Abstract
In a recently published study, Xu et al. used two surface markers, CLSTN2 and PTPRO, to generate highly purified donor dopaminergic neurons and achieved stable and predictable therapeutic outcomes by transplantation into the brain of PD animal models (Xu et al., 2022).
In a recently published study, Xu et al. used two surface markers, CLSTN2 and PTPRO, to generate highly purified donor dopaminergic neurons and achieved stable and predictable therapeutic outcomes by transplantation into the brain of PD animal models (Xu et al., 2022).
Impediments to clinical translation in Parkinson’s disease stem cell therapy
Parkinson’s disease (PD) is a neurodegenerative disorder primarily involving the progressive loss of mDA (midbrain dopaminergic) neurons that project from the substantia nigra to the striatum. Although non-motor symptoms contribute to the disease in many patients, it is this loss of mDA neurons that leads to prominent and disabling motor dysfunctions such as bradykinesia, rigidity, and tremors. In a pioneering study in the 1980s, Bjorklund et al. showed that embryonic mDA neural tissue obtained from fetal ventral mesencephalon (VM), when transplanted into the striatum of unilaterally 6-hydroxydopamine-treated rats, could reverse the symptoms of dopamine depletion in this model of PD (Barker et al., 2013). Although this demonstrated that graft-induced recovery of motor function was possible, human fetal mesencephalic cells remain a resource severely limited by both logistical and ethical considerations; moreover, the nature of the donor dissection often resulted in contamination by serotonergic neurons that have been postulated to underlie the graft-induced dyskinesia that limited beneficial outcomes in clinical trials (Li and Li, 2021). To identify more practical cell sources and to avoid the obstacles related to using fetal tissue, increasing attention has been paid to utilizing human pluripotent stem cells (hPSCs) due to their potentially unlimited availability and capacity to differentiate into most adult cell types. An abundance of evidence has been provided to show that pluripotent stem cell-derived mDA progenitors are able to survive and differentiate into mature dopamine neurons in animal models of PD (Parmar et al., 2020) and may improve symptoms in patients with PD (Schweitzer et al., 2020). Thus, hPSCs hold promise for the development of cell transplantation as a therapy for PD.
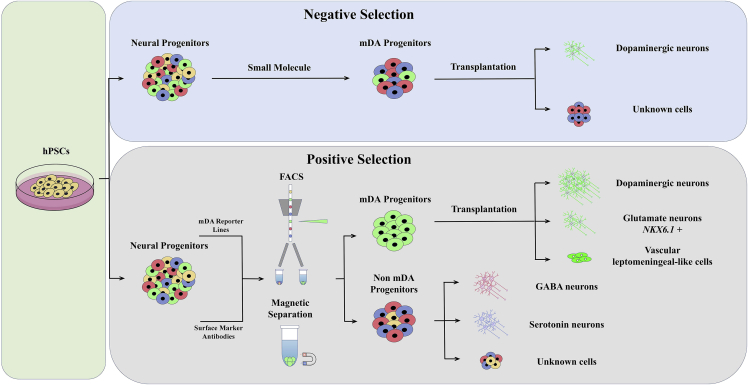
However, there remain some significant problems in this field: since fully mature mDA neurons do not survive after harvesting or transplantation, investigators have turned to mDA progenitors, which survive much better and may be better able to locate and connect to their normal pre- and postsynaptic circuit elements even in adult brain. However, the more immature the mDA progenitors, the greater their potential to proliferate after implantation, form tumors, develop into unwanted cell types, or make inappropriate synaptic connections; such off-target effects also result in a significant loss of efficiency of the therapy. The yield of mDA neurons in the grafts is typically low, and different batches or cell lines have high variability in this respect. The detailed composition of the implanted progenitor cell population and its final products in the resulting grafts is not entirely defined. All these concerns constitute significant impediments to the strict demands of clinical application. One possible solution to address these issues would be to transplant a dopaminergic cell population with defined molecular characteristics that identify its commitment to the desired ventral mDA neuronal fate. If, among such defined characteristics, corresponding cell surface markers can be identified, these might be leveraged using specific antibodies to these markers to isolate the cells by techniques such as fluorescence-activated cell sorting (FACS) or the use of magnetic beads, reducing the numbers of undesired cell types and enhancing the production of mDA neurons (Figure 1). Genetic modification and cell surface marker approaches have been used to isolate mDA progenitors from VM tissues in such a manner (Bye et al., 2015; Jonsson et al., 2009). Previous studies have proposed several such cell surface markers (CORIN, LRTM1, IAP, FoLR1, et al.) that could be used to enrich ventral mDA precursors. However, some of these markers are expressed in brain areas outside the ventral midbrain. For example, CORIN is expressed not only in ventral midbrain but also in non-dopaminergic hindbrain and spinal cord. In current mDA progenitor differentiation protocols, mesencephalic floor plate markers LMX1A, FOXA2, and OTX2 are commonly used to verify the mDA identity of progenitors in vitro before grafting, but these VM markers are also co-expressed in diencephalic progenitors of the subthalamic nucleus and therefore do not exclusively identify the mDA domain (Kirkeby et al., 2017). Thus, the identification of more specific surface markers would be a major boon to clinical translation. To solve these problems, Xu et al. have established a method to identify suitable markers using a unique and systematic strategy and demonstrated the potential of this technique to produce highly purified mDA progenitors that can achieve stable and predictable therapeutic outcomes in animal models (Xu et al., 2022).
Figure 1.
Main strategies for enrichment hPSC-derived ventral mDA progenitors
Enriching mDA progenitors from hPSC products
The search for surface markers can be divided into two processes. The first involves identification of authentic dopaminergic neuronal precursor cells and their distinction from off-target cells during their differentiation in vitro. The next comprises the search for genes that are differentially expressed in these mDA progenitors in order to identify correlating surface markers that can be used for cell selection strategies.
By depicting the single-cell molecular atlas during mDA neuron differentiation, Xu et al. found that the process of hPSC-based mDA neuron differentiation in vitro closely recapitulated the development of adjacent fetal brain regions including ventral midbrain, isthmus, and ventral hindbrain in vivo. In particular, the mid-hindbrain regional identities and heterogeneity of the cells were established at very early stages of differentiation, 8 days after starting differentiation (stage I), while the specification of mDA progenitors and neurons occurred at mid to late stages of differentiation. As the development of the neural tube proceeds, two signaling centers are established: the isthmus, which demarcates the midbrain-hindbrain boundary (MHB), and the floor plate (FP), which regulates ventral identities. During these and subsequent developmental stages, the combined regulation of transcription factors and morphogens from the isthmus and the FP orchestrates multiple functions, including the regional identity of the VM and the specification and proliferation of mDA progenitors, as well as the differentiation and survival of mDA neurons (Arenas et al., 2015). Other researchers have similarly found that the formation of the caudal VM MHB was important in the differentiation of mDA neurons, and a high representation of genes expressed in the caudal VM and MHB region was positively associated with high mDA neuron yield in vivo (Kirkeby et al., 2017). Xu et al. report that cellular diversity in the cultures rose in parallel with mDA neuron differentiation and mesencephalic progenitors, then further split into three progenitor subclusters, including mDA progenitors. Off-target cells primarily comprised metencephalic-like cells, MHB-like cells, and non-mDA progenitors, which could differentiate into various non-mDA neurons, vascular leptomeningeal-like cells (VLMCs), and others.
After utilizing differential gene-expression analysis on the mDA progenitor cluster at stages III and IV, Xu et al. identified CLSTN2 and PTPRO as two surface markers specific for mDA progenitors. Moreover, fluorescence in situ hybridization (FISH) for CLSTN2 mRNA in the mouse embryo at embryonic day 11.5 (E11.5) and E12.5 showed that CLSTN2 is specifically expressed in the VM, which co-expressed FP markers FOXA2 and LMX1A. This CLSTN2 expression pattern provides a potential explanation for the greater specificity of CLSTN2 compared with other surface markers such as CORIN, IAP, and LRTM1, which localize in E12.5 mouse mesencephalon both to the FP and to the basal plate or alar plate.
Finally, by generating hPSC lines in which tdTomato was inserted into the C terminus of either the CLSTN2 or PTPRO gene, Xu et al. isolated CLSTN2+ or PTPRO+ progenitors and found that these progenitors gave rise to high yields of TH+ mDA neurons both during in vitro differentiation and after in vivo transplantation. Implanted CLSTN2- or PTPRO-enriched progenitors resulted in smaller grafts but denser dopaminergic innervation compared with unsorted cells. Moreover, compared with the cell doses required in other studies to achieve beneficial effects (typically 100,000–200,000 mDA progenitors for transplantation per mouse), only 7,500 sorted CLSTN2+ progenitors sufficed to produce similar improvements in PD model mice.
The main attraction of stem cell therapy for PD is potential access to unlimited quantities of pure, stable mDA progenitors for transplantation. Batch variability and vaguely defined final products are clearly a major obstacle to clinical application. In other studies, although all VM-patterned cell batches were routinely checked for high co-expression of the VM markers LMX1A, FOXA2, and OTX2 prior to grafting (80%), the in vivo outcome in terms of number of mDA neurons has varied considerably between experiments. Other surface markers have been used to reduce the proportion of off-target cells developing in vivo in grafts, but they have not resulted in well-defined cell compositions in the grafts. Xu et al. showed that using their markers and selection technique, grafts from different batches of surface marker-sorted groups (CLSTN2+ or PTPRO+) had strikingly similar neuronal compositions. Sorted progenitors could stably generate grafts consisting of about 70% mDA neurons and about 30% NKX6-1+ glutamatergic neurons. This method also depleted most undesired neuronal subtypes, including BARHL1+ and NKX2-1+ glutamatergic neurons, that have been reported as lateral mesencephalic contaminating populations in VM-patterned hESCs both in vitro and in vivo (Kirkeby et al., 2017), implying more stable and predictable graft outcomes.
Discussion
Clinical research aimed at PD cell replacement therapy has now developed over more than three decades. Among concerns with the original fetal mesencephalic tissue source that forms the proof of principle for current stem cell-based strategies is that it contained other midbrain cell populations in addition to dopamine cells. Stem cell-based products, because of the possibility of artificially guided differentiation toward a desired target product, have the theoretical potential to provide greater purity than surgically harvested fetal VM tissue. However, previous studies have shown that using current methodology, stem cell-derived dopamine progenitor products still contain substantial populations of off-target cells, resulting in reduced efficacy and significant safety concerns.
To address these concerns related to cell diversity, there are two basic strategies: negative selection via removal of unwanted cells or positive selection via harvesting of the target cells (Figure 1). Undifferentiated hPSCs can be selectively eliminated from among differentiated mDA progenitors using small molecules, such as the survivin inhibitor quercetin (BIRC5) (Song et al., 2020). To eliminate residual neural stem cells, γ-secretase inhibitor or gamma irradiation have been applied (Katsukawa et al., 2016). To positively select desired cells, effective cell surface marker identification is critical. Xu et al. have identified CLSTN2 or PTPRO as two cell surface markers that can potentially be used in this way. However, they have based their cell sorting methodology on genetic modification of these two markers rather than on direct application of selective antibodies. While this is a scientifically elegant demonstration of the specifying potential of these markers, genetic modification of this sort would introduce significant complications to clinical application. Discussion of the justification for their chosen approach and its relevance to the clinical translation that the work is ultimately designed to facilitate would have been welcome. Once appropriate sorting antibodies against these two markers are established, this could become a practical method for producing better defined, safer, and more uniform mDA neurons for clinical PD cell therapy.
Graft volume is another important parameter affecting the safety of PD cell therapy that has received less attention than it perhaps deserves. As discussed above, it is the inability of mature mDA neurons to survive transplantation that has required the use of dopamine progenitors for cell therapy; but although committed to an mDA neuronal fate, progenitors still have limited proliferation ability, which, together with similar limited proliferation of other cell types in the graft, results in variable degrees of enlargement of the graft volume after implantation. An overly large graft could produce sufficient “mass effect” in the human brain to be clinically detrimental. In most reported clinical cases of cell therapy for PD, the transplantation sites have been in the striatum, rather than the substantia nigra, and thus are in the normal efferent target area for mDA neurons rather than in their normal SNpc (substantia nigra pars compacta) endogenous location. This choice of target has been mandated both by the higher surgical risk of the midbrain target and by its presumably lesser tolerance to accommodating mass effect.
The implications of ectopic or isotopic graft placement for restoration of normal neuronal circuits are a matter of ongoing study (Adler et al., 2019; Cardoso et al., 2018). A recent report suggested that nigral grafts receive extensive presynaptic inputs from the same, appropriate brain regions as do endogenous mDA neurons, while striatal grafts receive presynaptic input of similar transmitter type but from different anatomic regions than normal (Xiong et al., 2021). The clinical implications of such altered synaptic input are not well understood, but an anatomic placement that promotes restoration of normal circuitry seems likely to be beneficial. If the graft volume can be reduced while maintaining efficacy, as demonstrated by Xu et al. using CLSTN2 or PTPRO sorting, it may be possible to transplant these cells isotopically into the normal substantia nigra location to facilitate restoration of impaired network circuits in a more physiological fashion, representing yet another potential benefit of isotopic approach (Moriarty et al., 2022).
Finally, clinical therapeutic products in general, and cell therapy products in particular, must establish and satisfy a set of quality control and release criteria for clinical grade cell product manufacturing prior to regulatory approval and safe clinical use. The current lack of predictive markers that correlate reliably with postimplantation safety and efficacy are a challenge to clinical translation. Effective predictive markers could overcome batch-to-batch variation problems by providing a rapid and accurate screening tool to identify “good” batches quickly.
The current work provides new strategies to predict cell fate after transplantation as well as to obtain stable and predictable cell therapy results. It thus represents a major advance toward more effective and safer cell therapy for PD.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32170807), the Natural Science Foundation of Shanghai (21ZR1406300), and the Office of Global Partnerships (Key Projects Development Fund). We also thank members of the Parkinson Disease and Cell Therapy Laboratory for their discussion and suggestions.
Conflict of interest
The authors declare no competing interests.
Contributor Information
Jeffrey S. Schweitzer, Email: jschweitzer1@mgh.harvard.edu.
Bin Song, Email: binsong@fudan.edu.cn.
References
- Adler A.F., Cardoso T., Nolbrant S., Mattsson B., Hoban D.B., Jarl U., Wahlestedt J.N., Grealish S., Bjorklund A., Parmar M. hESC-derived dopaminergic transplants integrate into basal Ganglia circuitry in a preclinical model of Parkinson's disease. Cell Rep. 2019;28:3462–3473.e5. doi: 10.1016/j.celrep.2019.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas E., Denham M., Villaescusa J.C. How to make a midbrain dopaminergic neuron. Development. 2015;142:1918–1936. doi: 10.1242/dev.097394. [DOI] [PubMed] [Google Scholar]
- Barker R.A., Barrett J., Mason S.L., Bjorklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson's disease. Lancet Neurol. 2013;12:84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- Bye C.R., Jonsson M.E., Bjorklund A., Parish C.L., Thompson L.H. Transcriptome analysis reveals transmembrane targets on transplantable midbrain dopamine progenitors. Proc. Natl. Acad. Sci. USA. 2015;112:E1946–E1955. doi: 10.1073/pnas.1501989112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso T., Adler A.F., Mattsson B., Hoban D.B., Nolbrant S., Wahlestedt J.N., Kirkeby A., Grealish S., Bjorklund A., Parmar M. Target-specific forebrain projections and appropriate synaptic inputs of hESC-derived dopamine neurons grafted to the midbrain of parkinsonian rats. J. Comp. Neurol. 2018;526:2133–2146. doi: 10.1002/cne.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M.E., Ono Y., Bjorklund A., Thompson L.H. Identification of transplantable dopamine neuron precursors at different stages of midbrain neurogenesis. Exp. Neurol. 2009;219:341–354. doi: 10.1016/j.expneurol.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Katsukawa M., Nakajima Y., Fukumoto A., Doi D., Takahashi J. Fail-safe therapy by gamma-ray irradiation against tumor formation by human-induced pluripotent stem cell-derived neural progenitors. Stem Cells Dev. 2016;25:815–825. doi: 10.1089/scd.2015.0394. [DOI] [PubMed] [Google Scholar]
- Kirkeby A., Nolbrant S., Tiklova K., Heuer A., Kee N., Cardoso T., Ottosson D.R., Lelos M.J., Rifes P., Dunnett S.B., et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson's disease. Cell Stem Cell. 2017;20:135–148. doi: 10.1016/j.stem.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Y., Li W. Postmortem studies of fetal grafts in Parkinson's disease: what lessons have we learned? Front. Cell Dev. Biol. 2021;9:666675. doi: 10.3389/fcell.2021.666675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty N., Gantner C.W., Hunt C.P.J., Ermine C.M., Frausin S., Viventi S., Ovchinnikov D.A., Kirik D., Parish C.L., Thompson L.H. A combined cell and gene therapy approach for homotopic reconstruction of midbrain dopamine pathways using human pluripotent stem cells. Cell Stem Cell. 2022;29:434–448.e5. doi: 10.1016/j.stem.2022.01.013. [DOI] [PubMed] [Google Scholar]
- Parmar M., Grealish S., Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat. Rev. Neurosci. 2020;21:103–115. doi: 10.1038/s41583-019-0257-7. [DOI] [PubMed] [Google Scholar]
- Schweitzer J.S., Song B., Herrington T.M., Park T.Y., Lee N., Ko S., Jeon J., Cha Y., Kim K., Li Q., et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson's disease. N. Engl. J. Med. 2020;382:1926–1932. doi: 10.1056/NEJMoa1915872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B., Cha Y., Ko S., Jeon J., Lee N., Seo H., Park K.J., Lee I.H., Lopes C., Feitosa M., et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson's disease models. J. Clin. Invest. 2020;130:904–920. doi: 10.1172/JCI130767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M., Tao Y., Gao Q., Feng B., Yan W., Zhou Y., Kotsonis T.A., Yuan T., You Z., Wu Z., et al. Human stem cell-derived neurons repair circuits and restore neural function. Cell Stem Cell. 2021;28:112–126.e6. doi: 10.1016/j.stem.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., He H., Gao Q., Zhou Y., Wu Z., Zhang X., Sun L., Hu G., Guan Q., You Z., et al. Human midbrain dopaminergic neuronal differentiation markers predict cell therapy outcome in a Parkinson's disease model. J. Clin. Invest. 2022 doi: 10.1172/JCI156768. [DOI] [PMC free article] [PubMed] [Google Scholar]