Abstract
Studies by His from 1868 to 1904 delineated the critical role of the dorsal roof plate in the development of the hindbrain choroid plexus, and of the rhombic lips in the development of hindbrain auditory centers. Modern molecular studies have confirmed these observations and placed them in a mechanistic context. Expression of the transcription factor Lmx1a/b is crucial to the development of the hindbrain choroid plexus, and also regulates the expression of Atoh1, a transcription factor that is essential for the formation of the cochlear hair cells and auditory nuclei. By contrast, development of the vestibular hair cells, vestibular ganglion and vestibular nuclei does not depend on Lmx1a/b. These findings demonstrate a common dependence on a specific gene for the hindbrain choroid plexus and the primary auditory projection from hair cells to sensory neurons to hindbrain nuclei. Thus, His’ conclusions regarding the origins of specific hindbrain structures are borne out by molecular genetic experiments conducted more than a hundred years later.
Keywords: Brainstem, Cochlear nuclei, Ear, Cochlear hair cells
Introduction
Wilhelm His Sr (1831–1904) invented the microtome (His, 1870), described the histology of brain and body development (His, 1880), and introduced a new anatomical nomenclature (His, 1895). He described and defined the ‘Zwischenstrang’ [neural crest (His, 1868)], the rhombencephalon, and some of the principal features of the brain anlage [floor plate, basal plate, alar plate, roof plate, sulcus limitans, isthmus (His, 1890, His, 1904)]. Most notably, His pointed out early on that the geniculate ganglion of cranial nerve VII does not derive from the ‘Zwischenstrang’, thus indicating a different developmental origin for what he referred to as ‘visceral ganglia‘. It was only later that the true origins of such ganglia as well as of the ear anlage were identified as placodes (O’Neill et al., 2012, Schlosser, 2017; Von Kupffer, 1891). His defined specific longitudinal domains within the rhombencephalon based on external and internal features, and identified the rhombic lip as the source of migratory cell populations giving rise to major dorsal and ventral nuclei, including the pontine nuclei (‘Brueckenkerne‘), the superior and inferior olives, and the cochlear nuclei (de No, 1981, Held, 1893, Malmierca, 2015, Muniak et al., 2016).
Our review will concentrate on the insights provided by His into the development of the human hindbrain with an emphasis on its longitudinal regionalization and on the role of the dorsal plate and rhombic lip (‘Rautenlippe’) in the development of the rhombencephalic choroid plexus and the auditory projection. We extend an excellent previous review (Ray and Dymecki, 2009) by adding more recent molecular insights into the role of specific critical genes (Chizhikov et al., 2021, Elliott et al., 2021, Glover et al., 2018).
His’ observations on the hindbrain were based on human embryos
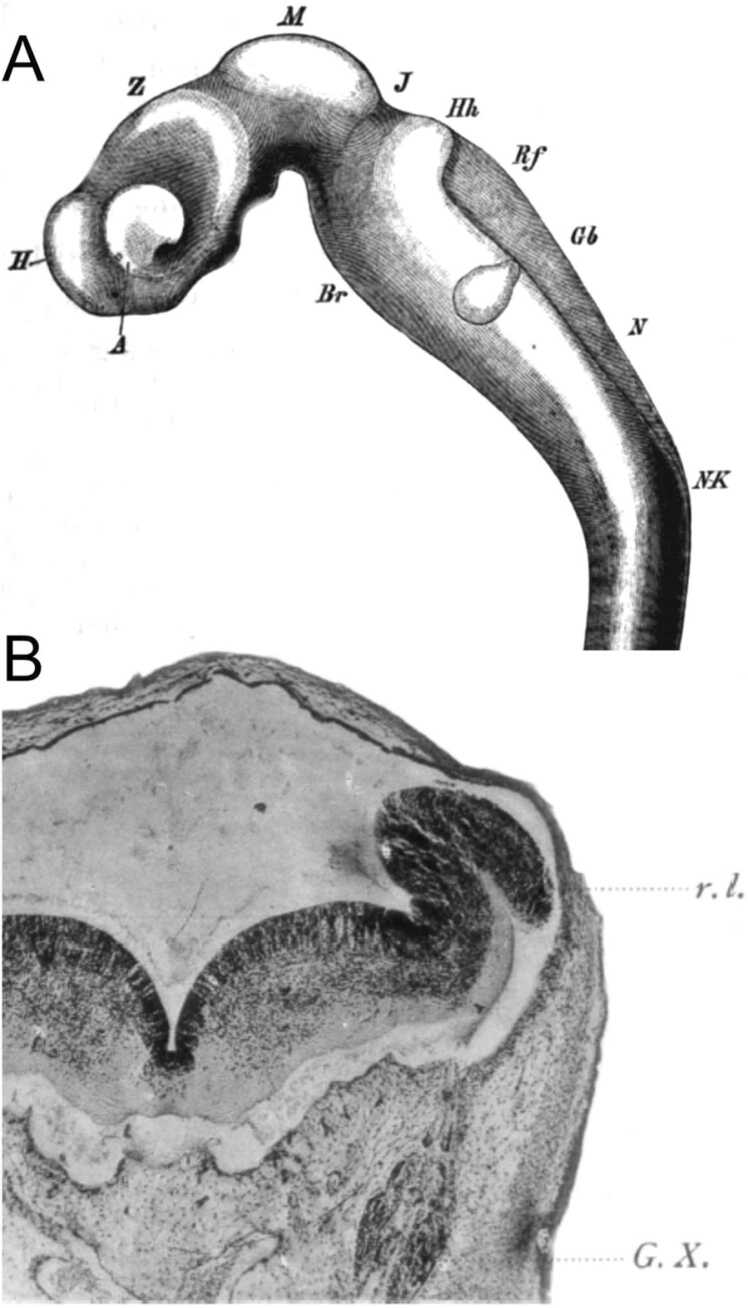
His was a member of the ‘Entwicklungsmechanik’ school whose aim was to explain how the shape and form of the brain arise during development. His noted that the brain undergoes significant shape changes that open the 4th ventricle. His basic working hypothesis was that the neural tube behaves like a rubber tube cut dorsally. He described how the roof of the 4th ventricle forms the choroid plexus and how its shape depends on the narrowing of the ventricular floor at the calamus scriptorius (Fig. 1). To His, the lateral recesses of the 4th ventricle were formed by the force of the pons pushing against the medulla oblongata: the form of the ‘Rautenhirn’ then emerging as a tube fully closed at the isthmus and obex but with an increasingly wider gap in between, stretching the thin, overlying roof plate.
Fig. 1.
A whole mount of the brain (A) and coronal section through the rhombencephalon (B) of a human embryo. Abbreviations: A, Augenblase, eye; Br, Brueckenkruemmung, pons; Gb, Gehorblase, ear; H, Hemisphaerenhirn, telencephalon; Hh, Hinterhirn, pons; J, Isthmus; M, Mittelhirn, midbrain; N, Nachhirn, medulla oblongata; NK, Nachhirnkruemmung, bridge of spinal cord; Rf, Rautenfeld, rhombomeric choroid plexus; Z, Zwischenhirn, diencephalon; rl = rhombic lip; G.X. = ganglion of the vagus nerve. Taken from His (1897).
His noted that in transverse sections through the rhombencephalon of the human embryo the extremes of the alar plates are demarcated by a longitudinal pial sulcus, forming what he called the ‘Rautenlippen‘, or rhombic lips (Fig. 1B). Much of his subsequent work focused on neuron populations that he believed to originate from the rhombic lips. He realized, based purely on histological evidence, that several hindbrain neuron populations were generated through tangential migrations of neuroblasts, delaminating from the rhombic lips. His was the first to correctly identify these migratory cells as they traverse the rhombencephalon.
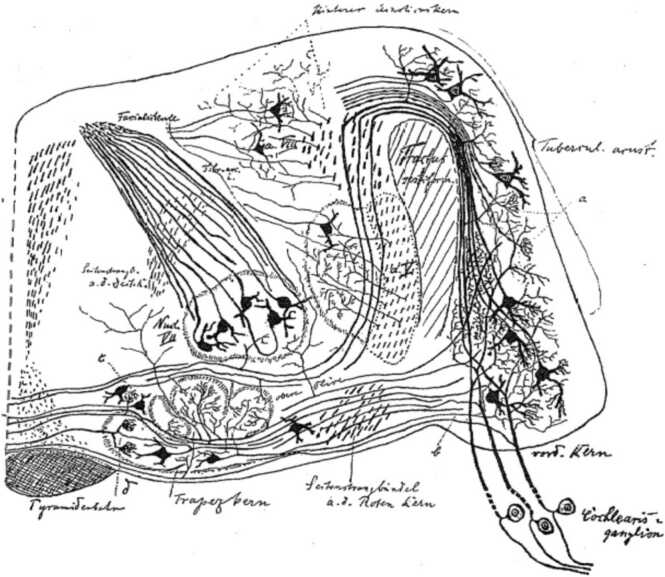
Following his early insights into the distinct origins of cranial and spinal sensory ganglia, His provided a detailed reconstruction of a ventral view of the human embryonic brainstem that highlighted all nerve fiber roots and many of their associated central nuclei. He also drew some of the central nuclei he postulated to originate from the rhombic lip, such as the pons and superior olivary nuclei (‘Brueckenkern’, ‘obere Olive’; Fig. 2 (de No, 1981; Held, 1893)). The development of the auditory centers was of particular interest to him, as well as to his son (His Jr, 1889). However, His Sr did not consider the different auditory and vestibular subnuclei and relied on the more sophisticated work of Ramon y Cajal and Gustav Retzius for insights into the peripheral and central projections of auditory afferents as they relate to these subnuclei and to the cerebellum (Retzius, 1884, y Cajal, 1911). His noted in particular that the above-mentioned nuclei as well as the rhombic lip and the choroid plexus of the fourth ventricle are features unique to the hindbrain, as elaborated in detail by de No (de No, 1981).
Fig. 2.
Silver-stained coronal section of the hindbrain of a cat showing the innervation of the cochlear nuclei (Tuberculum) by afferents of the cochlear ganglion (Cochlearis ganglion), and second-order projections from the anterior ventral cochlear nucleus (PVCN, root Kern) to the superior olives (Trapezkern). Taken from Held (1893).
His explicitly defined distinct longitudinal regions of the brainstem: a) the ‘Schaltstueck’ (an intermediate segment between the spinal cord proper and the tip of the calamus scriptorius where the rhombic lips start to diverge); b) the calamus scriptorius region containing the gracile and cuneate nuclei; c) the medulla oblongata (‘Nachhirn’); d) the pons (‘Bruecke’ or ‘Hinterhirn’) and e) the isthmus. Throughout this region, the roof plate (‘Deckplatte’) in his description is a thin epithelial sheet that elaborates the choroid plexus of the fourth ventricle. His noted that the alar plate (‘Fluegelplatte’) and basal plate (‘Grundplatte’) develop into various partially identifiable nuclei (Fig. 1B), with the left and right basal plates connected by the diminutive midline floor plate (‘Bodenplatte’).
Thus, His described the hindbrain as being composed of longitudinal domains of different character, each containing specific sets of afferent inputs terminating in more or less defined central nuclei. He noted that some nuclei were established at specific sites along the longitudinal axis by migratory neuroblasts derived from the rhombic lip. Later work conducted mainly by the American school of functional neuroanatomists led Herrick to consider these domains as ‘neomorphs’ of the hindbrain, and he accorded them status as ‘special’ columns restricted to the rhombencephalon (Glover et al., 2018, Herrick, 1948).
Below we will expand on Wilhelm His Sr.’s observations by describing how his hindbrain regions correspond to molecular discontinuities in longitudinal progenitor domains in the dorsal hindbrain, and relating these to the neuromeric origins of the choroid plexus and the primary auditory nuclei (see Section 2 and 3; and (Fritzsch and Elliott, 2017, Hernandez-Miranda et al., 2017, Millen et al., 2014, Mishima et al., 2009, Ray and Dymecki, 2009). We pinpoint the transcription factor Lmx1a/b as a key regulator of the development of these hindbrain structures, and further describe the role of additional transcription factors that specify the cochlear hair cells and sensory neurons and the hindbrain auditory nuclei. Most of the information presented has been obtained from studies on mice, both wild type and various genetic mutant lines.
The longitudinal regionalization of the hindbrain
The rostrocaudal extent of the hindbrain (Fig. 1A) is defined by visible external morphological features in craniate vertebrates. The sulcus isthmus encompasses the midbrain/hindbrain boundary (MHB) and adjacent isthmus, and the caudal limit of the calamus scriptorius sets the hindbrain/spinal cord boundary (Fritzsch and Glover, 2006, Watson et al., 2017). Of these two boundaries, the MHB is best understood in a molecular context. The MHB forms through a sequential and partially overlapping expression of specific genes. For example, Lmx1b (Millen et al., 2014, Mishima et al., 2009) is needed to stabilize the expression of Wnt1 (McMahon and Bradley, 1990) and to upregulate and maintain the expression of Fgf8 (Guo et al., 2007; Lee et al., 1997, Watson et al., 2017), which initiates the proliferation of neuronal progenitors specific to the isthmus.
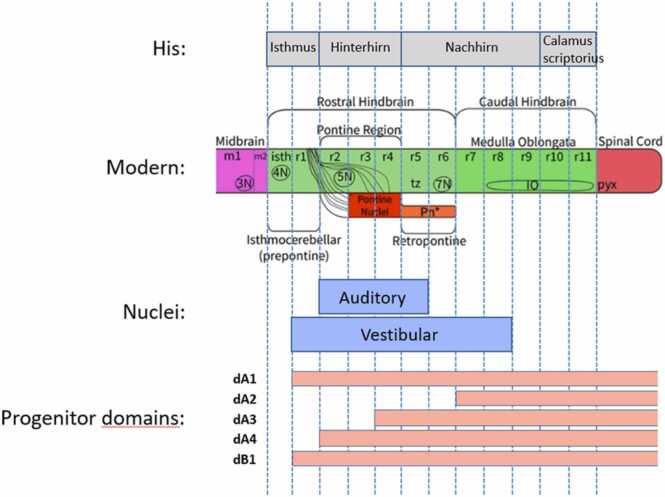
Between these two extremes lie His’ calamus scriptorius region (containing the gracile and cuneate nuclei), ‘Nachhirn’ (medulla oblongata), and ‘Hinterhirn’ (pons), which he evidently distinguished in large part based on the shape and appearance of the 4th ventricle (Fig. 1A). The differential specification of their constituent neuron populations is driven by a complex interaction of signaling systems and transcription factors in which retinoic acid, fibroblast growth factors (Fgf8) and Hox genes play pivotal roles (Glover et al., 2006, Parker and Krumlauf, 2017). A similar mechanism, albeit without the elements that instate physical segmentation, is likely to underlie the specification of the cryptic pseudorhombomeres which continue in sequence from the last true rhombomere (rhombomere 6, r6) to pseudorhombomere 11 (Tomás-Roca et al., 2016). Based on a comparison of His’ drawings and contemporary studies of overt and cryptic hindbrain segmentation, the first rhombomere lies within what His described as the isthmus, r2-r4 correspond to the Hinterhirn (pons), r5 and r6 to the upper part of the Nachhirn (medulla oblongata), and r7-r11 to the lower part of the Nachhirn and the calamus scriptorius region (Fig. 3). A recent study indicates that, in its modern definition, the isthmus is a domain separate and immediately rostral to r1 (thus receiving the monicker “r0”), and specified by the expression of Fgf8 [(Watson, et al., 2017)].
Fig. 3.
The relationship between His’ hindbrain regions, modern hindbrain neuromeric organization, the auditory and vestibular nuclear complexes, and the 5 most dorsal progenitor domains. Modified after (Diaz and Glover, 2021, Diek et al., 2022).
The Hinterhirn, or pons, is of particular interest, since recent work has shown that its traditional definition, based on the outward ventral bulge associated with the basilar pontine nuclei, does not represent a core division of the neural tube, but is rather a ventral appendix to the rhombencephalon (Nieuwenhuys and Puelles, 2015, Puelles and Ferran, 2012). Recent studies have shown that the pontine nuclei originate from the rhombic lips through migration, with the neurons eventually settling on the ventral surface of the hindbrain several rhombomeres more rostral. Moreover, their final location along the series of rhombomeres varies among species, and supernumerary pontine nuclei, generated through genetic manipulation of migratory mechanisms, can settle at ectopic ventral locations (Di Bonito et al., 2017; Di Meglio et al., 2013).
Hindbrain dorsoventral patterning is an elaboration of spinal cord dorsoventral patterning
The hindbrain rhombomeres and pseudorhombomeres exhibit the same basic pattern of dorsoventral gene expression as the spinal cord (Diek et al., 2022, Hernandez-Miranda et al., 2017, Lai et al., 2016), but with several features that are unique to r0-r7. One example is Wnt gene expression, which shows greater variation in the rhombencephalon than in the spinal cord (Merzdorf and Forecki, 2018), thereby influencing the shape changes associated with IVth ventricle formation. Wnt genes play a major role in dorsoventral patterning by establishing, with BMPs, a dorsal signaling gradient that interacts with an opposing ventral signaling gradient established by Shh and Gli. Wnt genes and their downstream targets are therefore instrumental in specifying dorsal hindbrain structures such as the choroid plexus and neuron populations such as the oculomotor and trochlear motoneurons (Jahan et al., 2021).
Complex interactions among a set of basic Helix-Loop-Helix (bHLH) genes establish a series of dorsal progenitor domains (dA1-dA4 and dB1-dB4, see Fig. 3). These domains arise through cross-repressive reciprocal interactions among genes that are selectively expressed at different dorsal levels, which sharpens their mutual expression boundaries (Lai, et al., 2016). Such interactions define the boundaries of the most dorsal progenitor domains dA1-dA4, characterized by the expression of (in sequence starting from the roof plate) Atoh1, Neurog1/2, Ascl1 and Ptf1a, each in combination with Olig3. Additional transcription factors such as Phox2b contribute to this hindbrain-specific dorsal patterning. The end result is that the hindbrain shares with the spinal cord six dorsal progenitor domains, but has two additional dorsal progenitor domains not found in the spinal cord (Hernandez-Miranda et al., 2017, Lunde et al., 2019).
In addition, roof plate development is regulated by Lmx1a/b and Gdf7, whose expression is triggered by high BMP and Wnt levels. Recent work has shown that the unique dorsal morphology of the hindbrain and the formation of the rhombencephalic choroid plexus depend on these two genes (Glover et al., 2018, Lee et al., 2000, Mishima et al., 2009). For example, in Lmx1a/b double null mice, the choroid plexus never forms, and the dorsal part of the hindbrain is transformed into a rostral elongation of the spinal cord (Chizhikov et al., 2021; Elliott et al., 2021).
Several features of gene expression lead to rhombomere-specific modifications of the dorsal progenitor domains. Ptf1a is expressed in apparent hindbrain-specific duplicate variants (Hernandez-Miranda et al., 2017), a situation that results in an alteration of dorsal cell fates in r0-r7 in Ptfi1a null mice (Diek et al., 2022, Iskusnykh et al., 2016, Lowenstein et al., 2021). Specifically, there is a loss of Neurog1/2 in r1-r6 and a partial loss of Ascl1 in r1-r3 (Hernandez-Miranda et al., 2017). A unique combinatorial interaction of Atoh1, Neurog1/2, Olig3 and Ptf1a, among other genes (Lowenstein et al., 2021, Pan et al., 2009), defines the cerebellum (Fig. 4). A delayed expression of Neurod1 adds to the interaction by providing a cerebellum-specific negative feedback of Atoh1 (Kersigo et al., 2021). This helps to establish the rostral limit of the auditory nuclei, whose development depends on a higher level of Atoh1 expression (Pan et al., 2009). Lmx1a/b, Fgf8 and Wnt1 are also important in regulating cerebellar development (Glover et al., 2018, Jahan et al., 2021, Watson et al., 2017).
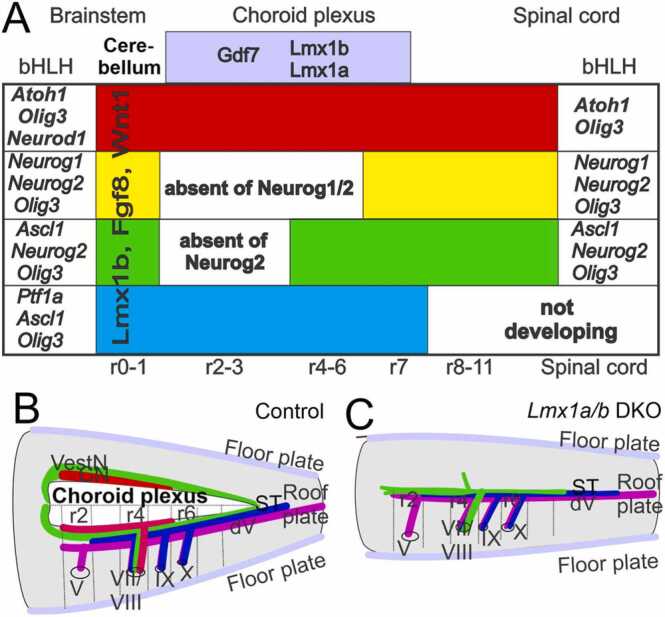
Fig. 4.
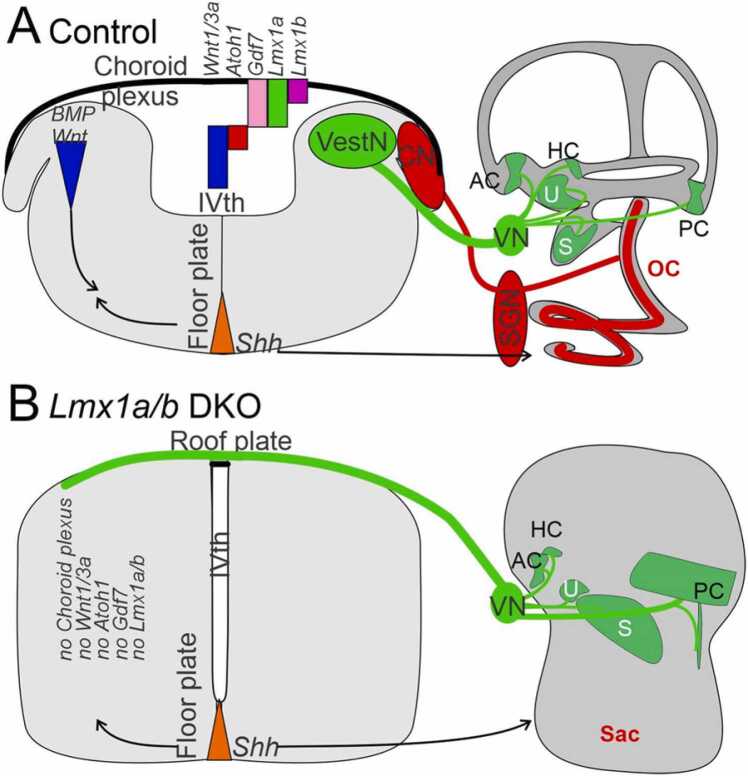
Dorsal development of the hindbrain depends on Lmx1a/b. (A) The choroid plexus originates from r2 to r7 (light purple) and depends on the expression of Lmx1a/b and Gdf7. The bHLH genes Atoh1, Neurog1, Neurog2, Neurod1, Ascl1, Olig3 and Ptf1a are expressed combinatorially in different dorsoventral domains with different longitudinal extents. The domain expressing the combination of Atoh1, Olig3 and Neurod1 (dA1, red) is continuous from the spinal cord up to the cerebellum. The domain expressing the combination of Neurog1, Neurog2 and Olig 3 (dA2, yellow) is interrupted in r2–6 where expression of Neurog1 and 2 is absent. The domain expressing the combination of Ascl1, Neurog2 and Olig3 (dA3, green) is interrupted at r2-3 where expression of Neurog2 is absent. The domain expressing the combination of Ptf1a, Ascl2 and Olig3 (dA4, blue) is continuous from r7 up to the cerebellum (Ptf1a expression is absent more caudally). All of these genes as well as Lmx1b, Fgf8 and Wnt1 are expressed in the cerebellum at r0-r1. (B, C) Depictions of structures and axon projections in hindbrains that have been slit along the ventral midline and folded out. (B) Normal hindbrain. (C) Hindbrain lacking Lmx1a/b expression (Lmx1a/b DKO). In this case, the choroid plexus and auditory nuclei do not form, the roof plate remains narrow, and vestibular sensory neurons project axons across it. Abbreviations: dV, trigeminal axons; ST, solitary tract axons; V, VII, VIII, IX, X, afferent fibers. Reprinted with permission from (Chizhikov et al., 2021; Elliott et al., 2021; Hernandez-Miranda et al., 2017).
Atoh1 and Olig3 are expressed in the spinal cord, the hindbrain and the cerebellum (Bermingham et al., 2001, Farago et al., 2006, Fritzsch et al., 2006, Hernandez-Miranda et al., 2017, Pan et al., 2009). Complete knockout of Atoh1 expression using Wnt1-cre upstream of Atoh1 (Wang et al., 2005) leads to the loss of all hindbrain neurons that depend on Atoh1/Olig3, leaving only the choroid plexus (Elliott et al., 2017). In contrast, some Atoh1-positive neurons develop in Olig3-null mice, indicating that Olig3 is less critical than Atoh1 in mediating dorsal hindbrain neurogenesis (Lowenstein et al., 2021). Lack of Gdf7 (Lee et al., 2000) or Lmx1a/b expression (Mishima et al., 2009) abolishes Atoh1 expression and leads to the same phenotype (Fig. 4, Fig. 5).
Fig. 5.
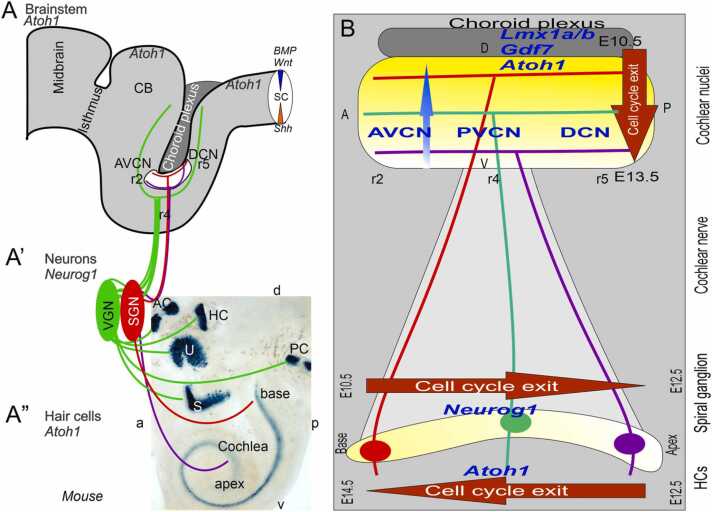
Cochlear and auditory projection development depends on the expression of specific genes. The development of spiral ganglion neurons (SGN; A’) depends on the expression of Neurog1. Their projection extends from the cochlea (A”, B) and ends in a topologically organized projection in the auditory nuclei (AVCN, PVCN, DCN) whose development depends on the expression of Atoh1 (A, B). Also shown are the vestibular ganglion neurons (VGN, A’), which project from the 5 vestibular sensory endorgans (anterior, posterior and horizontal semicircular canals, AC, PC, HC, and utricle and saccule, U, S; A”) to the central vestibular nuclei and cerebellum (CB; A). SGNs proliferate in a spatiotemporal gradient from the base to the apex of the cochlea during the embryonic period E10.5-E12.5 (B). Their central projections develop topologically from dorsal to ventral positions within the central auditory nuclei over approximately the same period (E10.5.E13.5; B). Later, hair cells proliferate in a gradient from the apex to the base of the cochlea during the period E12.5-E14.5 (B), and contact the peripheral terminals of the SGNs. AC, anterior crista; AVCN, anteroventral cochlear neurons; CB, cerebellum; aLL, pLL, anterior/posterior lateral line neurons; DCN, dorsal cochlear neurons; HC, horizontal crista; PC, posterior crista; r2/4/6, rhombomeres; S, saccule; SC, spinal cord; U, utricle. Modified from (Filova et al., 2022, Fritzsch et al., 2019, Macova et al., 2019, Nichols et al., 2008).
In the absence of Lmx1a/b, projections to the cerebellum are perturbed, including unusual central vestibular and solitary tract projections, which are free to cross the dorsal midline due to the aberrantly closed roof plate [Fig. 4, Fig. 6; (Elliott et al., 2021)]. Neither electroreception nor auditory projections reach the cerebellum (Fig. 4), although in other specific gene mutations auditory fibers are perturbed but can be transiently traced to the cerebellum (Schmidt and Fritzsch, 2019).
Fig. 6.
Central projections of auditory and vestibular sensory afferents depend on proper development of the dorsal hindbrain. In Lmx1a/b DKO mice vestibular neurons (VN) project dorsally in the hindbrain as in control mice, whereas auditory projections fail to develop (A, B). However, in Lmx1a/b DKO mice, vestibular projections cross the midline through the roof plate, which is continuous across the midline due to the absence of the choroid plexus (B). Choroid plexus development depends on the expression of Atoh1, Gdf7 and Wnt1/3a which is lost in the absence of Lmx1a/b expression (A, B). AC, HC, PC, anterior, horizontal, posterior cristae; CN, cochlear nuclei; S, saccule; SGN, spiral ganglion neurons; ST, solitary tact; U, utricle; VestN, vestibular nuclei; VN, vestibular ganglion neurons. Reprinted with permission from (Chizhikov et al., 2021, Elliott et al., 2021, Glover et al., 2018, Lee et al., 2000).
In summary, the development of dorsal hindbrain structures including the auditory nuclei depends on complex interactions among specific bHLH genes. Lack of expression of the key gene Lmx1a/b leads to a loss of the choroid plexus and of Atoh1 expression. The latter effect impacts on the expression of other dorsal patterning genes (Neurog1, Neurog2, Neurod1, Olig3, Ascl1 and Ptf1a) resulting in additional perturbance of the development of dorsal hindbrain nuclei.
Generation of cochlear sensory neurons depends on Eya1, Sox2, Neurog1 and Neurod1
Spiral ganglion neurons (SGNs) have been classified into type Ia, Ib, Ic and type II (Elliott et al., 2021, Petitpré et al., 2022). The development of SGNs depends on a set of genes that collectively regulate both proliferation and specification. In contrast, expression of a different set of genes defines vestibular ganglion neurons (Sun et al., 2022) that also depend on BDNF and TrkB for normal development and viability (Elliott et al., 2021).
In the earliest stage of neurogenesis, Eya1 interacts with Brg1 to initiate pro-neurosensory development (Xu et al., 2021). In the absence of Eya1 there is no neuronal development whatsoever, leading to formation of an inner ear in which neither neurons nor hair cells differentiate (Xu, et al., 2021). A crucial next step is the initiation of Sox2 expression, which is needed to upregulate Neurog1 (Kageyama et al., 2019, Riddiford and Schlosser, 2016). Neurog1 (Ma et al., 2000), Pax2 and Lmx1a/b (Bouchard et al., 2010, Chizhikov et al., 2021) are all essential for SGN development (Fig. 3), but the effect of knocking out or knocking down these and other genes is weaker in the vestibular ganglion than in the cochlear ganglia. Early-differentiating vestibular hair cells and sensory neurons are generated in the absence of Sox2 and Neurog1, whereas their later-differentiating auditory counterparts are not (Dvorakova et al., 2020). A complete loss of SGNs, but only a partial loss of VGNs, occurs in the absence of Pax2 (Bouchard, et al., 2010), Gata3 (Duncan and Fritzsch, 2013), Lmx1a/b (Chizhikov, et al., 2021), Fgfr2 (Pirvola et al., 2000), Shh (Riccomagno et al., 2002) and Dicer (Kersigo et al., 2011). Partial loss of VGNs occurs in the absence of Fgf10 (Pauley et al., 2003) and Foxg1 (Hwang et al., 2009, Pauley et al., 2006). In Sox10 null mice, VGNs develop virtually normally whereas SGNs become disorganized (Mao et al., 2014). In the absence of Erb2 expression nearly all SGNs are lost but VGNs are only reduced (Morris et al., 2006).
Following the initial formation of neurons triggered by Eya1, Sox2, Pax2 and Neurog1/2, further neuronal differentiation is regulated by another set of genes, starting with Neurod1 (Alsina, 2020, Macova et al., 2019) and followed by Isl1, Foxg1, Pou4f1 and Phox2b (Alsina, 2020, Filova et al., 2022, Moody and LaMantia, 2015, Sun et al., 2022) and their interactions with Shh, BMPs and Wnts (Muthu et al., 2019). Regional regulation of distinct VGN and SGN populations is further defined by downstream genes involved in distinct patterns of innervation (Sun, et al., 2022). For example, the expression of Calbindin, Calretinin, Pou4f1 and Peripherin distinguish connections from the inner and outer hair cells (Elliott et al., 2021, Petitpré et al., 2022, Petitpré et al., 2018, Shrestha et al., 2018, Sun et al., 2018).
Additional interactions regulate differentiation of the different SGN classes. Neurod1 (Jahan et al., 2010) interacts with Nhlh1/2 (Krüger et al., 2004), Isl1 (Filova, et al., 2022), and Ebf2 (Petitpré, et al., 2022) to initiate the formation of type I and II SGNs (Petitpré et al., 2022, Shrestha et al., 2018, Sun et al., 2018). Several other genes, including Gata3, Zfhx2 and Dpf1, interact to regulate the differentiation of the other three SGN classes (type Ia, type Ib, type II) (Appler et al., 2013, Duncan and Fritzsch, 2013, Karis et al., 2001, Luo et al., 2013, Petitpré et al., 2022), all of which depend additionally on the expression of Cux2 and Pou4f2. Id2 regulates expression of Calretinin and Pcdh20 in the differentiation of type Ia SGNs. Runx1 regulates expression of Lypd1, Calbindin, Calretinin, and Pou4f1 in the differentiation of type Ib SGNs (Huang et al., 2001, Petitpré et al., 2022, Shrestha et al., 2018, Sun et al., 2018).
A spatiotemporal pattern of SGN development has been demonstrated in mammals (de No, 1981), with differentiation first of basal turn neurons that innervate the anteroventral, posteroventral, and dorsal cochlear nuclei (AVCN, PVCN, DCN) followed, after a delay, by differentiation of apical turn neurons (Filova et al., 2022, Fritzsch et al., 2019, Schmidt and Fritzsch, 2019) (Fig. 5). SGN neurons develop prior to the cochlear hair cells and central auditory nuclei. SGNs can establish central projections even in the absence of target hair cells (Elliott et al., 2017, Kersigo and Fritzsch, 2015), but expression of Neurod1, Wnts, Fzd, Npr2 and Ephrins is required for proper targeting of the central projections (Duncan et al., 2019, Macova et al., 2019, Milinkeviciute and Cramer, 2021, Schmidt and Fritzsch, 2019). Additional work is needed to characterize selectively the development of the central terminations of the different SGN classes (Filova et al., 2022, Filova et al., 2022, Muniak et al., 2016, Muniak and Ryugo, 2014).
In summary, the SGNs are generated and diversified through the action of a set of genes downstream of Neurog1. Shortly after their generation, SGNs innervate their target hair cells, but they can differentiate and establish their central projections even in the absence of these peripheral targets. Segregation of the central projections follows a spatiotemporal, topological sequence that is also dependent on the expression of specific genes by the neurons of the central auditory nuclei (Fig. 5).
Hair cell differentiation depends on several genes including Lmx1a/b
Mechanosensory hair cells are utilized in vestibular, cochlear, lateral line, electroreceptor and Merkel cell-mediated somatosensory signaling (Chagnaud et al., 2017, Elliott et al., 2021). Evolutionary evidence suggests that hair cells derived from the unicellular choanoflagellates (Arendt et al., 2016, Fritzsch and Straka, 2014), through a transformation of the single flagellum surrounded by villi of choanoflagellates into the kinocilium/stereocilia bundle that distinguishes hair cells. The specification of inner ear hair cells begins with the actions of Eya1/Six1 (Ahmed et al., 2012), Pax2/8 (Bouchard, et al., 2010), Shh (Muthu, et al., 2019), BMPs (Ohyama et al., 2010) and Wnts (Wright et al., 2015) during formation of the otocyst. Upregulation of Sox2 (Dvorakova, et al., 2020) sets up hair cell differentiation within the otic sensory epithelium. This depends on interactions between Atoh1 and Neurod1 (Filova et al., 2020), Pou4f3 (Li et al., 2020, Xiang et al., 2003), Gfi1 (Hertzano et al., 2004), Srrm/Rest (Nakano et al., 2020) and Barhl1 (Chellappa et al., 2008).
Lmx1a null mutant mice (Huang et al., 2018, Koo et al., 2009, Nichols et al., 2020, Nichols et al., 2008, Steffes et al., 2012) and various human LMX1A mutations (Lee et al., 2020, Oziębło et al., 2022, Schrauwen et al., 2018, Wesdorp et al., 2018) exhibit auditory and vestibular defects that can be linked to impaired hair cell differentiation. Lmx1a null mouse mutants appear to be completely deaf (Steffes et al., 2012), and humans with LMX1A mutations exhibit partial hearing loss (Lee et al., 2022, Schrauwen et al., 2018, Wesdorp et al., 2018). Lmx1a/b double KO mice completely lack the cochlea (Chizhikov et al., 2021, Elliott et al., 2021) (Fig. 5).
The differentiation of functionally mature hair cells depends on several genes that encode key functional proteins. The stereocilia are interconnected by tip link proteins such as PCDH15 and CDH23, which regulate hair cell morphogenesis during differentiation and function as the mechanical transducers for opening the mechanoelectrical transduction channels (METs) in mature hair cells. MET opening permits endolymphatic potassium to enter and depolarize the hair cells (Elliott et al., 2018). METs are composed in part by the transmembrane proteins Tmc1 and, transiently, Tmc2 (Marcovich and Holt, 2020, Shibata et al., 2016). Auditory hair cells in mammals depend further on the expression of Vangl2, Dvl1, Celsr1 and Gal2, regulated through the planar cell polarity (PCP) pathway (Tarchini et al., 2013). Emx2 and Jag1 are both required for the normal development of outer hair cells (Holley et al., 2010, Jiang et al., 2017).
Summary and Conclusion
In summary, Wilhelm His Sr. not only laid the foundation for understanding the neural crest as the origin of much of the peripheral sensory nervous system (Glover et al., 2018), but also contributed profound insight into the importance of the adjacent rhombic lip in the formation of central neuron populations novel to the hindbrain. He thus made seminal contributions to our understanding of the evolutionary elaboration of a basic spinal cord organization into the more complex and functionally diverse hindbrain region. Contemporary molecular studies have defined the critical dependence of central hindbrain nuclei and specific cell types on the expression of particular genes (including Atoh1, Neurog1/2, Olig3, Ascl1 and Ptf1a for central nuclei, Neurog1 for neurons, and Atoh1 for hair cells) (Hernandez-Miranda et al., 2017, Lunde et al., 2019). Development of dorsal structures such as the roof plate and choroid plexus depends on the expression of Lmx1a/b, BMPs and Wnts (Chizhikov et al., 2021, Elliott et al., 2021, Glover et al., 2018). In the absence of Lmx1a/b the choroid plexus and auditory nuclei do not form (Fig. 6). The elegant and detailed anatomical descriptions that His made of hindbrain-specific specializations are thus being validated at the molecular level over a century later.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
"Compliance with Ethical Statements"
N/A.
Conflict of interest statement
The corresponding author states that there are no conflicts of interest.
Acknowledgements
This work was supported by the NIH (R01 AG060504).
Contributor Information
Joel C. Glover, Email: joel.glover@medisin.uio.no.
Bernd Fritzsch, Email: bernd-fritzsch@uiowa.edu.
References
- Ahmed M., Xu J., Xu P.-X. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development. 2012;139:1965–1977. doi: 10.1242/dev.071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsina B. Mechanisms of cell specification and differentiation in vertebrate cranial sensory systems. Curr. Opin. Cell Biol. 2020;67:79–85. doi: 10.1016/j.ceb.2020.08.006. [DOI] [PubMed] [Google Scholar]
- Appler J.M., Lu C.C., Druckenbrod N.R., Yu W.M., Koundakjian E.J., Goodrich L.V. Gata3 is a critical regulator of cochlear wiring. J. Neurosci. 2013;33:3679–3691. doi: 10.1523/JNEUROSCI.4703-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D., Musser J.M., Baker C.V., Bergman A., Cepko C., Erwin D.H., Pavlicev M., Schlosser G., et al. The origin and evolution of cell types. Nat. Rev. Genet. 2016;17:744–757. doi: 10.1038/nrg.2016.127. [DOI] [PubMed] [Google Scholar]
- Bermingham N.A., Hassan B.A., Wang V.Y., Fernandez M., Banfi S., Bellen H.J., Fritzsch B., Zoghbi H.Y. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- Bouchard M., de Caprona D., Busslinger M., Xu P., Fritzsch B. Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev. Biol. 2010 doi: 10.1186/1471-213X-10-89. 10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnaud B.P., Engelmann J., Fritzsch B., Glover J.C., Straka H. Sensing external and self-motion with hair cells: a comparison of the lateral line and vestibular systems from a developmental and evolutionary perspective. Brain, Behav. Evol. 2017;90:98–116. doi: 10.1159/000456646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa R., Li S., Pauley S., Jahan I., Jin K., Xiang M. Barhl1 regulatory sequences required for cell-specific gene expression and autoregulation in the inner ear and central nervous system. Mol. Cell. Biol. 2008;28:1905–1914. doi: 10.1128/MCB.01454-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov V.V., Iskusnykh I.Y., Fattakhov N., Fritzsch B. Lmx1a and Lmx1b are Redundantly Required for the Development of Multiple Components of the Mammalian Auditory System. Neuroscience. 2021;452:247–264. doi: 10.1016/j.neuroscience.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bonito M., Studer M., Puelles L. Nuclear derivatives and axonal projections originating from rhombomere 4 in the mouse hindbrain. Brain Struct. Funct. 2017;222:3509–3542. doi: 10.1007/s00429-017-1416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio T., Kratochwil C.F., Vilain N., Loche A., Vitobello A., Yonehara K., Hrycaj S.M., Roska B., et al. Ezh2 orchestrates topographic migration and connectivity of mouse precerebellar neurons. Science. 2013;339:204–207. doi: 10.1126/science.1229326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz C., Glover J.C. The vestibular column in the mouse: a rhombomeric perspective. Front. Neuroanat. 2021:15. doi: 10.3389/fnana.2021.806815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diek D., Smidt M.P., Mesman S. Molecular organization and patterning of the medulla oblongata in health and disease. Int. J. Mol. Sci. 2022;23:9260. doi: 10.3390/ijms23169260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J.S., Fritzsch B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PloS One. 2013;8 doi: 10.1371/journal.pone.0062046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J.S., Fritzsch B., Houston D.W., Ketchum E.M., Kersigo J., Deans M.R., Elliott K.L. Topologically correct central projections of tetrapod inner ear afferents require Fzd3. Sci. Rep. 2019;9:10298. doi: 10.1038/s41598-019-46553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorakova M., Macova I., Bohuslavova R., Anderova M., Fritzsch B., Pavlinkova G. Early ear neuronal development, but not olfactory or lens development, can proceed without SOX2. Dev. Biol. 2020;457:43–56. doi: 10.1016/j.ydbio.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K.L., Fritzsch B., Duncan J.S. Evolutionary and Developmental Biology Provide Insights Into the Regeneration of Organ of Corti Hair Cells. Front. Cell. Neurosci. 2018;12:252. doi: 10.3389/fncel.2018.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K.L., Kersigo J., Pan N., Jahan I., Fritzsch B. Spiral ganglion neuron projection development to the hindbrain in mice lacking peripheral and/or central target differentiation. Front. Neural Circuits. 2017;11:25. doi: 10.3389/fncir.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K.L., Kersigo J., Lee J.H., Yamoah E.N., Fritzsch B. Sustained Loss of Bdnf affects peripheral but not central vestibular targets. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.768456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K.L., Pavlínková G., Chizhikov V.V., Yamoah E.N., Fritzsch B. Development in the Mammalian auditory system depends on transcription factors. Int. J. Mol. Sci. 2021;22:4189. doi: 10.3390/ijms22084189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K.L., Kersigo J., Lee J.H., Jahan I., Pavlinkova G., Fritzsch B., Yamoah E.N. Developmental changes in peripherin-eGFP expression in spiral ganglion neurons. Front. Cell. Neurosci. 2021;15:181. doi: 10.3389/fncel.2021.678113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago A.F., Awatramani R.B., Dymecki S.M. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–218. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Filova I., Bohuslavova R., Tavakoli M., Yamoah E.N., Fritzsch B., Pavlinkova G. Early deletion of neurod1 alters neuronal lineage potential and diminishes neurogenesis in the inner ear. Front Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.845461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filova I., Dvorakova M., Bohuslavova R., Pavlinek A., Elliott K.L., Vochyanova S., Fritzsch B., Pavlinkova G. Combined Atoh1 and Neurod1 deletion reveals autonomous growth of auditory nerve fibers. Mol. Neurobiol. 2020;57:5307–5323. doi: 10.1007/s12035-020-02092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filova I., Pysanenko K., Tavakoli M., Vochyanova S., Dvorakova M., Bohuslavova R., Smolik O., Fabriciova V., et al. ISL1 is necessary for auditory neuron development and contributes toward tonotopic organization. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2207433119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B., Glover J. (2006), Evolution of the Deuterostome Central Nervous System: An Intercalation of Developmental Patterning Processes with Cellular Specification Processes-2.01.
- Fritzsch B., Straka H. Evolution of vertebrate mechanosensory hair cells and inner ears: toward identifying stimuli that select mutation driven altered morphologies. J. Comp. Physiol. A. 2014;200:5–18. doi: 10.1007/s00359-013-0865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B., Elliott K.L. Gene, cell, and organ multiplication drives inner ear evolution. Dev. Biol. 2017;431:3–15. doi: 10.1016/j.ydbio.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B., Elliott K.L., Pavlinkova G. Primary sensory map formations reflect unique needs and molecular cues specific to each sensory system. F1000Research. 2019:8. doi: 10.12688/f1000research.17717.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B., Pauley S., Feng F., Matei V., Nichols D. The molecular and developmental basis of the evolution of the vertebrate auditory system. Int. J. Comp. Psychol. 2006;19:1–25. [Google Scholar]
- Glover J.C., Renaud J.S., Rijli F.M. Retinoic acid and hindbrain patterning. Dev. Neurobiol. 2006;66:705–725. doi: 10.1002/neu.20272. [DOI] [PubMed] [Google Scholar]
- Glover J.C., Elliott K.L., Erives A., Chizhikov V.V., Fritzsch B. Wilhelm His’ lasting insights into hindbrain and cranial ganglia development and evolution. Dev. Biol. 2018;444:S14–S24. doi: 10.1016/j.ydbio.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Qiu H.-Y., Huang Y., Chen H., Yang R.-Q., Chen S.-D., Johnson R.L., Chen Z.-F., et al. Lmx1b is essential for Fgf8 and Wnt1 expression in the isthmic organizer during tectum and cerebellum development in mice. Development. 2007;134:317–325. doi: 10.1242/dev.02745. [DOI] [PubMed] [Google Scholar]
- Held H. Die centrale gehörleitung. Arch. Anat. Physiol. Anat. Abt. 1893;17:201–248. [Google Scholar]
- Hernandez-Miranda L.R., Müller T., Birchmeier C. The dorsal spinal cord and hindbrain: From developmental mechanisms to functional circuits. Dev. Biol. 2017;432:34–42. doi: 10.1016/j.ydbio.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Herrick C.J. The brain of the tiger salamander. Ambystoma tigrinum. 1948 [Google Scholar]
- Hertzano R., Montcouquiol M., Rashi-Elkeles S., Elkon R., Yucel R., Frankel W.N., Rechavi G., Moroy T., et al. Transcription profiling of inner ears from Pou4f3(ddl/ddl) identifies Gfi1 as a target of the Pou4f3 deafness gene. Hum. Mol. Genet. 2004;13:2143–2153. doi: 10.1093/hmg/ddh218. [DOI] [PubMed] [Google Scholar]
- His W. Untersuchungen über die erste Anlage des Wirbelthierleibes: die erste Entwickelung des Hühnchens im Ei. FCW Vogel. 1868 [Google Scholar]
- His W. Beschreibung eines Mikrotoms. Arch. für Mikrosk. Anat. 1870;6:229–232. [Google Scholar]
- His W. Anatomie menschlicher embryonen. FCW Vogel. 1880 [Google Scholar]
- His W., Jr Zur Entwicklungsgeschichte des Acoustico-Facialis Gebietes beim Menschen. Arch. F. Anat. U. Physiol. 1889;supp:1 [Google Scholar]
- His W. (1890) Histogenese und Zusammenhang der Nervenelemente.
- His W. Die anatomische nomenclatur: nomina anatomica. Veit. 1895 [Google Scholar]
- His W. Address upon the development of the brain. Trans. R. Acad. Med. Irel. 1897;15 [Google Scholar]
- His W. Die Entwickelung des menschlichen Gehirns während der ersten Monate. S. Hirzel. 1904 [Google Scholar]
- Holley M., Rhodes C., Kneebone A., Herde M.K., Fleming M., Steel K.P. Emx2 and early hair cell development in the mouse inner ear. Dev. Biol. 2010;340:547–556. doi: 10.1016/j.ydbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E.J., Liu W., Fritzsch B., Bianchi L.M., Reichardt L.F., Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Hill J., Yatteau A., Wong L., Jiang T., Petrovic J., Gan L., Dong L., et al. Reciprocal Negative Regulation Between Lmx1a and Lmo4 Is Required for Inner Ear Formation. J. Neurosci. 2018;38:5429–5440. doi: 10.1523/JNEUROSCI.2484-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C.H., Simeone A., Lai E., Wu D.K. Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Dev. Dyn. 2009;238:2725–2734. doi: 10.1002/dvdy.22111. [DOI] [PubMed] [Google Scholar]
- Iskusnykh I.Y., Steshina E.Y., Chizhikov V.V. Loss of Ptf1a leads to a widespread cell-fate misspecification in the brainstem, affecting the development of somatosensory and viscerosensory nuclei. J. Neurosci. 2016;36:2691–2710. doi: 10.1523/JNEUROSCI.2526-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I., Kersigo J., Pan N., Fritzsch B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010;341:95–110. doi: 10.1007/s00441-010-0984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I., Kersigo J., Elliott K.L., Fritzsch B. Smoothened overexpression causes trochlear motoneurons to reroute and innervate ipsilateral eyes. Cell Tissue Res. 2021;384:59–72. doi: 10.1007/s00441-020-03352-0. [DOI] [PubMed] [Google Scholar]
- Jiang T., Kindt K., Wu D.K. Transcription factor Emx2 controls stereociliary bundle orientation of sensory hair cells. Elife. 2017;6 doi: 10.7554/eLife.23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R., Shimojo H., Ohtsuka T. Dynamic control of neural stem cells by bHLH factors. Neurosci. Res. 2019;138:12–18. doi: 10.1016/j.neures.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Karis A., Pata I., van Doorninck J.H., Grosveld F., de Zeeuw C.I., de Caprona D., Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J. Comp. Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kersigo J., Fritzsch B. Inner ear hair cells deteriorate in mice engineered to have no or diminished innervation. Front. Aging Neurosci. 2015;7:33. doi: 10.3389/fnagi.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersigo J., D'Angelo A., Gray B.D., Soukup G.A., Fritzsch B. The role of sensory organs and the forebrain for the development of the craniofacial shape as revealed by Foxg1–cre‐mediated microRNA loss. Genesis. 2011;49:326–341. doi: 10.1002/dvg.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersigo J., Gu L., Xu L., Pan N., Vijayakuma S., Jones T., Shibata S.B., Fritzsch B., et al. Effects of Neurod1 Expression on Mouse and Human Schwannoma Cells. Laryngoscope. 2021;131:E259–e270. doi: 10.1002/lary.28671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S.K., Hill J.K., Hwang C.H., Lin Z.S., Millen K.J., Wu D.K. Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Dev. Biol. 2009;333:14–25. doi: 10.1016/j.ydbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger M., Ruschke K., Braun T. NSCL-1 and NSCL-2 synergistically determine the fate of GnRH-1 neurons and control necdin gene expression. Embo J. 2004;23:4353–4364. doi: 10.1038/sj.emboj.7600431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H.C., Seal R.P., Johnson J.E. Making sense out of spinal cord somatosensory development. Development. 2016;143:3434–3448. doi: 10.1242/dev.139592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.J., Dietrich P., Jessell T.M. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- Lee S., Danielian P.S., Fritzsch B., McMahon A.P. Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development. 1997;124:959–969. doi: 10.1242/dev.124.5.959. [DOI] [PubMed] [Google Scholar]
- Lee S.Y., Han J.H., Carandang M., Kim M.Y., Kim B., Yi N., Kim J., Kim B.J., et al. Novel genotype-phenotype correlation of functionally characterized LMX1A variants linked to sensorineural hearing loss. Hum. Mutat. 2020;41:1877–1883. doi: 10.1002/humu.24095. [DOI] [PubMed] [Google Scholar]
- Lee S.Y., Soon Yoo H., Hee Han J., Hee Lee D., Soo Park S., Hwan Suh M., Ho Lee J., Oh S.H., et al. Novel Molecular Genetic Etiology of Asymmetric Hearing Loss: Autosomal-Dominant LMX1A Variants. Ear Hear. 2022 doi: 10.1097/AUD.0000000000001237. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang T., Ramakrishnan A., Fritzsch B., Xu J., Wong E.Y.M., Loh Y.E., Ding J., et al. Dynamic changes in cis-regulatory occupancy by Six1 and its cooperative interactions with distinct cofactors drive lineage-specific gene expression programs during progressive differentiation of the auditory sensory epithelium. Nucleic Acids Res. 2020;48:2880–2896. doi: 10.1093/nar/gkaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein E.D., Rusanova A., Stelzer J., Hernaiz-Llorens M., Schroer A.E., Epifanova E., Bladt F., Isik E.G., et al. Olig3 regulates early cerebellar development. Elife. 2021;10 doi: 10.7554/eLife.64684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde A., Okaty B.W., Dymecki S.M., Glover J.C. Molecular profiling defines evolutionarily conserved transcription factor signatures of major vestibulospinal neuron groups. Eneuro. 2019:6. doi: 10.1523/ENEURO.0475-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X.J., Deng M., Xie X., Huang L., Wang H., Jiang L., Liang G., Hu F., et al. GATA3 controls the specification of prosensory domain and neuronal survival in the mouse cochlea. Hum. Mol. Genet. 2013;22:3609–3623. doi: 10.1093/hmg/ddt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Anderson D.J., Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J. Assoc. Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macova I., Pysanenko K., Chumak T., Dvorakova M., Bohuslavova R., Syka J., Fritzsch B., Pavlinkova G. Neurod1 is essential for the primary tonotopic organization and related auditory information processing in the midbrain. J. Neurosci. 2019;39:984–1004. doi: 10.1523/JNEUROSCI.2557-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca M.S. The Rat Nervous System. Elsevier,; 2015. Auditory system; pp. 865–946. [Google Scholar]
- Mao Y., Reiprich S., Wegner M., Fritzsch B. Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PloS One. 2014;9 doi: 10.1371/journal.pone.0094580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovich I., Holt J.R. Evolution and function of Tmc genes in mammalian hearing. Curr. Opin. Physiol. 2020;18:11–19. [Google Scholar]
- McMahon A.P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Merzdorf C., Forecki J. Zic family. Springer,; 2018. Amphibian Zic Genes; pp. 107–140. (vol.) [DOI] [PubMed] [Google Scholar]
- Milinkeviciute G., Cramer K.S. In: Fritzsch B., editor. vol. 2. Elsevier; 2021. Development of the ascending auditory pathway; pp. 337–353. (The Senses). [Google Scholar]
- Millen K.J., Steshina E.Y., Iskusnykh I.Y., Chizhikov V.V. Transformation of the cerebellum into more ventral brainstem fates causes cerebellar agenesis in the absence of Ptf1a function. Proc. Natl. Acad. Sci. 2014;111:E1777–E1786. doi: 10.1073/pnas.1315024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y., Lindgren A.G., Chizhikov V.V., Johnson R.L., Millen K.J. Overlapping function of Lmx1a and Lmx1b in anterior hindbrain roof plate formation and cerebellar growth. J. Neurosci. 2009;29:11377–11384. doi: 10.1523/JNEUROSCI.0969-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody S.A., LaMantia A.-S. vol. 111. Elsevier,; 2015. Transcriptional regulation of cranial sensory placode development; pp. 301–350. (Current Topics in Developmental Biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.K., Maklad A., Hansen L.A., Feng F., Sorensen C., Lee K.-F., Macklin W.B., Fritzsch B. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006;1091:186–199. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniak M.A., Ryugo D.K. Tonotopic organization of vertical cells in the dorsal cochlear nucleus of the CBA/J mouse. J. Comp. Neurol. 2014;522:937–949. doi: 10.1002/cne.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniak M.A., Connelly C.J., Suthakar K., Milinkeviciute G., Ayeni F.E., Ryugo D.K. The primary auditory neurons of the mammalian cochlea, vol. Springer,; 2016. Central projections of spiral ganglion neurons; pp. 157–190. [Google Scholar]
- Muthu V., Rohacek A.M., Yao Y., Rakowiecki S.M., Brown A.S., Zhao Y.-T., Meyers J., Won K.-J., et al. Genomic architecture of Shh-dependent cochlear morphogenesis. Dev. (Camb., Engl. ) 2019;146:dev181339. doi: 10.1242/dev.181339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y., Wiechert S., Fritzsch B., Bánfi B. Inhibition of a transcriptional repressor rescues hearing in a splicing factor–deficient mouse. Life Sci. Alliance. 2020;3 doi: 10.26508/lsa.202000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D.H., Pauley S., Jahan I., Beisel K.W., Millen K.J., Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334:339–358. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D.H., Bouma J.E., Kopecky B.J., Jahan I., Beisel K.W., He D.Z.Z., Liu H., Fritzsch B. Interaction with ectopic cochlear crista sensory epithelium disrupts basal cochlear sensory epithelium development in Lmx1a mutant mice. Cell Tissue Res. 2020;380:435–448. doi: 10.1007/s00441-019-03163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R., Puelles L. Springer; 2015. Towards a New Neuromorphology. [Google Scholar]
- de No R.L. Raven Press; 1981. The Primary Acoustic Nuclei. [Google Scholar]
- O’Neill P., Mak S.-S., Fritzsch B., Ladher R.K., Baker C.V. The amniote paratympanic organ develops from a previously undiscovered sensory placode. Nat. Commun. 2012;3:1041. doi: 10.1038/ncomms2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T., Basch M.L., Mishina Y., Lyons K.M., Segil N., Groves A.K. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J. Neurosci. 2010;30:15044–15051. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oziębło D., Lee S.Y., Leja M.L., Sarosiak A., Bałdyga N., Skarżyński H., Kim Y., Han J.H., et al. Update on CD164 and LMX1A genes to strengthen their causative role in autosomal dominant hearing loss. Hum. Genet. 2022;141:445–453. doi: 10.1007/s00439-022-02443-y. [DOI] [PubMed] [Google Scholar]
- Pan N., Jahan I., Lee J.E., Fritzsch B. Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg (Atoh1-cre) recombination and granule cell requirements for Neurod1 for differentiation. Cell Tissue Res. 2009;337:407–428. doi: 10.1007/s00441-009-0826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H.J., Krumlauf R. Segmental arithmetic: summing up the Hox gene regulatory network for hindbrain development in chordates. Wiley Interdiscip. Rev.: Dev. Biol. 2017:6. doi: 10.1002/wdev.286. [DOI] [PubMed] [Google Scholar]
- Pauley S., Lai E., Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev. Dyn.: Off. Publ. Am. Assoc. Anat. 2006;235:2470–2482. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S., Wright T.J., Pirvola U., Ornitz D., Beisel K., Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev. Dyn.: Off. Publ. Am. Assoc. Anat. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitpré C., Wu H., Sharma A., Tokarska A., Fontanet P., Wang Y., Helmbacher F., Yackle K., et al. Neuronal heterogeneity and stereotyped connectivity in the auditory afferent system. Nat. Commun. 2018;9:3691. doi: 10.1038/s41467-018-06033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitpré C., Faure L., Uhl P., Fontanet P., Filova I., Pavlinkova G., Adameyko I., Hadjab S., et al. Single-cell RNA-sequencing analysis of the developing mouse inner ear identifies molecular logic of auditory neuron diversification. Nature. Communications. 2022;13:1–15. doi: 10.1038/s41467-022-31580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U., Spencer-Dene B., Xing-Qun L., Kettunen P., Thesleff I., Fritzsch B., Dickson C., Ylikoski J. FGF/FGFR-2 (IIIb) signaling is essential for inner ear morphogenesis. J. Neurosci. 2000;20:6125–6134. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L., Ferran J.L. Concept of neural genoarchitecture and its genomic fundament. Front. Neuroanat. 2012;6 doi: 10.3389/fnana.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R.S., Dymecki S.M. Rautenlippe Redux—toward a unified view of the precerebellar rhombic lip. Curr. Opin. Cell Biol. 2009;21:741–747. doi: 10.1016/j.ceb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzius G. Das Gehörorgan der Reptilien, der Vögel und der Säugethiere. Samson &; Wallin: 1884. pp. 1–368. [Google Scholar]
- Riccomagno M.M., Martinu L., Mulheisen M., Wu D.K., Epstein D.J. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16:2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford N., Schlosser G. Dissecting the pre-placodal transcriptome to reveal presumptive direct targets of Six1 and Eya1 in cranial placodes. Elife. 2016;5 doi: 10.7554/eLife.17666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. From so simple a beginning–what amphioxus can teach us about placode evolution. Int. J. Dev. Biol. 2017;61:633–648. doi: 10.1387/ijdb.170127gs. [DOI] [PubMed] [Google Scholar]
- Schmidt H., Fritzsch B. Npr2 null mutants show initial overshooting followed by reduction of spiral ganglion axon projections combined with near-normal cochleotopic projection. Cell Tissue Res. 2019:1–18. doi: 10.1007/s00441-019-03050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen I., Chakchouk I., Liaqat K., Jan A., Nasir A., Hussain S., Nickerson D.A., Bamshad M.J., et al. A variant in LMX1A causes autosomal recessive severe-to-profound hearing impairment. Hum. Genet. 2018;137:471–478. doi: 10.1007/s00439-018-1899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S.B., Ranum P.T., Moteki H., Pan B., Goodwin A.T., Goodman S.S., Abbas P.J., Holt J.R., et al. RNA interference prevents autosomal-dominant hearing loss. Am. J. Hum. Genet. 2016;98:1101–1113. doi: 10.1016/j.ajhg.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B.R., Chia C., Wu L., Kujawa S.G., Liberman M.C., Goodrich L.V. Sensory neuron diversity in the inner ear is shaped by activity. Cell. 2018;174:1229–1246. doi: 10.1016/j.cell.2018.07.007. e1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffes G., Lorente-Cánovas B., Pearson S., Brooker R.H., Spiden S., Kiernan A.E., Guénet J.L., Steel K.P. Mutanlallemand (mtl) and Belly Spot and Deafness (bsd) are two new mutations of Lmx1a causing severe cochlear and vestibular defects. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Babola T., Pregernig G., So K.S., Nguyen M., Su S.-S.M., Palermo A.T., Bergles D.E., et al. Hair cell mechanotransduction regulates spontaneous activity and spiral ganglion subtype specification in the auditory system. Cell. 2018;174:1247–1263. doi: 10.1016/j.cell.2018.07.008. e1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wang L., Zhu T., Wu B., Wang G., Luo Z., Li C., Wei W., et al. Single-cell transcriptomic landscapes of the otic neuronal lineage at multiple early embryonic ages. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110542. [DOI] [PubMed] [Google Scholar]
- Tarchini B., Jolicoeur C., Cayouette M. A molecular blueprint at the apical surface establishes planar asymmetry in cochlear hair cells. Dev. Cell. 2013;27:88–102. doi: 10.1016/j.devcel.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Tomás-Roca L., Corral-San-Miguel R., Aroca P., Puelles L., Marín F. Crypto-rhombomeres of the mouse medulla oblongata, defined by molecular and morphological features. Brain Struct. Funct. 2016;221:815–838. doi: 10.1007/s00429-014-0938-y. [DOI] [PubMed] [Google Scholar]
- Von Kupffer C The development of the cranial nerves of vertebrates. J. Comp. Neurol. 1891;1:246–264. [Google Scholar]
- Wang V.Y., Rose M.F., Zoghbi H.Y. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Watson C., Shimogori T., Puelles L. Mouse Fgf8–Cre‐LacZ lineage analysis defines the territory of the postnatal mammalian isthmus. J. Comp. Neurol. 2017;525:2782–2799. doi: 10.1002/cne.24242. [DOI] [PubMed] [Google Scholar]
- Wesdorp M., de Koning Gans P.A.M., Schraders M., Oostrik J., Huynen M.A., Venselaar H., Beynon A.J., van Gaalen J., et al. Heterozygous missense variants of LMX1A lead to nonsyndromic hearing impairment and vestibular dysfunction. Hum. Genet. 2018;137:389–400. doi: 10.1007/s00439-018-1880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K.D., Rogers A.A.M., Zhang J., Shim K. Cooperative and independent functions of FGF and Wnt signaling during early inner ear development. BMC Dev. Biol. 2015;15:1–15. doi: 10.1186/s12861-015-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M., Maklad A., Pirvola U., Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003;4:2. doi: 10.1186/1471-2202-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Li J., Zhang T., Jiang H., Ramakrishnan A., Fritzsch B., Shen L., Xu P.X. Chromatin remodelers and lineage-specific factors interact to target enhancers to establish proneurosensory fate within otic ectoderm. PNAS. 2021;118:1–12. doi: 10.1073/pnas.2025196118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- y Cajal S.R. Histologie du système nerveux de l'homme & des vertébrés: Cervelet, cerveau moyen, rétine, couche optique, corps strié, écorce cérébrale générale & régionale, grand sympathique. A. Maloine. 1911 [Google Scholar]