotics in patients with hematologic malignancies during allogeneic hematopoietic stem cell transplantation (allo-HSCT) is associated with profound changes in intestinal microbiota composition, particularly with loss of organisms belonging to the Clostridia class and Bacteroidetes phylum (122–126). These changes are associated with lower bacterial diversity, increased risk of systemic infection with multi-drug resistant bacteria, higher rates of graft-versus-host disease (GVHD), and GVHD-related mortality (122–126).
Figure 2.
Spectrum of microbiome-specific interventions for the treatment of cancer.
Table 2.
List of clinical trials evaluating microbiome therapeutics in cancer.
| Sponsor and trial information | Nature of product | Clinical trial information | Dose | Antibiotic conditioning | Enrollment status | ORR/DCR | TRAE |
|---|---|---|---|---|---|---|---|
| FMT | |||||||
| Baruch et al. (142) | Donor (melanoma PD-1 responder) | PD-1 primary and secondary refractory melanoma | Colonoscopic FMT (day 0), then oral FMT capsules (day 1 and day 12), repeated every 2 weeks along with nivolumab. Nivolumab 240 mg every 2 weeks. | Yes | Not active | 30% | No moderate-to-severe irAEs |
| Davar et al. (51) | Donor (melanoma PD-1 responder) | PD-1 secondary refractory melanoma | Single responder-derived FMT (day 1). Pembrolizumab 200 mg every 3 weeks. | No | Not active | 20%/40% | 3 grade 3 irAEs (2 instances fatigue, 1 peripheral motor neuropathy) |
| NCT04729322 | Donor (dMMR–PD-1 responder) | dMMR patients following progression on PD-1 | Cycle 1: FMT induction via colonoscopy (day 5), then capsules on days 1, 8, 15. Cycles 2+: FMT capsules on day 1 every 3 weeks. | Metronidazole, vancomycin, neomycin | Active, enrolling | Not reported | Not reported |
| NCT04130763 | Healthy donors with microbiome profiles similar to PD-1 responders | GI cancers following progression on PD-1 | Cycle 1: FMT capsules for 1 week as induction. Cycles 2: FMT capsule maintenance. | None | Active, enrolling | Not reported | Not reported |
| MiMic (NCT03772899) | Healthy donor | PD-1 naïve metastatic melanoma in combination with nivolumab | FMT induction preimmunotherapy. FMT maintenance with nivolumab or pembrolizumab. | None | Active, enrolling | Not reported | Not reported |
| Complete consortia products | |||||||
| MCGRAW (NCT03817125, Seres) | Orally bioavailable encapsulated consortia of commensal bacteria |
|
SER-401 capsule once daily for 7 days (lead-in), then daily. Nivolumab per label. | Vancomycin, 5 days | Active, not enrolling | Not reported | Not reported |
| PICASSO (NCT03772899, MaaT Pharma) | Healthy donor–derived full-spectrum microbiome therapeutic (MaaT033) |
|
MaaT033 every 3 weeks (weeks 0–9), then every 12 weeks (weeks 15–23); total 7 infusions. | None | Active, enrolling | Not reported | Not reported |
| Synthetic bacterial consortia | |||||||
| VE800 (Vedanta) | Orally bioavailable LBP containing 11 distinct nonpathogenic, nontoxigenic, commensal bacterial strains | Select histologies following failure of standard therapy in combination with pembrolizumab (NCT04208958):
|
VE800 5 capsules loading, then 2 capsules daily. Nivolumab per label. | Vancomycin, 5 days | Active, not enrolling | Not reported | Not reported |
| Monoclonal microbial candidates | |||||||
| Miyarisan Pharmaceutical | Orally bioavailable bifidogenic Clostridium butyricum strain MIYAIRI588 (CBM588) | Advanced RCC in combination with nivolumab and ipilimumab (NCT03829111) (143) | CBM588 890 mg twice daily. | None | Completed | 58% (nivo/ipi + CBM588) vs. 20% (nivo/ipi) | Grade 3/4 AEs: 52% (nivo/ipi + CBM588) vs. 50% (nivo/ipi) |
| Advanced RCC in combination with nivolumab and cabozantinib (NCT05122546) | None | Active, enrolling | Not reported | Not reported | |||
| Evelo | Orally bioavailable monoclonal microbial derived from single clone of Bifidobacterium spp. | PD-1 refractory melanoma in combination with pembrolizumab (NCT03595683) | EDP1503 capsules twice daily for 14 days (lead-in), then twice daily; each capsule contains ≥ 7.5 x 1010CFUs. Pembrolizumab per label. | None | Suspended | Not reported | Not reported |
Multiple histologies following failure of standard therapy in combination with pembrolizumab (NCT03775850):
|
None | Active, not enrolling | Not reported | Not reported | |||
| NCT03637803 (4d Pharma) | Orally bioavailable LBP containing Enterococcus gallinarum flagellin, which has TLR5 stimulatory properties | Select histologies following failure of standard therapy in combination with pembrolizumab (NCT03775850):
|
MRx0518 capsules twice daily. Pembrolizumab per label. | None | Active, not enrolling | Not reported | Not reported |
| Oncomimics | |||||||
| ROSALIE, NCT04116658 (Enterome) | EO2401: multipeptide (3) vaccine containing peptides (oncomimic), which are strongly homologous but not identical to key tumor antigens in select tumors | Glioblastoma following failure of standard therapy in 3 cohorts:
|
EO2401 multiple doses. Nivolumab and bevacizumab per label. | None | Active, not enrolling | Not reported | Not reported |
| Prebiotics, postbiotics, probiotics, and dietary interventions | |||||||
| NCT05220124 (Tianjin Medical University Second Hospital, Tianjin, China) | Live combined (Bifidobacterium spp., Lactobacillus spp., and Enterococcus spp.) probiotic capsules | Prospective phase IV randomized trial of immunotherapy ± live combined probiotic capsules in platinum-ineligible metastatic urothelial cancer | Live combined probiotic capsules 420 mg twice daily for 3–4 cycles. | None | Active, enrolling | Not reported | Not reported |
| NCT04699721 (Xiangya Hospital of Central South University, Hunan, China) | Unnamed probiotic | Phase I trial of nivolumab with carboplatin and paclitaxel and probiotic in resectable NSCLC | Not stated | None | Active, enrolling | Not reported | Not reported |
| EDEN, NCT04866810 (NIH) | Dietary and exercise intervention | Randomized phase II study of anti–PD-L1 immunotherapy ± dietary and exercise intervention in cancer patients | Plant-based, high-fiber diet and exercise (150 minutes moderate-intensity, 75 minutes high-intensity weekly) | None | Active, enrolling | Not reported | Not reported |
| DIET (NCT04645680) | Isocaloric high-fiber diet | Randomized, double-blind phase II study evaluating high-fiber diet in patients with resectable stage III/IV melanoma following definitive surgery | Isocaloric high-fiber diet (Arm 1) vs. isocaloric control diet (Arm 2) | None | Active, enrolling | Not reported | Not reported |
| NCT03700437 | Intermittent fasting diet | Nonrandomized pilot phase II study of intermittent fasting diet in patients with stage III NSCLC following definitive chemoimmunotherapy (CIT) | Chemolieve®, a plant-based FMD that provides ∼300 calories/fasting day | None | Active, enrolling | Not reported | Not reported |
| CETOREIN (NCT04316520) | Ketogenic diet | Nonrandomized pilot phase II study of ketogenic diet in patients with advanced RCC receiving immunotherapy | Ketogenic diet | None | Active, enrolling | Not reported | Not reported |
Abbreviations: CFU, colony-forming units; dMMR, deficient mismatch repair; FMD, fasting mimicking diet; GE, gastroesophageal; LBP, live bacterial product; NHL, non-Hodgkins lymphoma; nivo/ipi, nivolumab/ipilimumab; NSCLC, non–small cell lung cancer; MSS CRC, microsatellite stable colorectal carcinoma; TNBC, triple-negative breast cancer; RCC, renal cell carcinoma.
The effects of dietary intake upon cancer incidence, progression, and therapeutic outcomes are broad and all-encompassing. However, compelling epidemiologic associations are undermined by inconsistent dietary data collection across studies precluding deeper mechanistic insights, as reviewed in detail elsewhere (127). Across studies, the intake of insoluble dietary fiber (DF), which is not digested but instead fermented into SCFAs by gut bacteria, is associated with increased production of CD103+DCs, enhanced differentiation of activated CD8+T cells into effector cells with memory phenotype (128, 129), and improved outcomes to anti–PD-1 ICI therapy in patients with cancer (130, 131). Specifically, increased intake DF (defined as 20 g/day) was associated with significantly improved PFS in a retrospective analysis of ICI-treated patients with melanoma. The most pronounced benefit was observed in patients with sufficient DF intake and no probiotic use (131).
Multiple studies are evaluating prebiotics (molecules that promote growth of beneficial microbes), probiotics (live microorganisms with putative benefit), and/or postbiotics (microbial-derived molecules) during cancer treatment. Prebiotics, including insoluble DF, demonstrate promise in preclinical models of melanoma and colon cancer, and several clinical trials are evaluating this in patients with melanoma (DIET - NCT04645680, EDEN - NCT04866810), NSCLC (NCT03700437), and RCC (NCT04316520; refs. 132, 133). Interestingly, one preclinical study has recently shown that natural polyphenol castalgin exerts antitumor activity in multiple mouse tumor models to overcome resistance to anti–PD-1 by modulating gut microbiota (134). There is currently a lack of experimental evidence for the use of postbiotics, at least in the context of cancer. A prospective clinical trial compared high-fiber (20 g/day) to high fermented food (6 servings/day) diets in healthy adults and found the latter was associated with increased microbial diversity and reduced systemic inflammation during the 10-week dietary intervention (135). These data suggest that, while the human microbiome is relatively recalcitrant to rapid diet-induced remodeling, specific changes introduced gradually may be leveraged to improve human health.In the context of metabolic disease, an inpatient crossover study compared a ketogenic with an isocaloric control diet in adult men without diabetes but with overweight or obesity. The KD increased Bacteroidetes and Proteobacteria and reduced Actinobacteria and Firmicutes, resulting in decreased intestinal Th17 cell levels (99). KDs in patients with cancer are feasibile and efficient in inducing ketosis (136–140), but the effects of KDs on anticancer treatments have been variable, with favorable effects in patients with breast cancer undergoing neoadjuvant chemotherapy (137) but no effects on reirradiated malignant glioma (138).
The efficacy of FMT in treating ICI-resistant cancers was demonstrated in two clinical trials (51, 141). Both trials evaluated the response of patients with PD-1 refractory melanoma [either primary (51) or secondary (141) to anti–PD-1 therapy combined with FMT obtained from individual long-term responders (R; R-FMT)]. The studies differed in the number of long-term responder donors (2 vs. 8), nature of host microbiota depletion (antibiotics vs. polyethylene glycol-electrolyte laxative), number of patients treated (10 vs. 16), and number of R-FMT administered (multiple vs. single). Both studies demonstrated that R-FMT effectively shifted the microbiome composition toward taxa favoring anti–PD-1 efficacy and reported that R-FMT was associated with increased intratumoral and peripheral antitumor immunity. Davar and colleagues found donor engraftment in 10 of 15 recipients that was sufficient to induce response in 6 patients. In contrast, complete donor engraftment was not required to ameliorate refractory Clostridium difficilecolitis (rCDI; ref. 142). A recent study indicated that antibiotic duration and FMT dosing frequency influences engraftment in non–germ-free mice (143), suggesting that the intestinal dysbiosis observed in PD-1–resistant melanoma may be more “refractory” than rCDI and thus require more extended dosing.
Rationally designed bacterial consortia, monoclonal microbial candidates and bacterial peptides (oncomimics) are being evaluated in combination with ICIs across multiple indications (Table 2). Tanoue and colleagues used a preclinical system previously used to characterize Treg-inducing bacteria from healthy human donor stool to isolate a consortium of 11 bacterial strains. These induced IFNγ-producing CD8+ T cells in the lamina propria and systemically in a CD103+DC- and MHC class I–dependent manner (144). This and other consortia (MET-4, NuBiyota) are being studied in clinical trials in combination with anti–PD-1 in advanced cancer. Other strains being investigated include E. gallinarum (MRx0518), B. animalis lactis (EDP1503), and C. butyricum (CBM588). A phase II trial evaluating the butyrate-producing bacterium Clostridium butyricum (CBM588) in combination with nivolumab/ipilimumab reported that the addition of CBM588 increased response rates in patients with advanced intermediate- and poor- risk RCC (145). Patients treated with CMB588 had a higher response rate than controls (58% vs. 20%), but, surprisingly, the control arm performed poorly when compared with historical controls (20% vs. 42% in CheckMate 214) and the CBM588-treated patients did not exhibit a pharmacodynamic signal indicating increased bifidogenic bacteria. Synthetic biology—or the specific engineering of bacteria for a defined purpose—offers another therapeutic approach. This approach was used, preclinically, for the controlled production and intratumoral release of nanobodies targeting PD-1/CTLA-4 (146) and CD47 (147) within the tumor microenvironment.
Conclusion
The gut microbiome plays an established and critical role in regulating antitumor immunity and responses to ICI in patients with cancer; mounting evidence suggests the intratumoral microbiome may do the same. Preclinical studies identified some mechanisms underlying the immune and antitumor effects of specific bacteria, but more studies are needed to elucidate how gut bacteria augment or impede antitumor immunity in humans. Uncovering these mechanisms will require scaled evaluations of gut microbiota in large and deeply phenotyped cancer cohorts integrating patient-level information (outcome, irAE incidence) and the many variables that may influence gut microbiome composition both at the patient (diet, medication use) and cohort (geography, ethnicity) levels. Uniform sample collection and processing methods are also necessary.
Clinical trials evaluating FMT in refractory melanoma indicate this new modality may be used to modulate gut microbiota in cancer. Additional well-designed interventional clinical trials in patients with melanoma are required to confirm these results. Others are needed to generate preliminary data for additional cancers where ICI efficacy has been linked to gut microbiota (NSCLC, RCC, etc.). It remains to be seen whether defined consortia can produce the same benefits as FMT-based therapies. Finally, mechanistic studies in mouse tumor models will be needed to help validate hypotheses raised by studies in patients with cancer.
Acknowledgments
This work is supported by NIH/NCI grants R01CA222203, R01CA257265, and P50CA257265, and the James W. and Frances G. McGlothlin Chair in Melanoma Immunotherapy Research to H.M. Zarour.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Authors' Disclosures
D. Davar reports grants from Arcus, Checkmate Pharmaceuticals, CellSight Technologies, Immunocore, Merck, and Tesaro/GSK and other support from Checkmate Pharmaceuticals, Finch, Shionogi, and Vedanta Biosciences outside the submitted work; in addition, D. Davar has a patent for 63/124,231 pending and a patent for 63/208,719 pending. H.M. Zarour reports grants from NIH/NCI during the conduct of the study as well as grants from Bristol-Myers Squibb, grants and personal fees from Checkmate Pharmaceuticals and GlaxoSmithKline, and personal fees from Vedanta outside the submitted work; in addition, H.M. Zarour has a patent for 63/124,231 pending and a patent for 63/208,719 pending.
References
- 1. Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P.. Human T cell responses against melanoma. Annu Rev Immunol 2006;24:175–208. [DOI] [PubMed] [Google Scholar]
- 2. Boon T, Old LJ. Cancer tumor antigens. Curr Opin Immunol 1997;9:681–3. [DOI] [PubMed] [Google Scholar]
- 3. Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol 2005;175:6169–76. [DOI] [PubMed] [Google Scholar]
- 4. Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res 2012;72:887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDermott DF, Lee J-L, Szczylik C, Donskov F, Malik J, Alekseev BY, et al. Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (accRCC): Results from cohort A of KEYNOTE-427. J Clin Oncol 2018;36 Suppl 15:4500. [Google Scholar]
- 7. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong PF, Wei W, Smithy JW, Acs B, Toki MI, Blenman KRM, et al. Multiplex quantitative analysis of tumor-infiltrating lymphocytes and immunotherapy outcome in metastatic melanoma. Clin Cancer Res 2019;25:2442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362:eaar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol 2018;36:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daillere R, Vetizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, et al. Enterococcus hirae and barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016;45:931–43. [DOI] [PubMed] [Google Scholar]
- 15. Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013;342:971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberti MP, Yonekura S, Duong CPM, Picard M, Ferrere G, Tidjani Alou M, et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med 2020;26:919–31. [DOI] [PubMed] [Google Scholar]
- 18. Uribe-Herranz M, Rafail S, Beghi S, Gil-de-Gomez L, Verginadis I, Bittinger K, et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J Clin Invest 2020;130:466–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bessell CA, Isser A, Havel JJ, Lee S, Bell DR, Hickey JW, et al. Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. JCI Insight 2020;5:e135597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest 2007;117:2197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi Y, Zheng W, Yang K, Harris KG, Ni K, Xue L, et al. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med 2020;217:e20192282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015;350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Griffin ME, Espinosa J, Becker JL, Luo JD, Carroll TS, Jha JK, et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science 2021;373:1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yonekura S, Terrisse S, Alves Costa Silva C, Lafarge A, Iebba V, Ferrere G, et al. Cancer induces a stress ileopathy depending on B-adrenergic receptors and promoting dysbiosis that contribute to carcinogenesis. Cancer Discov 2022;12:1128–51. [DOI] [PubMed] [Google Scholar]
- 26. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- 29. Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018;29:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsikala-Vafea M, Belani N, Vieira K, Khan H, Farmakiotis D., et al. Use of antibiotics is associated with worse clinical outcomes in patients with cancer treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Int J Infect Dis 2021;106:142–54. [DOI] [PubMed] [Google Scholar]
- 31. Pinato DJ, Gramenitskaya D, Altmann DM, Boyton RJ, Mullish BH, Marchesi JR, et al. Antibiotic therapy and outcome from immune-checkpoint inhibitors. J Immunother Cancer 2019;7:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tinsley N, Zhou C, Tan G, Rack S, Lorigan P, Blackhall F, et al. Cumulative antibiotic use significantly decreases efficacy of checkpoint inhibitors in patients with advanced cancer. Oncologist 2020;25:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang XZ, Gao P, Song YX, Xu Y, Sun JX, Chen XW, et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology 2019;8:e1665973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol 2019;5:1774–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28:1368–79. [DOI] [PubMed] [Google Scholar]
- 36. Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia 2017;19:848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters BA, Wilson M, Moran U, Pavlick A, Izsak A, Wechter T, et al. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med 2019;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, et al. The Diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in chinese patients with NSCLC. J Thorac Oncol 2019;14:1378–89. [DOI] [PubMed] [Google Scholar]
- 39. Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaifaoui M, Mimpen I, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res 2020;8:1243–50. [DOI] [PubMed] [Google Scholar]
- 40. Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med 2022;28:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol 2020;78:195–206. [DOI] [PubMed] [Google Scholar]
- 42. Peng Z, Cheng S, Kou Y, Wang Z, Jin R, Hu H, et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol Res 2020;8:1251–61. [DOI] [PubMed] [Google Scholar]
- 43. Gharaibeh RZ, Jobin C.. Microbiota and cancer immunotherapy: in search of microbial signals. Gut 2019;68:385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaikh FY, White JR, Gills JJ, Hakozaki T, Richard C, Routy B, et al. A uniform computational approach improved on existing pipelines to reveal microbiome biomarkers of non-response to immune checkpoint inhibitors. Clin Cancer Res 2021;27:2571–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thomas AM, Manghi P, Asnicar F, Pasolli E, Armanini F, Zolfo M, et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med 2019;25:667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med 2019;25:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ascierto PA, Long GV. Progression-free survival landmark analysis: a critical endpoint in melanoma clinical trials. Lancet Oncol 2016;17:1037–9. [DOI] [PubMed] [Google Scholar]
- 48. Ferrara R, Pilotto S, Caccese M, Grizzi G, Sperduti I, Giannarelli D, et al. Do immune checkpoint inhibitors need new studies methodology? J Thorac Dis 2018;10Suppl 13:S1564–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee KA, Thomas AM, Bolte LA, Bjork JR, de Ruijter LK, Armanini F, et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med 2022;28:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McCulloch JA, Davar D, Rodrigues RR, Badger JH, Fang JR, Cole AM, et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med 2022;28:545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021;371:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol 2018;16:e2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dwiyanto J, Hussain MH, Reidpath D, Ong KS, Qasim A, Lee SWH, et al. Ethnicity influences the gut microbiota of individuals sharing a geographical location: a cross-sectional study from a middle-income country. Sci Rep 2021;11:2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gorvitovskaia A, Holmes SP, Huse SM. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome 2016;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Knights D, Ward TL, McKinlay CE, Miller H, Gonzalez A, McDonald D, et al. Rethinking “enterotypes”. Cell Host Microbe 2014;16:433–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang R, Walker AR, Datta S.. Unraveling city-specific signature and identifying sample origin locations for the data from CAMDA MetaSUB challenge. Biol Direct 2021;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pasolli E, Asnicar F, Manara S, Zolfo M, Karcher N, Armanini F, et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell 2019;176:649–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Karcher N, Pasolli E, Asnicar F, Huang KD, Tett A, Manara S, et al. Analysis of 1321 Eubacterium rectale genomes from metagenomes uncovers complex phylogeographic population structure and subspecies functional adaptations. Genome Biol 2020;21:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Truong DT, Tett A, Pasolli E, Huttenhower C, Segata N.. Microbial strain-level population structure and genetic diversity from metagenomes. Genome Res 2017;27:626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chai JN, Peng Y, Rengarajan S, Solomon BD, Ai TL, Shen Z, et al. Helicobacter species are potent drivers of colonic T cell responses in homeostasis and inflammation. Sci Immunol 2017;2:eaal5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019;364:1179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 2014;510:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, et al. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 2018;554:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li Y, Tinoco R, Elmen L, Segota I, Xian Y, Fujita Y, et al. Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5(-/-) mice. Nat Commun 2019;10:1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Laute-Caly DL, Raftis EJ, Cowie P, Hennessy E, Holt A, Panzica DA, et al. The flagellin of candidate live biotherapeutic Enterococcus gallinarum MRx0518 is a potent immunostimulant. Sci Rep 2019;9:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zheng JH, Nguyen VH, Jiang SN, Park SH, Tan W, Hong SH, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med 2017;9:eaak9537. [DOI] [PubMed] [Google Scholar]
- 68. Fluckiger A, Daillere R, Sassi M, Sixt BS, Liu P, Loos F, et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 2020;369:936–42. [DOI] [PubMed] [Google Scholar]
- 69. Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017;551:512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015;42:344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kaplan CW, Ma X, Paranjpe A, Jewett A, Lux R, Kinder-Haake S, et al. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect Immun 2010;78:4773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kalaora S, Nagler A, Nejman D, Alon M, Barbolin C, Barnea E, et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 2021;592:138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cekic C, Linden J.. Purinergic regulation of the immune system. Nat Rev Immunol 2016;16:177–92. [DOI] [PubMed] [Google Scholar]
- 74. Allard B, Allard D, Buisseret L, Stagg J.. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol 2020;17:611–29. [DOI] [PubMed] [Google Scholar]
- 75. Wang T, Gnanaprakasam JNR, Chen X, Kang S, Xu X, Sun H, et al. Inosine is an alternative carbon source for CD8(+)-T-cell function under glucose restriction. Nat Metab 2020;2:635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020;369:1481–9. [DOI] [PubMed] [Google Scholar]
- 77. Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018;360:eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Maslowski KM, Mackay CR.. Diet, gut microbiota and immune responses. Nat Immunol 2011;12:5–9. [DOI] [PubMed] [Google Scholar]
- 79. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013;504:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446–50. [DOI] [PubMed] [Google Scholar]
- 81. Kespohl M, Vachharajani N, Luu M, Harb H, Pautz S, Wolff S, et al. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4(+) T cells. Front Immunol 2017;8:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Park J, Goergen CJ, HogenEsch H, Kim CH. Chronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosis. J Immunol 2016;196:2388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 2015;8:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dupraz L, Magniez A, Rolhion N, Richard ML, Da Costa G, Touch S, et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal gammadelta T cells. Cell Rep 2021;36:109332. [DOI] [PubMed] [Google Scholar]
- 86. Coutzac C, Jouniaux JM, Paci A, Schmidt J, Mallardo D, Seck A, et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun 2020;11:2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A, et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat Commun 2021;12:4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab 2021;33:988–1000. [DOI] [PubMed] [Google Scholar]
- 89. Botticelli A, Vernocchi P, Marini F, Quagliariello A, Cerbelli B, Reddel S, et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med 2020;18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open 2020;3:e202895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Klein MS, Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, et al. Metabolomic modeling to monitor host responsiveness to gut microbiota manipulation in the BTBR(T+tf/j) mouse. J Proteome Res 2016;15:1143–50. [DOI] [PubMed] [Google Scholar]
- 92. Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Shearer J.. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism 2016;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes 2008;32:1720–4. [DOI] [PubMed] [Google Scholar]
- 95. Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC, et al. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 2016;16:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ferrere G, Tidjani Alou M, Liu P, Goubet AG, Fidelle M, Kepp O, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight 2021;6:e145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dai X, Bu X, Gao Y, Guo J, Hu J, Jiang C, et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol Cell 2021;81:2317–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Goldberg EL, Shchukina I, Asher JL, Sidorov S, Artyomov MN, VD Dixit, et al. Ketogenesis activates metabolically protective gammadelta T cells in visceral adipose tissue. Nat Metab 2020;2:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 2020;181:1263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fan S, Zhang H, Chen X, Lu L, Xu L, Xiao M, et al. Cloning, characterization, and production of three alpha-L-fucosidases from Clostridium perfringens ATCC 13124. J Basic Microbiol 2016;56:347–57. [DOI] [PubMed] [Google Scholar]
- 101. Wright DP, Rosendale DI, Robertson AM.. Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol Lett 2000;190:73–9. [DOI] [PubMed] [Google Scholar]
- 102. Luke NR, Sauberan SL, Russo TA, Beanan JM, Olson R, Loehfelm TW, et al. Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect Immun 2010;78:2017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Frank M, Hennenberg EM, Eyking A, Runzi M, Gerken G, Scott P, et al. TLR signaling modulates side effects of anticancer therapy in the small intestine. J Immunol 2015;194:1983–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bhatt AP, Pellock SJ, Biernat KA, Walton WG, Wallace BD, Creekmore BC, et al. Targeted inhibition of gut bacterial beta-glucuronidase activity enhances anticancer drug efficacy. Proc Natl Acad Sci. 2020;117:7374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kurita A, Kado S, Matsumoto T, Asakawa N, Kaneda N, Kato I, et al. Streptomycin alleviates irinotecan-induced delayed-onset diarrhea in rats by a mechanism other than inhibition of beta-glucuronidase activity in intestinal lumen. Cancer Chemother Pharmacol 2011;67:201–13. [DOI] [PubMed] [Google Scholar]
- 106. Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017;357:1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mohindroo C, Hasanov M, Rogers JE, Dong W, Prakash LR, Baydogan S, et al. Antibiotic use influences outcomes in advanced pancreatic adenocarcinoma patients. Cancer Med 2021;10:5041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pal SK, Li SM, Wu X, Qin H, Kortylewski M, Hsu J, et al. Stool bacteriomic profiling in patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor-tyrosine kinase inhibitors. Clin Cancer Res 2015;21:5286–93. [DOI] [PubMed] [Google Scholar]
- 109. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2016;7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med 2021;27:1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 2020;182:655–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Suresh K, Naidoo J, Zhong Q, Xiong Y, Mammen J, de Flores MV, et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest 2019;129:4305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang Y, Ma W, Abu-Sbeih H, Jiang Z-D, DuPont HL., et al. Fecal microbiota transplantation (FMT) for immune checkpoint inhibitor induced–colitis (IMC) refractory to immunosuppressive therapy. J Clin Oncol 2020;38 Suppl 15:3067. [Google Scholar]
- 114. Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med 2018;24:1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Koch P, del Valle F, Berdel WE, Willich NA, Reers B, Hiddemann W, et al. Primary gastrointestinal non-Hodgkin's lymphoma: II. Combined surgical and conservative or conservative management only in localized gastric lymphoma–results of the prospective German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001;19:3874–83. [DOI] [PubMed] [Google Scholar]
- 116. Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, et al. Commensal microbiota promote lung cancer development via gammadelta T cells. Cell 2019;176:998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Le Noci V, Guglielmetti S, Arioli S, Camisaschi C, Bianchi F, Sommariva M, et al. Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: a strategy to promote immunosurveillance against lung metastases. Cell Rep 2018;24:3528–38. [DOI] [PubMed] [Google Scholar]
- 118. Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017;358:1443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov 2018;8:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Meisel M, Hinterleitner R, Pacis A, Chen L, Earley ZM, Mayassi T, et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature 2018;557:580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Vicente-Duenas C, Janssen S, Oldenburg M, Auer F, Gonzalez-Herrero I, Casado-Garcia A, et al. An intact gut microbiome protects genetically predisposed mice against leukemia. Blood 2020;136:2003–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med 2012;209:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012;55:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant 2014;20:640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, et al. Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant 2015;21:1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 2016;8:339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Steck SE, Murphy EA. Dietary patterns and cancer risk. Nat Rev Cancer 2020;20:125–38. [DOI] [PubMed] [Google Scholar]
- 128. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014;20:159–66. [DOI] [PubMed] [Google Scholar]
- 129. Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8(+) T cells. Immunity 2019;51:285–97. [DOI] [PubMed] [Google Scholar]
- 130. Nomura M, Nagatomo R, Inoue K, Doi K, Shimizu J, Baba K, et al. 1249P - Association of SCFA in gut microbiome and clinical response in solid cancer patients treated with andi-PD-1 antibody. Ann Oncol 2019;30:v509. [Google Scholar]
- 131. Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021;374:1632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Li Y, Elmen L, Segota I, Xian Y, Tinoco R, Feng Y, et al. Prebiotic-induced anti-tumor immunity attenuates tumor growth. Cell Rep 2020;30:1753–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhang SL, Mao YQ, Zhang ZY, Li ZM, Kong CY, Chen HL, et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics 2021;11:4155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Messaoudene M, Pidgeon R, Richard C, Ponce M, Diop K, Benlaifaoui M, et al. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov 2022;12:1070–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021;184:4137–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zahra A, Fath MA, Opat E, Mapuskar KA, Bhatia SK, Ma DC, et al. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the university of iowa experience of two phase 1 clinical trials. Radiat Res 2017;187:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Khodabakhshi A, Akbari ME, Mirzaei HR, Mehrad-Majd H, Kalamian M, Davoodi SH, et al. Feasibility, safety, and beneficial effects of mct-based ketogenic diet for breast cancer treatment: a randomized controlled trial study. Nutr Cancer 2020;72:627–34. [DOI] [PubMed] [Google Scholar]
- 138. Voss M, Wagner M, von Mettenheim N, Harter PN, Wenger KJ, Franz K, et al. ERGO2: a prospective, randomized trial of calorie-restricted ketogenic diet and fasting in addition to reirradiation for malignant glioma. Int J Radiat Oncol Biol Phys 2020;108:987–95. [DOI] [PubMed] [Google Scholar]
- 139. Klement RJ, Champ CE, Kammerer U, Koebrunner PS, Krage K, Schafer G, et al. Impact of a ketogenic diet intervention during radiotherapy on body composition: III-final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res 2020;22:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Schreck KC, Hsu FC, Berrington A, Henry-Barron B, Vizthum D, Blair L, et al. Feasibility and biological activity of a ketogenic/intermittent-fasting diet in patients with glioma. Neurology 2021;97:e953–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021;371:602–9. [DOI] [PubMed] [Google Scholar]
- 142. Staley C, Kelly CR, Brandt LJ, Khoruts A, Sadowsky MJ., et al. Complete microbiota engraftment is not essential for recovery from recurrent clostridium difficile infection following fecal microbiota transplantation. mBio 2016;7:e01965–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gopalakrishnan V, Dozier EA, Glover MS, Novick S, Ford M, Morehouse C, et al. Engraftment of bacteria after fecal microbiota transplantation is dependent on both frequency of dosing and duration of preparative antibiotic regimen. Microorganisms 2021;9:1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019;565:600–5. [DOI] [PubMed] [Google Scholar]
- 145. Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med 2022;28:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gurbatri CR, Lia I, Vincent R, Coker C, Castro S, Treuting PM, et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci Transl Med 2020;12:eaax0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chowdhury S, Castro S, Coker C, Hinchliffe TE, Arpaia N, Danino T., et al. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med 2019;25:1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]