Summary
There is significant variation in the response to adversity, with a substantial proportion of individuals displaying psychological resilience. Epigenetic mechanisms are hypothesised to be one molecular pathway of how experiences can become biologically embedded and contribute to individual differences in resilience. However, not much is known regarding the role of epigenetics in the development of psychological resilience. In this review, we propose a new conceptual model for the different functions of epigenetic mechanisms in psychological resilience. The model considers 1) the initial establishment of the epigenome, 2) epigenetic modification due to protective environmental exposures across life, 3) the role of protective factors in counteracting adverse influences, and 4) genetic moderation of environmentally induced epigenetic modifications. After reviewing empirical evidence for the various components of the model, we identify research areas which should be prioritized and discuss practical implications of the proposed model for epigenetic research on resilience.
Keywords: Epigenetics, DNA methylation, Genetics, Resilience, Mental Health, Developmental Psychopathology
In the face of adversity some individuals develop stress-related disorders such as depression or post-traumatic stress disorder (PTSD). However, a significant proportion exhibit psychological resilience defined broadly as the maintenance of good mental health despite exposure to adversity.1 A better understanding of the development of resilience is of significant clinical benefit in the prevention and treatment of stress-related disorders.
Introduction to Resilience
Although resilience is generally considered the capacity of an individual to overcome and bounce back from adversity, the exact definition and associated means of measuring it differ considerably. It can be conceptualised in three ways: 1) as a dynamic and malleable process, 2) as a stable trait, and 3) as an outcome in response to adversity. In line with contemporary thinking, we consider resilience to be a dynamic process or complex function of numerous individual (e.g. genetic factors) and social-environmental factors (e.g. social support) which allows an individual to maintain good psychological health despite significant adversity. 2,3 Importantly, resilience reflects not simply the absence of risk factors but includes also the influence of protective factors which promote positive adaptation. Although resilience has become a common feature of mental health research, the majority of psychiatric studies tend to focus on resilience-reducing or risk-conferring factors, often overlooking the contribution of resilience-promoting factors.
Given the varied definitions, resilience has been measured in a multitude of ways. A common method is through binary segregation of those that have succumbed to a single mental health disorder at a single point in time compared to those that have not.4 Others consider a more nuanced longitudinal approach and utilise complex statistical models to identify discrete, longitudinal trajectories of mental health following adversity.1 Further methods focus less on the outcome and more on individual attributes which can contribute to resilience in an individual.3 Given the complexity of resilience, its assessment remains a challenge due to the fact that individuals may be resilient in one psychological domain, while vulnerable to another, and resilient at one time, but not another.
How do these social-environmental factors become biologically embedded throughout the lifespan and cause long-term changes to the body’s biology that ultimately impact psychiatric outcomes? Epigenetic mechanisms are hypothesised to be one important molecular pathway by which this occurs.5,6 There is now a growing body of research that supports the hypothesis that adverse environments impact the epigenome and that epigenetic differences can distinguish vulnerable and resilient individuals.5-7 However, the specific role of protective environments and associated epigenetic mechanisms that contribute to the development of resilience are often overlooked.
Our aim in this paper is to consider the multiple roles that epigenetic mechanisms might play in psychological resilience, considering the complex relationships with environmental factors and genetics across the lifespan. In particular, we will pay particular attention to resilience-promoting factors. After introducing epigenetics, we present a theoretical model based on theory and empirical literature which outlines three specific ways in which epigenetics could contribute to the development of psychological resilience. We then use this model to assess the current state of research, highlight areas which remain to be thoroughly investigated and provide suggestions for future research.
Introduction to Epigenetics
Whilst the genome remains relatively stable throughout life, the expression of its genes is highly variable. This variability is partially controlled by epigenetic mechanisms, a diverse group of mitotically heritable and long lasting but reversible molecular changes, providing an important layer of control.8 Born out of this diversity, there is some disagreement as to exactly which mechanisms comprise epigenetics, but the best understood and most commonly-researched, particularly in relation to mental health, are DNA methylation, histone modification and non-coding RNAs (ncRNAs) (outlined in Panel 1 and fully described in the appendix, page 1). Acquiring, maintaining and eliminating these various modifications allows for a dynamic and multi-level system of control, impacting all stages of gene expression.9
Panel 1: The three major classes of epigenetic modification.
DNA methylation
The addition or removal of methyl groups directly to the DNA code which impact the tightness of DNA packaging. A common feature in gene promoters, this effectively supresses expression of the associated gene.
Histone modification
The addition or removal of small chemical groups (e.g. methyl groups) to the histone proteins which form the scaffold for DNA. Depending on the specific modification this modulates the tightness of DNA packaging and consequently the expression of nearby genes.
Non-coding RNAs
Diverse species of RNA which can impact the many stages of gene expression from transcription to post-translational modification. The most commonly studied species in psychiatric research is micro RNAs which act by binding to messenger RNAs to prevent them from being translated into proteins.
The epigenome is modifiable in response to a variety of environmental factors including diet, physical health, and psychological trauma, allowing long-term adaptive changes to gene expression.10,11 However, epigenetic changes also occur as a function of normal development (e.g. cellular differentiation). As a result, epigenetic patterns tend to be cell- and tissue-specific. While human post-mortem brain tissue studies have identified epigenetic signatures of resilience,12 most human studies rely on more easily accessible tissues such as blood, saliva and buccal cells.13 The extent to which these mirror the nervous system epigenome is unclear, although there is evidence that peripheral changes in DNA methylation can reflect those occurring centrally, particularly in psychiatric relevant genes,14 and can also reflect systemic changes relevant to resilience, such as inflammation.15
A Conceptual Model for the Role of Epigenetics in Resilience
A wide range of epigenetic differences has been observed in individuals with good mental health compared to those with psychiatric disorders.5,16,17 These differences can arise in various ways, but some are thought to be influenced by the environment and could mediate the impact of subsequent adversity on mental health.18 As described above, epigenetic mechanisms have the potential to record the experience of various life events in a lasting manner. The majority of epigenetic research in psychiatry to date has focused on the impact of specific adverse events on the epigenome and how epigenetic differences translate into the development of single psychiatric disorders. These relationships have been covered in numerous reviews.7,17-19 However, much less is known regarding how epigenetics are impacted by protective and positive aspects of the environment and how this contributes to the development of psychological resilience. Additionally, research often fails to account for the main effects of genetic variation as well as genetic moderation of environmental influences on epigenetics. This is particularly important as individuals have been shown to exhibit variable sensitivity to both adverse and protective events.20
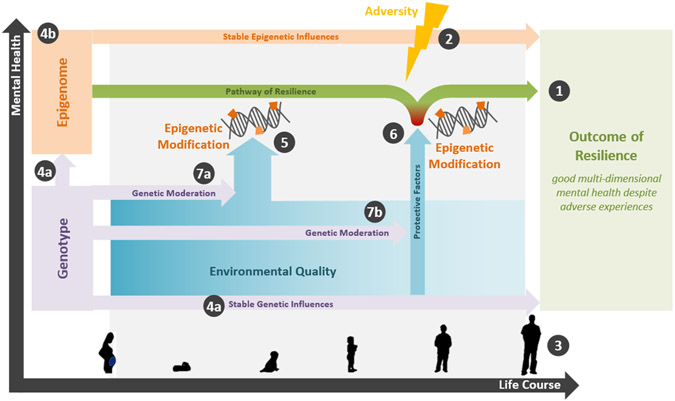
Hence, we propose a conceptual model for three specific roles of epigenetics in the development of psychological resilience across the lifespan, building upon existing research and theories on the relationships between adversity, epigenetics, and mental health outcomes. Our theoretical model is depicted in Figure 1. At the model’s core lies a pathway of resilience resulting in good multidimensional mental health (1), defined by the presence of adversity (2). The resilient outcome can be influenced by epigenetics in at least three ways, which occur throughout the lifespan (3). Firstly, an epigenetic signature of resilience may be partially present from conception, determined by genetic variation (4a) or inherited directly (4b). Secondly, some aspects of the epigenome are more changeable and modified by the environment, particularly during early development (5). Thirdly, specific protective factors during exposure to adversity impact how amenable the epigenome is to this negative event (6). Finally, genetic factors also play an important role, directly influencing resilience (7a) and moderating the effects of the environment on the epigenome (7b). It is necessary to consider all of these factors in order to understand the multiple potential ways that epigenetic factors contribute to resilience. In the remainder of this review, we will summarise the literature that concerns each aspect of this model.
Figure 1. Theoretical model outlining the contribution of epigenetics in the development of psychological resilience.
This model focusses on the development of multidimensional resilience (1), defined by the presence of an adverse event (2). This resilient outcome can be influenced by epigenetics in three key ways, which occur at different stages throughout the lifespan (3). Firstly, resilience-associated epigenetic differences may be determined by genetic variation (4a) or inherited from previous generations (4b). Secondly, the epigenome can be modified by the environment, particularly during early life (5). Thirdly, specific protective factors during adversity exposure will affect how modified the epigenome is (6). Genetic factors can directly influence resilience itself (7a) and can moderate the effects that the environment and protective factors have on the epigenome (7b).
Review of Empirical Evidence
Epigenetic Differences between Psychiatric and Resilient Outcomes
Epigenetic differences have been observed in presumably resilient individuals when comparing unaffected individuals to those with psychiatric disorders, including PTSD, depression, and anxiety disorders. This has been thoroughly examined elsewhere.16,17,21,22 The majority of research has focussed on differential DNA methylation in peripheral tissues such as blood and have identified resilience-associated differences in a variety of genes relating to immune function, neuronal plasticity, stress regulation and neurotransmission as well as others with unclear mechanisms of action. However, results are not always consistent and with the advent of hypothesis-free epigenome-wide studies, findings are often not replicated, particularly in the most carefully controlled studies.23 Although studied far less frequently, some case-control differences in histone modifications and miRNA expression have also been associated with psychopathology.22
Although these studies have attempted to identify epigenetic signatures of resilience, their approach often focusses on the absence of a single psychiatric outcome and rarely considers the presence of adversity. While not necessarily identifying resilience as we define it here, this approach has identified some similar epigenetic correlates of resilience between different mental health outcomes, although this is commonly due to the overlap in selected and studied candidate genes. For example, elevated DNA methylation of a gene important for neuronal development (BDNF) 4,24 and increased biological age estimated from a defined selection of DNA methylation loci have been observed in individuals with various psychopathologies.12,25 Other findings differ considerably between disorders, which may result from methodological differences, represent mechanisms more closely aligned to the development of specific symptoms or may result from a greater burden of adversity.
In an attempt to reconcile these differences, more recent studies have attempted to identify DNA methylation signatures of multi-dimensional resilience within the same cohort. One such study did not identify shared DNA methylation differences across different psychopathologies in the blood of World Trade Centre survivors23, while a second found multiple differentially methylated regions in resilient at-risk Brazilian adolescents when considering multi-dimensional psychopathology.26 Hence, evidence for the existence of a general resilient epigenome is currently not conclusive.
Importantly, while epigenetic markers may differentiate resilient individuals from those with mental illness, it is not entirely clear when and how these differences arise. They may represent epigenetic differences present from conception, or those that accumulate during development as well as those triggered by acute traumatic events and downstream coping mechanisms. The literature concerning these various possibilities is discussed below.
Inheritance of Epigenetic Resilience
Although epigenetic modifications are amenable to change throughout the lifespan, a certain proportion is set at conception and during the earliest stages of development. This early determination of the epigenome can be due to inherited genetic variation or direct inheritance of epigenetic marks themselves.
Genetic Determination of Epigenetic Variation
Genetic variation is a significant contributor to the epigenome present at conception. While monozygotic twins tend to have similar epigenetic profiles, at least at birth,27,28 and allele-specific patterns can be passed from parent to child,29 epigenetic profiles can differ substantially between individuals with different ancestral backgrounds.30 The genome can directly influence the epigenome in numerous ways. For example, genetic variation can directly impact the specific sites at which DNA methylation can occur, 31 and may also impact the efficacy of sequence-dependent actions of ncRNAs.32
Significant progress has been made in the discovery of genome-wide significant risk and protective genetic variants for psychopathology, but the majority lie within non-coding regions with unclear functional significance.33,34 In many cases, resilience-associated genetic variation is hypothesised to impact gene expression potentially via epigenetic intermediates. For example, psychiatric disease associated SNPs have been found to be enriched for multiple methylation quantitative trait loci (mQTL),35,36 while SNPs in the FKBP5 gene, a key component of the stress response, have been associated with the risk of depression as well as the gene’s methylation state.37,38 Therefore, genetic variation is likely one important source of epigenetic resilience. Disentangling genetic from epigenetic effects is a complex task but statistical approaches have been developed, and successfully applied to QTLs.39,40 Similar approaches are needed to achieve the same for phenotypes like resilience.
Multi-Generational Transmission of Epigenetic Variation
A second way by which epigenetic marks can be inherited is a direct mechanism, independent of genetic variation. Although a controversial subject in mammalian biology, studies indicate that this direct epigenetic inheritance occurs in some animal models.41,42 This can occur via inter-generational transmission whereby germline cells or the developing foetus is subject to the same environment as the parent. More interesting is the potential for transmission of epigenetic information to further generations not present at the time of exposure (i.e. trans-generational inheritance). These phenomena may allow molecular adaptation to the environment to be passed from parent to child and, importantly, suggests that the parental environment can impact the health of subsequent generations.
To date, the majority of pre-clinical research in this area has focussed on the epigenetic inheritance of traumatic experiences which elicits increased psychopathology in offspring. In rodents, various stress paradigms can produce depressive and anxiety-like behaviours in multiple subsequent generations and is correlated with specific epigenetic marks hypothesised to elicit their effect through neurodevelopmental or endocrinological mechanisms.43,44 The specific molecular mechanisms permitting this inheritance remain to be elucidated and it is unclear whether these specific epigenetic signatures are consistently conserved at the cellular level throughout the generations or are re-established in each subsequent generation.
Currently, the existence of transgenerational epigenetic inheritance in humans has yet to be proven. True transgenerational inheritance is difficult to ascertain due to the number of generations necessary and the confounding due to genetic inheritance and shared pre- and postnatal environments affected by psychopathology. Multiple human studies have indicated that traumas such as combat exposure, forced displacement, genocide exposure, and low maternal bonding may be passed on inter-generationally, and have been associated with both psychopathology in children and DNA methylation in the FKBP5 gene.45,46 Currently, the sole indications that the effects of damaging environments may be passed trans-generationally come from rare and non-replicable cohorts, and have yet to identify corresponding epigenetic changes.42
Importantly, the majority of research in this area has focussed on epigenetic inheritance of adversity, which correlates with reduced resilience in subsequent generations. While this indicates that psychological resilience could be degraded due to inherited epigenetic marks, it is unclear whether resilience could also be promoted through similar mechanisms. Resilience-promoting influences such as exercise and environmental enrichment have been shown to reduce depressive-, anxiety- and fear-related behaviours in at least one subsequent generation in mice,47,48 but further research will be needed to ascertain whether epigenetic modifications allow this inheritance of resilience and whether similar processes occur in humans.
Environmental Influences on Epigenetic Resilience
While some epigenetic marks are inherited or set during organismal development, many exhibit plasticity in response to the environment, allowing molecular adaptation throughout life. While monozygotic twins have relatively similar DNA methylation and histone acetylation patterns during their early years, large differences are observed in later life suggesting a substantial input from the environment during subsequent years.49,50
Environmental Quality is Associated with Epigenetic Changes
There is a large body of evidence that indicates that the epigenome is susceptible to adversity, including prenatal stress, early life trauma, maltreatment, and social stress, reviewed elsewhere.5,25,51,52 The epigenome is particularly sensitive during early developmental stages and resulting epigenetic changes last far beyond the time of adversity.5 Although epigenetic outcomes of these events are often studied in isolation, evidence exists for cumulative and longitudinal effects. Time since adversity can moderate the magnitude of epigenetic change,53 while repeated or varied sources of adversity may accumulate to produce larger epigenetic responses.54,55 These adversity-inflicted epigenetic changes have been correlated with later sensitivity to adversity and consequently reduced resilience.5
Less well understood is the impact that protective or positive environmental factors have on the epigenome. While the absence of negative or detrimental factors is expected to shift the trajectory of mental health towards resilience, it is also important to understand which factors directly promote resilience itself. Parental care is one protective factor which has been found to promote resilience alongside epigenetic changes. Research investigating the epigenetic and psychological consequences of naturally-occurring variation in mothering behaviours in rats found that high quality care resulted in offspring with more robust stress hormone responses and reduced depressive- and anxiety-like behaviours alongside reduced DNA methylation at the gene encoding a key receptor in the glucocorticoid stress system (Nr3c1) alongside increased histone acetylation in hippocampal tissue.56 In humans, preliminary research suggests that aspects of parental care such as breastfeeding and physical contact are also associated with decreased methylation of N3CR1, as well as BDNF, and increased methylation of the pro-inflammatory gene TNF.57
Positive aspects of the environment such as cognitive stimulation, healthy diet, and exercise may also promote resilience through epigenetic mechanisms. Environmental enrichment during early development in mice has been found to alter miRNA expression and histone acetylation at the Bdnf gene58,59 and also rectified epigenetic changes induced by inherited trauma.59 Similarly, individual dietary components, such as dietary phytochemicals, have been found to reduce depressive-like behaviours through epigenetic mechanisms targeting systemic inflammation and neuronal plasticity in mice.60 Although challenging to translate into humans, healthy lifestyle factors such as moderate physical activity, dietary quality and cognitive stimulation are associated with resilience-building.61 Little work has been conducted to investigate the impact of these factors upon the epigenome, but minimal alcohol intake, fruit and vegetable consumption, and moderate exercise have been associated with a lower epigenetic age, a resilience-associated epigenetic factor.62,63 Further research is needed to understand whether this has a downstream impact upon resilience.
Psychotherapeutic interventions may also impact the epigenome alongside their therapeutic effect. For example, changes in DNA methylation of candidate stress-related genes (FKBP5, SLC6A4) have been observed in patients undergoing psychological treatments for PTSD, phobia, or anxiety symptoms.64-66 In a more recent hypothesis-free epigenome-wide approach, treatment-responsive individuals exhibited reduction of PTSD symptoms alongside differential DNA methylation in multiple genes.67 However, although epigenetic changes occur alongside symptom reduction, it is not clear whether the psychological therapies rectify the biological “scarring” of prior negative experiences or have their own independent promotive effect.
Importantly, while negative aspects of the environment tend be thought of as sensitising individuals to further adversity, some negative factors may actually increase resilience through a so-called steeling effect.68,69 For example, in mice certain mild stress paradigms appear to promote resilience in later life.70-72 Similarly, in a recent cohort study, Brazilian children who experienced prenatal maternal violence exhibited increased resilience to postnatal maternal violence compared to those that only experienced violence postnatally.73 Differentially methylated sites were identified in the FKBP5 and NR3C1 genes suggesting a prenatally-derived stress-adaptive mechanism to overcome subsequent adversity. Although this preliminary work suggests that negative life experiences can promote later resilience through epigenetic changes, more work is needed to confirm these findings and to better understand the threshold at which such experiences become detrimental to resilience.
Protective Environmental Influences on Epigenetic Resilience during Adversity Exposure
As outlined above, numerous reports show that environmental influences can alter the epigenome. However, individuals exhibit variability in this response. Some intra-individual variation in the epigenetic response may well be due to specific protective factors, acting concurrently to dampen or counteract the adversity. Individual and social-environmental factors such as good emotional regulation, strong social support, and good familial relationships have been shown to promote resilience.74 Little work has been conducted on this, but limited human studies indicate that specific protective factors can moderate the epigenetic outcomes of adversity. For example, the impact of perinatal depression upon the offspring’s DNA methylation of stress-related genes NR3C1 and SLC6A4 has been found to be moderated by the quality of maternal care.57 Additionally, mothers’ cognitive appraisal of a natural disaster during pregnancy moderated the impact of the disaster upon methylation of the inflammation-related LTA gene in children.75 However, further work is necessary to confirm and expand upon the specific protective nature of maternal care and cognitive appraisal on the epigenome.
Genetic Moderation of the Environment and Epigenetic Processes
A large body of evidence indicates an important role for genetics in various stress-related disorders, with a substantial overlap between different dimensional outcomes suggesting some shared “resilience loci”.33,34,76,77 As outlined above, some of these genetic factors may contribute to resilience through direct effects upon the epigenome. In addition, genetic factors may moderate the impact of the environment upon the epigenome.
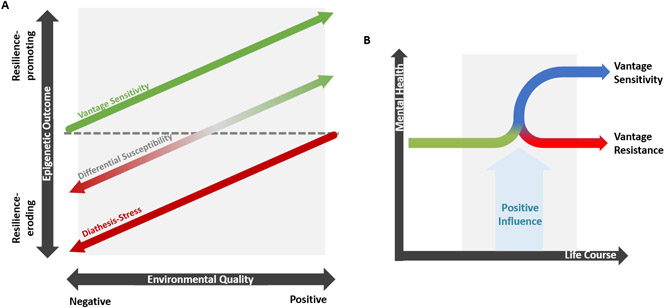
Although the environment can have both “scarring” and “steeling” effects upon the epigenome, there is large variation in response to these experiences. This disparity may be explained by sensitivity-conferring genetic variants that increase vulnerability to negative environments but may also promote favourable outcomes in response to positive exposures. This represents individual differences in Environmental Sensitivity – the degree to which people respond to both negative and positive environments (Figure 2a).78-82 Research has shown that Environmental Sensitivity is a measurable, common, complex, heritable, and polygenic trait 83.
Figure 2. Models of Environmental Sensitivity and Vantage Sensitivity.
(A) Illustration of the three models of Environmental Sensitivity: Vantage Sensitivity, Differential Susceptibility and Diathesis-Stress. These models describe the inter-individual differences in response to both positive and negative environmental factors, and have been applied to epigenetic changes in the context of resilience. Diathesis-Stress describes individual differences in response to negative factors whereas Vantage Sensitivity describes variability regarding positive factors. Differential Susceptibility represents a combination of diathesis-stress and vantage sensitivity with heightened sensitivity to both negative and positive experiences. Figure adapted from Pluess, 2015.78 (B) The Vantage Sensitivity model which outlines the differential variation in benefit specifically from positive influences. Figure adapted from de Villiers et al. 2018.98
A growing number of studies provide evidence that genetics can moderate the impact of negative environmental factors on epigenetic modifications.84 For example, epigenome-wide neonate DNA methylation has been shown to result from an interaction between genetic variation in nearby cis variants and prenatal factors such as maternal smoking, maternal depression, and gestational age.85 Individual genetic variants in the FKBP5 gene have also been shown to moderate the impact of adversities such as childhood maltreatment or the synthetic glucocorticoid dexamethasone on FKBP5 DNA methylation.86-88
Of particular relevance to resilience building is the observation that people differ substantially in their response to the positive effects of nurturing experiences as described by Vantage Sensitivity (Figure 2b).20 As of yet, little research has been conducted to understand genetic moderation of protective influences on the epigenome, although it is possible that variants which moderate adversity may also moderate positive factors. Polygenic scores for Environmental Sensitivity have been shown to moderate the beneficial outcomes of cognitive behavioural therapy on anxiety symptoms,89 and genome-wide and single locus variation in the SCL6A4 gene the impact of good parenting quality on youth psychopathology.89,90 Interestingly, individuals with the highly sensitive SCL6A4 genotype, did not manifest increased susceptibility to poor parenting suggesting that this variant may specifically provide Vantage Sensitivity rather than general Environmental Sensitivity.91 However, it remains to be seen whether these psychological outcomes are due to epigenetic changes and genetic moderators of the impact of positive factors on epigenetics have yet to be identified.
Discussion and Directions for Future Research
Here we have presented a theoretical model describing the multiple ways in which epigenetics may contribute to the long term development of psychological resilience. The epigenome is initially defined by direct or indirect inheritance, but is subject to change due to environmental influences during early and later development. This plasticity could be moderated by genetically-derived individual differences in Environmental Sensitivity. The resulting epigenetic profile of resilience therefore reflects an accumulation of these individual and social-environmental factors, and alongside stable genetic influences allows for the successful adaptation to adversity and the maintenance of good mental health. While moderate evidence exists for several aspects of this model, we wish to highlight areas which are currently lacking and make practical suggestions for future research (see Panel 2).
Panel 2: Practical Suggestions for Future Research.
| Research Aim | Practical Suggestion |
|---|---|
| Measure resilience in a consistent and appropriate way |
|
| Characterise epigenetic changes throughout the lifespan |
|
| Understand the interplay between genetics and epigenetics |
|
| Consider a wider variety of epigenetic mechanisms in an unbiased and genome-wide manner |
|
| Identify the biological mechanisms underpinning epigenetically-derived resilience |
Firstly, the concept of resilience in epigenetic research should be studied in its own right, rather than simply as a disease-free state.2 While some studies solely study people that have experienced adversity,23 or consider the severity of adversity,92 others do not. Therefore, identified epigenetic differences may reflect true resilience in some cases, whilst in others they may simply reflect the presence or severity of adversity. Additionally, the consideration of multiple psychiatric outcomes will allow us to understand the general mechanisms that underlie psychological resilience more broadly, rather than the development of specific sets of mental health symptoms. To this end, adversity must be taken into account and, where appropriate, multi-dimensional psychiatric outcomes should be considered.
Secondly, we must widen our search for environmental factors that promote resilience through epigenetic changes. There is moderate evidence that various forms of adversity can impact the epigenome and consequently resilience, although results are not always consistent and studies rarely consider all three factors. However, there has been little investigation into how positive or protective aspects of the environment could have their own independent or counteracting actions in the epigenetic development of resilience. Furthermore, while it is suggested that reduced resilience may be inherited through inter-generational transmission of epigenetics, this is far from proven in humans and it is unknown whether such mechanisms can promote resilience. Future lines of enquiry should take genetic variation into account to fully appreciate the degree to which the environment can impact the epigenome in different genetic contexts.
Thirdly, the longitudinal nature of the trajectory towards epigenetic change and resilience must be fully considered, taking into account the developmental context and time since exposure. It is also critical to assess the direction of causality between environmental factors, epigenetic changes and psychiatric outcomes as this is rarely done. Hence, it is crucial to adopt a life course perspective utilising longitudinal studies with repeated assessments allowing the investigation of within-person epigenetic changes across different developmental periods and critically before the development of pathological outcomes. Additionally, environmental factors are typically studied in isolation. While informative, such events are unlikely to occur alone in naturalistic settings and have been shown to have cumulative effects on the epigenome and may impact the epigenome and resilience in the form of developmental cascades, impacting the response to further events.54,55 This has particular relevance for epigenetics, which is defined by continuous and highly plastic changes, for example through varying ncRNA expression or the methylation of multiple loci in a region.
Finally, the search for epigenetic factors involved in resilience must be expanded to include a wider variety of mechanisms. As highlighted in this review, the vast majority of studies in relation to epigenetics and resilience aim to identify differential DNA methylation. While this approach has generated a wealth of knowledge, we know relatively little about the involvement of other epigenetic mechanisms including ncRNA expression, ncRNA modification and histone modifications, as well as more recently discovered effects of DNA methylation.93,94 As these modifications accumulate and interact with one-another to produce tightly regulated changes in gene expression, it is not sufficient to only study differences in single DNA methylation loci.9
Although our model aims to encapsulate and summarise the key components involved in the development of resilience, we acknowledge that further factors may be involved. For example, genetic variation may moderate the impact of epigenetic changes on resilience.86 We have also not considered in-depth the specific biological mechanisms by which changes to the epigenome result in psychological resilience. This is particularly challenging given that the majority of research solely studies epigenetics in peripheral tissues. Although clear etiological processes can be assigned to certain epigenetic changes such as those impacting the systemic stress response system (FKBP5, NR3C1), hypothesis-free epigenome-wide studies increasingly highlight epigenetic changes without clear downstream effects.
In conclusion, we have outlined the complex mechanisms by which aspects of the environment can become biologically embedded through epigenetic changes and contribute to the development of psychological resilience. We have outlined the research areas which are currently lacking strong evidence and highlighted strategies that will improve future work. In particular, we hope that this review has emphasized certain themes. Firstly, the importance of clearly defining resilience in well-designed epigenetic studies. Secondly, broadening our study of the role of the environment on epigenetics and resilience to include protective and positive factors, the inheritance of epigenetic resilience and the role of genetic moderation informed by theories of Environmental Sensitivity. Thirdly, appreciating the longitudinal and cumulative effects of the environment on the epigenome and resilience. Finally, improving and expanding upon the commonly studied epigenetic mechanisms.
Supplementary Material
Acknowledgments
The writing of this manuscript was supported by funding from the Eunice Shriver National Institute of Child Health & Human Development (NICHD; R01HD083387). We sincerely thank Professors Elisabeth Binder and Vardhman Rakyan for their invaluable comments and feedback in the development of this manuscript.
Footnotes
Search strategy and selection criteria
References for this review were identified through searches of PubMed for articles published up to May 31, 2020. We did not apply any additional time restrictions, but we largely focussed on papers published within the past 5 years. We searched article titles and abstracts for the specific keywords (and combinations thereof): “resilien*”, “mental health”, “depress*”, “anxi*”, “trauma*”, “stress”, “advers*”, “environment”, “inherit*”, “epigen*”, “methyl*”, “histone”, “RNA”. We reviewed the articles resulting from these searches and the relevant references cited in those articles. Given the number of studies that certain keyword combinations yielded, we restricted our selection of papers to peer-reviewed papers that covered unique areas of our proposed model. We supplemented these search results with important publications from our personal literature libraries. Only articles published in English were included.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Demelza Smeeth, Department of Biological and Experimental Psychology, School of Biological and Chemical Sciences, Queen Mary University of London, London, UK.
Stephan Beck, UCL Cancer Institute, Paul O’Gorman Building, University College London, London, UK.
Elie G Karam, Department of Psychiatry and Clinical Psychology, Balamand University, St Georges Hospital University Medical Center, Institute for Development, Research, Advocacy and Applied Care (IDRAAC), Lebanon.
Michael Pluess, Department of Biological and Experimental Psychology, School of Biological and Chemical Sciences, Queen Mary University of London, London, UK.
References
- 1.Galatzer-Levy IR, Huang SH, Bonanno GA. Trajectories of resilience and dysfunction following potential trauma: A review and statistical evaluation. Clinical Psychology Review 2018; 63: 41–55. [DOI] [PubMed] [Google Scholar]
- 2.Ungar M, Theron L. Resilience and mental health: how multisystemic processes contribute to positive outcomes. The Lancet Psychiatry 2020; 7: 441–8. [DOI] [PubMed] [Google Scholar]
- 3.Ungar M Designing resilience research: Using multiple methods to investigate risk exposure, promotive and protective processes, and contextually relevant outcomes for children and youth. Child Abuse & Neglect 2019; 96: 104098. [DOI] [PubMed] [Google Scholar]
- 4.Kim TY, Kim SJ, Chung HG, Choi JH, Kim SH, Kang JI. Epigenetic alterations of the BDNF gene in combat-related post-traumatic stress disorder. Acta Psychiatr Scand 2017; 135: 170–9. [DOI] [PubMed] [Google Scholar]
- 5.Barker ED. Epigenetics, Early Adversity and Child and Adolescent Mental Health. Psychopathology 2018; 51: 71–5. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS. Allostasis and the Epigenetics of Brain and Body Health Over the Life Course: The Brain on Stress. JAMA Psychiatry 2017; 74: 551–2. [DOI] [PubMed] [Google Scholar]
- 7.Schiele MA, Gottschalk MG, Domschke K. The applied implications of epigenetics in anxiety, affective and stress-related disorders - A review and synthesis on psychosocial stress, psychotherapy and prevention. Clinical Psychology Review 2020; 77: 101830. [DOI] [PubMed] [Google Scholar]
- 8.Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nature Reviews Genetics 2016; 17: 487–500. [DOI] [PubMed] [Google Scholar]
- 9.Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity 2010; 105: 4–13. [DOI] [PubMed] [Google Scholar]
- 10.Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nature Reviews Genetics 2012; 13: 97–109. [DOI] [PubMed] [Google Scholar]
- 11.Aristizabal MJ, Anreiter I, Halldorsdottir T, et al. Biological embedding of experience: A primer on epigenetics. PNAS 2020; 117: 23261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han LKM, Aghajani M, Clark SL, et al. Epigenetic Aging in Major Depressive Disorder. Am J Psychiatry 2018; 175: 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Sante J, Ismaylova E, Nemoda Z, et al. Peripheral DNA methylation of HPA axis-related genes in humans: Cross-tissue convergence, two-year stability and behavioural and neural correlates. Psychoneuroendocrinology 2018; 97: 196–205. [DOI] [PubMed] [Google Scholar]
- 14.Braun PR, Han S, Hing B, et al. Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Translational Psychiatry 2019; 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 2014; 140: 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiele MA, Domschke K. Epigenetics at the crossroads between genes, environment and resilience in anxiety disorders. Genes, Brain and Behavior 2018; 17: e12423. [DOI] [PubMed] [Google Scholar]
- 17.Zannas AS, Provençal N, Binder EB. Epigenetics of Posttraumatic Stress Disorder: Current Evidence, Challenges, and Future Directions. Biological Psychiatry 2015; 78: 327–35. [DOI] [PubMed] [Google Scholar]
- 18.Dudley KJ, Li X, Kobor MS, Kippin TE, Bredy TW. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neuroscience & Biobehavioral Reviews 2011; 35: 1544–51. [DOI] [PubMed] [Google Scholar]
- 19.Jiang S, Postovit L, Cattaneo A, Binder EB, Aitchison KJ. Epigenetic Modifications in Stress Response Genes Associated With Childhood Trauma. Front Psychiatry 2019; 10. DOI: 10.3389/fpsyt.2019.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pluess M Vantage Sensitivity: Environmental Sensitivity to Positive Experiences as a Function of Genetic Differences. Journal of Personality 2017; 85: 38–50. [DOI] [PubMed] [Google Scholar]
- 21.Barker ED, Walton E, Cecil CAM. Annual Research Review: DNA methylation as a mediator in the association between risk exposure and child and adolescent psychopathology. Journal of Child Psychology and Psychiatry 2018; 59: 303–22. [DOI] [PubMed] [Google Scholar]
- 22.Penner-Goeke S, Binder EB. Epigenetics and depression. Dialogues Clin Neurosci 2019; 21: 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuan P-F, Waszczuk MA, Kotov R, et al. An epigenome-wide DNA methylation study of PTSD and depression in World Trade Center responders. Transl Psychiatry 2017; 7: e1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Januar V, Ancelin M-L, Ritchie K, Saffery R, Ryan J. BDNF promoter methylation and genetic variation in late-life depression. Transl Psychiatry 2015; 5: e619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf EJ, Maniates H, Nugent N, et al. Traumatic Stress and Accelerated DNA Methylation Age: A Meta-Analysis. Psychoneuroendocrinology 2018; 92: 123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spindola LM, Santoro ML, Pan PM, et al. Detecting multiple differentially methylated CpG sites and regions related to dimensional psychopathology in youths. Clinical Epigenetics 2019; 11: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ajami M, Sadeghian MH, Soleimani M, et al. Comparison of miRNA Profiles of Cord Blood Stem Cells in Identical and Fraternal Twins. Cell J 2019; 21: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Karlsson R, Lampa E, et al. Epigenetic influences on aging: a longitudinal genome-wide methylation study in old Swedish twins. Epigenetics 2018; 13: 975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDaniell R, Lee B-K, Song L, et al. Heritable Individual-Specific and Allele-Specific Chromatin Signatures in Humans. Science 2010; 328: 235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kader F, Ghai M. DNA methylation-based variation between human populations. Mol Genet Genomics 2017; 292: 5–35. [DOI] [PubMed] [Google Scholar]
- 31.Heijmans BT, Kremer D, Tobi EW, Boomsma DI, Slagboom PE. Heritable rather than age-related environmental and stochastic factors dominate variation in DNA methylation of the human IGF2/H19 locus. Hum Mol Genet 2007; 16: 547–54. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Zhang F, Li T, et al. MirSNP, a database of polymorphisms altering miRNA target sites, identifies miRNA-related SNPs in GWAS SNPs and eQTLs. BMC Genomics 2012; 13: 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serretti A, Fabbri C. Shared genetics among major psychiatric disorders. The Lancet 2013; 381: 1339–41. [DOI] [PubMed] [Google Scholar]
- 34.Parens E, Matthews L, Appelbaum PS. Polygenic risk scores, prediction of psychiatric disorders, and the health of all of us. The Lancet Psychiatry 2020; 7: 481. [DOI] [PubMed] [Google Scholar]
- 35.Ciuculete DM, Boström AE, Voisin S, et al. A methylome-wide mQTL analysis reveals associations of methylation sites with GAD1 and HDAC3 SNPs and a general psychiatric risk score. Transl Psychiatry 2017; 7: e1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciuculete DM, Voisin S, Kular L, et al. meQTL and ncRNA functional analyses of 102 GWAS-SNPs associated with depression implicate HACE1 and SHANK2 genes. Clin Epigenetics 2020; 12: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klinger-König J, Hertel J, Van der Auwera S, et al. Methylation of the FKBP5 gene in association with FKBP5 genotypes, childhood maltreatment and depression. Neuropsychopharmacology 2019; 44: 930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tozzi L, Farrell C, Booij L, et al. Epigenetic Changes of FKBP5 as a Link Connecting Genetic and Environmental Risk Factors with Structural and Functional Brain Changes in Major Depression. Neuropsychopharmacology 2018; 43: 1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannon E, Weedon M, Bray N, O’Donovan M, Mill J. Pleiotropic Effects of Trait-Associated Genetic Variation on DNA Methylation: Utility for Refining GWAS Loci. The American Journal of Human Genetics 2017; 100: 954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi T, Wu Y, Zeng J, et al. Identifying gene targets for brain-related traits using transcriptomic and methylomic data from blood. Nature Communications 2018; 9: 2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez MF, Lehner B. Intergenerational and transgenerational epigenetic inheritance in animals. Nature Cell Biology 2019; 21: 143–51. [DOI] [PubMed] [Google Scholar]
- 42.Horsthemke B A critical view on transgenerational epigenetic inheritance in humans. Nature Communications 2018; 9: 2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ambeskovic M, Babenko O, Ilnytskyy Y, Kovalchuk I, Kolb B, Metz GAS. Ancestral Stress Alters Lifetime Mental Health Trajectories and Cortical Neuromorphology via Epigenetic Regulation. Sci Rep 2019; 9. DOI: 10.1038/s41598-019-42691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weaver ICG, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci 2004; 7: 847–54. [DOI] [PubMed] [Google Scholar]
- 45.Grasso DJ, Drury S, Briggs-Gowan M, et al. Adverse childhood experiences, posttraumatic stress, and FKBP5 methylation patterns in postpartum women and their newborn infants. Psychoneuroendocrinology 2020; 114: 104604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yehuda R, Daskalakis NP, Bierer LM, et al. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biological Psychiatry 2016; 80: 372–80. [DOI] [PubMed] [Google Scholar]
- 47.Yeshurun S, Short AK, Bredy TW, Pang TY, Hannan AJ. Paternal environmental enrichment transgenerationally alters affective behavioral and neuroendocrine phenotypes. Psychoneuroendocrinology 2017; 77: 225–35. [DOI] [PubMed] [Google Scholar]
- 48.Short AK, Yeshurun S, Powell R, et al. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl Psychiatry 2017; 7: e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA 2005; 102: 10604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong CCY, Caspi A, Williams B, et al. A longitudinal study of epigenetic variation in twins. Epigenetics 2010; 5: 516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cunliffe VT. The epigenetic impacts of social stress: how does social adversity become biologically embedded? Epigenomics 2016; 8: 1653–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramo-Fernández L, Schneider A, Wilker S, Kolassa I-T. Epigenetic Alterations Associated with War Trauma and Childhood Maltreatment. Behavioral Sciences & the Law 2015; 33: 701–21. [DOI] [PubMed] [Google Scholar]
- 53.Dunn EC, Soare TW, Zhu Y, et al. Sensitive Periods for the Effect of Childhood Adversity on DNA Methylation: Results From a Prospective, Longitudinal Study. Biol Psychiatry 2019; 85: 838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parade SH, Parent J, Rabemananjara K, et al. Change in FK506 binding protein 5 (FKBP5) methylation over time among preschoolers with adversity. Dev Psychopathol 2017; 29: 1627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunn EC, Soare TW, Raffeld MR, et al. What life course theoretical models best explain the relationship between exposure to childhood adversity and psychopathology symptoms: recency, accumulation, or sensitive periods? Psychol Med 2018; 48: 2562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fish EW, Shahrokh D, Bagot R, et al. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci 2004; 1036: 167–80. [DOI] [PubMed] [Google Scholar]
- 57.Provenzi L, Brambilla M, Minico GS di, Montirosso R, Borgatti R. Maternal caregiving and DNA methylation in human infants and children: Systematic review. Genes, Brain and Behavior 2020; 19: e12616. [DOI] [PubMed] [Google Scholar]
- 58.Branchi I, Karpova NN, D’Andrea I, Castrén E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci Lett 2011; 495: 168–72. [DOI] [PubMed] [Google Scholar]
- 59.McCreary JK, Erickson ZT, Hao Y, Ilnytskyy Y, Kovalchuk I, Metz GAS. Environmental Intervention as a Therapy for Adverse Programming by Ancestral Stress. Sci Rep 2016; 6: 37814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Hodes GE, Zhang H, et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun 2018; 9. DOI: 10.1038/s41467-017-02794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laird KT, Krause B, Funes C, Lavretsky H. Psychobiological factors of resilience and depression in late life. Transl Psychiatry 2019; 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quach A, Levine ME, Tanaka T, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY) 2017; 9: 419–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao W, Ammous F, Ratliff S, et al. Education and Lifestyle Factors Are Associated with DNA Methylation Clocks in Older African Americans. Int J Environ Res Public Health 2019; 16. DOI: 10.3390/ijerph16173141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bishop JR, Lee AM, Mills LJ, et al. Methylation of FKBP5 and SLC6A4 in Relation to Treatment Response to Mindfulness Based Stress Reduction for Posttraumatic Stress Disorder. Front Psychiatry 2018; 9: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stoffel M, Aguilar-Raab C, Rahn S, et al. Effects of Mindfulness-Based Stress Prevention on Serotonin Transporter Gene Methylation. PPS 2019; 88: 317–9. [DOI] [PubMed] [Google Scholar]
- 66.Roberts S, Keers R, Breen G, et al. DNA methylation of FKBP5 and response to exposure-based psychological therapy. Am J Med Genet B Neuropsychiatr Genet 2019; 180: 150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vinkers CH, Geuze E, van Rooij SJH, et al. Successful treatment of post-traumatic stress disorder reverses DNA methylation marks. Molecular Psychiatry 2019; : 1–8. [DOI] [PubMed] [Google Scholar]
- 68.Liu RT. A developmentally informed perspective on the relation between stress and psychopathology: When the problem with stress is that there is not enough. J Abnorm Psychol 2015; 124: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rutter M Resilience as a dynamic concept. Dev Psychopathol 2012; 24: 335–44. [DOI] [PubMed] [Google Scholar]
- 70.Hassan AM, Jain P, Reichmann F, et al. Repeated predictable stress causes resilience against colitis-induced behavioral changes in mice. Front Behav Neurosci 2014; 8. DOI: 10.3389/fnbeh.2014.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suo L, Zhao L, Si J, et al. Predictable Chronic Mild Stress in Adolescence Increases Resilience in Adulthood. Neuropsychopharmacology 2013; 38: 1387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peña CJ, Nestler EJ, Bagot RC. Environmental Programming of Susceptibility and Resilience to Stress in Adulthood in Male Mice. Front Behav Neurosci 2019; 13. DOI: 10.3389/fnbeh.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serpeloni F, Radtke KM, Hecker T, et al. Does Prenatal Stress Shape Postnatal Resilience? – An Epigenome-Wide Study on Violence and Mental Health in Humans. Front Genet 2019; 10. DOI: 10.3389/fgene.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gartland D, Riggs E, Muyeen S, et al. What factors are associated with resilient outcomes in children exposed to social adversity? A systematic review. BMJ Open 2019; 9: e024870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao-Lei L, Elgbeili G, Massart R, Laplante DP, Szyf M, King S. Pregnant women’s cognitive appraisal of a natural disaster affects DNA methylation in their children 13 years later: Project Ice Storm. Transl Psychiatry 2015; 5: e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brainstorm Consortium, Anttila V, Bulik-Sullivan B, et al. Analysis of shared heritability in common disorders of the brain. Science 2018; 360. DOI: 10.1126/science.aap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maul S, Giegling I, Fabbri C, Corponi F, Serretti A, Rujescu D. Genetics of resilience: Implications from genome-wide association studies and candidate genes of the stress response system in posttraumatic stress disorder and depression. Am J Med Genet B Neuropsychiatr Genet 2020; 183: 77–94. [DOI] [PubMed] [Google Scholar]
- 78.Pluess M Individual Differences in Environmental Sensitivity. Child Development Perspectives 2015; 9: 138–43. [Google Scholar]
- 79.Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull 2009; 135: 885–908. [DOI] [PubMed] [Google Scholar]
- 80.Aron EN, Aron A, Jagiellowicz J. Sensory Processing Sensitivity: A Review in the Light of the Evolution of Biological Responsivity. Personality and Social Psychology Review 2012; published online Jan 30. DOI: 10.1177/1088868311434213. [DOI] [PubMed] [Google Scholar]
- 81.Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology 2011; 23: 7–28. [DOI] [PubMed] [Google Scholar]
- 82.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology 2005; 17: 271–301. [DOI] [PubMed] [Google Scholar]
- 83.Assary E, Zavos HMS, Krapohl E, Keers R, Pluess M. Genetic architecture of Environmental Sensitivity reflects multiple heritable components: a twin study with adolescents. Molecular Psychiatry 2020; : 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niitsu K, Rice MJ, Houfek JF, Stoltenberg SF, Kupzyk KA, Barron CR. A Systematic Review of Genetic Influence on Psychological Resilience. Biological Research For Nursing 2019; 21: 61–71. [DOI] [PubMed] [Google Scholar]
- 85.Teh AL, Pan H, Chen L, et al. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res 2014; 24: 1064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klengel T, Mehta D, Anacker C, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci 2013; 16: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wiechmann T, Röh S, Sauer S, et al. Identification of dynamic glucocorticoid-induced methylation changes at the FKBP5 locus. Clin Epigenetics 2019; 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tyrka AR, Ridout KK, Parade SH, Paquette A, Marsit CJ, Seifer R. Childhood maltreatment and methylation of FK506 binding protein 5 gene (FKBP5). Development and Psychopathology 2015; 27: 1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keers R, Coleman JRI, Lester KJ, et al. A Genome-Wide Test of the Differential Susceptibility Hypothesis Reveals a Genetic Predictor of Differential Response to Psychological Treatments for Child Anxiety Disorders. PPS 2016; 85: 146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hankin BL, Nederhof E, Oppenheimer CW, et al. Differential susceptibility in youth: evidence that 5-HTTLPR x positive parenting is associated with positive affect ‘for better and worse’. Transl Psychiatry 2011; 1: e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brody GH, Beach SRH, Chen Y-F, et al. Perceived discrimination, serotonin transporter linked polymorphic region status, and the development of conduct problems. Dev Psychopathol 2011; 23: 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Snijders C, Krauskopf J, Pishva E, et al. Circulating Serum MicroRNAs as Potential Diagnostic Biomarkers of Posttraumatic Stress Disorder: A Pilot Study. Front Genet 2019; 10. DOI: 10.3389/fgene.2019.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu X, Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet 2017; 18: 517–34. [DOI] [PubMed] [Google Scholar]
- 94.Engel M, Eggert C, Kaplick PM, et al. The Role of m6A/m-RNA Methylation in Stress Response Regulation. Neuron 2018; 99: 389–403.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caspi A, Houts RM, Belsky DW, et al. The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clin Psychol Sci 2014; 2: 119–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Edgar RD, Jones MJ, Meaney MJ, Turecki G, Kobor MS. BECon: a tool for interpreting DNA methylation findings from blood in the context of brain. Transl Psychiatry 2017; 7: e1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bell CG, Lowe R, Adams PD, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biology 2019; 20: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Villiers B, Lionetti F, Pluess M. Vantage sensitivity: a framework for individual differences in response to psychological intervention. Soc Psychiatry Psychiatr Epidemiol 2018; 53: 545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.