Abstract
Chronic wound healing remains a challenging medical problem affecting society, which urgently requires anatomical and functional solutions. Adipose-derived stem cells (ADSCs), mesenchymal stem cells with self-renewal and multiple differentiation ability, play essential roles in wound healing and tissue regeneration. The exosomes from ADSCs (ADSC-EXOs) are extracellular vesicles that are essential for communication between cells. ADSC-EXOs release various bioactive molecules and subsequently restore tissue homeostasis and accelerate wound healing, by promoting various stages of wound repair, including regulating the inflammatory response, promoting wound angiogenesis, accelerating cell proliferation, and modulating wound remodeling. Compared with ADSCs, ADSC-EXOs have the advantages of avoiding ethical issues, being easily stored, and having high stability. In this review, a literature search of PubMed, Medline, and Google Scholar was performed for articles before August 1, 2022 focusing on exosomes from ADSCs, chronic wound repair, and therapeutic potential. This review aimed to provide new therapeutic strategies to help investigators explore how ADSC-EXOs regulate intercellular communication in chronic wounds.
Keywords: adipose-Derived stem cells (ADSCs), exosome (EXO), chronic wounds, wound healing, therapeutic potential
Background
The skin, the largest organ in humans, is a natural physical barrier against external stimulation (1). Loss of the balance between humans and the environment as a result of illness or trauma may result in substantial skin damage or even death (2, 3). Chronic wounds are long-lasting wounds that fail to achieve complete anatomical and functional repair through the normal healing process after 1 month of clinical treatment (4, 5). Chronic wounds, including vascular ulcers (venous and arterial ulcers), pressure ulcers, and diabetic foot ulcers, have complex pathogenesis and long disease courses, and are associated with high disability rates (6). Common features of chronic wounds include persistent bacterial biofilms, defective re-epithelization, decreased angiogenesis, and delayed extracellular matrix (ECM) remodeling (7, 8). Approximately 2.5% of the population in the United States is affected by chronic wounds (9, 10). According to conservative estimates, nearly $32 billion is spent on wound care, thus placing substantial pressure on the economy and healthcare system (11).
Many advanced therapies have been advocated as being effective for chronic wounds, such as negative pressure wound therapy; hyperbaric oxygen treatment; and biophysical, biological, and bioengineered therapies (4). Adipose-derived stem cells (ADSCs) are considered the most advantageous therapy for present-day regenerative medicine, given the abundant sources of adipose tissue, and the cells' outstanding proliferative ability and convenient isolation. ADSCs secrete paracrine factors and differentiate into multiple lineages (12, 13).
Exosomes from ADSCs (ADSC-EXOs) are small, single-membrane nanovesicles released by ADSCs through paracrine secretion, and are enriched in proteins, lipids, and nucleic acids (14–16) (Figure 1). As critical mediators of intercellular communication, they can alter the behaviors of recipient cells by transmitting signals and transporting molecules into target cells. Recent studies have shown that ADSC-EXOs had therapeutic effects in many aspects of disease, including wound healing (17, 18), organ diseases (19, 20), neurodegenerative diseases (21, 22), and cancer (23, 24). In regeneration and wound healing, ADSC-EXOs modulate persistent inflammation, angiogenesis, and ECM reconstruction. ADSC-EXO have functions resembling those of the parental stem cells, but are safer and more efficient in clinical applications, thus decreasing cell transplantation risks (25, 26).
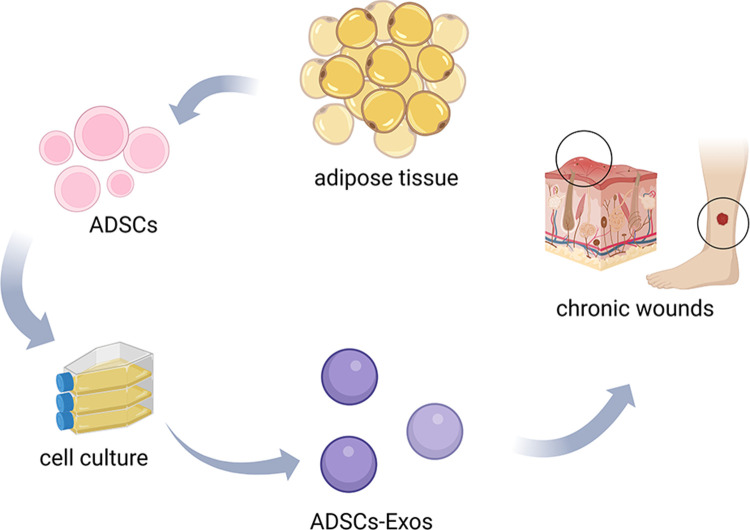
Figure 1.
The production and applications of ADSCs-EXOs. Extracted from adipose tissue, ADSCs were collected and processed to obtain ADSCs-EXOs by cell culture. ADSCs-EXOs can apply to the treatment of chronic wound healing.
Adipose-derived stem cells
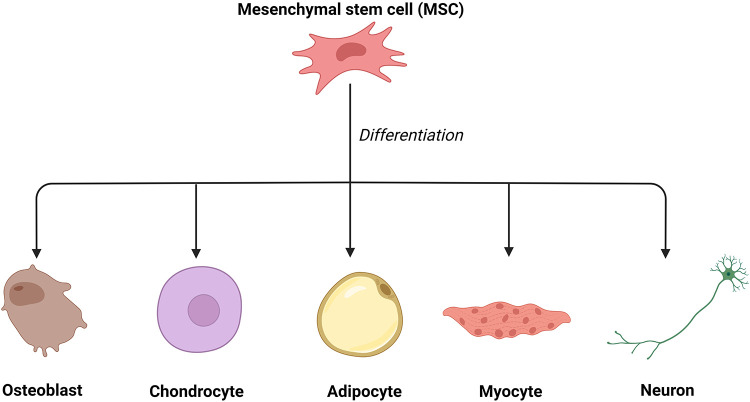
Mesenchymal stem cells (MSCs) have been a promising tool in tissue engineering and regenerative medicine (27, 28) since bone marrow mesenchymal stem cells (BMMSCs) were first discovered by Alexander Friedenstein in the late 1960s (29). MSCs have been successfully applied in corneal regeneration (30), wound healing, and skin rejuvenation (12, 28, 31). In their earlier studies, Friedenstein and colleagues demonstrated that MSCs, possibly derived from the mesoderm, can differentiate into various mesenchymal tissue lineages, such as osteoblasts, chondrocytes, adipocytes, myoblasts, and even neurons (32, 33) (Figure 2). In addition, these pluripotent cells can be isolated from a variety of tissues, such as adipose tissue, muscle, blood vessels, skin, and bone marrow (34, 35).
Figure 2.
The differentiation of mesenchymal stem cells. Mesenchymal stem cells can differentiate into various mesenchymal tissue lineages, such as osteoblasts, chondrocytes, adipocytes, myoblasts, and even neurons.
Among all these mesenchymal stem cells, ADSCs appear to be the most advantageous for clinical applications. Compared with other types of tissues, adipose tissue is available in relatively large quantities in humans. Furthermore, many ADSCs can be isolated from adipose tissue. Prior studies have indicated that 500 times more stem cells can be harvested from adipose tissue than from equal amounts of bone marrow (36, 37). In comparison with BMMSCs, ADSCs are relatively easily obtained, owing to their subcutaneous localization. Furthermore, patients tend to feel more comfortable with less donor site morbidity (12, 38). Moreover, ADSCs have higher proliferation ability than BMMSCs (39).
In recent studies, the roles of ADSCs in wound repair treatment have been confirmed. For example, ADSCs are crucial in wound repair in diabetic foot ulcers by enhancing VEGFR3-mediated lymphangiogenesis (40). In addition, ADSCs prevent scar formation and promote wound repair in skin-deficient mice by activating the PI3K/Akt pathway (41). However, barriers to the use of ADSCs must be addressed, such as their potential oncologic properties (42). Interestingly, ADSC-EXOs, the main factors through which ADSCs exert their biological effects (43), cannot actively contribute to tumorigenesis as adipose cell-free derivatives (44).
Characteristic of exosomes
Extracellular vesicles (EVs) are membrane-contained vesicles secreted by cells from multiple organisms (45). On the basis of their contents, size, and membrane composition, three primary subgroups of EVs have been defined: apoptotic bodies, microvesicles, and exosomes (46) (Figure 3). EVs generally refer to vesicles ranging from 150 to 1,000 nm released by budding from the plasma membrane (PM) (47). The term “exosomes” was initially used to describe 40–1,000-nm vesicles released by all types of cultured cells and exhibiting 5′-nucleotidase activity (48). Nevertheless, in the late 1980s, this term was used for small (30–100-nm) vesicles of endocytic origin, which are released outside cells as a result of the fusion of multivesicular bodies (MVBs) with the PM during reticulocyte differentiation (47, 49). Exosomes can be isolated from most biological fluids and cell types, such as saliva, urine, semen, nasal lavage fluid, plasma, and serum (50–53).
Figure 3.
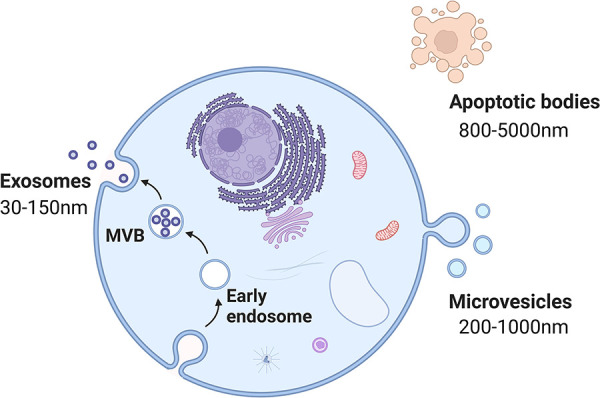
The biogenesis of each subtype of EVs. Apoptotic bodies (800–5,000 nm in size) are the result of the disintegration of apoptotic cells. Microvesicles (200–1,000 nm in size) arise from the plasma membrane. Exosomes (30–150 nm in size) originate from endosome.
Exosomes contain many functional proteins, mRNAs, microRNAs (miRNAs), and complete organelles (Figure 2), which are released into the cytoplasm and mediate communication among target cells through surface membrane proteins (54, 55).
As the primary mediators of information transmission, miRNAs regulate the genes of recipient cells through self-degradation and re-expression; consequently, exosomes secreted by different cells vary in their biological functions (56). Current research on stem cell paracrine factors has indicated that exosomes secrete thousands of nutritional factors, such as stem cell factors, insulin-like growth factor I, vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGF-β) (57). The uptake of exosomes by recipient cells occurs by fusion with the cell membrane, endocytosis, or receptor-ligand interaction. Adhesion-associated molecules on the surfaces of exosomes, such as glycoproteins, exosomal tetraspanin complexes, and integrins, determine the types of recipient cells (16, 58, 59).
Inflammatory functions of ADSC-EXOs
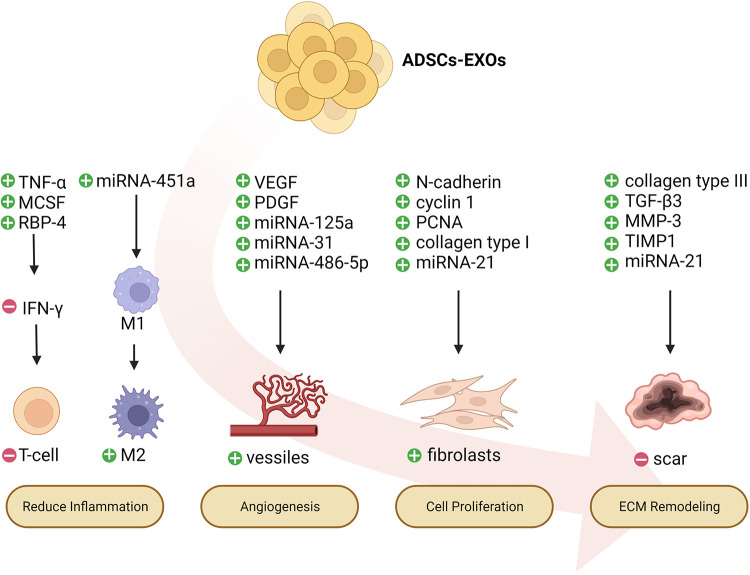
ADSC-EXOs have immunomodulatory and anti-inflammatory effects, which remove necrotic tissue and pathogenic microorganisms from wounds, and consequently control local damage (60–62). ADSC-EXOs inhibit the differentiation and activation of T cells, thus inhibiting the release of the inflammatory factor IFN-γ and the proliferation of T cells (63, 64). ADSC-EXOs decrease adipose inflammation and obesity by regulating the phenotypic polarization of macrophages (65). Moreover, miRNAs, small, endogenous, non-coding nucleotides contained in ADSC-EXOs, play major roles in regulating metabolism and cell growth (66). One study has found that miRNA-451a enriched in ADSC-EXOs successfully promotes M1-to-M2 polarization of macrophages by downregulating macrophage migration inhibitory factor (MIF) (67). MIF is a pleiotropic pro-inflammatory mediator that participates in immune regulation in vivo (68). Some studies have indicated that inhibition of MIF suppresses the activation of macrophages and the expression of inflammatory factors, such as NO, tumor necrosis factor-alpha (TNF-α), and IL-6, thereby decreasing inflammatory responses and ameliorating arthritis and articular cartilage injury (68–70). Furthermore, ADSC-EXOs release many immunomodulatory proteins, such as TNF-α, macrophage colony-stimulating factor (MCSF), and retinol-binding protein 4 (RBP-4) (71). Macrophages and proteolytic enzymes are released after the destruction of cells, and subsequently digest cell debris and necrotic tissue, thus providing a suitable environment for wound repair (72–74). ADSC-EXOs have been found to contribute to wound healing in rats with diabetic foot ulcers, particularly in the presence of overexpression of nuclear factor erythroid 2-associated factor 2 (NRF2). The expression of oxidative stress-associated proteins and inflammatory cytokines is diminished (75). ADSC-EXOs have comparable properties to those of their parent cells. They can improve graft retention by upregulating early inflammation and angiogenesis (76). Similarly, ADSC-EXOs upregulate the expression of macrophage inflammatory protein-1α and monocyte chemoattractant protein-1, thus promoting early inflammation (76). Further research on immunomodulation and anti-inflammation of ADSC-EXOs in chronic wounds is needed.
Angiogenesis regulation by ADSC-EXOs
Another function of ADSC-EXOs in wound repair is promoting angiogenesis, a dynamic process delivering sufficient nutrients and oxygen to the tissue. Emerging new capillaries, macrophages, and loose connective tissue contribute to granulation tissue formation. The elevated glucose levels in patients with diabetes can destroy the balance between vessel growth and maturation (77). In chronic wounds, perturbations in vascular integrity decrease vascularity and capillary density (78). VEGF, angiopoietin, fibroblast growth factor (FGF), and transforming growth factor (TGF) are key angiogenic cytokines in wound angiogenesis. Among these factors, VEGF-A is considered one of the most potent angiogenic factors in wounds (79). This protein, which is produced by many cells such as macrophages, binds its receptors on endothelial cells and subsequently induces migration, proliferation, and vessel growth. Mice deficient in VEGF-A 6, 7, or Flk1 8 succumb to a lack of angiogenesis early in development (80–82). ADSC-EXOs possess a higher ability to enhance angiogenesis in fat grafting by regulating VEGF/VEGF-R signaling and activating the protein kinase A (PKA) signaling pathway (83, 84). In addition to growth factors that mediate wound healing, such as VEGF-A and platelet-derived growth factor BB (PDGF-BB), ADSC-EXOs are enriched in miRNA-125a and miRNA-31 (85). ADSC-EXOs transfer miRNA-125a and miRNA-31 cargo into vascular endothelial cells (VECs), and consequently downregulate the expression of angiogenesis inhibitor Delta-like ligand 4 (DLL4), thereby promoting VEC proliferation and angiogenesis (86). Lu et al. have found that ADSC-EXOs containing miRNA-486-5p promote angiogenesis and accelerate cutaneous wound healing (87). In addition, ADSC-EXOs inhibit the overexpression of the anti-angiogenic gene hypoxia-inducible factor-1 (HIF-1) after chronic wound injury (88). We propose that ADSC-EXOs significantly affect angiogenesis, a possibility requiring further investigation.
Proliferation and ADSC-EXOs
ADSC-EXOs have a beneficial effect in accelerating cell proliferation, which is crucial for the treatment of chronic wounds. For example, exosomes from miRNA-199-3p-modified ADSCs contribute to the proliferation and migration of endothelial tip cells (89). Moreover, ADSC-EXOs facilitate osteosarcoma progression by increasing COLGALT2 expression in osteosarcoma cells (90). In the cell proliferation stage, fibroblasts stimulate wound healing by proliferating and synthesizing large amounts of ECM components, such as collagen and elastic fibers, under the stimulation of trauma (78). ADSC-EXOs are internalized by fibroblasts and affect cell migration, proliferation, and collagen synthesis by promoting gene expression of N-cadherin, cyclin 1, proliferating cell nuclear antigen, and collagen type I and III. In a dependent manner, higher doses of exosomes can achieve faster migration rates (91). Choi et al. have found that ADSC-EXOs promote the proliferation and differentiation of dermal fibroblasts through microRNAs inhibiting genes including NPM1, PDCD4, CCL5, and NUP62, thereby contributing to the regeneration of skin fibroblasts (92). Moreover, miRNA-21 is highly expressed in adipose-derived stem cell exosomes and has been found to enhance the migration and proliferation of HaCaT cells by increasing matrix metalloproteinase-9 expression through the PI3K/AKT pathway (93).
ECM remodeling and ADSC-EXOs
ADSC-EXOs regulate remodeling of the ECM. During the wound remodeling stage, fibroblasts differentiate into myofibroblasts, and the granulation tissue gradually becomes fibrotic; collagen gradually increases; the wound begins to contract; and scar tissue is eventually formed. ADSC-EXOs promote collagen remodeling through the synthesis of collagen types I and III in early stages of wound healing. In late stages, they inhibit collagen formation and decrease scarring (94). ADSC-EXOs prevent fibroblast-to-myofibroblast differentiation by increasing the ratio of collagen III to collagen I, as well as the ratio of TGF-β3 to TGF-β1. Moreover, ADSC-EXOs increase the expression of matrix metalloproteinase-3 (MMP3) in skin dermal fibroblasts by activating the ERK/MAPK pathway, thus resulting in a high ratio of MMP3 to tissue inhibitor of metalloprotease 1 (TIMP1), and facilitating remodeling of the ECM and diminished scarring during wound healing (92). More research on wound remodeling is needed to achieve the goals of clinical application of ADSC-EXOs.
Wound healing is a complex and dynamic physiological process that can generally be divided into four highly integrated and overlapping stages: hemostasis, inflammation, proliferation, and remodeling. These phases and their biophysiological functions must occur in an appropriate sequence at a specific time. Otherwise, interruptions, abnormalities, or prolongations in the process may result in delayed or chronic wound non-healing. ADSC-EXOs are extensively involved in the above-mentioned wound repair process through their release of various bioactive molecules (95, 96).
All types of wounds may begin as small cuts and have the potential to evolve into chronic wounds. The repair process of chronic wounds usually begins with a normal acute wound. Similar features can be found in chronic wounds, although they are classified into different categories according to etiology. Persistent infections, excessive levels of proinflammatory cytokines, as well as senescent cells that do not respond to reparative stimuli lead to chronic wounds. Definitive evidence has indicated that wound dressing along with ADSC-EXOs alleviates diabetic and infectious wound healing (97) (Figure 4).
Figure 4.
Potential mechanisms of ADSCs-EXOs regulating wound healing. ADSCs-EXOs might accelerate chronic wound repair by up-regulating early inflammation, promoting angiogenesis, enhancing proliferation, and regulating extracellular matrix (ECM) remodeling.
Challenges and prospects
The exosome field has advanced remarkably rapidly. Extensive evidence has indicated that ADSC-EXOs have robust effects on multiple stages of chronic wound repair tissue regeneration as critical mediators of intercellular communication. For example, in a diabetic mouse model of delayed wound healing, ADSC-EXOs enhance skin collagen production, angiogenesis, and cell proliferation; inhibit apoptosis; promote skin barrier function repair; and decrease inflammation in skin lesions (98). Given these properties, ADSC-EXOs may have promise in applications in chronic wound repair, skin anti-aging therapy, and scarless cutaneous repair. ADSC-EXOs show positive effects in preventing skin aging through protecting human dermal fibroblasts (HDFs) against ultraviolet B-induced photoaging damage (99, 100). Wang and colleagues have demonstrated that ADSC-EXOs promote wound repair in diabetic mice during angiogenesis and remodeling (101). In genetic therapy, ADSC-EXOs have been found to be a new therapeutic target for curing PD in patients (102).
Despite the positive results obtained in pre-clinical studies, multiple issues must be considered before clinical application of ADSC-EXOs. First, animal models for research are unable to fully reproduce the complexity of human chronic wounds, and clinical trials are scarce. Pigs or guinea pigs have skin structure somewhat similar to that in humans, thus providing a better animal model than mice in ADSC-EXO studies. Second, because they induce both effective pro-tumor and antitumor immune responses, ADSC-EXOs must be very carefully assessed in terms of safety and efficacy. Finally, technical issues such as extraction and purification of ADSC-EXOs must be simplified. The delivery and application methods are also worthy of consideration to achieve the development of large quantities of ADSC-EXOs capable of long-term storage of for clinical applications. Finally, although ADSC-EXOs appear to be a promising therapy for multiple diseases, further investigation and comprehensive information on isolating and identifying ADSC-EXOs is needed for widespread applications in clinical practice.
Conclusion
In summary, chronic wound repair is a well-orchestrated process involving numerous factors participating in a sequence of steps. ADSC-EXOs appear to be a potential therapeutic agent for chronic wounds by promoting various stages of wound healing, including decreased oxidative stress, increased neo-vascularization, enhanced collagen deposition, and less scarring. With continuing discoveries in this field, ADSC-EXOs involved in various biological functions are expected to hold promise for treating a wide variety of disorders.
Acknowledgments
The Figure was created in the web application BioRender.com (Available online: Biorender.com, accessed on 1 August 2022).
Funding
This work was funded by National Natural Science Foundation of China (No. 82172205), National Natural Science Foundation of China (No. 82272276), GuangDong Basic and Applied Basic Research Foundation (Nos. 2021A1515011453, 2022A1515012160), Special Fund for Science and Technology Innovation Strategy of Guangdong Province (No. 2020A1515011402), Medical Research Fund of Guangdong Province (No. A2020322) and Research Grant of Key Laboratory of Regenerative Medicine, Ministry of Education, Jinan University (No. ZSYXM202209).
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. (2002) 12(4):390–9. [PubMed] [Google Scholar]
- 2.Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. (2018) 16(3):143–55. 10.1038/nrmicro.2017.157 [DOI] [PubMed] [Google Scholar]
- 3.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. (2011) 9(4):244–53. 10.1038/nrmicro2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. (2015) 4(9):560–82. 10.1089/wound.2015.0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue M, Zhao R, Lin H, Jackson C. Delivery systems of current biologicals for the treatment of chronic cutaneous wounds and severe burns. Adv Drug Delivery Rev. (2018) 129:219–41. 10.1016/j.addr.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech. (2014) 7(11):1205–13. 10.1242/dmm.016782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y-Z, Gou M, Da L-C, Zhang W-Q, Xie H-Q. Mesenchymal stem cells for chronic wound healing: current status of preclinical and clinical studies. Tissue Eng Part B Rev. (2020) 26(6):555–70. 10.1089/ten.teb.2019.0351 [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Yang B, Tedesco A, Lebig EGD, Ruegger PM, Xu K, et al. High levels of oxidative stress and skin microbiome are critical for initiation and development of chronic wounds in diabetic mice. Sci Rep. (2019) 9(1):19318. 10.1038/s41598-019-55644-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones RE, Foster DS, Longaker MT. Management of chronic wounds-2018. JAMA. (2018) 320(14):1481–2. 10.1001/jama.2018.12426 [DOI] [PubMed] [Google Scholar]
- 10.Sen CK. Human wound and its burden: updated 2020 compendium of estimates. Adv Wound Care. (2021) 10(5):281–92. 10.1089/wound.2021.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. (2018) 21(1):27–32. 10.1016/j.jval.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 12.Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. (2011) 11(57):160–70. [PubMed] [Google Scholar]
- 13.Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—a review. Biotechnol Adv. (2018) 36(4):1111–26. 10.1016/j.biotechadv.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. (2021) 11(7):3183–95. 10.7150/thno.52570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. (2019) 88:487–514. 10.1146/annurev-biochem-013118-111902 [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. (2019) 1871(2):455–68. 10.1016/j.bbcan.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi R, Jin Y, Hu W, Lian W, Cao C, Han S, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. (2020) 318(5):C848–56. 10.1152/ajpcell.00041.2020 [DOI] [PubMed] [Google Scholar]
- 18.Shao M, Jin M, Xu S, Zheng C, Zhu W, Ma X, et al. Exosomes from long noncoding RNA-Gm37494-ADSCs repair spinal cord injury via shifting microglial M1/M2 polarization. Inflammation. (2020) 43(4):1536–47. 10.1007/s10753-020-01230-z [DOI] [PubMed] [Google Scholar]
- 19.Shen W, Zhao X, Li S. Exosomes derived from ADSCs attenuate sepsis-induced lung injury by delivery of circ-fryl and regulation of the miR-490-3p/SIRT3 pathway. Inflammation. (2022) 45(1):331–42. 10.1007/s10753-021-01548-2 [DOI] [PubMed] [Google Scholar]
- 20.Cao S, Huang Y, Dai Z, Liao Y, Zhang J, Wang L, et al. Circular RNA mmu_circ_0001295 from hypoxia pretreated adipose-derived mesenchymal stem cells (ADSCs) exosomes improves outcomes and inhibits sepsis-induced renal injury in a mouse model of sepsis. Bioengineered. (2022) 13(3):6323–31. 10.1080/21655979.2022.2044720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Jin M, Ji M, Zhang W, Liu A, Wang T. Hypoxic pretreatment of adipose-derived stem cell exosomes improved cognition by delivery of circ-Epc1 and shifting microglial M1/M2 polarization in an Alzheimer's Disease mice model. Aging (Albany NY). (2022) 14(7):3070–83. 10.18632/aging.203989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X, Pan J, Li Y, Jiang Y, Zheng H, Shi R, et al. Extracellular vesicles from adipose-derived stem cells promote microglia M2 polarization and neurological recovery in a mouse model of transient middle cerebral artery occlusion. Stem Cell Res Ther. (2022) 13(1):21. 10.1186/s13287-021-02668-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lou G, Chen L, Xia C, Wang W, Qi J, Li A, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. (2020) 39(1):4. 10.1186/s13046-019-1512-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang Z, Liu H, Zhang Y, Xiong L, Zeng Z, He X, et al. Cyr61 from adipose-derived stem cells promotes colorectal cancer metastasis and vasculogenic mimicry formation via integrin α β. Mol Oncol. (2021) 15(12):3447–67. 10.1002/1878-0261.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. (2018) 8(1):237–55. 10.7150/thno.21945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vu NB, Nguyen HT, Palumbo R, Pellicano R, Fagoonee S, Pham PV. Stem cell-derived exosomes for wound healing: current status and promising directions. Minerva Med. (2021) 112(3):384–400. 10.23736/S0026-4806.20.07205-5 [DOI] [PubMed] [Google Scholar]
- 27.Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res. (2014) 163(4):399–408. 10.1016/j.trsl.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 28.Nae S, Bordeianu I, Stăncioiu AT, Antohi N. Human adipose-derived stem cells: definition, isolation, tissue-engineering applications. Rom J Morphol Embryol. (2013) 54(4):919–24. [PubMed] [Google Scholar]
- 29.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. (2008) 2(4):313–9. 10.1016/j.stem.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saccu G, Menchise V, Giordano C, Delli Castelli D, Dastrù W, Pellicano R, et al. Regenerative approaches and future trends for the treatment of corneal burn injuries. J Clin Med. (2021) 10(2):317. 10.3390/jcm10020317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanson SE, Gutowski KA, Hematti P. Clinical applications of mesenchymal stem cells in soft tissue augmentation. Aesthet Surg J. (2010) 30(6):838–42. 10.1177/1090820X10386364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. (1997) 276(5309):71–4. 10.1126/science.276.5309.71 [DOI] [PubMed] [Google Scholar]
- 33.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. (2001) 7(6):259–64. 10.1016/S1471-4914(01)02016-0 [DOI] [PubMed] [Google Scholar]
- 34.Panchalingam KM, Jung S, Rosenberg L, Behie LA. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: a review. Stem Cell Res Ther. (2015) 6:225. 10.1186/s13287-015-0228-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couto P S, Rotondi MC, Bersenev A, Hewitt CJ, Nienow AW, Verter F, et al. Expansion of human mesenchymal stem/stromal cells (hMSCs) in bioreactors using microcarriers: lessons learnt and what the future holds. Biotechnol Adv. (2020) 45:107636. 10.1016/j.biotechadv.2020.107636 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Khan D, Delling J, Tobiasch E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. Sci World J. (2012) 2012:793823. 10.1100/2012/793823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong WK, Sugii S. Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol. (2013) 45(6):1083–6. 10.1016/j.biocel.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 38.Ding D-C, Chou H-L, Hung W-T, Liu H-W, Chu T-Y. Human adipose-derived stem cells cultured in keratinocyte serum free medium: Donor’s Age does not affect the proliferation and differentiation capacities. J Biomed Sci. (2013) 20:59. 10.1186/1423-0127-20-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barba M, Cicione C, Bernardini C, Michetti F, Lattanzi W. Adipose-derived mesenchymal cells for bone regereneration: state of the art. Biomed Res Int. (2013) 2013:416391. 10.1155/2013/416391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Wei T, He Z. ADSCs enhance VEGFR3-mediated lymphangiogenesis via METTL3-mediated VEGF-C mA modification to improve wound healing of diabetic foot ulcers. Mol Med. (2021) 27(1):146. 10.1186/s10020-021-00406-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Bai X, Zhao B, Li Y, Zhang Y, Li Z, et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. (2018) 370(2):333–42. 10.1016/j.yexcr.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 42.Behr B, Ko SH, Wong VW, Gurtner GC, Longaker MT. Stem cells. Plast Reconstr Surg. (2010) 126(4):1163–71. 10.1097/PRS.0b013e3181ea42bb [DOI] [PubMed] [Google Scholar]
- 43.Praveen Kumar L, Sangeetha K, Ranjita M, Vijayalakshmi S, Rajagopal K, Verma RS. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. (2019) 46:1–9. 10.1016/j.cytogfr.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 44.Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. (2020) 11(1):312. 10.1186/s13287-020-01831-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. (2015) 4:27066. 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. (2013) 2. 10.3402/jev.v2i0.20389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. (2014) 30:255–89. 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- 48.Trams EG, Lauter CJ, Salem N, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. (1981) 645(1):63–70. 10.1016/0005-2736(81)90512-5 [DOI] [PubMed] [Google Scholar]
- 49.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. (1987) 262(19):9412–20. 10.1016/S0021-9258(18)48095-7 [DOI] [PubMed] [Google Scholar]
- 50.Lässer C, O'Neil SE, Ekerljung L, Ekström K, Sjöstrand M, Lötvall J. RNA-containing exosomes in human nasal secretions. Am J Rhinol Allergy. (2011) 25(2):89–93. 10.2500/ajra.2011.25.3573 [DOI] [PubMed] [Google Scholar]
- 51.Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. (2009) 69(2):159–67. 10.1002/pros.20860 [DOI] [PubMed] [Google Scholar]
- 52.Pisitkun T, Shen R-F, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. (2004) 101(36):13368–73. 10.1073/pnas.0403453101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caby M-P, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. (2005) 17(7):879–87. 10.1093/intimm/dxh267 [DOI] [PubMed] [Google Scholar]
- 54.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. (2007) 9(6):654–9. 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- 55.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. (2008) 10(12):1470–6. 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. (2010) 107(14):6328–33. 10.1073/pnas.0914843107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X-Y, Zheng Z-H, Li X-Y, Guo J, Zhang Y, Li H, et al. Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: response and correction of immunological anomalies. Curr Pharm Des. (2013) 19(27):4893–9. 10.2174/13816128113199990326 [DOI] [PubMed] [Google Scholar]
- 58.Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. (2012) 44(9):1574–84. 10.1016/j.biocel.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 59.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. (2010) 70(4):1668–78. 10.1158/0008-5472.CAN-09-2470 [DOI] [PubMed] [Google Scholar]
- 60.Chang C-L, Sung P-H, Chen K-H, Shao P-L, Yang C-C, Cheng B-C, et al. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am J Transl Res. (2018) 10(4):1053–70. [PMC free article] [PubMed] [Google Scholar]
- 61.Seo Y, Kim H-S, Hong I-S. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. (2019) 2019:5126156. 10.1155/2019/5126156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cargnoni A, Papait A, Masserdotti A, Pasotti A, Stefani FR, Silini AR, et al. Extracellular vesicles from perinatal cells for anti-inflammatory therapy. Front Bioeng Biotechnol. (2021) 9:637737. 10.3389/fbioe.2021.637737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. (2014) 5:556. 10.3389/fimmu.2014.00556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolandi Z, Mokhberian N, Eftekhary M, Sharifi K, Soudi S, Ghanbarian H, et al. Adipose derived mesenchymal stem cell exosomes loaded with miR-10a promote the differentiation of Th17 and Treg from naive CD4 T cell. Life Sci. (2020) 259:118218. 10.1016/j.lfs.2020.118218 [DOI] [PubMed] [Google Scholar]
- 65.Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. (2018) 67(2):235–47. 10.2337/db17-0356 [DOI] [PubMed] [Google Scholar]
- 66.Yu X, Odenthal M, Fries JWU. Exosomes as miRNA carriers: formation-function-future. Int J Mol Sci. (2016) 17(12):2028. 10.3390/ijms17122028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li R, Li D, Wang H, Chen K, Wang S, Xu J, et al. Exosomes from adipose-derived stem cells regulate M1/M2 macrophage phenotypic polarization to promote bone healing via miR-451a/MIF. Stem Cell Res Ther. (2022) 13(1):149. 10.1186/s13287-022-02823-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Gu R, Jia J, Hou T, Zheng LT, Zhen X. Inhibition of macrophage migration inhibitory factor (MIF) tautomerase activity suppresses microglia-mediated inflammatory responses. Clin Exp Pharmacol Physiol. (2016) 43(11):1134–44. 10.1111/1440-1681.12647 [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Xu L, Zhang Z, Zhang Z, Zheng L, Li D, et al. Structure-activity relationships and anti-inflammatory activities of N-carbamothioylformamide analogues as MIF tautomerase inhibitors. J Chem Inf Model. (2015) 55(9):1994–2004. 10.1021/acs.jcim.5b00445 [DOI] [PubMed] [Google Scholar]
- 70.Zhang Z, Zhang R, Li L, Zhu L, Gao S, Lu Q, et al. Macrophage migration inhibitory factor (MIF) inhibitor, Z-590 suppresses cartilage destruction in adjuvant-induced arthritis via inhibition of macrophage inflammatory activation. Immunopharmacol Immunotoxicol. (2018) 40(2):149–57. 10.1080/08923973.2018.1424896 [DOI] [PubMed] [Google Scholar]
- 71.Kranendonk MEG, Visseren FLJ, van Balkom BWM, Nolte-'t Hoen ENM, van Herwaarden JA, de Jager W, et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). (2014) 22(5):1296–308. 10.1002/oby.20679 [DOI] [PubMed] [Google Scholar]
- 72.Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. (2017) 79:593–617. 10.1146/annurev-physiol-022516-034356 [DOI] [PubMed] [Google Scholar]
- 73.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. (2010) 30(3):245–57. 10.1055/s-0030-1255354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. (1985) 76(5):2003–11. 10.1172/JCI112200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X, Xie X, Lian W, Shi R, Han S, Zhang H, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. (2018) 50(4):1–17. 10.1038/s12276-018-0058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen B, Cai J, Wei Y, Jiang Z, Desjardins HE, Adams AE, et al. Exosomes are comparable to source adipose stem cells in fat graft retention with up-regulating early inflammation and angiogenesis. Plast Reconstr Surg. (2019) 144(5):816e–27e. 10.1097/PRS.0000000000006175 [DOI] [PubMed] [Google Scholar]
- 77.Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic wound-healing science. Medicina (Kaunas). (2021) 57(10):1072. 10.3390/medicina57101072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin P. Wound healing–aiming for perfect skin regeneration. Science. (1997) 276(5309):75–81. 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- 79.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. (2003) 9(6):669–76. 10.1038/nm0603-669 [DOI] [PubMed] [Google Scholar]
- 80.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. (1996) 380(6573):435–9. 10.1038/380435a0 [DOI] [PubMed] [Google Scholar]
- 81.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. (1995) 376(6535):62–6. 10.1038/376062a0 [DOI] [PubMed] [Google Scholar]
- 82.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. (1996) 380(6573):439–42. 10.1038/380439a0 [DOI] [PubMed] [Google Scholar]
- 83.Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. (2019) 109:59–68. 10.1016/j.biocel.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 84.Xue C, Shen Y, Li X, Li B, Zhao S, Gu J, et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells Dev. (2018) 27(7):456–65. 10.1089/scd.2017.0296 [DOI] [PubMed] [Google Scholar]
- 85.Hoang DH, Nguyen TD, Nguyen H-P, Nguyen X-H, Do PTX, Dang VD, et al. Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and xeno-free condition. Front Mol Biosci. (2020) 7:119. 10.3389/fmolb.2020.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. (2016) 129(11):2182–9. 10.1242/jcs.170373 [DOI] [PubMed] [Google Scholar]
- 87.Lu Y, Wen H, Huang J, Liao P, Liao H, Tu J, et al. Extracellular vesicle-enclosed miR-486-5p mediates wound healing with adipose-derived stem cells by promoting angiogenesis. J Cell Mol Med. (2020) 24(17):9590–604. 10.1111/jcmm.15387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, et al. Adipose-derived stem cells induce angiogenesis via microvesicle transport of miRNA-31. Stem Cells Transl Med. (2016) 5(4):440–50. 10.5966/sctm.2015-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du L, Li G, Yang Y, Yang G, Wan J, Ma Z, et al. Exosomes from microRNA-199-3p-modified adipose-derived stem cells promote proliferation and migration of endothelial tip cells by downregulation of semaphorin 3A. Int J Clin Exp Pathol. (2018) 11(10):4879–88. [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y, Chu Y, Li K, Zhang G, Guo Z, Wu X, et al. Exosomes secreted by adipose-derived mesenchymal stem cells foster metastasis and osteosarcoma proliferation by increasing COLGALT2 expression. Front Cell Dev Biol. (2020) 8:353. 10.3389/fcell.2020.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. (2016) 6:32993. 10.1038/srep32993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Choi EW, Seo MK, Woo EY, Kim SH, Park EJ, Kim S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp Dermatol. (2018) 27(10):1170–2. 10.1111/exd.13451 [DOI] [PubMed] [Google Scholar]
- 93.Yang C, Luo L, Bai X, Shen K, Liu K, Wang J, et al. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch Biochem Biophys. (2020) 681:108259. 10.1016/j.abb.2020.108259 [DOI] [PubMed] [Google Scholar]
- 94.Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HMA, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. (2017) 7(1):13321. 10.1038/s41598-017-12919-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. (2010) 89(3):219–29. 10.1177/0022034509359125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. (2003) 83(3):835–70. 10.1152/physrev.2003.83.3.835 [DOI] [PubMed] [Google Scholar]
- 97.Shiekh PA, Singh A, Kumar A. Data supporting exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Data Brief. (2020) 31:105671. 10.1016/j.dib.2020.105671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao B, Zhang X, Zhang Y, Lu Y, Zhang W, Lu S, et al. Human exosomes accelerate cutaneous wound healing by promoting collagen synthesis in a diabetic mouse model. Stem Cells Dev. (2021) 30(18):922–33. 10.1089/scd.2021.0100 [DOI] [PubMed] [Google Scholar]
- 99.Guo S, Wang T, Zhang S, Chen P, Cao Z, Lian W, et al. Adipose-derived stem cell-conditioned medium protects fibroblasts at different senescent degrees from UVB irradiation damages. Mol Cell Biochem. (2020) 463(1–2):67–78. 10.1007/s11010-019-03630-8 [DOI] [PubMed] [Google Scholar]
- 100.Li L, Ngo HTT, Hwang E, Wei X, Liu Y, Liu J, et al. Conditioned medium from human adipose-derived mesenchymal stem cell culture prevents UVB-induced skin aging in human keratinocytes and dermal fibroblasts. Int J Mol Sci. (2019) 21(1):49. 10.3390/ijms21010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang J, Yi Y, Zhu Y, Wang Z, Wu S, Zhang J, et al. Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2020) 34(1):124–31. 10.7507/1002-1892.201903058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Q, Wang Z, Xing H, Wang Y, Guo Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s Disease. Mol Ther Nucleic Acids. (2021) 23:1334–44. 10.1016/j.omtn.2021.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]