Abstract
Objective
To develop a nomogram to predict preterm birth before 28 weeks in pregnant women undergoing cervical cerclage.
Study design
We retrospectively studied the medical records on pregnant women who underwent cervical cerclage in January 2016 to September 2020. We developed the model from a development cohort in Women's Hospital, Zhejiang university, School of medicine, which randomly divided by 7:3 into training cohort for nomogram development, and internal validation cohort to confirm the model's performance. We then tested the nomogram in an external validation cohort over a similar period. The Harrell's C-index, calibration curve, decision curve analyses (DCA) were performed to assess the model.
Results
528 patients formed the development cohort, and 97 patients formed the external validation cohort. The model initially incorporated 10 baseline variables, while 5 variables were estimated in the nomogram at last: history of prior second-trimester loss, use of in-vitro fertilization (IVF), cervical dilation at cerclage, C-reactive protein (CRP) and platelet-lymphocyte ratio (PLR). The nomogram achieved good concordance indexes of 0.82(95%CI 0.77–0.88), 0.80(95%CI 0.72–0.88) and 0.79 (95%CI 0.68–0.90) in the training, internal and external validation cohort, respectively. And the nomogram had well-fitted calibration curves. Decision curve analysis demonstrated that the nomogram was clinically useful.
Conclusions
The well-performed nomogram graphically represents the risk factors and a pre-operative predicted model in predicting the risk of preterm birth at <28 weeks in singleton pregnant women undergoing cervical cerclage. The model can provide a useful guide for clinicians and patients in making appropriate clinical decisions.
Keywords: Cervical insufficiency, Cervical cerclage, Prognosis, Nomogram, Singleton, IVF, Cervical dilation, Platelet-lymphocyte ratio
Cervical insufficiency; Cervical cerclage; Prognosis; Nomogram; Singleton; IVF; Cervical dilation; Platelet-lymphocyte ratio.
1. Introduction
Preterm birth is still the leading course of neonatal morbidity and mortality, despite continuous progress in obstetrics and neonatology [1]. Preterm birth is defined as infants born alive before 37 weeks of pregnancy, while its lower boundary of gestational weeks depends on the medical skills of neonatology at different countries. Infants born before 28 weeks, namely extremely preterm (EPT) birth, have the highest mortality with reported death rates of approximately 50% primarily [2, 3]. Owing to development in newborn intensive care, many large prospective studies have shown improved survival for EPT infants [4, 5]. Recent study has shown that the survival rates at 28 weeks ranged from 89-97% at different regions [6], which means most babies born after 28 weeks could survive. Therefore, obstetricians would make effort to prolong gestational weeks to 28 weeks.
Among all the possible reasons of preterm birth, cervical insufficiency accounts for 0.5%–2% of preterm birth and is implicated in 15–25% of second-trimester pregnancy losses [7, 8]. Cervical cerclage has been proved to be the most efficient intervention to pregnant women diagnosed as cervical insufficiency since it was fist applied by Shirodkar and McDonald 50 years ago [7]. As the American College of Obstetrics and Gynecology has defined, cervical cerclage is performed elective-prophylactically based on history (≥2 times prior second-trimester pregnancy losses with no or minimal mild symptoms), or therapeutically indicated on ultrasound (with a prior spontaneous preterm birth and cervical length ≤25mm by transvaginal ultrasound scans) or in emergency, based on physical examination (cervical dilation on physical examination) [9]. Cervical cerclage is placed to give mechanical support to the cervix and keep the cervix closed, thereby to reduce the risk of preterm birth. It is said that compared with no cerclage, pregnant women with cerclage were less likely to give birth before 37, 34 (average RR 0.77, 95% CI 0.66 to 0.89; 9 studies, 2415 women) and 28 completed gestational weeks [10].
However, cervical cerclage is associated with complications such as sepsis, premature rupture of membranes, premature labor, cervical bleeding which may probably result in miscarriage [11]. Even with prophylactic cerclage, only 73.9% pregnant women delivered beyond 36 weeks, while as for emergency cerclage, the rate was only 23.5% [12]. How to choose suitable candidates to cerclage is still a bewilderment in clinical practice. Therefore, we aimed at identifying possible factors associated with EPT birth after cervical cerclage, and to develop a nomogram to predict the exact likelihood of prolonging gestations after 28 weeks for prospective decision making.
2. Material and methods
We developed and validated the predicting model in a retrospective multicenter study in Zhejiang Province in China from January 2016 to September 2020. This retrospective cohort study examined records of cervical cerclage from Women's Hospital, Zhejiang university, School of medicine, Huzhou Maternal and Child Health Care Hospital and Fuyang People's Hospital. The first one was a provincial tertiary hospital in Zhejiang Province, while the latter two were county hospitals. The study was approved by the ethical committee of Zhejiang university (IRB-20200160-R) and written informed consent was obtained from all patients.
We included all women with successful transvaginal cervical cerclage done at 12–26 weeks of gestation in singleton pregnancies, both prophylactical, therapeutical and emergency cerclage. Women who had transabdominal cerclage or preconceptional cerclage were excluded. And women who failed in cerclage, such as preterm premature rupture of membranes (PPROM) during cerclage placement were exclude. Women who had to terminate pregnancy due to fetal malformation, still birth or other iatrogenic indications that jeopardize the health of the mother or fetus such as severe preeclampsia, fetal growth restriction and hemorrhea of placenta previa were excluded. Women with missing data in their medical records were also excluded.
All the procedures were conducted in accordance with the American College of Obstetricians and Gynecologists (ACOG) practice bulletin [9]. All women included underwent the same protocol, as follows: after admission, preoperative examinations including white blood cell counts (WBC), C-reactive protein (CRP), microbiological assessment of cervical culture and vaginal content (eg. bacteria, fungi, mycoplasma, chlamydia) and evaluation of cervical dilatation, were performed. The degree of cervical dilatation and remaining cervical length were estimated by ultrasound examination, and would be reconfirmed by speculum examination. Then a transvaginal cerclage by the McDonald procedure with one single stitch (four trends of No. 10 silk thread) was performed in the operating room under epidural anesthesia. In cases of bulging membranes, a Cook catheter [13] or a uterine balloon tamponade described by our team [14] was used to reposition the prolapsed membranes. Standard treatment such as bed rest, antibiotic, steroid usage and tocolysis were given to patients during hospitalization. Ritodrine hydrochloride, indomethacin or nifedipine were given as prophylactic tocolysis when needed during perioperative period. After discharge, women were asked to decrease their activity and received every two weeks' monitors of cervical length. The stitch would be generally removed around the 37 weeks’ gestation.
Medical records were reviewed and the following data was evaluated: maternal demographic characteristics such as age, pregestational body mass index (BMI), use of in-vitro fertilization (IVF), gravidity, parity, history of prior second-trimester loss, history of hysteroscopy, cervical surgery, or uterine malformation, history of polycystic ovary syndrome (PCOS) and intervals from prior pregnancy. Clinical characteristics were cervical dilation and length, gestational age at cerclage, results of microbiological tests and blood tests, pregnancy complications such as gestational diabetes mellitus (GDM), PPROM, placental abruption and hypertensive disorders in pregnancy. GDM was diagnosed based on the International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria: GDM is diagnosed if any of the 75g OGTT plasma glucose values during 24–28 gestational weeks ≥5.1 mmol/L at fasting, 10.0 mmol/L at 1h, and 8.5mmol/at 2h. The pregnancy outcome concerned in the study was whether delivered before 28 weeks of gestation.
2.1. Statistical analyses
Continuous variables were presented as median (range/interquartile range). Categorical variables were presented as numbers and percentages. Fisher's exact test or chi-squared test for categorical variables and Wilcoxon-Mann-Whitney test for continuous variables were applied when appropriate.
All patients enrolled in Women's Hospital, Zhejiang university, School of medicine were included in development cohort, which randomly divided by 7:3 into training cohort for nomogram development, and internal validation cohort to confirm the model's performance. We also externally validated the final model in patients enrolled in Huzhou Maternal and Child Health Care Hospital and Fuyang People's Hospital. The significance of each variable in the training group was assessed by univariate logistic regression for investigating the independent risk factors of EPT birth, presented as odds ratio (OR) with the 95% of confidence interval (CI). Multicollinearity among the variables was screened according to the variance inflation factor (VIF) (VIF>5 was considered strong collinearity). A backward stepwise elimination approach was applied to select independent variables for the multivariable logistic regression. A nomogram was formulated based on the results of multivariate logistic regression analysis and by using statistical software (rms in R; http://www. r-ptoject.org). For model performance, we assessed the discrimination (the ability to differentiate between the prediction and outcome) and calibration (the discrepancy between predicted and observed outcomes). To quantify the model discrimination, we calculated the Harrell's concordance index (C index) as the area under the receiver operating characteristic curve (AUC) with 95% CI. The calibration was evaluated by calibration plots, accompanied by the Hosmer–Lemeshow goodness-of-fit test. The calibration slope was also calculated, with 1 being the ideal value.
The model internal and external validation was accessed by bootstrapped 500 resampling to quantify overoptimism. Receiver operating characteristic curve (ROC) was used to calculate the optimal cutoff value by maximizing the Youden index (i.e., sensitivity + specificity- 1), and then the sensitivity, specificity, predictive values and likelihood ratios were estimated.
Decision curve analysis (DCA) was conducted to determine the clinical usefulness of the nomogram by quantifying the net benefits at different threshold probabilities in the validation dataset [15].
All tests were two sided with a significance level set as p < 0.05. The analyses were performed using Statistical Package for Social Sciences for Windows, version 22.0 (IBM SPSS, Chicago, 1L, USA) and R statistical software, version 4.0.3.
3. Results
3.1. Clinical characteristics in the study
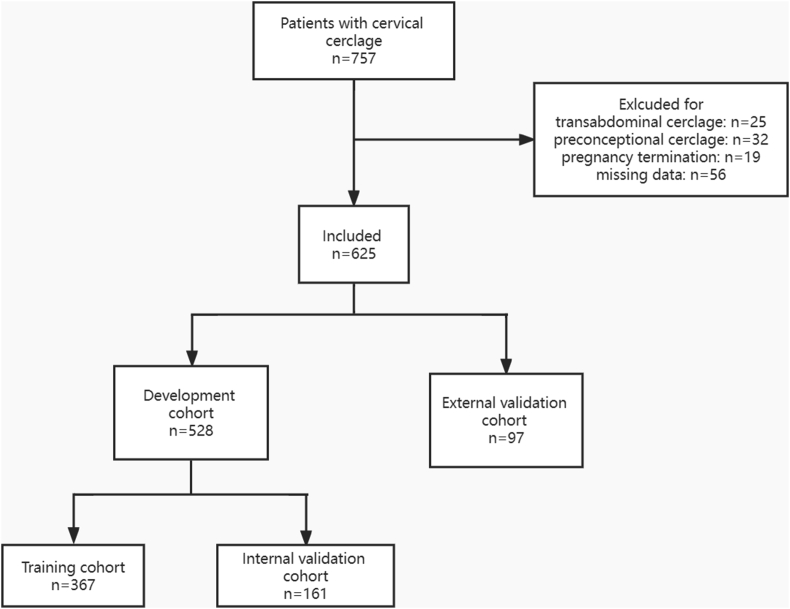
During the study period, there were 847 patients successfully underwent cervical cerclage in the three hospitals in Zhejiang Province, China. Among them, 32 patients had preconceptional cerclage, 25 patients had transabdominal cerclage, and 19 patients had to terminate pregnancy due to fetal malformation. 56 patients with missing data were also excluded from analysis. Overall, 625 patients who met the inclusion criteria were considered for further analyses. Among them, 528 patients form Women's Hospital, Zhejiang university, School of medicine, formed the development cohort, and 97 patients from Huzhou Maternal and Child Health Care Hospital and Fuyang People's Hospital, formed the external validation cohort. The flowchart of the study is shown in Figure 1. Several characteristics, such as gravidity, history of prior second-trimester loss, use of IVF, intervals from prior pregnancy, gestational week at cerclage and delivery, cervical length and the incidence of PPROM showed significant difference between development and external validation cohort. Details are shown in Table S1.
Figure 1.
Flowchart of the study.
In development cohort, median age (range) at surgery was 31 (19, 48) years old. 397 patients received prophylactical cerclage and 131patients received non-selective cerclage. Median gestational week at cerclage was 16 (11, 25) weeks and at delivery was 37 (14, 42) weeks. In total, 102 (19.32%) patients gave birth before 28 weeks of gestation. Of all the participants, 367 and 161 patients were divided into the training and internal validation cohorts, respectively. The clinical characteristics and pregnancy outcomes were almost similar between the training and validation cohorts. Details between training and internal validation cohort are shown in Table S2.
3.2. Development of an EPT-predicting nomogram
All variables were initially included in the univariate regression analysis as shown in Table 1. Eight items showed statistically significant difference: pregestational BMI, use of IVF, History of prior second-trimester loss, cervical length and dilation, neutrophil-lymphocyte ratio (NLR), CRP and PLR before cerclage. We also involved gravidity and intervals from prior pregnancy (both p-value <0.1) besides eight items above in multivariable logistic regression. VIF of all variables involved were less than 5.
Table 1.
Univariate Logistic Regression Analysis of risk factors associated with EPT in women undergoing cerclage in the training cohort.
| Variable | Deliver <28w (n = 63) | Deliver≥28w (n = 304) | OR (95%CI) | P-value | VIF | |
|---|---|---|---|---|---|---|
| Age, y | 32 (22, 41) | 31 (19, 48) | 1.02 (0.97, 1.08) | 0.414 | 2.93 | |
| ≥35 y, n (%) | 18 (28.57%) | 74 (24.34%) | 1.24 (0.68, 2.28) | 0.481 | 2.75 | |
| <35 y, n (%) | 45 (71.43%) | 230 (75.66%) | Reference | |||
| Gravidity | 3 (0, 9) | 3 (1, 9) | 0.93 (0.78, 1.11) | 0.934 | 5.31 | |
| Parity | 0 (0, 2) | 0 (0, 3) | 0.60 (0.35, 1.03) | 0.062 | 2.43 | |
| Pregestational BMI, kg/m2 | 23.43 (12.02, 39.35) | 22.27 (14.17, 34.05) | 1.07 (1.00. 1.15) | 0.049 | 1.19 | |
| IVF | Yes, n (%) | 14 (22.22%) | 37 (12.17%) | 2.06 (1.04, 4.10) | 0.039 | 1.22 |
| No, n (%) | 49 (77.78%) | 267 (87.83%) | Reference | |||
| History of prior early abortion, n | 0 (0, 7) | 0 (0, 5) | 1.10 (0.88, 1.37) | 0.398 | 3.98 | |
| History of prior second-trimester loss, n | 1 (0, 4) | 1 (0, 3) | 1.72 (1.26, 2.35) | 0.001 | 1.89 | |
| History of preterm birth, n | 0 (0, 1) | 0 (0, 2) | 0.52 (0.21. 1.28) | 0.153 | 1.40 | |
| History of hysteroscopic surgery, n | 0 (0, 5) | 0 (0, 5) | 1.23 (0.91, 1.67) | 0.175 | 1.22 | |
| History of corn biopsy | Yes, n (%) | 2 (3.17%) | 14 (4.61%) | 0.68 (0.15, 3.07) | 0.615 | 1.17 |
| No, n (%) | 61 (96.83%) | 290 (95.39%) | Reference | |||
| PCOS | Yes, n (%) | 1 (1.59%) | 10 (3.29%) | 0.47 (0.60–3.77) | 0.481 | 1.07 |
| No, n (%) | 62 (98.41%) | 294 (96.71%) | Reference | |||
| History of surgery for uterine malformation/fibroids | Yes, n (%) | 1 (1.59%) | 2 (0.66%) | 2.44 (0.22–27.28) | 0.470 | 1.10 |
| No, n (%) | 62 (98.41%) | 302 (99.34%) | Reference | |||
| History of failure of cervical cerclage | Yes, n (%) | 5 (7.94%) | 10 (3.29%) | 2.53 (0.84, 7.69) | 0.101 | 1.05 |
| No, n (%) | 58 (92.06%) | 294 (96.71%) | Reference | |||
| Intervals from prior pregnancy | No prior pregnancy | 10 (15.87%) | 33 (10.86%) | Reference | 0.050 | 1.29 |
| 0–6 month | 6 (9.52%) | 22 (7.24%) | 0.90 (0.29, 2.83) | |||
| 6–12 month | 18 (28.57%) | 52 (17.11%) | 1.14 (0.47, 2.78) | |||
| >12 month | 29 (46.03%) | 197 (64.80%) | 0.49 (0.22, 1.09) | |||
| Gestational weeks at cerclage, wk | 17 (12, 24) | 16 (11, 25) | 1.02 (0.95, 1.09) | 0.542 | 2.12 | |
| Cervical length, cm | 1.80 (0, 4.30) | 2.50 (0, 5.70) | 0.68 (0.54, 0.85) | 0.001 | 2.46 | |
| Cervical dilation, cm | 0 (0, 8) | 0 (0, 6) | 1.95 (1.54, 2.43) | <0.001 | 1.58 | |
| WBC before cerclage, ∗10ˆ9/L# | 9.90 (6.20, 15.50) | 9.69 (4.7, 17.80) | 1.01 (0.89, 1.15) | 0.862 | 1.80 | |
| CRP before cerclage, mg/L# | 4.90 (0.60, 39.10) | 3.40 (0.10, 33.70) | 1.11 (1.05, 1.16) | <0.001 | 1.24 | |
| NLR before cerclage | 5.11 (2.55, 9.30) | 4.56 (1.00, 17.92) | 1.17 (1.02, 1.34) | 0.023 | 2.54 | |
| PLR before cerclage | 155.63 (84, 348.18) | 138.35 (25.09, 464) | 1.01 (1.00, 1.01) | 0.001 | 2.19 | |
| Microbiological infection | Yes, n (%) | 19 (30.16%) | 89 (29.28%) | 1.04 (0.58, 1.89) | 0.889 | 1.08 |
| No, n (%) | 44 (69.84%) | 215 (70.72%) | Reference | |||
Abbreviations: EPT, extremely preterm birth; BMI, body mass index; IVF, in-vitro fertilization; WBC, white blood cells; CRP, C-reactive protein; PCOS, polycystic ovary syndrome; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; OR, odds ratio; CI, confidence interval; VIF, variance inflation factor.
∗P value < 0.05.
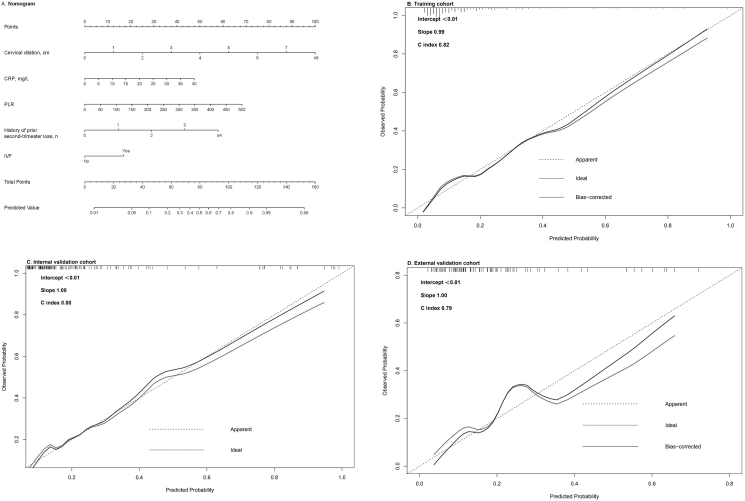
On multivariate analysis, five variables were identified as independently predictors of EPT birth after cerclage: use of IVF, history of prior second-trimester loss, cervical dilation at cerclage, CRP and PLR before cerclage. Details are shown in Table 2. These five variables were used to form an EPT birth predicting nomogram (Figure 2A).
Table 2.
Multivariate Logistic regression analysis of EPT in women undergoing cerclage in the training cohort.
| Variable | OR (95%CI) | P-value | VIF |
|---|---|---|---|
| History of prior second-trimester loss | 2.56 (1.71–3.82) | <0.001∗∗ | 1.04 |
| Cervical dilation, cm | 2.26 (1.74–2.92) | <0.001∗∗ | 1.07 |
| IVF | 2.81 (1.20–6.59) | 0.018∗ | 1.03 |
| CRP before cerclage | 1.07 (1.01, 1.15) | 0.030∗ | 1.10 |
| PLR before cerclage | 1.01 (1.00, 1.02) | 0.001∗∗ | 1.03 |
Abbreviations: EPT, extremely preterm birth; IVF, in-vitro fertilization; CRP, C-reactive protein; PLR, platelet-lymphocyte ratio; OR, odds ratio; CI, confidence interval; VIF, variance inflation factor.
∗P < 0.05, ∗∗P < 0.01.
Figure 2.
Nomogram for predicting the risk of preterm birth before 28 gestational weeks and its predictive performance. A, Nomogram to estimate the risk of EPT birth in pregnant women underwent cervical cerclage. Instructions: to use the nomogram, find the position of each variable on the corresponding axis, draw a line to the points axis for the number of points of each variable, add up the points of each variable to a total points and determine the corresponding probability of EPT-birth. B, Validation of the predictive performance of the nomogram in training cohort. C, Validation of the predictive performance of the nomogram in the internal validation cohort. D, Validation of the predictive performance of the nomogram in the external validation cohort. EPT, extremely preterm; CRP, C-reactive protein; PLR, platelet-lymphocyte ratio; IVF, in-vitro fertilization.
3.3. Validation and performance of the nomogram
The nomogram demonstrated good accuracy in predicting EPT birth after cervical cerclage, with a C index of 0.82 (95%CI 0.77–0.88). The Hosmer-Lemeshow test yielded a nonsignificant statistic (P = 0.67), which suggested that there was no departure from perfect fit. In addition, the calibration plots showed good agreement on the predicted and observed risks, with a calibration slop of 0.99 (Figure 2B).
In the internal validation cohort, the nomogram showed good discrimination with a C-index of 0.80 (95%CI 0.72–0.88). The Hosmer-Lemeshow test yielded a nonsignificant statistic (P = 0.16) and the calibration slop was 1.00 (Figure 2C).
In the external validation cohort, good calibration was observed with a C-index of 0.79 (95%CI 0.68–0.90). The Hosmer-Lemeshow test yielded a nonsignificant statistic (P = 0.10). The calibration curve showed good agreement with a calibration slop of 1.00 (Figure 2D).
The sensitivity, specificity, positive predictive value and negative predictive value of the model were 74.60%, 72.00%, 35.58% and 93.19% in the training cohort, 69.20%. 71.10%. 43.35% and 87.84% in the internal validation cohort, and 66.70%, 74.20%, 41.67% and 88.97% in the external validation cohort, respectively (Table 3).
Table 3.
Accuracy of the Nomogram for estimating the risk of EPT birth after cerclage.
| Variable | Training cohort | Internal validation cohort | External validation cohort |
|---|---|---|---|
| C index | 0.82 (95%CI 0.77–0.88) | 0.80 (95%CI 0.72–0.88) | 0.79 (95%CI 0.68–0.90) |
| Sensitivity, % | 74.60 | 69.20 | 66.70 |
| Specificity, % | 72.00 | 71.10 | 74.20 |
| Positive predictive value, % | 35.58 | 43.35 | 41.67 |
| Negative predictive value, % | 93.19 | 87.84 | 88.97 |
| Positive likelihood ratio | 2.67 | 2.39 | 2.59 |
| Negative likelihood ratio | 0.35 | 0.43 | 0.45 |
EPT: extremely preterm.
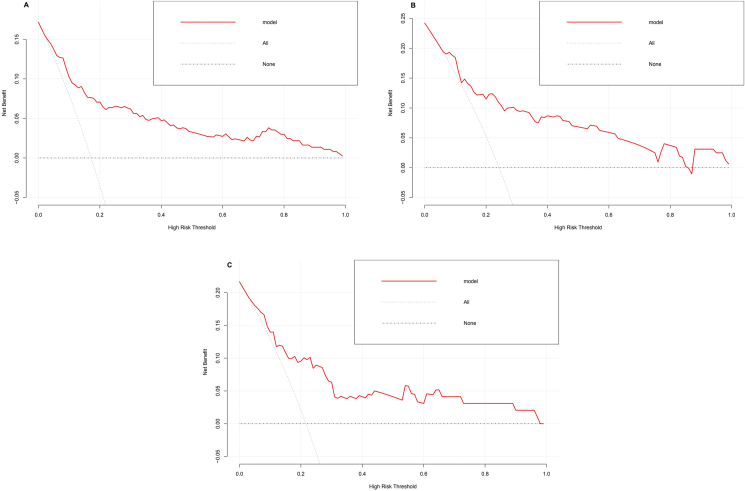
In addition, the DCA showed good positive net benefit in the nomogram which indicated a favorable clinical effect in singleton pregnancy patients with cerclage [Figure 3(A-C)].
Figure 3.
Decision curve analysis of the nomogram in the training cohort(A), internal validation cohort(B) and external validation cohort(C). The red line represents the prediction model. The gray dashed line assumes all patients have the outcome (deliver before 28). The horizontal line assumes no patients have the outcome. The preferred model is the one with the highest net benefit at any given threshold.
3.4. Comment
3.4.1. Principal findings
In the present study, we developed a well-performed and easy-used pre-operative nomogram for EPT birth after any type of cerclage from a huge multicenter study. We identified 5 risk factors as predictors associated with delivery at < 28 weeks which are easy to acquire: history of prior second-trimester loss, use of IVF, cervical dilation at cerclage, CRP and PLR before cerclage.
3.4.2. Results in the context of what is known
Among them, obstetric history, cervical dilation and infection index have been repeatedly conformed as risk factors of cerclage failure in many studies. Mian Pan et al. identified that cervical dilation and PLR as independent predictors of EP birth after cerclage in singleton pregnancies with a history of preterm birth and a sonographic short cervix [16]. Florent Fuchs et al. developed a scoring system for emergency cerclage based on obstetric history, cervical dilation, membranes bulging into the vagina and infection (WBC ≥13 600/mm3 or CRP >15 mg/L) to predict preterm delivery before 32 weeks [8]. Ruizhe Chen et al. found that history of prior preterm birth and second-trimester loss, CRP>5 mg/L and cervical dilation ≥3cm were independent risk factors of cerclage [17].
The variable “history of prior second-trimester loss” is usually associated with the diagnosis of cervical insufficiency, for which a prophylactic cerclage earlier in pregnancy might have been a better choice. As we involved all types of cerclage in our study, the variable “cervical dilation” could also indicate the type of cerclage patients received, when dilation was larger than 1cm, the patient might probably receive an emergency cerclage.
Infection was an important prognostic factor after cerclage. We compared WBC, CRP, NLR and PLR in the study. Recently, many studies have suggested NLR and PLR as subclinical inflammatory and immunologic markers in many obstetric syndromes [18, 19, 20]. In our study, we found both CRP and PLR as pre-operative predictors, which was in accordance with Mian Pan et al.’s results [16].
In the present study, we demonstrated that the use of IVF is an independent risk factor of preterm birth after cerclage. IVF has been reported to be associated with increased adverse pregnancy outcomes, such as cervical insufficiency [21, 22]. Wu Yaoqiu, et al. have firstly developed a nomogram to predict the individual cervical incompetence occurrence rate in IVF people [23]. This was probably due to more intrauterine surgical intervention during IVF procedure. A recent study conducted in infertile patients suggested that operative hysteroscopy prior to assisted reproductive technology cycle is significantly associated with cervical insufficiency between 13 and 27 weeks of gestation [24].
3.4.3. Clinical implications
For clinical use, one model suitable for any conditions of the same disease to any patients encountered is always easier to be accepted and applied by clinicians. Therefore, obstetricians could have priorities upon different patients and give different therapies according to different risk stratification from the model. For example, to patients with elective cerclage, obstetric history laid more emphasis on prognosis and to patients with emergency cerclage, cervical dilation has greater impact on prognosis. we evaluated valuables that were clinically relevant and routinely available to obstetricians, so the model could quickly provide certain information about prognosis before cerclage. Therefore, obstetricians could give different therapies according to different risk stratification from the model, for patients with higher risk of EPT birth, obstetricians could pay more attention.
In the present study, our nomogram had really high sensitivity, specificity and negative predictive value. Vanessa Ha et al. conducted a survey on pregnant women about their concerns about preterm birth prevention. The result showed that 50.2% women would not follow their healthcare provider's recommendation for cerclage for the uncertainty about the procedure and pregnancy outcomes after cerclage [25]. Therefore, the nomogram might serve as a useful tool for obstetricians to provide accurate and detailed information about the prognosis after cerclage, especially for those patients estimated as low risk of EPT birth as the negative predictive value of the model nearly 90%.
3.5. Strengths and limitations
The study has several strengths. First, our study includes a very large sample size. Second, to the best of our knowledge, this is the first nomogram for prediction of EPT birth under any indications of cervical cerclage for singleton pregnancies. Several studies have introduced scoring systems to identify risk factors associated with adverse pregnant outcomes of certain type of cerclage, mostly non-elective cerclage [8, 16, 26]. And most of them contained a really small sample size. In 2003, Anthony O Odibo et al. [27] first developed a model for identifying women receiving cerclage at risk for spontaneous preterm birth before 32 weeks. This study did not include laboratory data and it only described emergency cerclage without other characteristics. Terkildsen et al. [26] conducted the study in 116 pregnant women underwent emergency cerclage, and they only focused on variables of cervical examination, such as cervical length and dilation, without laboratory data. Florent Fuchs et al. [8] added WBC and CRP to reveal infection, but only contained 85 singleton pregnancies, while Mian Pan et al. [16] studied 96 singleton pregnancies with ultrasound-indicated cerclage.
Third, the parameters we involved in the model can be easily obtained by obstetricians as soon as patients were admitted in. Lastly, we have done external validation to evaluate the model, and demonstrated good accuracy in predicting EPT birth. Several variables involved in the nomogram, such as history of prior second-trimester loss, use of IVF, and other clinical characteristics like the incidence of PPROM showed significant difference between development and external validation cohort. Therefore, it means our model could be implemented in any obstetric unit.
The study had some limitations. First, this is a retrospective study. Data was based on electronic medical records and some information might be inexact and missing, for example, membranes bulging into vagina was not available in medical records. Second, our model did not consider the impact of medical therapy on pregnancy outcomes after cerclage. However, we also included patients from other hospitals as external validation cohort which might have different therapies, and still demonstrated good accuracy of our model.
4. Conclusions
We used 5 historical and clinical variables to develop and validate a user-friendly nomogram for prediction of preterm birth at < 28 weeks after cerclage. The nomogram could provide comprehensive and accurate prognostic information in any obstetric unit.
Declarations
Author contribution statement
Min Lv: Performed the experiments; Wrote the paper.
Cheng Chen: Analyzed and interpreted the data.
Liping Qiu: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Neng Jing, Mingming Wang and Baihui Zhao: Contributed reagents, materials, analysis tools or data.
Danqing Chen and Qiong Luo: Conceived and designed the experiments.
Funding statement
Dr. Qiong Luo was supported by National Key Research and Development Program of China [No. 2018YFC1004603].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the Staff at women's hospital, Zhejiang University for technical assistances and facility supports.
Contributor Information
Danqing Chen, Email: chendq@zju.edu.cn.
Qiong Luo, Email: luoq@zju.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Liu L., Oza S., Hogan D., et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet (London, England) 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyson J.E., Parikh N.A., Langer J., Green C., Higgins R.D. Intensive care for extreme prematurity--moving beyond gestational age. N. Engl. J. Med. 2008;358:1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Field D.J., Dorling J.S., Manktelow B.N., Draper E.S. Survival of extremely premature babies in a geographically defined population: prospective cohort study of 1994-9 compared with 2000-5. BMJ. 2008;336:1221–1223. doi: 10.1136/bmj.39555.670718.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel R.M., Kandefer S., Walsh M.C., et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N. Engl. J. Med. 2015;372:331–340. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norman M., Hallberg B., Abrahamsson T., et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;321:1188–1199. doi: 10.1001/jama.2019.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helenius K., Sjörs G., Shah P.S., et al. Survival in very preterm infants: an International comparison of 10 national neonatal networks. Pediatrics. 2017:140. doi: 10.1542/peds.2017-1264. [DOI] [PubMed] [Google Scholar]
- 7.Brown R., Gagnon R., Delisle M.F. No. 373-Cervical insufficiency and cervical cerclage. J. Obstet. Gynaecol. Can. 2019;41:233–247. doi: 10.1016/j.jogc.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs F., Senat M.V., Fernandez H., Gervaise A., Frydman R., Bouyer J. Predictive score for early preterm birth in decisions about emergency cervical cerclage in singleton pregnancies. Acta Obstet. Gynecol. Scand. 2012;91:744–749. doi: 10.1111/j.1600-0412.2012.01386.x. [DOI] [PubMed] [Google Scholar]
- 9.ACOG Practice Bulletin No.142 Cerclage for the management of cervical insufficiency. Obstet. Gynecol. 2014;123:372–379. doi: 10.1097/01.AOG.0000443276.68274.cc. [DOI] [PubMed] [Google Scholar]
- 10.Alfirevic Z., Stampalija T., Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst. Rev. 2017;6:Cd008991. doi: 10.1002/14651858.CD008991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harpham M.E., Algert C.S., Roberts C.L., Ford J.B., Shand A.W. Cervical cerclage placed before 14 weeks gestation in women with one previous midtrimester loss: a population-based cohort study. Aust. N. Z. J. Obstet. Gynaecol. 2017;57:593–598. doi: 10.1111/ajo.12635. [DOI] [PubMed] [Google Scholar]
- 12.Nelson L., Dola T., Tran T., Carter M., Luu H., Dola C. Pregnancy outcomes following placement of elective, urgent and emergent cerclage. J. Matern. Fetal Neonatal Med. 2009;22:269–273. doi: 10.1080/14767050802613199. [DOI] [PubMed] [Google Scholar]
- 13.Kanai M., Ashida T., Ohira S., Osada R., Konishi I. A new technique using a rubber balloon in emergency second trimester cerclage for fetal membrane prolapse. J. Obstet. Gynaecol. Res. 2008;34:935–940. doi: 10.1111/j.1447-0756.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- 14.Lv M., Zhao B., Chen Y., et al. Balloon tamponade for successful emergency cervical cerclage. J. Obstet. Gynaecol. Res. 2020;46:418–424. doi: 10.1111/jog.14186. [DOI] [PubMed] [Google Scholar]
- 15.Vickers A.J., Cronin A.M., Elkin E.B., Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med. Inf. Decis. Making. 2008;8:53. doi: 10.1186/1472-6947-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan M., Fang J.N., Wang X.X., Zhang J., Lin Z. Predictors of cerclage failure in singleton pregnancies with a history of preterm birth and a sonographic short cervix. Int. J. Gynaecol. Obstet. 2022;156(2):316–321. doi: 10.1002/ijgo.13640. [DOI] [PubMed] [Google Scholar]
- 17.Chen R., Huang X., Li B. Pregnancy outcomes and factors affecting the clinical effects of cervical cerclage when used for different indications: a retrospective study of 326 cases. Taiwan. J. Obstet. Gynecol. 2020;59:28–33. doi: 10.1016/j.tjog.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Sabre A., Sisti G., Gaither K. Neutrophil-to-Lymphocyte ratio and platelet-to-lymphocyte ratio in twins compared with singletons. South. Med. J. 2021;114:28–31. doi: 10.14423/SMJ.0000000000001190. [DOI] [PubMed] [Google Scholar]
- 19.Biyik I., Albayrak M., Keskin F. Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in missed abortion. Rev. Bras. Ginecol. Obstet. 2020;42:235–239. doi: 10.1055/s-0040-1709693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yücel B., Ustun B. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume, red cell distribution width and plateletcrit in preeclampsia. Pregnancy hypertension. 2017;7:29–32. doi: 10.1016/j.preghy.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Schieve L.A., Cohen B., Nannini A., et al. A population-based study of maternal and perinatal outcomes associated with assisted reproductive technology in Massachusetts. Matern. Child Health J. 2007;11:517–525. doi: 10.1007/s10995-007-0202-7. [DOI] [PubMed] [Google Scholar]
- 22.Declercq E., Luke B., Belanoff C., et al. Perinatal outcomes associated with assisted reproductive technology: the Massachusetts outcomes study of assisted reproductive technologies (MOSART) Fertil. Steril. 2015;103:888–895. doi: 10.1016/j.fertnstert.2014.12.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y., Liang X., Cai M., Gao L., Lan J., Yang X. Development and validation of a model for individualized prediction of cervical insufficiency risks in patients undergoing IVF/ICSI treatment. Reprod. Biol. Endocrinol. : RBE (Rev. Bras. Entomol.) 2021;19:6. doi: 10.1186/s12958-020-00693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gökçe A., Şükür Y.E., Özmen B., et al. The association between operative hysteroscopy prior to assisted reproductive technology and cervical insufficiency in second trimester. Arch. Gynecol. Obstet. 2021;303:1347–1352. doi: 10.1007/s00404-020-05863-1. [DOI] [PubMed] [Google Scholar]
- 25.Ha V., McDonald S.D. Pregnant women's preferences for and concerns about preterm birth prevention: a cross-sectional survey. BMC Pregnancy Childbirth. 2017;17:49. doi: 10.1186/s12884-017-1221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terkildsen M.F., Parilla B.V., Kumar P., Grobman W.A. Factors associated with success of emergent second-trimester cerclage. Obstet. Gynecol. 2003;101:565–569. doi: 10.1016/s0029-7844(02)03117-4. [DOI] [PubMed] [Google Scholar]
- 27.Odibo A.O., Farrell C., Macones G.A., Berghella V. Development of a scoring system for predicting the risk of preterm birth in women receiving cervical cerclage. J. Perinatol. 2003;23:664–667. doi: 10.1038/sj.jp.7211004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.