Abstract
Background
Computed tomography (CT) is commonly utilized in chronic obstructive pulmonary disease (COPD) for lung cancer screening and emphysema characterization. Computed tomography-morphometric analysis of body composition (muscle mass and adiposity) has gained increased recognition as a marker of disease severity and prognosis. This systematic review aimed to describe the CT-methodology used to assess body composition and identify the association of body composition measures and disease severity, health-related quality of life (HRQL), cardiometabolic risk factors, respiratory exacerbations, and survival in patients with COPD.
Methods
Six databases were searched (inception-September 2021) for studies evaluating adult COPD patients using thoracic or abdominal CT-muscle or adiposity body composition measures. The systematic review was conducted in accordance with the PRISMA guidelines.
Results
Twenty eight articles were included with 15,431 COPD patients, across all GOLD stages with 77% males, age range (mean/median 59–78 years), and BMI range 19.8–29.3 kg/m2. There was heterogeneity in assessment of muscle mass and adiposity using thoracic (n = 22) and abdominal (n = 8) CT-scans, capturing different muscle groups, anatomic locations, and adiposity compartments (visceral, subcutaneous, and epicardial). Low muscle mass and increased adiposity were associated with increased COPD severity measures (lung function, exercise capacity, dyspnea) and lower HRQL, but were not consistent across studies. Increased visceral adiposity (n = 6) was associated with cardiovascular disease or risk factors (hypertension, hyperlipidemia, and diabetes). Low muscle CSA was prognostic of respiratory exacerbations or mortality in three of six studies, whereas the relationship with increased intermuscular adiposity and greater mortality was only observed in one of three studies.
Conclusion
There was significant variability in CT-body composition measures. In several studies, low muscle mass was associated with increased disease severity and lower HRQL, whereas adiposity with cardiovascular disease/risk factors. Given the heterogeneity in body composition measures and clinical outcomes, the prognostic utility of CT-body composition in COPD requires further study.
Keywords: Lung disease, sarcopenia, tomography scanners, X-ray computed, body composition, chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of morbidity and mortality worldwide.1 Chronic obstructive pulmonary disease is a multi-systemic condition with several extrapulmonary manifestations, including alterations in body composition (muscle mass and adiposity) given underlying risk factors such as malnutrition, respiratory exacerbations, and physical inactivity.2–4 Sarcopenia (low muscle mass and function), affecting one-fifth of COPD patients, is associated with low physical function, increased disease severity and adverse clinical outcomes.5 Similarly, increased adiposity is associated with increased cardiometabolic risk factors and morbidity in COPD patients.6,7
A number of modalities have been utilized to assess body composition, including bio-electrical impedance (BIA), dual X-ray absorptiometry (DXA), ultrasound, magnetic resonance imaging and whole body computed tomography (CT).8 However, some of these body composition modalities have practical limitations in the clinical setting due to cost, timing and availability. It is for this reason that CT has gained increased recognition in the COPD population, specifically thoracic CT given its clinical application for characterization of parenchymal disease or lung cancer screening.9 The use of CT for assessment of body composition has been described in a number of pulmonary populations including idiopathic pulmonary fibrosis,10 lung transplantation,11 and lung cancer.12
The literature on body composition in COPD has evolved in recent years, with studies reporting measures of muscle mass and adiposity obtained from either thoracic or abdominal CT scans.6,7,13 As a result, there has been significant methodological variability in CT body composition assessments. Skeletal muscles have been characterized using single muscle (e.g. pectoralis muscle14 and erector spinae15,16) or multiple muscle groups.7,17 Furthermore, adipose tissue stores have included both thoracic and abdominal subcutaneous and visceral compartments, including mediastinal tissue.13,18,19 Although, low muscle mass and increased adiposity quantified using CT have generally been associated with lower exercise capacity, cardiometabolic risk factors, and lower survival in COPD patients, there has been significant heterogeneity in the strength of these associations across studies.6,7,16,20 Thus, a better understanding of CT body composition abnormalities in COPD may have important implications on management of cardiometabolic risk profile and prognosis.5 There is evidence that airflow obstruction is associated with metabolic syndrome, specifically central obesity, through a common process of systemic inflammation.21 Furthemore, given the high prevalence of cardiovascular disease in individuals with COPD, identifying modifiable risk factors such as obesity and increased adiposity may pose early interventional targets for cardiovascular risk reduction.22
Given the expanding literature in this area, we conducted a systematic review to: (1) Describe CT-based methodology used to assess body composition and (2) Identify the association of CT-based body composition measures with disease severity, health-related quality of life (HRQL), cardiometabolic risk factors, and clinical outcomes, specifically respiratory exacerbations and survival.
Methods
Study design
This systematic review aimed to assess studies evaluating the association of CT-based measures of body composition (muscle mass and adiposity) with clinical characteristics and outcomes in COPD patients. We conducted this review following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.23 Ethics approval was not sought given this was a systematic review. The protocol was registered with Open Science Framework on 26 October 2020 (https://osf.io/q7389).
Search strategy
A systematic literature search was conducted by an experienced medical librarian (A. O-C) capturing the topic of CT scans, skeletal muscle and adiposity in patients with COPD. Databases searched include Ovid MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Clinical Trials, CINAHL, and PubMed for non-Medline records. The search dates were from inception to 9 September 2021 (last update). For full details on search strategies for all databases, see supplementary appendix (Table 1S to 6S). Limits were applied for human and adult populations. Books and conference materials were excluded from EMBASE. Reference lists from included articles were also reviewed to assess for any additional relevant articles.
Eligibility criteria
We included full-text papers in English language of adult participants (≥ 18 years of age) with a clinical diagnosis of COPD. Studies had to have measures of either muscle or adiposity evaluated with thoracic or abdominal CT scan and at least one outcome measurement of interest described below (data extraction and synthesis section). All study types were included except for case series or reports.
Study selection
Two reviewers (CEO and DR) independently assessed all abstracts of relevant articles. Articles of interest were then retrieved for full-text evaluation if one of the two reviewers deemed the abstract eligible. If there were disagreements between the first two reviewers, a third reviewer was consulted (JMN) until consensus was reached.
Data extraction and synthesis
Two reviewers (JMN and CEO, SN, BE or KC) conducted the data abstraction following standardized criteria. The following data were abstracted: demographic characteristics, lung function, details on CT measures (anatomic location, muscles and adiposity compartments, and number of axial slices) as well as all terminology pertaining to ‘low muscle mass’ in the respective studies, as these cut offs are expected to change amongst studies. In addition, associations between CT body composition and clinical outcomes were abstracted. Specifically, the outcomes of interest were individual BODE index parameters (including body mass index (BMI), severity of obstruction, dyspnea and exercise capacity),24 HRQL (multi-dimensional patient reported measure capturing physical and/or psychosocial function),25 respiratory exacerbations (defined as acute worsening of symptoms of cough, phlegm or shortness of breath requiring antibiotics or corticosteroid management; severe exacerbations defined as those requiring emergency department visits or hospitalizations),26 all-cause survival, and cardiometabolic risk factors (i.e. hypertension, dyslipidemia, diabetes). Cardiovascular disease (i.e. myocardial infarction, stroke) was also captured given its known associations with body composition in COPD patients.27,28
Based on our previous experience with CT body composition measures in lung transplant candidates with significant heterogeneity in the methodology and cutoffs used to evaluate CT body composition measures,11 it was determined that a meta-analysis would not be feasible and was not planned for the current review. Descriptive statistics and ranges were used to describe the demographic and clinical characteristics of COPD patients in the included studies.
Quality assessment
Quality assessment for included articles in this systematic review were conducted using the National Heart, Lung, and Blood Institute Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.29 Two reviewers (JMN and CEO, SN, BE or KC) completed all quality appraisals, with disagreements being resolved by a third reviewer (DR) when necessary.
Results
Study selection and patient characteristics
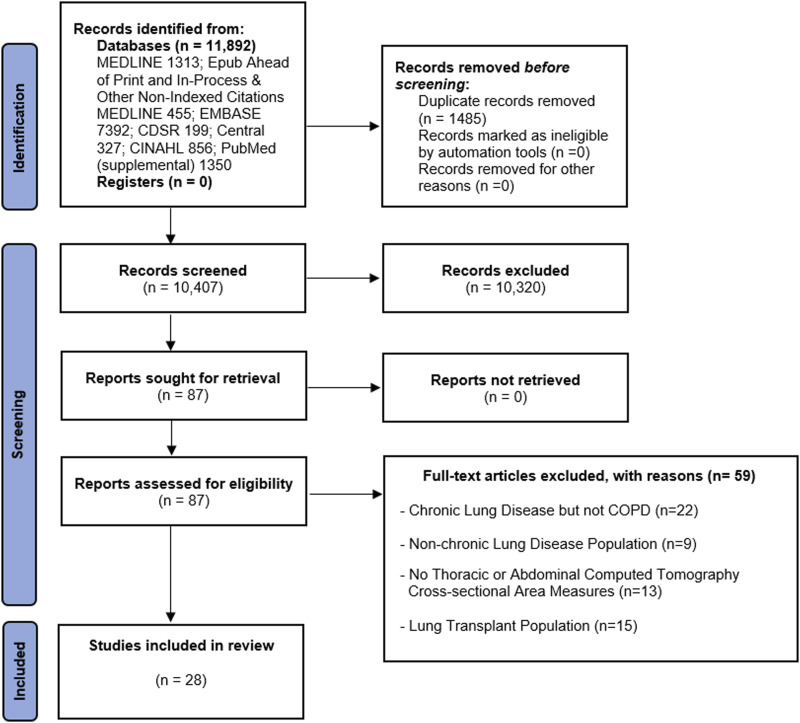
A total of 11,892 abstracts were identified with 87 full-text articles reviewed for eligibility, Figure 1. Of those, 28 articles were included in the systemic review.6,7,13–20,30–47 Selected studies were published between March 2010 and September 2021. A total of 15,431 individuals with COPD were included in the review.
Figure 1.
PRISMA flow diagram for systematic reviews of databases and registers.23
Chronic obstructive pulmonary disease patients in the selected studies comprised of 77% males, with a mean or median age range of 59–78 years old and BMI range of 19.8–29.3 kg/m2. Chronic obstructive pulmonary disease patients had good representation across GOLD stages and the most commonly described comorbidities were hypertension, diabetes, obesity, hyperlipidemia, and cardiovascular disease, as shown in Table 1. Patients in the majority of studies were current or former smokers, Table 1. There was international representation where the study was conducted: North America (n = 9), Europe (n = 7), Asia (n = 10), Australia (n = 1) and one-study was transcontinental.
Table 1.
Characteristics of patients with chronic obstructive pulmonary disease in the reviewed studies.
| Author (year) | N (% males) | Age, years* | BMI, kg/m2* | Lung function | Comorbidities; Smoking status (%); Pack-years smoked* | |
|---|---|---|---|---|---|---|
| FEV1% predicted* | GOLD stages, % | |||||
| Attaway (2021) | 60 (51) | 64.0 ± 6.9 | 26.3 ± 5.0 | 31 ± 10 | II: 4.3 III-IV: 95.7 |
Charlson comorbidity index
(CCI): 3.61±1.25 Current (CS) or former (FS): 98.3% Pack-years smoked (PYS): 52.9±30.7 |
| Ezponda (2021) | 174 (79) | 65 ± 8 | 27 ± 5 | 68±21 | I, II: 38,
47 III, IV: 1, 14 |
Comorbidities: NR CS: 39.5% PYS: 50 (IQR: 35–70) |
| Higashimoto (2021) | 38 (97) | Rehabilitation vs. None: 76.1 ± 7.1 vs. 76.7 ± 5.1 |
Rehabilitation vs.
None: 22.1 ± 3.4 vs. 23.1 ± 3.1 |
Rehabilitation vs. None: 51.4 ± 23.7 vs. 56.6 ± 17.0 |
Rehabilitation vs.
None: I – II: 41 vs. 59 III–IV: 59 vs. 41 |
Rehabilitation CCI versus None:
2 (1–3) vs. 3 (2–4) CS: 10.3% vs. 25.6%; FS: 89.7% vs. 74.4% PYS: 75.4 ± 35.5 vs. 64.5 ± 34.8 |
| Jeon (2021) | 492 (98) | 59.7 ± 7.3 | Wt: 69.1 ± 10.0 kg Ht: 1.7 ± 0.1 m |
NR | NR | HTN: 30.4%; CVD: 5.2%;
Diabetes:13.2%; hyperlipidemia: 4.8% Smoking status: NR PYS: ≥10 pack-years |
| Mason (2021) | Eclipse:
847 (63.6) COPDGene 2214 (50.5) |
ECLIPSE 61.8 ± 7.9 COPDGene: 59.8 ± 8.6 |
Eclipse: 26.5 ± 5.1 COPDGene: 29.1 ± 5.9 |
NR | NR | Comorbidities: NR CS: Eclipse- 38.4%, COPDGene −48.4% PYS: Eclipse: 45.8 ± 27.5 COPDGene: 42.8 ± 23.8 |
| Pishgar (2021) | 265 (61) | Died versus Survived: 75 ± 9 vs. 72 ± 9 | Died versus Survived: 28.2 ± 6.4 vs. 27.9 ± 5.1 | Died versus Survived: 75.2 ± 19.0 vs. 82.6 ± 18.3 | NR | Comorbidities:
NR Died versus Survived: CS: 26.5% vs. 14.4%; FS: 59.2% vs. 63.9% PYS: 45.3 ± 49.6 vs. 22.5 ± 26.3 |
| Shirahata (2021) | 36 (100) | 70.3 ± 5.8 | 21.9 ± 3.2 | 69.4 ± 24.4 (27.1–110.3) | I-II:
77.8 III-IV:22.2 |
Comorbidities, smoking status: NR PYS: 56.5 ± 23.3 (25–120) |
| Tashiro (2021) | 66 (96) | 71.1 ± 9.0 | 21.4 ± 3.8 | 60.2 ± 24.2 | I:
56.5 III: 43.5 |
Comorbidities, smoking status: NR PYS: 67.6 ± 33.0 |
| Zhi (2019) | All: 98 (72) Survived: 57 (74) Deceased: 41 (70) |
All:
78.0 (71.2–83.8) Survived: 77.0 (67.0–83.0) Deceased: 78.0 (73.0–84.0) |
NR | NR | NR | All: CCI- 2.00
(2.00–3.00) Survived: CCI- 2.00 (2.00–3.00) CS versus FS: 11 (14%) vs. 10 (13%) Deceased: CCI- 2.00 (2.00–3.00) CS versus FS: 14 (24%) vs. 10 (17%) PYS: NR |
| Sanders (2019) | 49 (33) | 59 (42–76) | 24.4 (23.4–25.5) | 30.3 (28.0–32.6) | NR | Comorbidities, smoking status:
NR PYS: 37 (31–43) |
| Coats (2018) | 144 (65) | GOLD stages: I: 66.4 ± 9.9 II+:63.9 ± 8.8 |
GOLD
stages: I: 26.3 ± 3.6 II+:27.4 ± 5.6 |
GOLD stages: I: 95 ± 12 II+: 64 ± 13 |
I:
48.6 II+: 51.4 |
GOLD
stages†, I, HBP: 16%; DM: 6%; PAD: 3%; CS: 21%; FS: 53%; PYS: 21 ± 24 II+,HBP:43%; DM: 9%; CAD: 9%; PAD:4% CS: 21%; FS: 53%; PYS: 37 ± 29 |
| Wallbridge (2018) | 20 (80) | 71.5 (62.3–78.8) | 23.5 (20.9–30.0) | 45 (34–74) | NR | Age-adjusted CCI: 5
(4–5) CS: 20% PYS: NR |
| Ju (2018) | 60 (97) | 71.6 ± 7.5 | 21.1 ± 3.4 | 54.1 ± 21.9 | I-II: 50 III-IV; 50 |
HBP: 28.3%; DM: 11.7% CS: 23.3%; FS: 76.7% PYS: 40.6 ± 16.6 |
| Martin (2017) | 511 (61) | 63.8 ± 6.9 | 24.4 ± 5.0 | 40.7 ± 15.3 | NR | CVD: 50%; HBP: 34%; DM: 6% CAC score: 540 ± 45 CS: 39%; FS: 61% PYS: 48.7 ± 1.1 |
| Martinez (2017) | 272 (56) | 64.7 ± 8.0 | 28.1 ± 5.6 | 59.0 ± 22.5 | I-II: 62.1 III-IV: 37.9 |
OB: 32.4%; DM: 11.0%; CVD: 22.1% Other CVD risk factors: 66.5% Musculoskeletal disease: 38.6% CS: 25.4% PYS: 51.1 ± 25.4 |
| McDonald (2017) | ECLIPSE‡ versus COPDGene‡: 1518 (64) vs. 3121 (56) |
ECLIPSE‡ versus
COPDGene‡: 64.0 (10) vs. 63.7 (12.8) |
ECLIPSE‡ versus COPDGene‡: NW: 36.3 vs. 30.3 |
ECLIPSE‡ versus
COPDGene‡: 47.4 (24.3) vs. 51.3 (30) |
Eclipse: ≥10 | ECLIPSE‡
versus COPDGene‡: CS: 35.6 vs. 39.5% PYS: ≥10 pack-years |
| Taka (2017) | 18 (100) | GOLD stages, I-II: 75 ± 2.9 III-IV: 68.1 ± 2.9 |
GOLD
stages, I-II: 20.0 ± 2.53 III-IV: 22.6 ± 2.75 |
GOLD stages, I-II: 95.1 ± 17.4 III-IV: 81.0 ± 12.8 |
I-II: 50 III-IV: 50 |
Comorbidities: NR CS or FS: 100% PYS: GOLD stages, I-II: 56.5 ± 23.0 III-IV: 59.9 ± 20.8 |
| Higami (2016) | 105 (92) | 73.1 ± 7.5 | 23.1 ± 2.76 | 66.9 ± 22.3 | I-II: 72.4 III-IV: 27.6 |
HBP: 37.1%; DM: 20.0%; CVD: 14.3%;
CS:19.1% PYS: 61.2 ± 31.4 |
| Tanimura (2016) | 130 (100) | 71.6 ± 8.4 | 21.4 ± 2.9 | 57.6 ± 20.3 | I-II: 64.6 III-IV: 35.4 |
CCI: 1.6 ± 0.9 CS: 18.5%; FS: 81.5% PYS: 70.0 ± 38.5 |
| Diaz (2015) | 1267 (55) | MI+ vs MI
-: 67 ± 8 vs. 64 ± 9 |
MI+ vs
MI -: 29 ± 6 vs. 28 ± 6 |
MI+
vs MI -: 56 ± 26 vs. 56 ± 23 |
NR | MI+ vs MI -: DM: 22 vs. 10% HBP: 63 vs. 52% OB: 36 vs. 30% CS: 33 vs. 36% PYS: 63 ± 32 vs. 51 ± 26 |
| Gaisi (2015) | 81 (78) | 64.3 ± 10.3 | 24.2 ± 5.8 | 28 (22–66) | I-II:
28 III-IV: 72 |
HBP: 63%; DM: 21%; DLD: 42%; OB: 15%; CS: 31%; PYS: 50.7 ± 22.2 |
| Diaz (2014) | 73 (62) | 62.0 (59.0–67.0) | 28.6 (23.9–32.6) | 43.5 (31.2–54.9) | NR | Comorbidities, smoking status: NR PYS: 44.0 (30.0–68.0) |
| Park (2014) | 98 (100) | GOLD stages, I: 67.0 ± 7.7 II: 71.0 ± 7.7 III: 70.1 ± 8.5 IV: 72.8 ± 10 |
GOLD
stages, I: 23.4 ± 2.8 II: 21.4 ± 3.9 III: 21.5 ± 2.6 IV:19.8 ± 2.9 |
GOLD stages, I: 94.8 ± 8.3 II:64.7 ± 8.1 III:41.3 ± 8.1 IV: 30.4 ± 8.3 |
I-II:
57.2 III-IV: 42.8 |
Comorbidities, smoking status: NR PYS: GOLD stages, I: 41.9 ± 10.7 II: 37.7 ± 20 III: 40.3 ± 16.5 IV: 44.0 ± 14.1 |
| ECLIPSE
versus COPDGene: 58 (58) vs. 484 (49) |
ECLIPSE versus COPDGene: 62.7 ± 6.1 vs. 64.6 ± 8.1 | ECLIPSE versus COPDGene: 26.6 ± 4.4 vs. 28.1 ± 6.3 | ECLIPSE versus COPDGene: 43.3 ± 14.9 vs. 48.7 ± 18.5 | COPDGene II: 49.2 III-IV: 50.8 |
ECLIPSE versus COPDGene:
CS: 34.5 vs.30.2% PYS: 49.7 ± 28.8 vs.54.9 ± 26.9 |
|
| Zagaceta (2013) | 171 (81) | 59.0 ± 7.0 | 26.9 ± 4.8 | 73.6 ± 21.9 | I-II: 80 III-IV: 20 |
CCI: 1 (1–2); HBP: 32.7%; DLD: 71%; DM: 14%; coronary calcium score: 2 (0–3); CS: 55%; PYS: 42 ± 17 |
| Van den Borst (2012) | 243 (58) | 73 ± 3.0 | 25.6 ± 4.6 | 63 ± 18 | NR | Comorbidities: NR CS: 27.6; FS: 54.7 PYS: 38 (9–57) |
| Furutate (2011) | 101 (100) | 69.0 (64.0–75.0) | 23.4 ± 3.2 | 58.6 ± 19.3 | I-II: 60.4 III-IV: 39.6 |
HBP: 49.5; DM: 14.9; DLD:
43.6; CCI: 2.8 ± 0.9 Smoking status: NR PYS: 70.0 (40.5–102.5) |
| Guerri (2010) | Non-fragile COPD versus fragile: 10 (50) vs. 10 (50) |
Non-fragile COPD versus fragile: 63.0 ± 8.0 vs. 69.0 ± 7.0 | Non-fragile COPD versus fragile: 27.0 ± 5.0 vs. 29.0 ± 3.0 | Non-fragile COPD versus fragile: 39.0 ± 10.0 vs. 36.0 ± 11.0 | NR | Non-fragile COPD
versus fragile: CCI: 2.3 ± 1.0 vs. 2.4 ± 1.4 CS: 40 vs. 30; FS: 60 vs. 70 PYS: NR |
*Data are reported as mean and standard deviation (mean ± SD) or median and interquartile range (25th-75th) in parentheses unless otherwise noted.
†= Data were extracted from figures using the Plot Digitizer software (version 2.6.9).
Abbreviations: BMI = body mass index; CAC = coronary artery calcification; CAD = coronary artery disease; CCI = charlson comorbidity index; CS = current smoker; CVD = cardiovascular disease; DLD = dyslipidemia; DM = diabetes mellitus; FS = former smoker; GOLD = global initiative for chronic obstructive lung disease; HBP = high blood pressure or hypertension; MI- = patients with copd but without myocardial infarction; MI+ = patients with copd and myocardial infarction; NR = not reported; NW = normal weight; OB = obesity; PAD = peripheral artery disease; PYS: pack-years smoked.
Quality assessment of included studies
Most studies were prospective (n = 18, 64%) and 12 of these were single-centered studies. The other 10 studies were retrospective or secondary analyses with three of the studies being single-centered. Most studies were appraised as good (n = 16, 57%) or fair (n=10, 36%) quality, and only two studies (7%) were characterized as poor (Table S7). Common strengths among the majority of studies included a clear description of study objectives, eligibility criteria and recruitment settings. However, four studies reported participation rates of less than 50% of eligible individuals.14,32,33,39 Additional limitations that were common across studies included a lack of sample size justification (n = 24, 86%) and inconsistent masking (either not described or unable to determine (n = 17, 61%). Furthermore, five studies (18%) had a loss of follow up greater than 20% 7,31,44–46 and four other studies failed to adjust for possible confounding.15,36,43,44
Methodological evaluation of skeletal muscle mass and adiposity
Skeletal muscle cross-sectional area (CSA) assessed with CT image analysis was evaluated in 22 studies (79% of included studies), with most using thoracic CT scans (n = 19). Of these studies, the most common muscle groups assessed were the paraspinal muscles (n = 13), pectoral muscles (n = 11), psoas/abdominal muscles (n = 7), and intercostals (n = 4), Table 2. A single CT CSA axial slice at each landmark was applied in the majority of studies, with four studies using multiple slices to assess muscle CSA; three studies assessed muscle measures using coronal CT slices. There was significant heterogeneity in the skeletal landmark and radiodensity (Hounsfield Unit) utilized for skeletal muscle. The reliability (inter or intra-observer agreement) for muscle CSA was very good to excellent in 11 of 22 studies that reported this measure, Table 2.
Table 2.
Skeletal muscle mass and adiposity tissue measurements in patients with chronic obstructive pulmonary disease.
| Author (year) | Skeletal muscle | Adipose tissue | Landmark/ROI | Number of slices | Radiodensity range (HU) for tissue segmentation | Reliability measures | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pectorals | Intercostals | Paraspinal | Latissimus dorsi | Psoas | Abdominals* | Subcutaneous | Visceral | Mediastinal | Epicardial | Intermuscular | |||||
| Attaway (2021) | X | X | Pectoral SM: above the aortic arch; paraspinal SM: lower aspect of T12 | 1 each landmark | SM: −50 to 90 | Interobserver R2: 0.75 to 0.76 |
|||||||||
| Ezponda (2021) | X | X | Psoas SM: L3; Paraspinal SM: T12 | 1 each landmark | NR | Interobserver# kappa: 0.82 | |||||||||
| Higashimoto (2021) | X | X | Pectoral SM: above the aortic arch; paraspinal: Lower aspect of T12 | 1 each landmark | NR | NR | |||||||||
| Jeon (2021) | X | X | X | X | X | X | X | L3 | 2 | Low attenuation SM: −29 to 29;
normal attenuation SM: 30 to 150 AT: −190 to −30 |
NR | ||||
| Mason (2021) | X | Above the aortic arch | 1 | NR | NRx` | ||||||||||
| Pishgar (2021) | X | X§ | X | Above the aortic
arch IMAT: Within pectoral SM |
1 | SM: −50 to
90 IMAT: HU in SAT was used to determine the individualized threshold for IMAT |
Interobserver¶ ICC: 0.91 to
0.99 Intra-observer ICC: 0.89 to 0.99 |
||||||||
| Shirahata (2021) | X | X | X♠ | Pectoral SM: above the aortic arch; paraspinal: T12; rectus abdominis: L1 | 1 each landmark | SM: −50 to 90 | Interobserver¶ ICC: 0.969
0.982 Intra-observer ICC: 0.984 to 0.994 |
||||||||
| Tashiro (2021) | X | X | Pectoral SM: above the aortic arch; paraspinal: Lower aspect of T12 | 1 each landmark | NR | NR | |||||||||
| Zhi (2019) | X | T12 | 1 | 1 | NR | ||||||||||
| Sanders (2019) | X | X | X† | X | X | L1 | 1 | SM: −29 to
150 AT: −190 to −30 |
SM: Inter CV: 1.3% | ||||||
| Coats (2018) | X | X | X | X | X | L4-L5 | NR | SM: −29 to
130 AT: −190 to −30 |
NR | ||||||
| Wallbridge (2018) | X | Lateral arc of the 1st rib‡ | 1 | SM: −29 to 150 | NR | ||||||||||
| Ju (2018) | X | Lateral arch of the 1st
rib‡; ROI for SM: 3rd-8th intercostal muscles (bilaterally) |
Multiple slices | SM: −29 to 100 | Interobserver¶ kappa: 0.76 | ||||||||||
| Martin (2017) | X | X | X | X | X | L2-L3 | 1 | SM: −29 to
130 AT: −190 to −30 |
Test-retest ICC: 0.99 to
1.00 Interobserver¶ ICC: 0.95 to 1.00 |
||||||
| Martinez (2017) | X | X§ | Above aortic arch | 1 | SM:
−50 to 90 AT: −200 to 0 |
NR for this study | |||||||||
| McDonald (2017) | X | Above aortic arch | 1 | SM: −50 to 90 | NR | ||||||||||
| Taka (2017) | X | T12 | 1 | NR | NR | ||||||||||
| Higami (2016) | X | X | SAT: Bottom right shoulder blade Epicardial AT: left main coronary artery |
1 each landmark | AT: Window width: −230 to −30; window level: −130 | NR | |||||||||
| Tanimura (2016) | X | X | T12 | 1 | NR | NR | |||||||||
| Diaz (2015) | X§ | X | SAT: Aortic arch VAT: L1 |
1 each landmark | SAT:
−200 to 0 VAT: −250 to −50 |
SAT & VAT: Interobserver# ICC: 0.99 | |||||||||
| Gaisi (2015) | X | X | Bifurcation of pulmonary trunk to end of myocardium | Multiple slices | AT: −190 to −30 | NR | |||||||||
| Diaz (2014) | X | X§ | Above the aortic arch, above the suprasternal notch of sternum | 1 each landmark | SM: −50 to
90 AT: −200 to 0 |
Interobserver¶ CCC: 0.818 to 0.994 Intra-observer test-retest measurement CCC: 0.697 to 1.00 |
|||||||||
| Park (2014) | X | X | X | Intercostals SM & IMAT:
Lateral arch of the bilateral 1st ribs‡; SM ROI: 3rd-8th muscles; IMAT ROI: 5th muscle Latissimus dorsi SM & IMAT ROI: T8 (axial plane) |
1 each landmark | SM: −29 to
100 IMAT: Mean HU within ROI |
Interobserver¶ kappa: 0.73 to 0.85 | ||||||||
| McDonald (2014) | X | Above aortic arch | 1 | SM: −50 to 90 | Interobserver¶ R2: 0.73 | ||||||||||
| Zagaceta (2013) | X | Center of the right pulmonary artery to the end of pericardial sac | Multiple slices | AT: −195 to −45 | Interobserver¶ CCC: 0.95 | ||||||||||
| Van den Borst (2012) | X | X | L4-L5 | 1 | NR | NR | |||||||||
| Furutate (2011) | X | X | Umbilicus | NR | NR | NR | |||||||||
| Guerri (2010) | X | X | X | X | Carina, iliac crest | 1 each landmark | SM: 0 to 100 | NR | |||||||
Symbols: * = Abdominals include the following muscles: rectus abdominis, external and internal obliques, transversus abdominis; † = Abdominals plus quadratus lumborum muscle; ‡ = computed tomography scans were obtained in the coronal plane; § = Subcutaneous adipose tissue anterior to the pectorals major and minor muscles; ¶ = interobserver reliability between two independent raters; # = Number of raters was not reported; ♠ = rectus abdominis alone.
Abbreviations AB = abdominals; AT = adipose tissue; CCC = concordance correlation coefficient; CI = confidence interval; CV = coefficient of variation; HU = hounsfield units; ICC = intraclass correlation coefficient; IMAT = intramuscular adipose tissue; L = lumbar vertebrae; NR = not reported; ROI = region of interest; SAT = subcutaneous adipose tissue; SM = skeletal muscle; T = thoracic vertebrae; VAT = visceral adipose tissue.
Adipose tissue was assessed in 14 studies (50%) with subcutaneous adiposity captured in the majority of studies (n = 11), whereas visceral (n = 6), mediastinal/epicardial (n = 3) and intermuscular (n = 4) were less commonly evaluated, as shown in Table 2. Thoracic adipose tissue (n = 9/14) was commonly evaluated at locations such as the aortic arch, bifurcation of the pulmonary artery or specific thoracic locations (i.e. first rib; third-eighth intercostal spaces) with a single cross-sectional slice, with the exception of two studies that had captured multiple slices. 33,36 For the abdominal imaging, there was variability in the vertebral lumbar area described ranging from L1 to L5, but one slice was utilized.
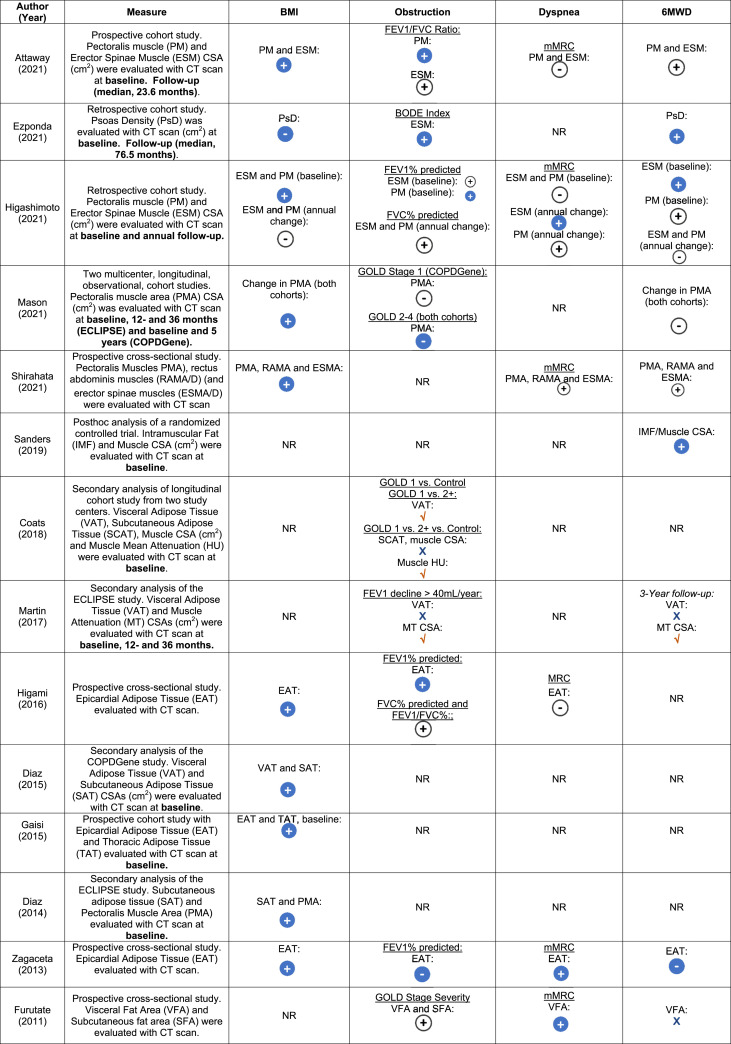
Relationships of muscle and adipose CT measurements with BODE index
Associations of skeletal muscle CSA or adiposity with individual BODE index measures (BMI, airway obstruction, dyspnea or exercise capacity) were described in 14 studies (50% of all studies),6,7,14,17-19,33,36,37 as shown in Table 3 and Table 8S. Muscle CSA had a low-moderate association with exercise capacity in four out of seven studies,6,17, 4,46 and its relationship with degree of airway obstruction was also mixed (not significant in four of seven studies).6,7,44,47 There was a low-moderate correlation or association between BMI and muscle CSA14,40,41,44,47 and both visceral and subcutaneous adiposity measures across five studies.14,18,19,33,36 Associations of adiposity with degree of airway obstruction, dyspnea, and 6MWD were mixed across the six-studies, as shown in Table 3 and Table 8S.6,7,17,19,36,37
Table 3.
Associations of muscle and adiposity with BODE index measurements in participants with chronic obstructive pulmonary disease.
 |
 :
association data not shown, significant (p <
.05);
:
association data not shown, significant (p <
.05);  :
association data not shown, not significant (p
> .05);
:
association data not shown, not significant (p
> .05);  : positive
association (p < .05);
: positive
association (p < .05);  : positive association (p
> .05);
: positive association (p
> .05); : negative
association (p < .05);
: negative
association (p < .05);  : negative
association (p >
.05).
: negative
association (p >
.05).
Abbreviations BMI = body mass index; BODE = body-mass index, airflow obstruction, dyspnea, and exercise; CSA = cross-sectional area; CT = computed tomography; FEV1 = forced expiratory volume in first second; FVC = forced vital capacity; GOLD = the global initiative for chronic obstructive lung disease; mMRC = modified medical research council; NR = not reported; NS = no significance; 6MWT/D = six meter walk test/distance.
Relationships of muscle and adipose CT measurements with quality of life
Health-related quality of life and their relationship with muscle CSA or adiposity measures were assessed in three studies (11% of all studies).6,16,35 McDonald et al. observed an inverse association between pectoralis muscle area and the total score on the St. George’s Respiratory Questionnaire (SGRQ) [β = −0.44 95% CI (−0.64 to −0.24) per 1 cm2 in pectoralis muscle CSA; p < .001], signifying improved HRQL.35 Similarly, Tanimura et al. observed a weak correlation between SGRQ and erector spinae muscles (r = − 0.35, p < .0001).16 Only one study described the association between HRQL and adiposity measures, with increased visceral adiposity associated with more favorable SGRQ scores (< 25 points, p = .049).6
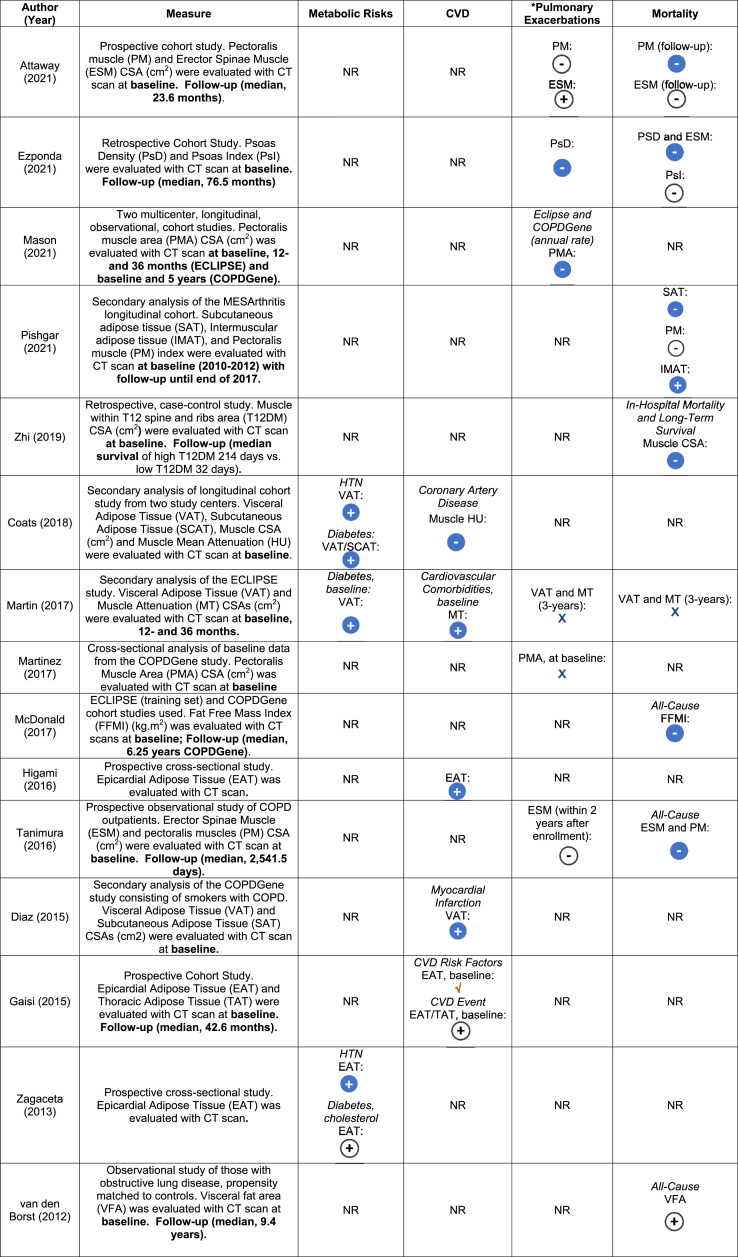
Relationship of muscle and adipose CT measurements with cardiometabolic risk factors
The relationship between muscle CSA and metabolic risk factors was evaluated in two (7%) studies. Coats et al. observed that increased muscle attenuation (greater muscle density) was associated with a decreased presence of coronary artery disease (CAD) at study enrollment [OR = .759 95% CI (0.662–.869), p < .001].7 Similarly, Martin and colleagues depicted that increased muscle attenuation was associated with a lower proportion of COPD patients with CAD.6
Metabolic risk factors, such as diabetes, hypertension, and hypercholesterolemia or presence of cardiovascular disease were assessed in six studies across different anatomical adipose locations, as shown in Table 4 and Table 9S. Visceral adiposity CSA was positively associated with metabolic risk factors, including hypertension and diabetes.7 Diaz et al. demonstrated that for those in the upper tertile of visceral adiposity tissue, the odds ratio for previous self-reported physician diagnosed myocardial infarction was 1.86 (95% CI (1.02–3.41, p = .04) at the time of baseline assessment.18 Gaisl et al. revealed that there was an increased number of cardiovascular disease risk factors with greater epicardial adipose tissue.33
Table 4.
Associations of muscle and adiposity measures with cardiovascular risk factors and clinical outcomes in participants with chronic obstructive pulmonary disease.
 |
 :
association data not shown, significant (p <
.05);
:
association data not shown, significant (p <
.05);  :
association data not shown, not significant (p
> .05);
:
association data not shown, not significant (p
> .05);  : positive
association (p < .05);
: positive
association (p < .05);  : positive association (p
> .05);
: positive association (p
> .05);  : negative
association (p < .05);
: negative
association (p < .05);  : negative
association (p >
.05).
: negative
association (p >
.05).
*Pulmonary Exacerbations Defined: Attaway (2021): ≥ 2 more exacerbation in prior year or > 1 hospital admission; Ezponda (2021): exacerbations in the 1-year prior to study enrollment; Mason (2021): Increase in respiratory symptoms needing antibiotics or systemic corticosteroids with severe event defined as emergency department visit or hospitalization. Martin (2017): moderate exacerbation requiring antibiotics or systemic corticosteroids, whereas severe exacerbation needing hospitalization. Martinez (2017): increased cough, phlegm or dyspnea > 48 h managed with antibiotics or systemic steroids in the year prior. Higami (2016): Moderate to severe exacerbations after 2-years of enrollment.
Abbreviations: BMI = body mass index; CSA = cross-sectional area; CT = computed tomography; CVD = cardiovascular disease; GOLD = global initiative for chronic obstructive lung disease; HTN = hypertension; HU = hounsfield unit; MD = mean difference; MT = muscle tissue; NR = not reported.
Associations of muscle and adipose CT measurements with clinical outcomes
Respiratory exacerbations
The association between muscle CSA and respiratory exacerbations was evaluated in six (21%) studies.16,32,38,40,46,47 Martinez et al. observed that CT measures of pectoralis muscle area (per 1 standard deviation) were associated with a 60% lower incidence of reported respiratory exacerbations in the year prior to CT body composition assessments, independent of demographics, lung function, and smoking history.32 Guerri et al. demonstrated that COPD patients with multiple exacerbations (≥ 4 in the previous year) had a lower intercostal muscle CSA than those with fewer exacerbations,38 Table 4 and Table 9S. Ezponda et al. demonstrated a weak inverse correlation between psoas muscle density and number of COPD exacerbations in the previous 1-year prior to study enrollment.46 Similarly, another study illustrated the frequency of respiratory exacerbations per year over 3 and 5 years, in two separate cohorts prospectively assessed, which was associated with loss of pectoralis CSA.40 However, in two studies the association between reduced erector spinae muscles CSA (r= −0.10)16 or pectoralis/erector spinae muscles were not significant based on number of exacerbations within 1-year of enrollment in both studies.47 Furthermore, Martin et al. did not observe an association between visceral adiposity tissue and rate of moderate to severe respiratory exacerbations over a 3-year period.6
Survival
The association between CT muscle CSA or adiposity and survival measures was evaluated in eight studies (29%).6,13,16,20,43,45-47 McDonald et al. demonstrated that a low fat free mass index derived from CT pectoral muscle area was associated with a 1.6 fold increase in mortality (p < .001) in 3121 COPD patients, adjusted for age, sex, race, smoking history, GOLD stage, and comorbidities (COPDGene cohort with a median follow-up of 6.25 years).20 Similarly, Tanimura et al. observed that erector spinae muscles CSA (cm2) was the strongest independent predictor of all cause mortality [HR 0.85 95% CI (.79–.92), p < .0001] over a median follow-up of 2542 days, along with modified Medical Research Council dyspnea, whereas age, BMI, forced expiratory volume in 1 s (FEV1), and pectoral muscle area were not significant.16 Attaway et al. demonstrated that higher pectoralis muscle CSA was associated with survival, but not erector spinae muscles over a median follow-up of 23.6 months.47 Similarly, Zhi,43 Pishgar,45 and Ezponda,46 demonstrated associations between muscle CSA and survival, as shown in Table 4 and 9S. Similarly, Pishgar et al. had shown that higher intermuscular adiposity was associated with increased mortality.45 van den Borst et al. demonstrated that abdominal visceral fat was associated with increased plasma interleukin-6 levels, a marker of increased mortality, but visceral fat was not directly associated with all cause-mortality in this cohort of COPD patients.13 Martin et al. did not observe an association between CT body composition measures and mortality in a cohort of 511 COPD patients.6
Discussion
This systematic review illustrates the clinical utility of CT muscle mass and adiposity measures in COPD patients. The majority of studies utilized thoracic CT measurements, whereas abdominal CT measures were applied less frequently. Despite variability in CT body composition measures, low muscle mass and increased adiposity were associated with lower FEV1%, exercise capacity, and increased dyspnea in several studies. Increased visceral and subcutaneous adiposity were associated with cardiovascular risk factors or disease in six studies. However, there was significant heterogeneity in the associations between body composition and clinical outcomes, such as COPD exacerbations and all-cause mortality, thus the prognostic utility of body composition measures requires further investigation.
Variability in the assessment of CT-based muscle and adiposity measures
In the present review, there was significant variability in the thoracic or abdominal landmarks utilized, muscles or adiposity tissues assessed. The majority of studies in COPD patients utilized thoracic CT scans (n = 22, 79%). Thoracic CT is clinically performed in COPD patients for assessment for pulmonary emboli, emphysema phenotyping, lung volume reduction surgery, and lung cancer screening,9 thus are readily available clinically for evaluation of body composition. Abdominal CT scans were often performed for research purposes and focused on the psoas, abdominal muscles or adipose tissue measures. To date, normative values for low muscle mass or abnormal adiposity CT measures have not been defined in COPD patients; however, these CT morphometric measures have been shown to have strong associations with more traditional measures of body composition such as BIA or DXA in COPD patients.20,35,41 Given the heterogeneity in CT body composition measures, automated segmentation techniques may facilitate the development of normative values with thoracic and abdominal CT measures,48 but prognostic utility of these cut-off values will need to be verified.
Relationship of body composition with BODE index measures
The associations of CT muscle mass and adiposity measures were commonly evaluated with individual BODE index parameters, which has been shown to predict mortality in COPD.24 Low muscle mass and increased adiposity were generally associated with increased airway obstruction, lower 6MWD, increased dyspnea severity, and low or increased BMI, respectively. The BODE index is multifactorial and observed to be associated with biomarkers of inflammation (TNF-alpha and leptin levels),49 physical inactivity,50 malnutrition, hypoxemia, and smoking,51 known risk factors for disease progression. Furthermore, BODE index has been shown to be responsive to pulmonary rehabilitation with greater than 70% of 83 COPD participants demonstrating > 1 point BODE index change, specifically in the indices of lung function, dyspnea, and exercise capacity, but no change in BMI.52 Thus, CT morphometric measures of body composition may provide additional insight into body composition changes that may not necessarily be captured with BMI.
In this review, CT muscle CSA was shown to be a more informative measure of disease severity than BMI in some studies.20,40 Furthermore, both visceral and subcutaneous compartments with CT had moderate correlations or associations with BMI.14,18,19,33,36 This is an important consideration given the increasing prevalence of obesity in COPD patients, 2,53 which may underestimate low muscle mass in the setting of preserved BMI and increased adiposity. Chronic obstructive pulmonary disease patients with sarcopenic obesity, which is prevalent in this population,37,54 are more likely to have lower 6MWD and higher systemic inflammatory burden compared to other body composition phenotypes, independent of age, sex, FEV1% and smoking history.55 Thus, CT morphometric analysis may allow identification of clinically important body composition phenotypes in COPD,4 which may help with further risk stratification.
Relationship of muscle mass and adiposity with clinical outcomes
Low muscle mass was associated with adverse clinical outcomes such as respiratory exacerbations32,38 and increased mortality16,20 in the majority of studies evaluating this outcome. As in other populations, muscle mass represents an element of physiologic reserve which may help combat respiratory exacerbations and infections, which are key contributors to morbidity and mortality in COPD.20,56,57 One of the known contributors to low muscle mass is systemic inflammation and those with increased fibrinogen or IL-6 levels were shown to have increased mortality.13,57,58 Furthermore, low muscle mass is a phenotypic criteria of malnutrition based on international consensus59 and has been shown to be associated with poor prognostic markers in COPD such as cachexia, muscle weakness60 and lower physical activity levels.60 Thus, quantification of muscle mass may help identify patients who may benefit from additional nutritional counselling and exercise training.61 It may also serve as a complementary measure of respiratory muscle evaluation, especially in those with frequent exacerbations.38
Chronic obstructive pulmonary disease patients have been shown to have greater visceral adiposity than healthy controls and increased cardiometabolic risk factors.6,13 Adipose tissue is known to be metabolically active with liberation of inflammatory mediators such as interleukin-6, tumor necrosis factor alpha, leptin, and adiponectin, which may have effects locally and systemically, and in turn increase risk for cardiometabolic risk factors in COPD. In the present review, visceral adiposity was associated with increased prevalence of diabetes and cardiovascular comorbidities, such as ischemic heart disease, congestive heart failure and cerebrovascular disease.6 Visceral adiposity has been demonstrated to be a more metabolically active tissue compared to subcutaneous tissue on abdominal CT scans.7,62 However, a unique adiposity tissue compartment is epicardial tissue evaluated in three studies,19,33,36 which is considered a visceral fat depot associated with coronary artery disease and cardiometabolic risk factors. Epicardial tissue is a unique CT morphometric measure as its anatomically intertwined with the myocardium and coronary arteries, and in fact shown to have a stronger association with cardiovascular risk factors compared to abdominal visceral adiposity in non obese patients.63,64 Furthermore, epicardial tissue is readily available from clinical thoracic CT scans and may potentially be utilized as a clinical risk factor for cardiovascular comorbidities, along with other known risk factors such as diet, physical inactivity, and corticosteroid use.65,66
Clinical implications of CT-body composition analysis
The present review highlights the clinical implications of CT morphometric analysis in the COPD population, which has become more readily available given establishment of lung cancer screening protocols.67 However, the optimal muscle or analytic technique for CT morphometric interpretation remains unclear. As the case with other traditional body composition measures (BIA and DXA),68 CT morphometric analysis has been shown to have stronger correlations with clinical outcomes than BMI. Mason et al. demonstrated that the change in CT skeletal muscle CSA to be 10-fold greater when compared to change in BMI.40 Consideration of CT skeletal muscle CSA or other traditional body composition measures may allow earlier rehabilitation and nutritional intervention opportunities rather than focusing on BMI or weight loss changes.69,70 However, even though CT morphometric analysis holds future promise as a prognostic marker that may help inform timing of transplantation, respiratory exacerbation risk, and survival in COPD, CT-body composition is not ready for clinical application at the present time. Methodological considerations that need to be addressed include development of normative reference values for muscle mass and adiposity, standardization of measurement techniques, and availability of automated methods for CT-body composition assessments in clinical settings. Nevertheless, the present review highlights the clinical implications of striving for routine CT-body composition assessment in COPD as it could allow assessment of body composition in large cohorts of patients.
Limitations
There are several limitations of this systematic review. Given the known significant variability in CT body composition measures in lung transplant candidates,11 a qualitative systematic review without a meta-analysis was undertaken in COPD patients. Secondly, there was heterogeneity in GOLD stage, comorbidities, and smoking history in over 15,000 COPD patients, which may in part explain some of the differences in body composition measures across studies. Furthermore, mechanisms of muscle atrophy and adiposity accumulation were not evaluated in the present review, neither were physical activity levels, nutrition, insulin resistance, or corticosteroid use, which are often associated with cardiometabolic risk factors.71 Finally, with the exception of one study by Martinez et al.,32 the peripheral measures of muscle size or strength were not reported, which are known to have important prognostic implications in COPD.72–74
Conclusion
CT-body composition has been commonly applied in the COPD literature. There was significant variability in CT measures of body composition. In several studies, low muscle mass was associated with disease severity, worse HRQL, and lower exercise capacity, whereas CT measures of adiposity were associated with cardiovascular disease or risk factors. However, the prognostic implications of CT-body composition measures on respiratory exacerbations and survival remains unclear given the significant heterogeneity in outcomes across studies. The present findings highlight the potential role for CT body composition assessments clinically as complementary markers of body composition, and potentially prognostic markers in the future. However, despite routine clinical availability of CT scans in the COPD population, there are a number of methodological considerations that will need to be undertaken before consideration of clinical application, including development of normative reference values and standardization and automatization of CT-body composition measurement techniques.
Supplemental Material
Supplemental Material for Computed tomography-based body composition measures in COPD and their association with clinical outcomes: A systematic review by John M Nicholson, Camila E Orsso, Sahar Nourouzpour, Brenawen Elangeswaran, Karan Chohan, Ani Orchanian-Cheff, Lee Fidler, Sunita Mathur and Dmitry Rozenberg in Chronic Respiratory Disease
Author contributions: JMN, CE.O, SN, BE, KC, A.OC, LF, SM, DR made substantial contributions to the conception and design of the work. JMN, SN and DR wrote the first draft of the manuscript and JMN, CE.O, SN, BE, KC, A.OC, LF, SM, DR revised the manuscript for important intellectual content. All authors made substantial contributions to the analysis or interpretation of data. All authors approved the manuscript and agree to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dmitry Rozenberg receives support from the Sandra Faire and Ivan Fecan Professorship in Rehabilitation Medicine.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Ani Orchanian-Cheff https://orcid.org/0000-0002-9943-2692
Dmitry Rozenberg https://orcid.org/0000-0001-8786-9152
References
- 1.Singh D, Miravitlles M, Vogelmeier C. Chronic obstructive pulmonary disease individualized therapy: tailored approach to symptom management. Adv Ther 2017; 34: 281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franssen FM, O'Donnell DE, Goossens GH, et al. Obesity and the lung: 5. Obesity and COPD. Thorax 2008; 63: 1110–1117. [DOI] [PubMed] [Google Scholar]
- 3.Mantoani LC, Dell'Era S, MacNee W, et al. Physical activity in patients with COPD: the impact of comorbidities. Expert Rev Respiratory Med 2017; 11: 685–698. [DOI] [PubMed] [Google Scholar]
- 4.Schols AM, Ferreira IM, Franssen FM, et al. Nutritional assessment and therapy in COPD: a European respiratory society statement. Eur Respir J 2014; 44: 1504–1520. [DOI] [PubMed] [Google Scholar]
- 5.Benz E, Trajanoska K, Lahousse L, et al. Sarcopenia in COPD: a systematic review and meta-analysis. Eur Respir Rev 2019; 28: 190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin M, Almeras N, Despres JP, et al. Ectopic fat accumulation in patients with COPD: an ECLIPSE substudy. Int J Chron Obstruct Pulmon Dis 2017; 12: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coats V, Despres JP, Almeras N, et al. Ectopic adiposity and cardiometabolic health in COPD. Int J Chron Obstruct Pulmon Dis 2018; 13: 3331–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fosbol MO, Zerahn B. Contemporary methods of body composition measurement. Clin Physiol Func Imag 2015; 35: 81–97. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt SP, Washko GR, Hoffman EA, et al. Imaging advances in chronic obstructive pulmonary disease. insights from the genetic epidemiology of chronic obstructive pulmonary disease (COPDGene) study. Am J Respir Crit Care Med 2019; 199: 286–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki Y, Yoshimura K, Enomoto Y, et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci Rep 2018; 8: 14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozenberg D, Orsso CE, Chohan K, et al. Clinical outcomes associated with computed tomography-based body composition measures in lung transplantation: a systematic review. Transpl Int 2020; 33: 1610–1625. [DOI] [PubMed] [Google Scholar]
- 12.Troschel AS, Troschel FM, Best TD, et al. Computed tomography–based body composition analysis and its role in lung cancer care. J Thorac Imaging 2020; 35: 91–100. [DOI] [PubMed] [Google Scholar]
- 13.van den Borst B, Gosker HR, Koster A, et al. The influence of abdominal visceral fat on inflammatory pathways and mortality risk in obstructive lung disease. Am J Clin Nutr 2012; 96: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz AA, Zhou L, Young TP, et al. Chest CT measures of muscle and adipose tissue in COPD: gender-based differences in content and in relationships with blood biomarkers. Acad Radiol 2014; 21: 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taka C, Hayashi R, Shimokawa K, et al. SIRT1 and FOXO1 mRNA expression in PBMC correlates to physical activity in COPD patients. Int J Chron Obstruct Pulmon Dis 2017; 12: 3237–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanimura K, Sato S, Fuseya Y, et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. Novel chest computed tomography–derived index for prognosis. Ann Am Thorac Soc 2016; 13: 334–341. [DOI] [PubMed] [Google Scholar]
- 17.Sanders KJC, Klooster K, Vanfleteren L, et al. CT-derived muscle remodelling after bronchoscopic lung volume reduction in advanced emphysema. Thorax 2019; 74: 206–207. [DOI] [PubMed] [Google Scholar]
- 18.Diaz AA, Young TP, Kurugol S, et al. Abdominal visceral adipose tissue is associated with myocardial infarction in patients with COPD. Chron Obstruct Pulm Dis 2015; 2: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higami Y, Ogawa E, Ryujin Y, et al. increased epicardial adipose tissue is associated with the airway dominant phenotype of chronic obstructive pulmonary disease. PloS One 2016; 11: e0148794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald MN, Diaz AA, Rutten E, et al. Chest computed tomography-derived low fat-free mass index and mortality in COPD. Eur Respir J 2017; 50: 1701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam KB, Jordan RE, Jiang CQ, et al. Airflow obstruction and metabolic syndrome: the Guangzhou Biobank Cohort Study. Eur Respir J 2010; 35: 317–323. [DOI] [PubMed] [Google Scholar]
- 22.Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis 2018; 12: 1753465817750524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 25.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995; 273: 59–65. [PubMed] [Google Scholar]
- 26.GOLD report (2022). https://goldcopd.org/2022-gold-reports-2/(last accessed September 8, 2022). [Google Scholar]
- 27.Mullerova H, Agusti A, Erqou S, et al. Cardiovascular comorbidity in COPD: systematic literature review. Chest 2013; 144: 1163–1178. [DOI] [PubMed] [Google Scholar]
- 28.Trinkmann F, Saur J, Borggrefe M, et al. Cardiovascular comorbidities in chronic obstructive pulmonary disease (COPD)-current considerations for clinical practice. J Clin Med 2019; 8: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Study quality assessment tools website. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (last accessed April 2, 2022). [Google Scholar]
- 30.Wallbridge P, Parry SM, Das S, et al. Parasternal intercostal muscle ultrasound in chronic obstructive pulmonary disease correlates with spirometric severity. Scient Rep 2018; 8: 15274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ju S, Lee SJ, Park MJ, et al. Clinical importance of cross-sectional area of intercostal muscles in patients with chronic obstructive pulmonary disease. Clin Respir J 2018; 12: 939–947. [DOI] [PubMed] [Google Scholar]
- 32.Martinez CH, Diaz AA, Meldrum CA, et al. Handgrip strength in chronic obstructive pulmonary disease. Associations with acute exacerbations and body composition. Ann Am Thorac Soc 2017; 14: 1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaisl T, Schlatzer C, Schwarz EI, et al. Coronary artery calcification, epicardial fat burden, and cardiovascular events in chronic obstructive pulmonary disease. PloS One 2015; 10: e0126613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park MJ, Cho JM, Jeon KN, et al. Mass and fat infiltration of intercostal muscles measured by CT histogram analysis and their correlations with COPD Severity. Acad Radiol 2014; 21: 711–717. [DOI] [PubMed] [Google Scholar]
- 35.McDonald ML, Diaz AA, Ross JC, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc 2014; 11: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zagaceta J, Zulueta JJ, Bastarrika G, et al. Epicardial adipose tissue in patients with chronic obstructive pulmonary disease. PloS One 2013; 8: e65593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furutate R, Ishii T, Wakabayashi R, et al. Excessive visceral fat accumulation in advanced chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2011; 6: 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerri R, Gayete A, Balcells E, et al. Mass of intercostal muscles associates with risk of multiple exacerbations in COPD. Mass Respir Med 2010; 104: 378–388. [DOI] [PubMed] [Google Scholar]
- 39.Jeon YJ, Han S, Park GM, et al. Intramuscular and intermuscular abdominal fat infiltration in copd: a propensity score matched study. Int J Chron Obstruct Pulmon Dis 2021; 16: 1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason SE, Moreta-Martinez R, Labaki WW, et al. Respiratory exacerbations are associated with muscle loss in current and former smokers. Thorax 2021; 76: 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirahata T, Sato H, Yogi S, et al. The product of trunk muscle area and density on the CT image is a good indicator of energy expenditure in patients with or at risk for COPD. Respir Res 2021; 22: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tashiro H, Takahashi K, Tanaka M, et al. Skeletal muscle is associated with exercise tolerance evaluated by cardiopulmonary exercise testing in Japanese patients with chronic obstructive pulmonary disease. Scient Rep 2021; 11: 15862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhi J, Shan Q, Liang L, et al. Low skeletal muscle area as a prognostic marker for chronic obstructive pulmonary disease in elderly patients admitted to ICU. Scient Rep 2019; 9: 19117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higashimoto Y, Shiraishi M, Sugiya R, et al. Effect of pulmonary rehabilitation on erector spinae muscles in individuals with COPD. Respir Care 2021; 66: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 45.Pishgar F, Shabani M, Quinaglia ACST, et al. Quantitative analysis of adipose depots by using chest CT and associations with all-cause mortality in chronic obstructive pulmonary disease: longitudinal analysis from mesarthritis ancillary study. Radiology 2021; 299: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ezponda A, Casanova C, Cabrera C, et al. Psoas muscle density evaluated by chest CT and long-term mortality in COPD patients. Arch Bronconeumol (Engl Ed) 2021. [DOI] [PubMed] [Google Scholar]
- 47.Attaway AH, Welch N, Yadav R, et al. Quantitative computed tomography assessment of pectoralis and erector spinae muscle area and disease severity in chronic obstructive pulmonary disease referred for lung volume reduction. COPD 2021; 18: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paris MT. Body composition analysis of computed tomography scans in clinical populations: the role of deep learning. Lifestyle Genom 2020; 13: 28–31. [DOI] [PubMed] [Google Scholar]
- 49.Gaki E, Kontogianni K, Papaioannou AI, et al. Associations between BODE index and systemic inflammatory biomarkers in COPD. COPD 2011; 8: 408–413. [DOI] [PubMed] [Google Scholar]
- 50.Jehn M, Schmidt-Trucksass A, Meyer A, et al. Association of daily physical activity volume and intensity with COPD severity. Respir Med 2011; 105: 1846–1852. [DOI] [PubMed] [Google Scholar]
- 51.Ansari K, Keaney N, Kay A, et al. Body mass index, airflow obstruction and dyspnea and body mass index, airflow obstruction, dyspnea scores, age and pack years-predictive properties of new multidimensional prognostic indices of chronic obstructive pulmonary disease in primary care. Ann Thoracic Med 2016; 11: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J 2005; 26: 630–636. [DOI] [PubMed] [Google Scholar]
- 53.Zewari S, Vos P, van den Elshout F, et al. Obesity in COPD: revealed and unrevealed issues. COPD 2017; 14: 663–673. [DOI] [PubMed] [Google Scholar]
- 54.van de Bool C, Rutten EP, Franssen FM, et al. Antagonistic implications of sarcopenia and abdominal obesity on physical performance in COPD. Eur Respir J 2015; 46: 336–345. [DOI] [PubMed] [Google Scholar]
- 55.Joppa P, Tkacova R, Franssen FM, et al. Sarcopenic obesity, functional outcomes, and systemic inflammation in patients with chronic obstructive pulmonary disease. J Am Med Directors Assoc 2016; 17: 712–718. [DOI] [PubMed] [Google Scholar]
- 56.Schols AM, Broekhuizen R, Weling-Scheepers CA, et al. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005; 82: 53–59. [DOI] [PubMed] [Google Scholar]
- 57.Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample. Am J Respir Crit Care Med 2006; 173: 79–83. [DOI] [PubMed] [Google Scholar]
- 58.Miller BE, Tal-Singer R, Rennard SI, et al. Plasma fibrinogen qualification as a drug development tool in chronic obstructive pulmonary disease. Perspective of the chronic obstructive pulmonary disease biomarker qualification consortium. Am J Respir Crit Care Med 2016; 193: 607–613. [DOI] [PubMed] [Google Scholar]
- 59.Cederholm T, Jensen GL, Correia M, et al. GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 2019; 10: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaes AW, Garcia-Aymerich J, Marott JL, et al. Changes in physical activity and all-cause mortality in COPD. Eur Respir J 2014; 44: 1199–1209. [DOI] [PubMed] [Google Scholar]
- 61.Maltais F, Decramer M, Casaburi R, et al. An official American thoracic society/European respiratory society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189: e15–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007; 116: 39–48. [DOI] [PubMed] [Google Scholar]
- 63.Taguchi R, Takasu J, Itani Y, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis 2001; 157: 203–209. [DOI] [PubMed] [Google Scholar]
- 64.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 2009; 30: 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cebron Lipovec N, Beijers RJ, van den Borst B, et al. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. COPD 2016; 13: 399–406. [DOI] [PubMed] [Google Scholar]
- 66.Chen W, Thomas J, Sadatsafavi M, et al. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med 2015; 3: 631–639. [DOI] [PubMed] [Google Scholar]
- 67.Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high-quality lung cancer screening: American College of chest physicians and American thoracic society policy statement. Chest 2015; 147: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schols AM, Soeters PB, Dingemans AM, et al. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993; 147: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 69.McDonald MN, Wouters EFM, Rutten E, et al. It’s more than low BMI: prevalence of cachexia and associated mortality in COPD. Respir Res 2019; 20: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rutten EP, Spruit MA, McDonald ML, et al. Continuous fat-free mass decline in COPD: fact or fiction? Eur Respir J 2015; 46: 1496–1498. [DOI] [PubMed] [Google Scholar]
- 71.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887. [DOI] [PubMed] [Google Scholar]
- 72.Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166: 809–813. [DOI] [PubMed] [Google Scholar]
- 74.Rozenberg D, Martelli V, Vieira L, et al. Utilization of non-invasive imaging tools for assessment of peripheral skeletal muscle size and composition in chronic lung disease: a systematic review. Respir Med 2017; 131: 125–134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Computed tomography-based body composition measures in COPD and their association with clinical outcomes: A systematic review by John M Nicholson, Camila E Orsso, Sahar Nourouzpour, Brenawen Elangeswaran, Karan Chohan, Ani Orchanian-Cheff, Lee Fidler, Sunita Mathur and Dmitry Rozenberg in Chronic Respiratory Disease