Abstract
We describe standardization of an enzyme-linked immunosorbent assay (ELISA) for measuring immunoglobulin G1 (IgG1) and IgG2 concentrations of antibodies to pneumococcal capsular polysaccharide (Pnc PS). The ELISA uses a human pneumococcal reference serum pool, lot 89-SF, as a reference. IgG1 and IgG2 concentrations were assigned to antibodies to Pnc PS serotypes 3, 6B, 14, 19F, and 23F in 89-SF by ELISA using affinity-purified monoisotypic IgG1 and IgG2 preparations. The sum of IgG1 and IgG2 concentrations in 89-SF agrees well with the previously assigned IgG concentrations. The IgG1 and IgG2 values in 89-SF were used to measure antibodies to Pnc PS 6B, 14, and 23F in adult pre- and postimmunization sera and the sum of IgG1 and IgG2 concentrations correlated well with the IgG values. Furthermore, the IgG2/IgG1 ratio did not affect the detection of IgG1, the isotype usually represented by a lower concentration.
Streptococcus pneumoniae is an important cause of respiratory infections and serious invasive diseases (6, 8, 13). It is known that antibodies to capsular polysaccharides (PS) of pneumococci provide protection against disease, and pneumococcal vaccines are developed to induce antibodies to PS. However, PS vaccines are not immunogenic in infants, and thus new saccharide-protein conjugate vaccines with improved immunogenicity are under evaluation (11).
Immunoglobulin G (IgG) subclass determinations are important for many reasons. Vaccination with plain PS such as pneumococcal PS (Pnc PS) elicits antibodies that are restricted to the rare IgG2 subclass in humans (3, 7, 22, 27), whereas antibodies to proteins are predominantly IgG1 (18, 25). Several studies have suggested that saccharide-protein conjugate vaccines can also induce antibodies of the IgG1 subclass (15, 23, 24), which is typical for responses to T-cell-dependent antigens. To study T-cell dependency of the response to conjugate vaccines, it would thus be informative to measure subclass responses as well. Furthermore, functional differences between subclasses (2, 5) and thus determination of IgG subclasses help to evaluate functional activity of antibodies to capsular PS. However, several reports show that it is not clear whether a particular IgG subclass is necessary for defense against encapsulated bacteria or if all subclasses provide equivalent levels of protection (12, 14, 28). Moreover, quantification of IgG subclass concentration is important in the diagnosis of individuals with specific antibody deficiencies (16).
Standardization of solid-phase methods for determination of subclass composition of antibodies has been difficult because properly standardized isotype-specific reagents and a standard serum with assigned weight-based units of different subclasses have been missing. IgG subclasses of antibodies measured by solid-phase methods have usually been expressed as enzyme immunoassay units of test sera at a specific optical density (OD) as compared to a standard serum. However, enzyme immunoassay units are not comparable except within well planned experiments because different subclass-specific second antibodies can have different affinities for the first antibody, leading to under- or overestimation of subclasses (25). Several methods have been used for quantitation of immunoglobulin isotypes, including measurement of concentrations of isotypes after purification of specific antibody (26) or measurement of specific antibody after physical separation of immunoglobulin isotypes (4). Myeloma proteins attached to plastic surfaces have been used as standards (10, 18, 20), but this method gives erroneously high results because of partial unavailability of epitopes (17). Moreover, purified isotype fractions have served as standards in solid-phase methods in which the affinity differences between different reagents have been corrected by coefficients (25, 26). Calibration of human reference sera can also be done by heterologous interpolation of the specific antibody response with dose-response curves generated with heterologous engineered human-mouse chimeric antibody (9).
An approach for measuring IgG subclass concentrations of anti-Pnc PS antibodies by an enzyme-linked immunosorbent assay (ELISA) is presented here. Comparison of ELISA results could be facilitated by using an international reference serum. Therefore, determination of anti-Pnc PS IgG1 and IgG2 antibody concentrations in a pneumococcal reference serum, lot 89-SF (21), allows interlaboratory standardization of subclass assays. Furthermore, it allows comparison of subclasses within a serotype and between serotypes. Finally, the IgG1 and IgG2 values of anti-Pnc PS 3, 6B, 14, 19F, and 23F present in reference serum 89-SF (21) determined in this study were used to quantitate IgG1 and IgG2 concentrations of antibodies to Pnc PS types 6B, 14, and 23F in sera of healthy adults after vaccination with the 23-valent polysaccharide vaccine or with one of the four conjugate vaccines. The sum of IgG1 and IgG2 concentrations was compared with the IgG values determined by a standard IgG ELISA.
MATERIALS AND METHODS
Sera.
Pneumococcal reference serum lot 89-SF is a pool of postvaccination adult sera. It was provided by Carl Frasch, CBER, Food and Drug Administration, Bethesda, Md. IgG antibody concentrations to Pnc PS in the reference serum have been previously described (21).
Adult sera were obtained from 46 healthy adults before and 1 month after vaccination (27a). Fourteen of them received one dose of 23-valent polysaccharide vaccine (Merck, Sharp & Dohme, West Point, Pa.), 10 of them received pentavalent oligosaccharide conjugate PncCRM (Lederle-Praxis Biologicals, West Henrietta, N.Y.) (1), 10 of them received PnT (PASTEUR-MERIEUX Sérums & Vaccins, Lyon, France) (19), and 12 of them received PncD (Connaught Laboratories Inc., Swiftwater, N.Y.) (19). Sera were stored at −20°C until tested.
Monoclonal antibodies against human IgG subclasses.
Mouse monoclonal antibodies to human IgG1 (clone HP6070) and IgG2 (clone HP6002) were purchased from Zymed Laboratories, Inc. (San Francisco, Calif.). The ascitic fluids containing antibodies to IgG1 (HP6070, 2C7), IgG2 (1E4), IgG3 (2F5), and IgG4 (IC2) were from I. Seppälä (HUCH Diagnostics, Helsinki, Finland).
Separation of IgG subclasses from human IgG by protein A-Sepharose chromatography.
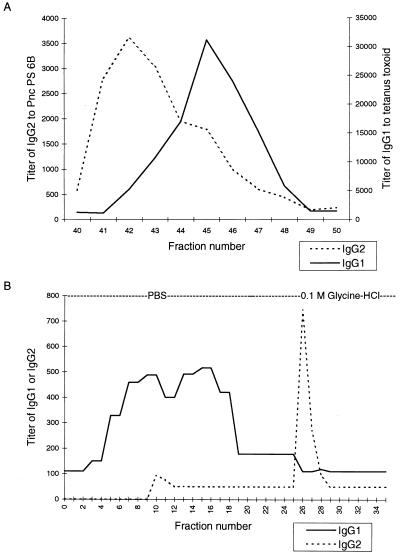
The separation of IgG subclasses from human IgG is summarized in Fig. 1. Two columns (5 mm by 21 cm) of protein A-Sepharose (Pharmacia Fine Chemicals, Uppsala, Sweden) were set in tandem and washed with 0.1 M sodium citrate buffer (pH 7.0) overnight. Eighty milligrams of a human immunoglobulin infusion substance known to contain anti-Pnc PS antibodies (Sandoglobulin; protein, 96% IgG; Sandoz, Sa Bale, Switzerland), filtered and neutralized with 1/10 volume of 1 M Tris-HCl (pH 7.6), was applied. Columns were washed with 0.1 M sodium citrate buffer (pH 7.0) until the first protein peak had appeared. A linear pH gradient from 0.1 M sodium citrate (pH 7.0) to 0.1 M sodium citrate (pH 3.0), monitored at 277 nm with high-performance liquid chromatography equipment (LKB, Bromma, Sweden), was then applied. Fractions were analyzed for protein content and, after neutralization with 1 M Tris-HCl (pH 8.3), for the distribution of IgG subclasses by ELISA (Fig. 2A). A Pnc PS type 6B ELISA was used for IgG2 and a tetanus toxoid (Tt) ELISA was used for IgG1 because it is known that anti-Pnc PS 6B antibodies are mainly of the IgG2 subclass and anti-Tt antibodies are mainly of the IgG1 subclass (3, 7, 21, 24, 26). Fractions with a higher ELISA activity of IgG1 than of IgG2 (fractions 45 to 48 in Fig. 2A) were pooled for further purification; this IgG1-enriched preparation was then applied to an anti-IgG2 column (see below) for fractionation of IgG1 and IgG2.
FIG. 1.
Steps in purification of monoisotypic IgG1 and IgG2 preparations and determination of IgG1 and IgG2 anti-Pnc PS in pneumococcal reference serum 89-SF. Those steps marked with a superscript “a” were followed by determination of IgG1 titer to Tt and IgG2 titer to Pnc PS 6B (Fig. 2) and subsequent pooling of the fractions into subclass preparations.
FIG. 2.
(A) Separation of IgG1 and IgG2 from human IgG by protein A-Sepharose chromatography. Each fraction was analyzed for titer of IgG1 antibody to Tt and for titer of IgG2 antibody to Pnc PS 6B. Fractions 45 to 48 were pooled (IgG1-enriched preparation) and purified further by anti-IgG2 affinity chromatography. (B) Purification of IgG1 and IgG2 by anti-IgG2 affinity chromatography. Fractions 4 to 19 and 26 to 31 were pooled to create an IgG1 preparation and an IgG2 preparation, respectively.
Purification of IgG1 and IgG2 preparations by anti-IgG2 affinity chromatography.
IgG1 and IgG2 preparations were derived from the IgG1-enriched preparation from protein A-Sepharose chromatography (Fig. 1). A mouse monoclonal antibody specific for an epitope common to human IgG2 and IgG4 (1E4) was used for affinity purification of IgG1 and IgG2 preparations. A total of 79.7 mg of the ascitic fluid, after ultracentrifugation at 178,000 × g for 20 min for removal of lipoproteins, was coupled to cyanogen bromide-activated Sepharose 4B (Pharmacia Biotech, Uppsala, Sweden) as described in the instructions of the manufacturer with a final matrix volume of 1.5 ml. A volume of 330 μl (protein, 5 mg) of protein A-Sepharose IgG1-enriched preparation was applied to the column. Fall-through fractions containing unbound IgG1 were collected by using phosphate-buffered saline (PBS). IgG2 was eluted by 0.1 M glycine-HCl (pH 4.0 to 3.0). Fractions were analyzed for protein content and pH and neutralized as described above. The purity of IgG1 and IgG2 in the affinity chromatography fractions (Fig. 2B) was analyzed by ELISA as described above. The mainly IgG1 and mainly IgG2 fractions were first pooled to create an IgG1 preparation (fractions 4 to 19) and an IgG2 preparation (fractions 26 to 31), respectively. Then, anti-Pnc PS antibodies in these preparations were analyzed for monoisotypic purity by IgG subclass (IgG1 to -4) ELISA (see below).
ELISA for anti-Pnc PS IgG antibodies.
A standard ELISA was performed as described earlier by Käyhty et al. (11) with one exception: a different conjugate, an alkaline-phosphate conjugated anti-human IgG (Sigma Immuno Chemicals, St. Louis, Mo.), was used.
ELISA for Pnc PS IgG subclass antibodies.
An ELISA specific for IgG subclasses was a four-layer modification of the ELISA described earlier by Käyhty et al. (11). Microtiter plates (MaxiSorp; Nunc, Roskilde, Denmark) were coated with Pnc PS (American Type Culture Collection, Rockville, Md.) as described earlier (11). A microtiter plate coated with pneumococcal C-polysaccharide (CPS; Statens Seruminstitut, Copenhagen, Denmark) in PBS (CPS, 1 μg/ml) was included in each assay to ensure the efficacy of CPS neutralization. Sera were first diluted 1:100 in 10% fetal bovine serum in PBS (10% FBS) containing 10 μg of CPS per ml, and, after 30 min of incubation at room temperature, threefold dilutions were made. Microtiter plates were incubated sequentially with mouse monoclonal antibodies to human IgG subclasses for 2 h at 37°C, with alkaline-phosphatase conjugated rabbit anti-mouse antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) at 22°C overnight, and with p-nitrophenyl phosphate (Sigma Diagnostics, St. Louis, Mo.) for 30 min at 37°C.
The anti-Pnc PS IgG1 and IgG2 concentrations were calculated by using the subclass-specific concentrations in the pneumococcal reference serum 89-SF (Food and Drug Administration, Bethesda, Md.) (21) determined in this study (Fig. 1). The results are given in micrograms per milliliter. The lower detection limits were 0.08, 0.06, 0.08, 0.20, and 0.06 μg/ml for IgG1 and 0.14, 0.18, 0.26, 0.20, and 0.18 μg/ml for IgG2 in assays of Pnc PS types 3, 6B, 14, 19F, and 23F, respectively. Half the concentration of the lower limit of detection was used in cases where the concentration remained below the detection limit. The interassay variation was monitored by running two in-house control serum samples on every plate. The coefficients of variance (CV) were 13.5 and 18.1% for PS 3 (n = 8), 13.2 and 11.9% for PS 6B (n = 12), 16.0 and 15.2% for PS 14 (n = 12), 16.0 and 24.0% for PS 19F (n = 8), and 15.3 and 20.4% for PS 23F (n = 11) in the IgG1 and IgG2 assays, respectively.
To analyze the monoisotypic purity of Pnc PS antibodies in the IgG1 and IgG2 preparations, the IgG and IgG1 to -4 antibody titers to Pnc PS 3, 6B, 14, 19F, and 23F in the preparations were determined in ELISAs specific for IgG and IgG1 to -4. Serial dilutions starting at 1:2 were done in 10% FBS containing 20 μg of CPS per ml for all assays. The end point titers were assigned as a reciprocal of the dilution that gave an OD of 0.3.
ELISA for Tt IgG1 antibodies.
Because IgG1 is a minor isotype in PS antibodies and is the predominant isotype in antibodies to proteins, we monitored the success in purifying the IgG1 fraction in chromatography by a Tt-specific ELISA. Tt (National Public Health Institute, Helsinki, Finland) in PBS (15 lf [limit of flocculation]/ml) was used for coating microtiter plates (Greiner, Frickenhausen, Germany) by incubation for 2 h at 37 or 4°C overnight. After six washes with saline plus 0.02% Tween 20 and 0.9% NaCl, samples diluted in 10% FBS were added to microtiter plates. The samples were incubated for 2 h at room temperature, after which the plates were washed as described above. Mouse anti-human IgG1 (HP6070) was diluted in 10% FBS and incubated for 1 h at room temperature. The plates were washed as described above, a substrate, 4-nitrophenylphosphate (1 mg/ml) in 0.02 M diethanolamine buffer (pH 10.0), containing 1 mM MgCl2, was added, and the plates were incubated for 30 min. The A405 was measured by an ELISA reader (Multiscan; Labsystems, Helsinki, Finland).
Statistical methods.
The correlation coefficients were calculated with the Excel 7.0 program by using log-transformed data.
RESULTS AND DISCUSSION
Isotypic purity of the IgG1 and IgG2 preparations.
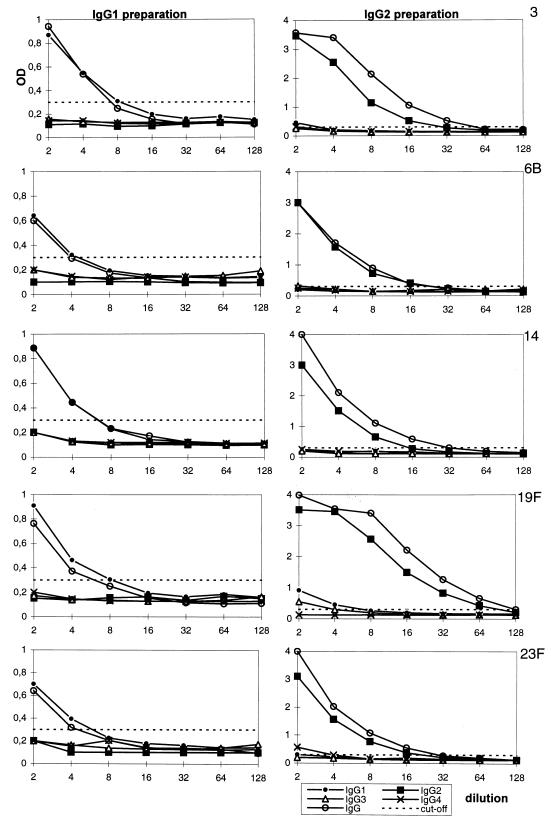
The aim of this study was to determine IgG1 and IgG2 concentrations of antibodies to pneumococcal serotypes in the internationally used pneumococcal reference serum pool lot 89-SF (21). In our approach, an IgG preparation known to contain antibodies to Pnc PS was fractionated into IgG1 and IgG2 preparations and then Pnc PS antibodies were determined (Fig. 1). The IgG1 and the IgG2 preparations were therefore run in ELISAs specific for IgG, IgG1, IgG2, IgG3, and IgG4 antibodies to Pnc PS types 3, 6B, 14, 19F, and 23F. To estimate the monoisotypic purity of the preparations, we compared titers of IgG1, IgG2, IgG3, and IgG4. Because different second antibodies can have different affinities and thus the titers of different subclasses cannot be directly compared, we used correction coefficients to correct the bias. The corrected titers obtained this way (Table 1) are comparable to each other regardless of the subclass because they are relative to the IgG reagent. The IgG and IgG1 to -4 curves and determination of the titers are illustrated in Fig. 3. The curve of the specific subclass titers was usually nearly identical to the curve of IgG titers. When the IgG1 preparation was titrated with anti-IgG and anti-IgG1 to -IgG4 antibodies, it was found that the titer of IgG1 was 1.25 times higher than the titer of IgG (mean of IgG1 determinations, all serotypes) and thus the IgG1 second antibody was 1.25 times more efficient in binding the first antibody than the IgG second antibody was. The titer of IgG1 (data not shown) was then divided by 1.25, the coefficient, to obtain the corrected titer (Table 1). It was easy to calculate the coefficient for IgG1 because no contaminating IgG2 to IgG4 antibodies were detected in the IgG1 preparation. Only antibodies to PS 19F had contaminating IgG4 with a low titer of 1. Because IgG4 did not yet have a coefficient, we made an assumption that the coefficient for IgG4 was 1. A titer of 1 for IgG4 obtained in this way had to be subtracted from the titer of anti-PS 19F IgG before calculation of the IgG1/IgG ratio. Thereafter, the coefficient for IgG2 was calculated. Because the IgG2 preparation was less pure than the IgG1 preparation, the IgG1, IgG3, and IgG4 titers had to be subtracted from the titer of IgG before calculation of the IgG2/IgG ratio. The correction coefficient for the IgG2 antibody was calculated to be 0.76 (mean of IgG2 determinations, all serotypes). A coefficient of 0.76 was then used to convert the titer of IgG2 (data not shown) to a corrected titer (Table 1). This calculation was based on the assumption that the coefficients for IgG3 and IgG4 were 1. In previous studies (23, 25), a higher detection efficiency was found for the IgG3 and IgG4 reagents than for the IgG1 reagent. This implies that the contamination by IgG3 or IgG4 antibodies cannot be seriously underestimated; more likely, it is slightly overestimated. The IgG1 and IgG2 data do not lose their validity because of this small uncertainty. After summing up the corrected titers of all subclasses, we found the mean contamination to be 3% in the IgG1 preparation and 10% in the IgG2 preparation (Table 1). Based on this, we conclude that these preparations were pure enough to be used with good accuracy as monoisotypic references.
TABLE 1.
Corrected titers of IgG1, IgG2, IgG3, and IgG4 and sum of the titers of IgG1 to -4 antibodies to Pnc PS 3, 6B, 14, 19F, and 23F in the affinity-purified IgG1 and IgG2 preparationsa
| Purified IgG subclass preparation | Pnc PS type | Corrected titer
|
Sum of titers of IgG1 to -4 | IgG subclass titer as % of the sum of IgG1 to -4 titers | |||
|---|---|---|---|---|---|---|---|
| IgG1 | IgG2 | IgG3 | IgG4 | ||||
| IgG1 | 3 | 6.4 | 0 | 0 | 0 | 6.4 | 100 |
| 6B | 3.2 | 0 | 0 | 0 | 3.2 | 100 | |
| 14 | 4.8 | 0 | 0 | 0 | 4.8 | 100 | |
| 19F | 6.4 | 0 | 0 | 1 | 7.4 | 86 | |
| 23F | 4.8 | 0 | 0 | 0 | 4.8 | 100 | |
| Meanb | 97 | ||||||
| IgG2 | 3 | 2.4 | 36.7 | 2 | 2 | 43.1 | 85 |
| 6B | 0 | 30.2 | 2 | 1 | 33.2 | 91 | |
| 14 | 0 | 19.7 | 0 | 0 | 19.7 | 100 | |
| 19F | 5.6 | 121.9 | 4 | 0 | 131.5 | 93 | |
| 23F | 1.6 | 27.5 | 1 | 4 | 34.1 | 81 | |
| Meanb | 90 | ||||||
The purity of the preparations was determined as a proportion of the titer of the specific subclass from the sum of the titers of IgG1 to -4 subclasses.
Proportion of the specific subclass (IgG1 or IgG2) from the sum of titers of IgG1 to -4 (mean of all Pnc PS types).
FIG. 3.
Titration of IgG and IgG1 to -4 antibodies in the IgG1 and IgG2 preparations to Pnc PSs 3, 6B, 14, 19F, and 23F. To calculate the correction coefficients for subclass-specific second antibodies, the end point titers of IgG and IgG1 to -4 were assigned as a reciprocal of the dilution that gave an OD of 0.3.
Assignment of IgG1 and IgG2 concentrations of Pnc PS antibodies to the reference serum 89-SF.
The monoisotypic preparations were then used as references in an ELISA to assign IgG1 and IgG2 concentrations of Pnc PS-specific antibodies in reference serum 89-SF. First, a standard ELISA was used in three or four separate experiments to measure the IgG concentration of antibodies to Pnc PS 3, 6B, 14, 19F, and 23F in the affinity-purified IgG1 and IgG2 preparations, and the mean values were equated with concentrations of IgG1 and IgG2 anti-Pnc PS present in the monoisotypic IgG1 and IgG2 preparations, respectively. Second, because of the limited supply of the IgG1 and IgG2 preparations, they were used as references in an IgG subclass-specific ELISA to determine the IgG1 and IgG2 Pnc PS concentrations in reference serum 89-SF (21). Again, each test was run at least three times and the means were assigned as the IgG subclass-specific concentrations (Table 2). CV for values assigned on different days usually remained below 20% (n = 3 to 4). The sum of IgG1 and IgG2 concentrations was consistent with the previously determined (21) IgG concentration to the same serotype and was 66 to 91% of the IgG concentration to serotypes 3, 6B, 14, 19F, and 23F (Table 2). Moreover, the IgG concentration determined earlier (21) may also contain IgG3 and IgG4 antibodies to Pnc PS that were not measured in the present study. The now-assigned values of IgG1 and IgG2 for antibodies to Pnc PS in reference serum 89-SF agree well with the previously assigned IgG values (21), which indicates that the IgG1 and IgG2 values for 89-SF have been reliably determined.
TABLE 2.
IgG1 and IgG2 antibody concentrations specific for Pnc PS 3, 6B, 14, 19F, and 23F in the pneumococcal reference serum lot 89-SF as determined by ELISA
| Pnc PS type | IgG1 concn (μg/ml)a (% CV) | IgG2 concn (μg/ml)a (% CV) | Sum of IgG1 and IgG2 concn (μg/ml)b | IgG concn assigned earlier (μg/ml)c |
|---|---|---|---|---|
| 3 | 0.41 ± 0.068 (16.5) | 1.65 ± 0.18 (11.0) | 2.06 (87) | 2.36 |
| 6B | 0.66 ± 0.036 (5.5) | 10.54 ± 1.95 (18.5) | 11.20 (66) | 16.90 |
| 14 | 2.87 ± 0.31 (10.7) | 21.23 ± 2.27 (10.7) | 24.11 (87) | 27.80 |
| 19F | 1.12 ± 0.39 (35.4) | 10.09 ± 0.89 (8.8) | 11.21 (86) | 13.00 |
| 23F | 0.73 ± 0.061 (8.3) | 6.66 ± 1.83 (27.6) | 7.39 (91) | 8.10 |
Antibody values are means ± standard deviations of three to four determinations.
Values do not include IgG3 and IgG4 concentrations. Values in parentheses represent sum of IgG1 and IgG2 concentrations relative to total IgG concentration as a percentage.
Data taken from reference 21.
Comparison of the IgG subclass-specific assay to the IgG-specific assay.
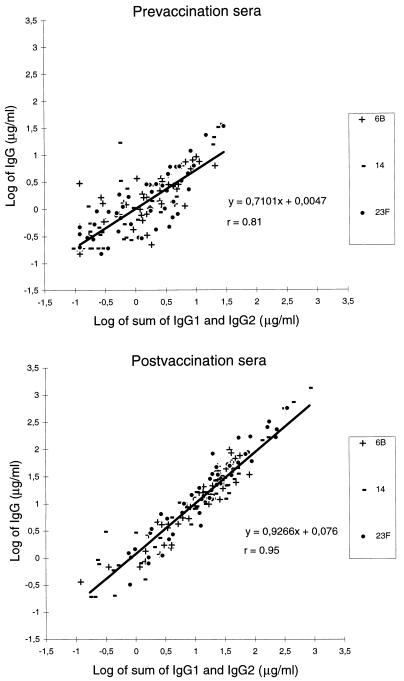
Adult pre- and postimmunization sera (27a) were quantitated for anti-Pnc PS 6B, 14, and 23F IgG1 and IgG2 concentrations by the IgG subclass-specific ELISA using the pneumococcal reference serum 89-SF as a reference with the anti-Pnc PS IgG1 and IgG2 values determined in this study (Table 2). The same sera were quantitated for IgG concentration by a standard ELISA using the pneumococcal serum pool 89-SF as a reference with the anti-Pnc PS IgG values determined earlier (21). The correlation between independently determined IgG concentrations and the sum of IgG1 and IgG2 concentrations measured by the IgG subclass-specific ELISA was determined. Correlation coefficients were 0.75, 0.84, and 0.80 for the preimmunization sera (n = 46) and 0.94, 0.96, and 0.95 for the postimmunization sera (n = 46) for anti-Pnc PS 6B, 14, and 23F, respectively (Fig. 4). Furthermore, a good correlation was observed for both IgG1-predominating (IgG2/IgG1, ≤1) and IgG2-predominating (IgG2/IgG1, >1) sera (r = 0.96, n = 19 and r = 0.93, n = 257, respectively; all serotypes together) when both all prevaccination and all postvaccination sera were combined. This suggests that there is no bias between the IgG assay and the IgG subclass assay and furthermore confirms that the IgG1 and IgG2 concentrations determined for 89-SF were reliably assigned.
FIG. 4.
Comparison of the sum of IgG1 and IgG2 concentrations determined by the IgG subclass ELISA with IgG concentrations of antibodies to Pnc PS 6B, 14, and 23F in adult pre- and postvaccination sera (all serotypes combined; n = 46 for each serotype).
Isotypic competition for binding to antigen.
We wanted to determine whether competition for antigen binding sites between IgG subclasses affects the isotype-specific results. The effect of excess IgG2 on the measurement of IgG1, the minor subclass in anti-PS IgG antibodies, was analyzed by mixing various amounts of an adult serum containing 22.77 μg of IgG2 antibodies and 0.04 μg of IgG1 antibodies, each per ml, to Pnc PS 14 (IgG2-predominating serum) and an adult serum containing 5.28 μg of IgG2 antibodies and 14.04 μg of IgG1 antibodies, each per ml, to Pnc PS 14 (IgG1-predominating serum) to produce the different IgG2/IgG1 ratios described in Table 3. Similar experiments were also performed with anti-Pnc PS IgG1- and IgG2-predominating sera for antigens 6B, 19F, and 23F. Suitable anti-Pnc PS 3-containing sera were not available. No inhibition of IgG1 binding to the antigen by IgG2 was found. Sera having an IgG2/IgG1 ratio between approximately 10 (range, 9.5 to 10.3) and 100 (range, 98.1 to 100.5) gave identical results in the IgG subclass ELISA specific for IgG1 (Table 3). As a result, the IgG subclass ELISA is not affected by competition for antigen binding sites between IgG subclasses, probably because PS antigens contain repetitive epitopes that offer multiple binding sites for antibodies. Therefore, values for IgG1, a rare subclass in antibodies to PS, were not underestimated.
TABLE 3.
Effect of excess serum IgG2 anti-Pnc PS on measurement of IgG1 anti-Pnc PS type 6B, 14, 19F, or 23Fa
| IgG2/IgG1 ratio | IgG1 concn (μg/ml) measured by IgG subclass ELISA
|
|||
|---|---|---|---|---|
| 6B | 14 | 19F | 23F | |
| <4b | 3.80 | 10.32 | 6.54 | 5.00 |
| 10 | 3.61 | 10.37 | 6.36 | 4.46 |
| 25 | 3.61 | 10.89 | 6.62 | 4.58 |
| 50 | 4.04 | 11.35 | 7.43 | 4.99 |
| 100 | 4.29 | 12.10 | 5.92 | |
Increasing amounts of IgG2-predominating adult sera were added to IgG1-predominating adult sera to produce the IgG2/IgG1 ratios described.
No excess serum IgG2 present. Results are the IgG1 anti-Pnc PS concentrations in the IgG1-predominating serum.
In conclusion, we have determined the IgG1 and IgG2 anti-Pnc PS concentrations in the pneumococcal reference serum. The standardized IgG subclass-specific ELISA correlated well with the standard IgG ELISA for the respective Pnc PS. These determinations can be used to measure IgG subclass concentrations of antibodies to Pnc PS.
ACKNOWLEDGMENTS
We thank Arja Vuorela, Hannele Lehtonen, Merja Anttila, and Leena Saarinen for excellent technical assistance and Pirjo Helena Mäkelä and Juhani Eskola for their helpful discussions during the writing of the manuscript.
REFERENCES
- 1.Åhman H, Käyhty H, Tamminen P, Vuorela A, Malinkoski F, Eskola J. Pentavalent pneumococcal oligosaccharide conjugate vaccine PncCRM is well-tolerated and able to induce an antibody response in infants. Pediatr Infect Dis J. 1996;15:134–139. doi: 10.1097/00006454-199602000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Amir J, Scott M G, Nahm M H, Granoff D M. Bactericidal and opsonic activity of IgG1 and IgG2 anticapsular antibodies to Haemophilus influenzae type b. J Infect Dis. 1990;162:163–171. doi: 10.1093/infdis/162.1.163. [DOI] [PubMed] [Google Scholar]
- 3.Barrett D J, Ayoub E M. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986;63:127–134. [PMC free article] [PubMed] [Google Scholar]
- 4.Bird P, Lowe J, Stokes R P, Bird A G, Ling N R, Jefferis R. The separation of human serum IgG into subclass fractions by immunoaffinity chromatography and assessment of specific antibody activity. J Immunol Methods. 1984;71:97–105. doi: 10.1016/0022-1759(84)90209-6. [DOI] [PubMed] [Google Scholar]
- 5.Bredius R G M, Driedijk P C, Schouten M F J, Weening R S, Out T A. Complement activation by polyclonal immunoglobulin G1 and G2 antibodies against Staphylococcus aureus, Haemophilus influenzae type b, and tetanus toxoid. Infect Immun. 1992;60:4838–4847. doi: 10.1128/iai.60.11.4838-4847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskola J, Takala A, Kela E, Pekkanen E, Kalliokoski R, Leinonen M. Epidemiology of invasive pneumococcal infections in children in Finland. JAMA. 1992;68:3323–3327. [PubMed] [Google Scholar]
- 7.Freijd A, Hammarström L, Persson M A A, Smith C I E. Plasma anti-pneumococcal antibody activity of the IgG class and subclasses in otitis prone children. Clin Exp Immunol. 1984;56:233–238. [PMC free article] [PubMed] [Google Scholar]
- 8.Gray B M, Converse G M, Dillon H C. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton R G. Application of engineered chimeric antibodies to the calibration of human antibody standards. Ann Biol Clin. 1990;49:242–248. [PubMed] [Google Scholar]
- 10.Hammarström L, Granström M, Oxelius V, Persson M A A, Smith C I E. IgG subclass distribution of antibodies against S. aureus teichoic acid and α-toxin in normal and immunodeficient donors. Clin Exp Immunol. 1984;55:593–601. [PMC free article] [PubMed] [Google Scholar]
- 11.Käyhty H, Åhman H, Rönnberg R-R, Tiilikainen R, Eskola J. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J Infect Dis. 1995;172:1273–1278. doi: 10.1093/infdis/172.5.1273. [DOI] [PubMed] [Google Scholar]
- 12.Käyhty H, Mäkelä O, Eskola J, Saarinen L, Seppälä I. Isotype distribution and bactericidal activity of antibodies after immunization with Haemophilus influenzae type b vaccines at 18-24 months of age. J Infect Dis. 1988;158:973–982. doi: 10.1093/infdis/158.5.973. [DOI] [PubMed] [Google Scholar]
- 13.Klein J O. The epidemiology of pneumococcal disease in infants and children. Rev Infect Dis. 1990;162:1316–1323. doi: 10.1093/clinids/3.2.246. [DOI] [PubMed] [Google Scholar]
- 14.Lortan J, Kaniuk A, Monteil M. Relationship of in vitro phagocytosis of serotype 14 Streptococcus pneumoniae to specific class and IgG subclass antibody levels in healthy adults. Clin Exp Immunol. 1993;91:54–57. doi: 10.1111/j.1365-2249.1993.tb03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mäkelä O, Péterfy F, Outschoorn I G, Richter A W, Seppälä I. Immunogenic properties of α(1→6) dextran, its protein conjugates, and conjugates of its breakdown products in mice. Scand J Immunol. 1984;19:541–550. doi: 10.1111/j.1365-3083.1984.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 16.Matter L, Wilhelm J A, Angehrn W, Skvaril F, Schopfer K. Selective antibody deficiency and recurrent pneumococcal bacteremia in a patient with Sjögren’s syndrome, hyperimmunoglobulinemia G, and deficiencies of IgG2 and IgG4. N Engl J Med. 1985;312:1039–1042. doi: 10.1056/NEJM198504183121607. [DOI] [PubMed] [Google Scholar]
- 17.Mattila P S. Quantitation of antibody isotypes in solid-phase assays. Comparison of myeloma protein and monoisotypic antibody standards. J Immunol Methods. 1985;83:43–53. doi: 10.1016/0022-1759(85)90056-0. [DOI] [PubMed] [Google Scholar]
- 18.Morell A, Roth-Wicky B, Skvaril F. Immunoglobulin G subclass restriction of antibodies against hepatitis B surface antigen. Infect Immun. 1983;39:565–568. doi: 10.1128/iai.39.2.565-568.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieminen T, Eskola J, Käyhty H. Pneumococcal conjugate vaccination in adults: circulating antibody secreting cell response and humoral antibody responses in saliva and in serum. Vaccine. 1998;16:630–636. doi: 10.1016/s0264-410x(97)00235-1. [DOI] [PubMed] [Google Scholar]
- 20.Persson M A A, Hammarström L, Smith C I E. Enzyme-linked immunosorbent assay for subclass distribution of human IgG and IgA antigen-specific antibodies. J Immunol Methods. 1985;78:109–121. doi: 10.1016/0022-1759(85)90334-5. [DOI] [PubMed] [Google Scholar]
- 21.Quataert S A, Kirch C S, Quackenbush Wiedl L J, Phipps D C, Strohmeyer S, Cimino C O, Skuse J, Madore D C. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarvas H, Rautonen N, Sipinen S, Mäkelä O. IgG subclasses of pneumococcal antibodies—effect of allotype G2m(n) Scand J Immunol. 1989;29:229–237. doi: 10.1111/j.1365-3083.1989.tb01120.x. [DOI] [PubMed] [Google Scholar]
- 23.Seppälä I, Sarvas H, Mäkelä O, Mattila P, Eskola J, Käyhty H. Human antibody responses to two conjugate vaccines of Haemophilus influenzae type b saccharides and diphtheria toxin. Scand J Immunol. 1988;28:471–479. doi: 10.1111/j.1365-3083.1988.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 24.Seppälä I, Pelkonen J, Mäkelä O. Isotypes of antibodies by plain dextran or a dextran-protein conjugate. Eur J Immunol. 1985;15:827–833. doi: 10.1002/eji.1830150816. [DOI] [PubMed] [Google Scholar]
- 25.Seppälä I J T, Routonen N, Sarnesto A, Mattila P A, Mäkelä O. The percentages of six immunoglobulin isotypes in human antibodies to tetanus toxoid: standardization of isotype-specific second antibodies in solid-phase assay. Eur J Immunol. 1984;14:868–875. doi: 10.1002/eji.1830140918. [DOI] [PubMed] [Google Scholar]
- 26.Shackelford P G, Granoff D M, Nelson S J, Scott M G, Smith D S, Nahm M H. Subclass distribution of human antibodies to Haemophilus influenzae type b capsular polysaccharide. J Immunol. 1987;138:587–592. [PubMed] [Google Scholar]
- 27.Siber G R, Schur P H, Aisenberb A C, Weitzman S A, Schiffman G. Correlation between serum IgG2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980;303:178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- 27a.Soininen, A., et al. Submitted for publication.
- 28.Weinberg G, Granoff D, Nahm M, Shackelford P. Functional activity of different IgG subclass antibodies against type b capsular polysaccharide of Haemophilus influenzae. J Immunol. 1986;136:4232–4236. [PubMed] [Google Scholar]