Summary:
Epilepsy surgery is the treatment of choice for patients with drug-resistant seizures. A timely evaluation for surgical candidacy can be life-saving for patients who are identified as appropriate surgical candidates, and may also enhance the care of non-surgical candidates through improvement in diagnosis, optimization of therapy, and treatment of comorbidities. Yet, referral for surgical evaluations is often delayed while palliative options are pursued, with significant adverse consequences due to increased morbidity and mortality associated with intractable epilepsy. The Surgical Therapies Commission of the International League Against Epilepsy (ILAE) sought to address these clinical gaps and clarify when to initiate a surgical evaluation. We conducted a Delphi consensus process with 61 epileptologists, epilepsy neurosurgeons, neurologists, neuro-psychiatrists, and neuropsychologists with a median of 22 years in practice, from 28 countries in all six ILAE world regions. After three rounds of Delphi surveys, evaluating 51 unique scenarios, we reached the following expert consensus recommendations: 1) Referral for a surgical evaluation should be offered to every patient with drug-resistant epilepsy (up to 70 years of age), as soon as drug-resistance is ascertained, regardless of epilepsy duration, sex, socioeconomic status, seizure type, epilepsy type (including epileptic encephalopathies), localization, and comorbidities [including severe psychiatric comorbidity like psychogenic non-epileptic seizures (PNES) or substance abuse] if patients are cooperative with management; 2) A surgical referral should be considered for older patients with drug-resistant epilepsy who have no surgical contraindication, and for patients (adults and children) who are seizure-free on 1–2 anti-seizure medications (ASM)s but have a brain lesion in non-eloquent cortex; 3) Referral for surgery should not be offered to patients with active substance abuse who are non-cooperative with management. We present the Delphi consensus results leading up to these expert consensus recommendations and discuss the data supporting our conclusions. High level evidence will be required to permit creation of clinical practice guidelines.
Keywords: epilepsy surgery, neuromodulation, drug-resistant epilepsy, public health, healthcare delivery, treatment
I-. Introduction:
In 2003, the American Academy of Neurology published guidelines stating that patients who continue to have disabling focal seizures with impaired awareness after appropriate antiseizure medication (ASM) trials should be considered for referral to undergo an evaluation for epilepsy surgery, but acknowledged the caveat that “criteria for failure of drug treatment have not been definitely established”1. In 2010, a taskforce of the International League Against Epilepsy (ILAE) addressed this uncertainty and defined drug-resistance as “failure of adequate trials of two tolerated and appropriately chosen [antiseizure medication (ASM)] schedules (whether as monotherapies or in combination) to achieve seizure freedom”2. Resective surgery can improve quality-of-life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy (DRE)3–6. Surgical evaluation is the most cost-effective approach to treat drug-resistant epilepsy, even when the likelihood of subsequent resection is less than 5% 7. Despite this evidence, referral for consideration of surgical therapy continues to be delayed as epilepsy duration still approximates two decades on average prior to initiation of a surgical work-up in adults, and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery. In parallel, the epilepsy surgery landscape is evolving to include therapeutic options such as laser interstitial thermal therapy (LiTT) with potentially less morbidity than resective surgery, and neuromodulation to treat patients not suitable for resective surgery. Identifying candidacy to any of these approaches starts with a surgical referral, so a timely evaluation is key.
The Surgical Therapies Commission of the ILAE set out to provide expert consensus recommendations for timing of pre-surgical evaluation, based on a rigorous Delphi process to achieve consensus involving subject-matter experts from all six ILAE world regions. Our goal was to provide clear, evidence-informed, objective, and clinically meaningful recommendations to guide any clinician involved in the care of people with epilepsy on when to refer patients of any age for evaluation of candidacy for epilepsy surgery.
II-. Critical Definitions and Concepts:
Distinction between Guidelines and Expert Consensus Statement:
The recommendations generated as part of this report are based on expert consensus opinion, which differ from a clinical practice guideline. Clinical practice guidelines provide evidence-based recommendations that are generated following a rigorous process including a systematic review, appraising the quality of the evidence and linking the evidence to the recommendations. Consensus recommendations are based on expert opinion and are used when there is limited evidence on a particular topic or where controversies exist, but where recommendations are needed.
Definition of drug-resistant epilepsy:
Rates of medication failure have been extensively explored to identify patients with DRE. In a seminal study by Kwan and Brodie in 2000, investigators examined ASM response in 470 patients with previously untreated epilepsy8. They found that of the entire cohort, 47%, 13% and 4% of individuals became seizure-free after the first, second and third or subsequent ASM, respectively. Thus, 36% of the original clinic-based cohort had ongoing disabling seizures despite maximal medical therapy. Considering these and other findings in adults9 and children10, 11, the ILAE Commission on Therapeutic Strategies proposed a definition of drug-resistance as failure to achieve sustained seizure freedom after adequate and well tolerated trials of two ASMs2.
In the last two decades, several new ASMs have been introduced, many with novel mechanisms of action and improved side effect profiles, but this definition of drug-resistance still stands12. In 2018, an investigation of 1795 people with newly diagnosed epilepsy found that 51%, 12%, and 4% of individuals achieved seizure freedom of 1 year or longer after a first, second, and third ASM regimen, respectively13. Only 2% of the entire cohort became seizure free with subsequent ASMs, and 36% of individuals suffered from persistent drug-resistant seizures. These findings emphasize that the likelihood of seizure-freedom with medical therapy alone is small in patients with documented DRE 13. In contrast, in a controlled trial of drug-resistant patients with temporal lobe epilepsy randomized to surgery or ASMs alone, no individuals who received maximal medical therapy achieved seizure freedom at two years, compared to 73% who underwent surgical resection 14. An RCT of children randomized to immediate surgical treatment versus continuation of medical treatment for 12 months (and later surgery) showed seizure freedom in 77% of the immediate surgery group after 12 months compared to only 7% of the medical group 15.
Value of referral to a tertiary epilepsy center beyond presurgical evaluation:
A referral for an epilepsy surgical evaluation is not equivalent to a commitment to undergo brain surgery. People with epilepsy have a lifelong brain disorder with localized or diffuse dysfunctional neuronal networks that result in seizures and other comorbidities. Specialized epilepsy care strives to promote the best possible quality-of-life through a comprehensive approach beyond trial-and-error choices of ASMs. Epilepsy centers offer a wide range of specialized diagnostic and therapeutic approaches with key benefits to our patients with uncontrolled seizures, even when surgical resection is not eventually pursued16. In fact, most patients with DRE do not end up undergoing surgery after referral17, but still benefit from comprehensive epilepsy care improving quality-of-life and lowering mortality18. A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.
An additional basic benefit of referral is to verify diagnosis. One third of patients with presumed DRE referred to epilepsy centers do not have epilepsy19, but are instead diagnosed after video-EEG with psychogenic non-epileptic seizures (PNES), which are associated with significant morbidity and mortality20. An early and accurate diagnosis of PNES can facilitate implementation of psychotherapy, lead to elimination of ASM, and improve outcomes. In parallel, for patients who do have epilepsy, recording their seizures in an epilepsy monitoring unit can be invaluable to help them understand their behavior during the event19.
Other key outcomes of a specialized evaluation are defining the etiology and type of epilepsy21. For example, the yield of an epilepsy MRI is directly related to hardware quality and imaging sequences but may be doubled by knowledge of the suspected epilepsy localization and experience of the neuroradiologist22. Lesions such as hippocampal sclerosis, cavernous malformation or glioneuronal tumors may warrant early surgical intervention14, 23. Complex or multifocal lesions or patients with nonlesional focal epilepsy on the other hand, require additional testing and may have a lower chance of seizure freedom. In general, resective surgical options are far more common and successful in focal epilepsy syndromes, particularly in individuals with an identified lesion24, while neuromodulation approaches are more commonly used in patients whose seizures originate in eloquent cortex, precluding resective surgery, those with poorly localized focal epilepsies or in those with generalized epilepsy syndromes25.
Surgical resective procedures, neuromodulation and ablative approaches
The landscape of non-pharmacological interventions to treat drug-resistant epilepsy continues to expand. A referral for a “surgical evaluation” can actually lead to a variety of interventions, and a specialized epilepsy program can identify the best options for any given patient. Traditional surgical procedures aiming for seizure-freedom include focal resections, multilobar resections and hemispherotomies depending on the etiology and the localization of the epileptogenic zone (EZ) that must be removed/disconnected to achieve seizure freedom26. The definition of the EZ is reached with the integration of seizure semiology, EEG, neuropsychological evaluation, and multimodal imaging. When a surgical resection is not possible due to bilateral, generalized or nonlocalized EZ or an EZ located in eloquent cortex, palliative procedures can be used, such as subpial transection of focal abnormalities, corpus callosotomy for disabling drop attacks, or neuromodulation including vagus nerve stimulation, deep brain stimulation, and responsive neurostimulation. These procedures rarely bring seizure freedom but can reduce seizure frequency and severity25.
Newer techniques, considered minimally invasive, include the stereotactic ablation of epileptogenic lesions or disconnection procedures27, 28. The two basic physical mechanisms of action currently in use are stereotactic radiosurgery (gamma knife, linear accelerators) and thermocoagulation (also known as thermotherapy or thermal ablation) where focused and controlled heat is applied to ablate tissue. The heating of the tissue can be achieved in three ways: focused ultrasound, stereotactic radiofrequency thermocoagulation (RF-TC) and LiTT27, 28. RF-TC is a less resource-intensive alternative to LiTT. Focused ultrasound also has a potential use for neuromodulation in epilepsies29. There is compelling evidence of efficacy for these emerging minimally invasive approaches30, 31, but recent meta-analyses suggest waning seizure-freedom over time across all types of epilepsy surgery, most noticeable in minimally invasive approaches 32 33. Overall, rigorous research is still needed to adequately resolve controversies regarding longterm risks and benefits.
III-. Current State of Referrals for Epilepsy Surgery
Current data on the timing of initiation of presurgical evaluations
Despite the emphasis placed on early intervention in focal epilepsy in the last decades, referral paths for presurgical evaluation have remained long, arduous and underutilized34, as epilepsy surgery is still considered by some pediatric and adult neurologists as a treatment of last resort35. Several studies have found a considerable delay in the referral of patients with focal epilepsy for presurgical evaluation36, with the mean latency between seizure onset and surgery amounting to 20 years in adults and five years in children37. The situation is more dramatic in the pediatric age group, where timely surgery can prevent otherwise irreversible neurocognitive decline38 and lead to long-term cognitive improvement39, 40. Two-thirds of children who had epilepsy surgery in the 2004 ILAE survey were younger than three years at epilepsy onset, but only a few of these children received surgery within two years41. Fortunately, considerable decrease in epilepsy duration to surgery has been noted over the last decades, as shown in a multicenter European epilepsy surgery study42, but early referral for pre-surgical evaluation is essential to support this encouraging trend.
Underutilization of surgery
In a Swedish study focusing on epileptogenic tumors and cavernomas 5, adults had a mean epilepsy duration of 13 years and children of five years, amounting to over a third of their lives, although all but one patient had an MRI-detectable lesion that eventually proved to be epileptogenic. Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults14, 43, and resective surgery in children44, is superior to continued ASMs both in terms of seizure freedom and improved quality-of-life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years45. Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates46, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.
Pediatric epilepsy surgery studies have shown trends for shorter epilepsy duration over time among surgical candidates47, 48, in line with the expansion and increasing utilization of pediatric epilepsy surgery in the last decades49.
Barriers and facilitators
Delayed referral may be partly attributed to temporary seizure remissions with new ASM trials, overestimation of surgical risks, underestimation of morbidity and mortality associated with ongoing seizures, and lack of access to appropriate healthcare50. Barriers to epilepsy surgery include lack of knowledge or misconceptions about surgical risks, negative behaviors or cultural issues and access issues. These barriers vary by region and setting and can originate from patients, their caregivers, clinicians or health systems51, 52. Table 1 provides examples of possible solutions to typical barriers.
Table 1:
Summary of barriers and facilitators of seeking epilepsy surgery evaluations.
| Barrier | Facilitator (Solutions) | |
|---|---|---|
| Physician | Lack of knowledge about: • Definition of drug-resistant epilepsy (DRE) • Role of epilepsy surgery • Indications for possible epilepsy surgery |
• Online tools to facilitate identification of possible candidates: e.g. www.toolsforepilepsy.com • EMR tools: • Machine learning techniques to identify DRE patients in electronic medical records (EMR) • Decision analysis tool, nomograms, etc. embedded in EMR • Computerized clinical practice guidelines • EMR prompts • Peer reviewed publications • Guidelines • Pay-per-performance models |
| Misconceptions about epilepsy surgery • Negative or ambivalent attitudes and perceptions about epilepsy surgery. • Deficient communication practices with patients regarding risk-benefit analysis of epilepsy surgery |
• Online educational tools • Self-management programs: e.g. Managing Epilepsy Well Network • Webinars and podcasts • Patient testimonial videos • Social media • Treatment of comorbidity (e.g. depression) |
|
| Person with epilepsy | Access and cost issues | • Mobile clinics • Telehealth • Multidisciplinary team including social worker to assist with identification of supportive services (e.g. transportation, health insurance) • Work with epilepsy organization (nonprofit, academic) to advocate for improved policies nationally to facilitate health coverage for epilepsy surgery |
| Health system and health resources | Team expertise – clinicians (epileptologists, epilepsy surgeons, neuropsychologists, intensivists, anesthesiologists), EEG technologists | • Building a multidisciplinary team • Maintaining/tracking volume and complexity of cases |
| Equipment – e.g. neuroimaging, neurophysiology | • Utilizing advanced diagnostics tools • Considering minimally invasive surgical techniques • Some epilepsy surgery interventions can be completed without needing invasive monitoring |
|
| Challenging coordination issues with referral center and epilepsy program | • Promote communication and collaboration between referring providers (e.g. community physician) and epilepsy specialists | |
| Cost | Improve access to epilepsy surgery via policy changes • Anti-discrimination policy • Exemption of transportation cost • Telehealth reimbursement policy • Patient-centered epilepsy care models • Affordability and access to insurance |
|
| LMICs – overwhelmed by existing burden of disease. | • Collaboration with high resource settings (e.g. visiting professorships, cross appointment of faculty experts interested in global health) • Cross region/country multidisciplinary rounds |
IV-. Methods
Working groups and participants
The Surgical Therapies Commission of the ILAE decided to pursue a systematic, inclusive, and rigorous process to generate expert consensus recommendations for referral for an epilepsy surgical evaluation. First, the Commission created a Recommendations Writing Group which included members from all the relevant professional groups including the Commission’s leadership, chairs of the Commission’s five taskforces (Pediatric Surgery Taskforce, Education Taskforce, Evidence Based Surgery Taskforce, Outcomes Taskforce, and Resource-Limited Countries Surgery Taskforce), and two members with epilepsy surgery expertise from each ILAE region nominated by that region’s Chair (Africa, Asia-Oceania, Eastern Mediterranean, Europe, Latin America and North America), except in Africa where only one member participated. We then created a Delphi Working Group to develop the initial Delphi questionnaire. Participants included the Chair of the ILAE Surgical Therapies Commission, a representative of the ILAE Executive Committee, a Delphi expert and the Chair of the ILAE Standards and Best Practice Council, an epileptologist with epidemiological and statistical expertise and a Young Epilepsy Section (YES) representative (neurosurgeon with health services research expertise).
Survey development, testing, and revisions
The Expert Consensus Recommendations Writing Group and the Delphi Working Group members participated in several online meetings to discuss the initial core elements for the questionnaire. The Delphi Working Group then generated the first Delphi questionnaire including criteria that may influence the decision to refer for an epilepsy surgery evaluation (e.g., sociodemographic, clinical history, therapies, EEG, imaging findings). The initial questionnaire was sent to the Writing Group members. Revisions were made based on their feedback. When answering questions, participants were asked to assume that potential surgical candidates had no surgical contraindications unless specified. They were asked to not base their answer on the resources available in their health system, but rather assume that surgical resources were available. Each criterion was rated on a 5-point Likert scale (Table 2). The pilot questionnaire was then revised by implementing additional suggestions from the Writing Group to generate a final questionnaire for the Delphi Process. The survey was hosted on Survey Monkey.
Table 2 –
Response options for Delphi questionnaire rounds 2–3
|
Irrespective
of all other patient characteristics: 6. I would never refer the patient for epilepsy surgery evaluation if this characteristic is present 7. I am unlikely to refer the patient for epilepsy surgery evaluation if this characteristic is present 8. I am not sure if this characteristic would influence my decision to refer the patient for epilepsy surgery evaluation 9. It is likely I would refer the patient for epilepsy surgery evaluation if this characteristic is present 10. I would always refer the patient for epilepsy surgery evaluation if this characteristic is present (This option also applies if this characteristic is irrelevant) If you choose options 2–4, please explain your choice and comment. For example, if you are uncertain about the relevance of age (answer 3), or you would likely not refer or refer (answer 2 or 4) based on certain age ranges (the very young or older adults) please state this. |
Delphi Process
Delphi panel members were selected to achieve broad representation of relevant clinical disciplines (adult and pediatric epileptologists, epilepsy neurosurgeons, neurologists, neuropsychiatrists and neuropsychologists) and all world regions. Thus, participants included all members of the ILAE Surgical Therapies Commission, plus the additional participants identified by the ILAE Regional Chairs (Total N=73 participants). For each of the three Delphi rounds, results were categorized as follows: 1) always/likely to refer (ratings #4–5) or irrelevant (i.e., would refer regardless of this criterion), 2) unsure (rating #3), 3) unlikely to refer or would never refer (ratings #1–2). Consensus was defined as having at least 66% of respondents in one of these categories (i.e., refer, unsure or never refer). Criteria without consensus were included in a subsequent round with revisions made according to the comments received from participants. The process was repeated (three rounds) until consensus was optimized.
Statistical Methods
We used parametric and non-parametric descriptive statistics to describe baseline participant demographics, including comparisons between those that would refer and those who would not. The results of the Delphi process were dichotomized into referral categories (‘always/likely’ and ‘never/unlikely’) and demographic characteristics were compared across these two groups using Kruskal-Wallis and Fisher’s exact tests for continuous and categorical variables, respectively, to investigate relationships between responder characteristics and their preferences.
Formulating the Expert Consensus Recommendations
The survey responses were converted into expert consensus recommendations as follows:
Consensus reached in the category of “always/likely to refer” = referral for a surgical evaluation “should be offered”.
Consensus reached in the category of “unlikely or never to refer” = referral for surgical evaluation “should not be offered”.
Consensus not reached but ≥50% answered “always” or “very likely” to refer = referral for surgical evaluation “should be considered”.
Consensus not reached and <50% agreement = “further research is needed”.
An initial draft of the expert consensus recommendations was created by the Delphi Working Group after the final Delphi round, which was then reviewed by the Writing Group and revised after further discussions.
Results
Participants and response rate
A total of 61 participants provided responses in at least one round of the Delphi process. Participants were comprised of epileptologists (n=23; 38%), epilepsy neurosurgeons (n=21; 34%), neurologists (n=14; 23%), neuropsychiatrists (n=1; 2%), and neuropsychologists (n=2; 3%) with a median of 22 years (interquartile range [IQR] 12–28) in practice. There was equal representation between those focusing on adult (n=24; 39%) or both adult and pediatric (n=25; 41%) with a minority whose practice was dedicated solely to pediatric epilepsy (n=12; 20%). Participants were from North America (n=18; 30%), Europe (n=17; 28%), Asia/Oceania (n=11; 18%), Latin America (n=8; 13%), the Eastern Mediterranean (n=4; 7%), and Africa (n=3; 4%). The majority of participants worked in a dedicated epilepsy center that offered surgery (n=54; 88%).
Delphi results
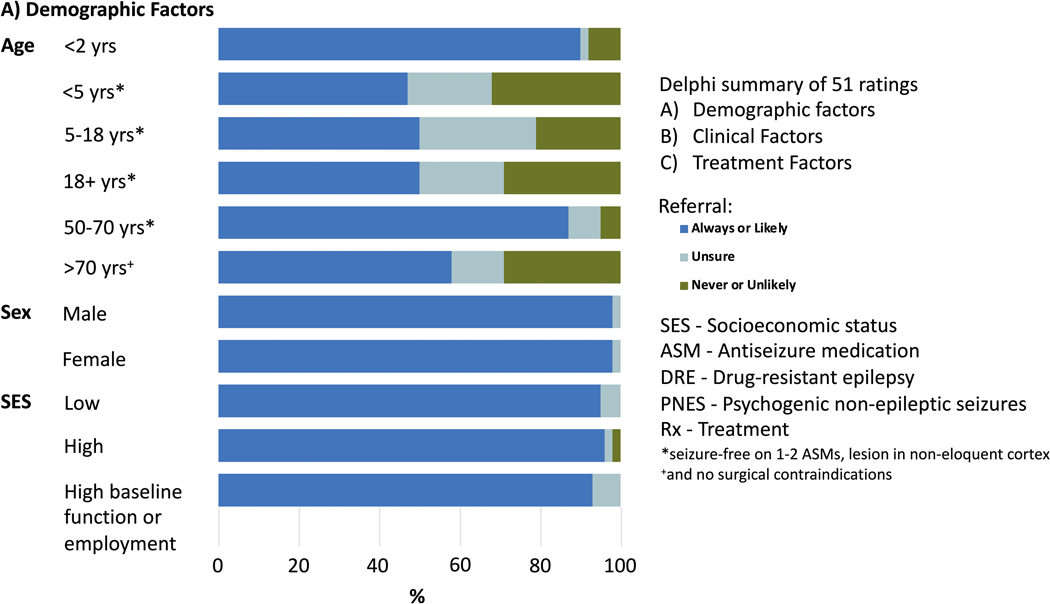
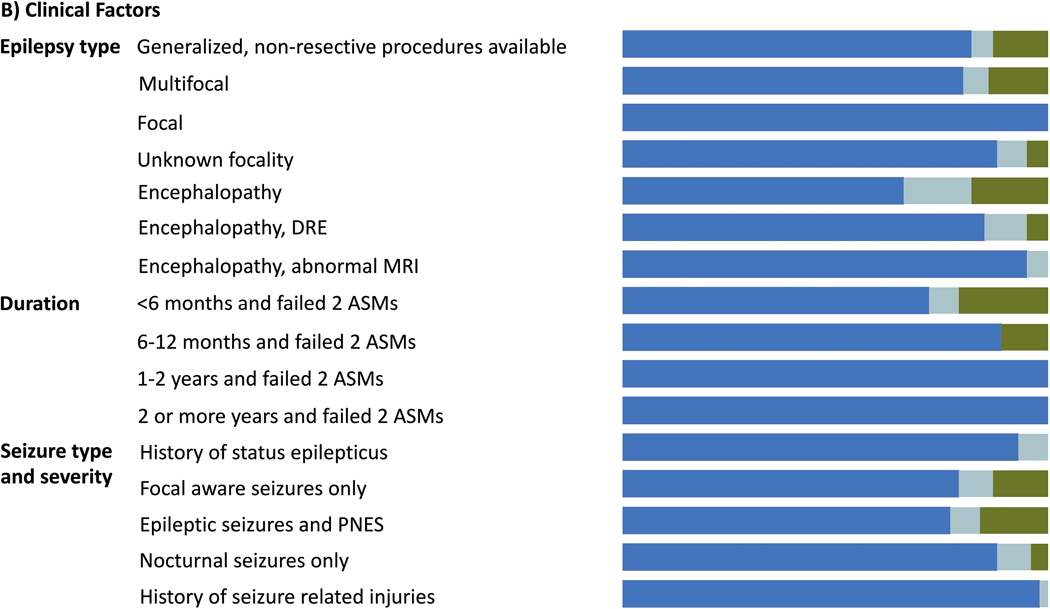
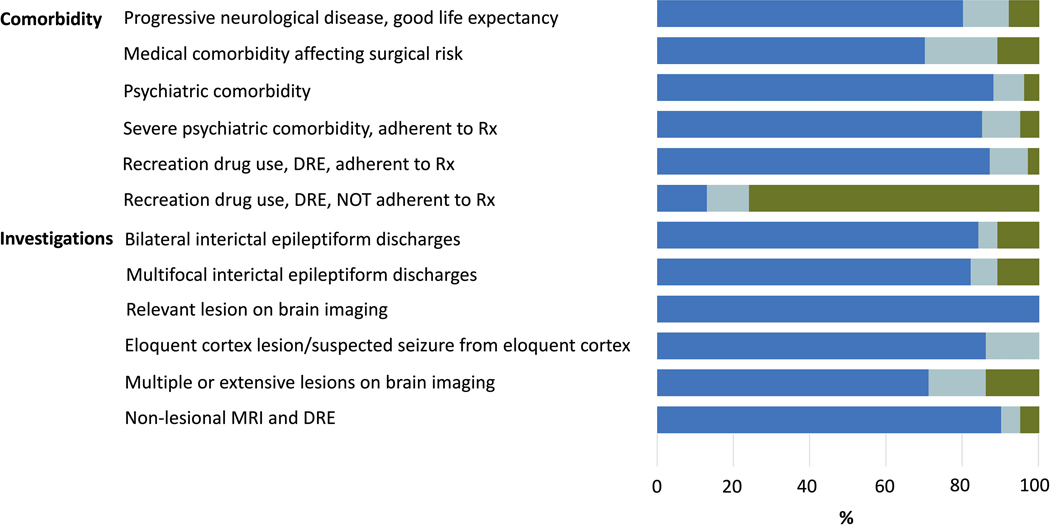
A total of 57 (93%) participants contributed to round 1 of the Delphi, 39 (64%) to round 2, and 38 (62%) to round 3. Consensus was reached in 30 of 38 scenarios in round 1 (79%). Criteria for which there was a lack of consensus were revised for clarity and included in the next round. Likewise, additional scenarios suggested by participants were included in the subsequent round. This resulted in 20 scenarios in round 2 (in which consensus was reached in 17; 85%) and 7 scenarios in round 3 (in which consensus was reached in 3; 43%). The final number of unique scenarios was 51, among which 45 (88.2%) had agreement. Of the 45 scenarios with agreements, 44 recommended a referral for an epilepsy surgery evaluation. Figure 1 shows the level of agreement for each scenario. Supplemental Table 1 lists in more details all scenarios with % agreement for each.
Figure 1.
legend: Delphi summary of 51 ratings based on A)-demographic factors, B) clinical factors, and C) treatment factors. Dark blue shades are for proportions where response was to always ro likely refer for presurgical evaluation; light blue for unsure, and green for never or unlikely. SES= Socioeconomic status; ASM= antiseizure medication; DRE= drug resistant epilepsy; PNES= psychogenic non-epileptic seizures; Rx= Treatment.
*seizure free on 1–2 ASMs, lesion in non-eloquent cortex +and no surgical contraindications.
Consensus reached in the category of “Always/Likely to refer”:
>66% responded that they would always/likely refer patients with:
-
Drug resistance: Once drug-resistance is established (failure to achieve sustained seizure remission despite two ASMs, as defined by ILAE), consensus was reached on referral regardless of age (up to 70 years old), sex, socioeconomic status, seizure type, epilepsy type (including epileptic encephalopathy), epilepsy duration, and epilepsy associated comorbidities including severe psychiatric comorbidities or substance use, if cooperative with management, and co-morbid PNES.
Consensus for referral in DRE was maintained regardless of epilepsy localization or the likelihood of candidacy for resective epilepsy surgery. In addition, our criteria for consensus to refer were still met accounting for:
Patient preference: 82% would always/likely refer a patient who is against surgery but willing to be evaluated and/or counseled further.
Therapies: 86% would always/likely refer patients with DRE even if they have not tried non-traditional therapies (e.g., ketogenic diet, cannabidiol). Similarly, more than 80% would still always/likely refer patients with DRE even if resective surgery is expected to only be ‘palliative’, or if patients had prior surgical resection but have ongoing drug-resistant seizures.
Number of ASMs: Survey responses re-enforced the general principle of surgical referral once drug-resistance is established. Consensus to refer was achieved in patients who failed an adequate trial of ≥2 ASMs, failed an adequate ASM trial due to side effects, or if they were non-adherent to ASMs but otherwise had documented DRE and were cooperative with management.
Of note, demonstrating drug-resistance was not a pre-requisite for surgical referral in lesional cases: 72% would always/likely refer patients who failed an adequate trial of one ASM (i.e., technically not meeting the definition of DRE) if they have a potentially epileptogenic lesion.
Consensus reached in the category to “not refer for a surgical evaluation”:
The only situation where a consensus was reached to withhold a surgical referral was the use/abuse of alcohol and/or recreational substances in patients with DRE who are not cooperative with management (76% unlikely/never refer; 11% unsure/no judgement; 13% always/likely refer).
No agreement reached, but a higher proportion of experts recommended to refer:
Although consensus as defined by >66% agreement was not reached in the following situations, a higher proportion would still always/likely refer:
Children of all age groups, and adults who are seizure-free on 1–2 ASMs, with a lesion in non-eloquent cortex (47% were always/likely to refer for children younger than 5 years, whereas 50% reported that they would always/likely refer for surgical evaluation in children 5 years or older, and in adults)
Patients of older age (>70 years) with no surgical contraindications (29% unlikely/never refer; 13% unsure/no judgement; 58% always/likely refer)
Patients who are non-adherent to ASM (29% unlikely/never refer; 32% unsure/no judgement; 49% always/likely refer)
No agreement reached, but a higher proportion of experts recommended not to refer:
49% of responders were unlikely or would never refer patients in whom an adequate trial of one tolerated and appropriately chosen and used ASM schedule failed to achieve sustained seizure freedom (49% unlikely/never refer; 7% unsure/no judgement; 44% always/likely refer).
Table 3 presents the final Expert Consensus Recommendations derived from these results.
Table 3:
Final Expert Consensus Recommendations of The Surgical Therapies Commission of the ILAE on timing of referral for an evaluation of candidacy for epilepsy surgery
| 1. Referral for a surgical evaluation should be offered to every patient with drug-resistant epilepsy (up to 70 years or younger) regardless of epilepsy duration, sex, socioeconomic status, seizure type, epilepsy type (including epileptic encephalopathies), comorbidities (including severe psychiatric comorbidity or substance abuse, if cooperative with management, and patients with both seizures and psychogenic non-epileptic seizures (PNES) and/or epilepsy localization. Specifically, a. Patients with DRE who may not appear to be appropriate candidates for resective surgery should be referred as other options may be offered. b. A patient’s reluctance to surgery should not preclude a referral if willing to be evaluated and/or counseled further. c. A surgical referral should not be delayed: i. If therapies other than seizure medications have not yet been tried; ii. if surgery is expected to be ‘palliative’; iii. if patient already had prior surgical resection but has ongoing drug-resistant seizures as either additional resection or other options might be offered; iv. if failure of adequate ASM trials was due to unacceptable side effects, v. if a patient with non-adherence to medical therapy previously demonstrated drug-resistance and is now otherwise cooperative with management. |
| 2. Referral for a surgical evaluation should not be offered for patients with drug-resistant epilepsy who use/abuse alcohol and/or recreational substances and are not cooperative with management. |
| 3. Referral for a surgical evaluation should be considered in: a. Patients 70 years or older with no surgical contraindications. b. Children and adults who are seizure-free on 1–2 ASMs, with a lesion in non-eloquent cortex. |
| 4. Further research is needed to clarify risk versus benefit balance of epilepsy surgery is needed for patients with ongoing seizures in the context of non-adherence to ASM without previously documented drug-resistance |
Discussion:
The Expert Consensus Recommendations presented in this document reflect the experience of medical and surgical epileptologists, neurosurgeons, and neuropsychologists/psychiatrists from around the world, as enabled by the Surgical Therapies Commission of the ILAE. Beyond expert opinions, our team followed a methodical Delphi process to optimize rigor, diversity, and inclusiveness.
The overarching theme is that patients need to be referred for a surgical evaluation as soon as drug-resistance is ascertained (Recommendation 1). The only scenario in which referral of patients with DRE was withheld was ongoing substance abuse with poor adherence with management (Recommendation 2). Several factors likely influenced these recommendations:
While many therapies offer seizure-remission, epilepsy surgery offers seizure-freedom:
Epilepsy surgery (whether through resection or ablation) is the only available potentially curative option, offering immediate and sustained seizure-freedom. In the context of resection, this is documented in three randomized clinical trials comparing resective surgery to medical therapy14, 43, 44, while troves of observational studies and meta-analyses show sustained seizure-freedom rates ranging from 40–50% a decade after extra-temporal procedures to 50–60% a decade after temporal lobe resections53–56. In the context of neuro-ablation, sustained seizure-freedom rates of 50–60% are observed 1–2 years after the procedure, and long-term data seem encouraging57. For patients with DRE, these odds of seizure-freedom after surgery need to be compared with the odds of seizure-freedom with ongoing medical therapy alone. In the seminal studies of response to medical therapy in newly diagnosed epilepsy8, 13, the percentage of responders to the 3rd or more ASM was 2%–4% if we use the percentage of the total cohort, versus 15% if we use the percentage of those who actually try the next medication (meaning the pool of non-responders to the 1st and 2nd medication). Either way, these numbers reflect the same drug-resistant cohort, only seen from different perspectives, and therefore should not alter the decision to refer as they remain significantly inferior to the odds of seizure-freedom with surgery. “Honeymoon” periods of intermittent remission do not modify the long-term outlook of this population. In one study of drug-resistant patients, the estimated cumulative probability of 12-month seizure remission was 33% at 7 years with adjustments of medical therapy, emphasizing the importance of expert management in ASM, offered in comprehensive epilepsy care programs. However, the risk for subsequent relapse was 71% at 5 years, highlighting the importance of surgery for definitive seizure-freedom58.
It is key to note that our Delphi expert panelists recommended a referral for surgical evaluation in those with DRE even if a patient was felt to be an unlikely candidate for resective surgery. This is because a specialized evaluation can further identify surgical candidates or other options for palliation in this challenging patient category. Uncontrolled open label extension studies demonstrated a 28% chance of achieving a 6-month remission at 9 years after initiation of responsive neuro-stimulation (RNS)59, and 18% achieved 6-month remission 7 years after anterior nucleus of the thalamus stimulation60. In the open-label extension studies for RNS, the median seizure reduction was 53%, 66%, and 75% at two, five, and nine years of follow-up, respectively, highlighting the potential value of these palliative therapies to aid in long-term management of non-surgical patients and improving their quality-of-life. Altogether, these data support the 2010 ILAE definition of drug-resistant epilepsy and suggest that although transient periods of seizure remission may occur, immediate and sustained seizure freedom with medical therapy or neuromodulation is unlikely after the failure of two ASMs. Initiating surgical evaluation as soon as drug-resistance is ascertained is key.
Delaying complete seizure-freedom by delaying surgery comes with consequences:
Cognitive Consequences of delaying surgery:
Delayed surgery can result in suboptimal cognitive outcomes for people with epilepsy via several mechanisms. First, delayed surgery fails to capitalize on the superior reorganization and compensatory capacities of the developing brain. The relocation of language abilities is just one characteristic of brain plasticity in young children; however, multiple windows of opportunity exist that allow the reorganization of specific cognitive functions as they mature at different timepoints through childhood and adolescence. These windows invariably narrow with age. In very young children, early surgery can prevent, halt or even reverse developmental arrest and regression of cognitive function40.
Second, delayed surgery fails to mitigate the impact of growing up with epilepsy. Seizures, subclinical EEG discharges and ASMs all adversely impact neurodevelopment38, 61. Growing up with a poorly understood and stigmatizing condition such as epilepsy also has an impact on educational and social development. People who grow up with epilepsy are set on a different trajectory for life. Surgery in adulthood does not reverse this trajectory, and difficulties adjusting to the ‘burden of normality’ following even successful surgery are common62. Surgery is the only intervention proven to be disease modifying, with seizure free children attaining psychosocial developmental milestones similar to healthy peers63. Recent studies provide evidence of progressive atrophy that is reversible after successful surgery, changes that are not seen when seizures are controlled with medication64. These data likely underlie the recommendation to consider surgery in children who have a resectable lesion at low risk from surgery, even when they are seizure-free (Recommendation 3b).
Whilst not contraindicated, surgery in later life is associated with lower cognitive reserves due to normal age-related declines in function and higher risk for postoperative cognitive decline, particularly among those with non-lesional epilepsy whose seizures arise from eloquent areas within the language-dominant hemisphere.65 Accelerated cognitive decline in some may also significantly increase the cognitive morbidity associated with surgery in adulthood. Sometimes it is too late to offer surgery as cognitive risks become too great, which would not have been the case earlier in the disease. Limited data exist on the cognitive outcomes of surgery in the elderly, likely underlying the lower degree of consensus in our recommendation of surgery for patients >70 years old (Recommendation 3a).
A comprehensive evaluation for epilepsy surgery in individuals with DRE includes neuropsychological evaluation to characterize cognitive and behavioral functioning and estimate the potential risks/benefits of surgery on cognitive and emotional functioning66. In children, cognitive risk depends on a range of factors including age at seizure onset and evaluation, seizure freedom, antiseizure medication load, and the extent of pre-operative damage more so than its lateralization or localization.67, 68 Cognitive functioning, in turn, has been shown to predict a child’s achievement of developmental milestones and longer-term psychosocial trajectory, which can be improved by surgery69. All of these factors must be carefully considered in the pre-operative neuropsychological evaluation in conjunction with the medical risks/benefits70 to determine the optimal treatment approach (e.g., resective surgery, LiTT, neuromodulation) for any given patient. The complexity of such an informed assessment needs to be done by experts in epilepsy surgery and further underscores why a referral for a surgical evaluation is critical.
Mortality:
Epilepsy surgery has the potential to reverse the most serious complication of epilepsy, that of excess mortality3, 5, 6. Compelling evidence indicates that uncontrolled epilepsy is associated with increased mortality rates, and equally compelling data demonstrate that surgery is associated with a reduction in excess mortality. The largest published series contrasted mortality in 1006 surgically treated patients with 104 non-surgically treated patients. Those who had surgery had a lower mortality rate (8.6 per 1,000 person-years (95% CI 6.58–11.15) than nonsurgical patients (25.3 per 1,000 person-years (95% CI 14.50–41.17) (p < 0.001). Seizure free patients had a mortality rate indistinguishable from that of the general population, and post-operative tonic-clonic seizure frequency was associated with increased mortality. Patients with persistent focal impaired awareness seizures had lower mortality than nonsurgical patients (p = 0.005); they showed a trend towards increased mortality risk compared with seizure-free patients (p = 0.08).6 A recent study reporting results in 590 surgical patients and a comparison group of 122 nonsurgical patients confirmed the reduction in mortality in surgical patients with lower all-cause and sudden unexplained death in epilepsy (SUDEP) related mortality. Time to SUDEP was longer in surgical patients, and 10 of 14 cases occurred more than 10 years after surgery3.
Seizure outcome implications of delayed surgery:
Worse outcomes with late surgery (frontal, temporal): In the last two decades, several observational studies, both in pediatric and adult cohorts, have suggested that longer epilepsy duration is associated with worse seizure outcomes after resective epilepsy surgery40, 55, 71, 72. This association has been reported in epilepsies related to epileptogenic lesions and epilepsies arising from the frontal, temporal, or posterior cortex, thus rendering possible confounding effects of referral patterns favoring the presence of a lesion or a specific lobar localization unlikely. A positive correlation between longer epilepsy duration and lower rates of postsurgical seizure freedom has also been established independently of age at surgery in pediatric cohorts55, 73. Additionally, delayed surgery has been recently shown to entail reduced chances of seizure- and ASM freedom for all lesions, with the sole exception of hippocampal sclerosis, in a multicentric pediatric and adult cohort24. Furthermore, two meta-analyses showed significant positive effects of early surgery on seizure-freedom, including both epilepsy durations as short as two years and very long durations of up to 20 years74, and an average delay to surgery of 2.8 years less for seizure-free patients compared to those with a less favorable outcome75. These data suggest that epileptogenic processes presenting with longer epilepsy duration decrease the chances of surgical success, independently of other predictors. Based on this assumption, recent studies suggest recommending surgery very early in the course of the disease, even for non-drug-resistant patients. For example, in certain lesional scenarios, such as epilepsy associated with cavernous malformations, surgery following two or less seizures has been associated with 95% seizure- and 79% ASM-freedom rates compared to 63% and 25% in patients with more than two seizures before surgery76. These observations likely contributed to our Delphi findings leading to Recommendation 3b.
Expected risks versus benefits analysis does not favor surgery in patients with active substance abuse:
Although there is some evidence that seizure outcomes are no different in those with active substance use disorder who have epilepsy surgery77, literature suggests increased perioperative surgical and anesthetic risk in this cohort.78Patients with active substance abuse are more likely to be non-adherent with their seizure medications79, and leave the hospital against medical advice80. This would further complicate already complex pre-surgical evaluations that require multiple inpatient tests and outpatient appointments, particularly in the subgroup of active substance users with documented nonadherence, thus our recommendation to delay surgical work-up until substance abuse is controlled and adherence with medical management is established (Recommendation 2).
Areas of further research and conclusion:
We were able to generate expert consensus recommendations in most scenarios; yet, we identified several scenarios where consensus could not be reached (Table 3), highlighting opportunities for future research, including situations where no consensus was reached, or situations where the strength of consensus did not reach 50% (e.g: surgical referral in very young children who are seizure-free on ASM but have a lesion in non-eloquent cortex).
The primary limitation of these recommendations is that they are based on the Delphi process for expert consensus generation, rather than evidence based guidelines, which require a high grade of evidence that is not available at present. Of note, the existing AAN guidelines now state that patients with drug-resistant epilepsy should be referred for consideration of epilepsy surgery. As such, the expert consensus recommendations presented here re-inforce and dissect the experts’ interpretation of the existing guidelines in specific clinical scenarios. Randomized clinical trials could theoretically investigate the risks vs benefits of epilepsy surgery for each of our >50 clinical scenarios to strengthen the credibility of our recommendations, but this is neither possible nor ethical given the overwhelming evidence and data presented in our discussion and underlying our recommendations. We hope this expert consensus report will reduce misconceptions and fill the knowledge gap about epilepsy surgery and, as a result, decrease time to surgery for those living with epilepsy who have ongoing seizures.
Supplementary Material
Key bullet points:
We present expert consensus recommendations generated through a Delphi Process designed by the Surgical Therapies Commission of the ILAE.
Referral for a surgical evaluation should be offered to every patient with epilepsy younger than 70 years of age as soon as drug-resistance is ascertained.
A surgical referral should be considered for older patients with drug-resistant epilepsy who have no surgical contraindication.
A surgical referral should be considered for patients who are seizure-free on 1–2 anti-seizure medications but have a brain lesion in non-eloquent cortex.
Referral for surgery should not be offered to patients with active substance abuse who are non-cooperative with management
Acknowledgment:
We acknowledge the following Delphi process participants: Mario Alonso Vanegas (Mexico); Marjan Asadollahi (Iran); Fabrice Bartolomei (France); Gretchen Birbeck (USA); Kees Braun (Netherlands); Sarat Chandra (India); Chun Kee Chung (South Korea); Youssef Comair (Lebanon); Thomas Czech (Austria); Olivier Delalande (France); Bertrand Devaux (France); Rei Enatsu (Japan); Ed Faught (USA); Martha Feucht (Austria); Stefano Francione (Italy); Dan Friedman (USA); William Gaillard (USA); Enrico Ghizoni (Brazil); Kensuke Kawai (Japan); Mark Keezer(Canada); Sonia Khan (Saudi Arabia); Nirmeen Kishk (Egypt);Vladimir Krylov (Russia); Katia Lin (Brazil); Pei Lin Lua (Malaysia); Kristina Malmgren (Sweden); Wirginia Maixner (Australia); Farrah Mateen (USA); Andrew McEvoy (United Kingdom); Zainal Muttaqin (Indonesia); Moosa Naduvil (USA); Jeff Ojemann (USA); Reda Ouazzani (Morocco); Cigdem Özkara (Turkey); Eliseu Paglioli (Brazil); Sally Rothemeyer (South Africa); John Rolston (USA); Felix Rosenow (Germany); Americo Sakamoto (Brazil); Laura Tassi (Italy); Sumeet Vadera (USA); Satsuki Watanbe (Japan); Natrujee Wiwattanadittakun (Thailand); Howard Weiner USA); Lily Wong-Kiesel (USA); Alice Yu (USA).
Disclosure: Dr. Cendes reports personal fees from UCB Pharma, United Medical, Eurofarma and Zodiac Pharma, and Institutional grants from Sao Paulo Research Foundation (FAPESP), and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), outside the submitted work; and member of the editorial boards of the following journals: (1) Neurology (2) Epilepsy Research (3) Epilepsia (Associate Editor), (4) Frontiers in Neurology (Specialty Chief Editor). CBJ has received unrestricted educational grants through UCB Canada Inc. and Eisai Inc. for work unrelated to this project. CBJ has received a Canadian Frailty Network Grant (Canadian Frailty Network (CAT2017–19) which is supported by the Government of Canada through the Networks Centres of Excellence (NCE) program for work unrelated to this project. Michael Sperling has received compensation for speaking at CME programs from Medscape, Projects for Knowledge, International Medical Press, and Eisai. He has consulted for Medtronic, Neurelis, and Johnson & Johnson. He has received research support from Eisai; Medtronic; Neurelis; SK Life Science; Takeda; Xenon; Cerevel; UCB Pharma; Janssen; and Engage Pharmaceuticals. He has received royalties from Oxford University Press and Cambridge University Press. JHC has received compensation to her institution for serving as a study investigator for GW Pharmaceuticals plc, Marinius Pharmaceuticals, Inc, Vitaflow (International) Limited, Zogenix and Stoke Therapeutics and as a speaker and advisory board member for GW Pharmaceuticals plc, Nutricia, Biocodex and Zogenix; has received research support from the National Institute of Health Research Great Ormond Street Hospital Biomedical Research Centre; has received grants from the Engineering and Physical Sciences Research Council, Epilepsy Research UK, Great Ormond Street Hospital Charity, the National Institute of Health Research, and the Waterloo Foundation; and holds an endowed chair at the University College London Great Ormond Street Institute of Child Health. NJ receives grant funding paid to her institution for grants unrelated to this work from NINDS (NIH U24NS107201, NIH IU54NS100064, 3R01CA202911–05S1, R21NS122389, R01HL161847). She is the Bludhorn Professor of International Medicine. She receives an honorarium for her work as an Associate Editor of Epilepsia. J receives research funding from the National Institutes of Health.
Footnotes
Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclaimer: This report was written by experts selected by the International League Against Epilepsy (ILAE) and was approved for publication by the ILAE. Opinions expressed by the authors, however, do not necessarily represent the policy or position of the ILAE
The remaining authors have no conflicts to declare.
References:
- 1.Engel J, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia. Jun 2003;44(6):741–51. doi: 10.1046/j.1528-1157.2003.48202.x [DOI] [PubMed] [Google Scholar]
- 2.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. Jun 2010;51(6):1069–77. doi: 10.1111/j.1528-1167.2009.02397.x [DOI] [PubMed] [Google Scholar]
- 3.Casadei CH, Carson KW, Mendiratta A, et al. All-cause mortality and SUDEP in a surgical epilepsy population. Epilepsy Behav. 07 2020;108:107093. doi: 10.1016/j.yebeh.2020.107093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Téllez-Zenteno JF, Dhar R, Hernandez-Ronquillo L, Wiebe S. Long-term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain. Feb 2007;130(Pt 2):334–45. doi: 10.1093/brain/awl316 [DOI] [PubMed] [Google Scholar]
- 5.Sperling MR, Feldman H, Kinman J, Liporace JD, O’Connor MJ. Seizure control and mortality in epilepsy. Ann Neurol. Jul 1999;46(1):45–50. doi: [DOI] [PubMed] [Google Scholar]
- 6.Sperling MR, Barshow S, Nei M, Asadi-Pooya AA. A reappraisal of mortality after epilepsy surgery. Neurology. May 24 2016;86(21):1938–44. doi: 10.1212/WNL.0000000000002700 [DOI] [PubMed] [Google Scholar]
- 7.Sheikh SR, Kattan MW, Steinmetz M, Singer ME, Udeh BL, Jehi L. Cost-effectiveness of surgery for drug-resistant temporal lobe epilepsy in the US. Neurology. Sep 8 2020;95(10):e1404–e1416. doi: 10.1212/wnl.0000000000010185. Epub 2020 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. Feb 03 2000;342(5):314–9. doi: 10.1056/NEJM200002033420503 [DOI] [PubMed] [Google Scholar]
- 9.Mohanraj R, Brodie MJ. Diagnosing refractory epilepsy: response to sequential treatment schedules. Eur J Neurol. Mar 2006;13(3):277–82. doi: 10.1111/j.1468-1331.2006.01215.x [DOI] [PubMed] [Google Scholar]
- 10.Geerts A, Arts WF, Stroink H, et al. Course and outcome of childhood epilepsy: a 15-year follow-up of the Dutch Study of Epilepsy in Childhood. Epilepsia. Jul 2010;51(7):1189–97. doi: 10.1111/j.1528-1167.2010.02546.x [DOI] [PubMed] [Google Scholar]
- 11.Berg AT, Levy SR, Testa FM, D’Souza R. Remission of epilepsy after two drug failures in children: a prospective study. Ann Neurol. May 2009;65(5):510–9. doi: 10.1002/ana.21642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: A summary of the Thirteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XIII). Epilepsia. February 2017;58(2):181–221. doi: 10.1111/epi.13634 [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Brodie MJ, Liew D, Kwan P. Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol. March 01 2018;75(3):279–286. doi: 10.1001/jamaneurol.2017.3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel J Jr., McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. Jama. Mar 7 2012;307(9):922–30. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for Drug-Resistant Epilepsy in Children. N Engl J Med. Oct 26 2017;377(17):1639–1647. doi: 10.1056/NEJMoa1615335 [DOI] [PubMed] [Google Scholar]
- 16.Sauro KM, Holroyd-Leduc J, Wiebe S, et al. Knowledge translation of an online tool to determine candidacy for epilepsy surgery evaluation. Neurol Clin Pract. Aug 2016;6(4):304–314. doi: 10.1212/CPJ.0000000000000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vakharia VN, Sparks RE, Li K, et al. Multicenter validation of automated trajectories for selective laser amygdalohippocampectomy. Epilepsia. 09 2019;60(9):1949–1959. doi: 10.1111/epi.16307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowerison MW, Josephson CB, Jetté N, et al. Association of Levels of Specialized Care With Risk of Premature Mortality in Patients With Epilepsy. JAMA Neurol. 11 January 2019;76(11):1352–1358. doi: 10.1001/jamaneurol.2019.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. Jun 2008;7(6):514–24. doi: 10.1016/S1474-4422(08)70108-X [DOI] [PubMed] [Google Scholar]
- 20.Nightscales R, McCartney L, Auvrez C, et al. Mortality in patients with psychogenic nonepileptic seizures. Neurology. August 11 2020;95(6):e643–e652. doi: 10.1212/WNL.0000000000009855 [DOI] [PubMed] [Google Scholar]
- 21.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 04 2017;58(4):512–521. doi: 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang I, Bernasconi A, Bernhardt B, et al. MRI essentials in epileptology: a review from the ILAE Imaging Taskforce. Epileptic Disord. Aug 01 2020;22(4):421–437. doi: 10.1684/epd.2020.1174 [DOI] [PubMed] [Google Scholar]
- 23.Jehi LE, Palmini A, Aryal U, Coras R, Paglioli E. Cerebral cavernous malformations in the setting of focal epilepsies: pathological findings, clinical characteristics, and surgical treatment principles. Acta Neuropathol. Jul 2014;128(1):55–65. doi: 10.1007/s00401-014-1294-y. Epub 2014 May 16. [DOI] [PubMed] [Google Scholar]
- 24.Lamberink HJ, Otte WM, Blümcke I, Braun KPJ. Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study. Lancet Neurol. Sep 2020;19(9):748–757. doi: 10.1016/s1474-4422(20)30220-9. [DOI] [PubMed] [Google Scholar]
- 25.Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L. Neuromodulation in epilepsy: state-of-the-art approved therapies. Lancet Neurol. Dec 2021;20(12):1038–1047. doi: 10.1016/S1474-4422(21)00300–8 [DOI] [PubMed] [Google Scholar]
- 26.Jehi L. The Epileptogenic Zone: Concept and Definition. Epilepsy Curr. Jan-Feb 2018;18(1):12–16. doi: 10.5698/1535-7597.18.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheikh SR, Nair D, Gross RE, Gonzalez-Martinez J. Tracking a changing paradigm and the modern face of epilepsy surgery: A comprehensive and critical review on the hunt for the optimal extent of resection in mesial temporal lobe epilepsy. Epilepsia. 09 2019;60(9):1768–1793. doi: 10.1111/epi.16310 [DOI] [PubMed] [Google Scholar]
- 28.Dorfer C, Rydenhag B, Baltuch G, et al. How technology is driving the landscape of epilepsy surgery. Epilepsia. 05 2020;61(5):841–855. doi: 10.1111/epi.16489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stern JM, Spivak NM, Becerra SA, et al. Safety of focused ultrasound neuromodulation in humans with temporal lobe epilepsy. Brain Stimul. 2021 Jul-Aug 2021;14(4):1022–1031. doi: 10.1016/j.brs.2021.06.003 [DOI] [PubMed] [Google Scholar]
- 30.Casseb RF, de Campos BM, Morita-Sherman M, et al. ResectVol: A tool to automatically segment and characterize lacunas in brain images. Epilepsia Open. Dec 2021;6(4):720–726. doi: 10.1002/epi4.12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C, Jermakowicz WJ, Chakravorti S, et al. Effects of surgical targeting in laser interstitial thermal therapy for mesial temporal lobe epilepsy: A multicenter study of 234 patients. Epilepsia. Jun 2019;60(6):1171–1183. doi: 10.1111/epi.15565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brotis AG, Giannis T, Paschalis T, Kapsalaki E, Dardiotis E, Fountas KN. A meta-analysis on potential modifiers of LITT efficacy for mesial temporal lobe epilepsy: Seizure-freedom seems to fade with time. Clin Neurol Neurosurg. Apr 20 2021;205:106644. doi: 10.1016/j.clineuro.2021.106644 [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Beg U, Padmanaban V, et al. A Systematic Review of Minimally Invasive Procedures for Mesial Temporal Lobe Epilepsy: Too Minimal, Too Fast? Neurosurgery. July 15 2021;89(2):164–176. doi: 10.1093/neuros/nyab125 [DOI] [PubMed] [Google Scholar]
- 34.Rydenhag B, Flink R, Malmgren K. Surgical outcomes in patients with epileptogenic tumours and cavernomas in Sweden: good seizure control but late referrals. J Neurol Neurosurg Psychiatry. Jan 2013;84(1):49–53. doi: 10.1136/jnnp-2012-302449 [DOI] [PubMed] [Google Scholar]
- 35.Erba G, Moja L, Beghi E, Messina P, Pupillo E. Barriers toward epilepsy surgery. A survey among practicing neurologists. Epilepsia. Jan 2012;53(1):35–43. doi: 10.1111/j.1528-1167.2011.03282.x [DOI] [PubMed] [Google Scholar]
- 36.de Flon P, Kumlien E, Reuterwall C, Mattsson P. Empirical evidence of underutilization of referrals for epilepsy surgery evaluation. Eur J Neurol. Apr 2010;17(4):619–25. doi: 10.1111/j.1468-1331.2009.02891.x [DOI] [PubMed] [Google Scholar]
- 37.Blumcke I, Spreafico R, Haaker G, et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N Engl J Med. Oct 26 2017;377(17):1648–1656. doi: 10.1056/NEJMoa1703784. [DOI] [PubMed] [Google Scholar]
- 38.Braun KPJ. Influence of epilepsy surgery on developmental outcomes in children. Eur J Paediatr Neurol. Jan 2020;24:40–42. doi: 10.1016/j.ejpn.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 39.Skirrow C, Cross JH, Owens R, et al. Determinants of IQ outcome after focal epilepsy surgery in childhood: A longitudinal case-control neuroimaging study. Epilepsia. 05 2019;60(5):872–884. doi: 10.1111/epi.14707 [DOI] [PubMed] [Google Scholar]
- 40.Kadish NE, Bast T, Reuner G, et al. Epilepsy Surgery in the First 3 Years of Life: Predictors of Seizure Freedom and Cognitive Development. Neurosurgery. June 01 2019;84(6):E368–E377. doi: 10.1093/neuros/nyy376 [DOI] [PubMed] [Google Scholar]
- 41.Harvey AS, Cross JH, Shinnar S, Mathern GW, Mathern BW, Taskforce IPESS. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. Jan 2008;49(1):146–55. doi: 10.1111/j.1528-1167.2007.01421.x [DOI] [PubMed] [Google Scholar]
- 42.Baud MO, Perneger T, Rácz A, et al. European trends in epilepsy surgery. Neurology. July 10 2018;91(2):e96–e106. doi: 10.1212/WNL.0000000000005776 [DOI] [PubMed] [Google Scholar]
- 43.Wiebe S, Eliasziw M, Matijevic S. Changes in quality of life in epilepsy: how large must they be to be real? Epilepsia. Jan 2001;42(1):113–8. doi: 10.1046/j.1528-1157.2001.081425.x [DOI] [PubMed] [Google Scholar]
- 44.Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for Drug-Resistant Epilepsy in Children. N Engl J Med. October 26 2017;377(17):1639–1647. doi: 10.1056/NEJMoa1615335 [DOI] [PubMed] [Google Scholar]
- 45.Jehi L, Friedman D, Carlson C, et al. The evolution of epilepsy surgery between 1991 and 2011 in nine major epilepsy centers across the United States, Germany, and Australia. Epilepsia. Oct 2015;56(10):1526–33. doi: 10.1111/epi.13116. Epub 2015 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berg AT, Langfitt J, Shinnar S, et al. How long does it take for partial epilepsy to become intractable? Neurology. Jan 28 2003;60(2):186–90. doi: 10.1212/01.wnl.0000031792.89992.ec [DOI] [PubMed] [Google Scholar]
- 47.Lamberink HJ, Boshuisen K, van Rijen PC, Gosselaar PH, Braun KP, (DCESP) DCESP. Changing profiles of pediatric epilepsy surgery candidates over time: A nationwide single-center experience from 1990 to 2011. Epilepsia. May 2015;56(5):717–25. doi: 10.1111/epi.12974 [DOI] [PubMed] [Google Scholar]
- 48.Belohlavkova A, Jezdik P, Jahodova A, et al. Evolution of pediatric epilepsy surgery program over 2000–2017: Improvement of care? Eur J Paediatr Neurol. May 2019;23(3):456–465. doi: 10.1016/j.ejpn.2019.04.002. Epub 2019 Apr 15. [DOI] [PubMed] [Google Scholar]
- 49.Pestana Knight EM, Schiltz NK, Bakaki PM, Koroukian SM, Lhatoo SD, Kaiboriboon K. Increasing utilization of pediatric epilepsy surgery in the United States between 1997 and 2009. Epilepsia. Mar 2015;56(3):375–81. doi: 10.1111/epi.12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jetté N, Sander JW, Keezer MR. Surgical treatment for epilepsy: the potential gap between evidence and practice. Lancet Neurol. 08 2016;15(9):982–994. doi: 10.1016/S1474-4422(16)30127-2 [DOI] [PubMed] [Google Scholar]
- 51.Roberts JI, Hrazdil C, Wiebe S, et al. Neurologists’ knowledge of and attitudes toward epilepsy surgery: a national survey. Neurology. Jan 13 2015;84(2):159–66. doi: 10.1212/WNL.0000000000001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solli E, Colwell NA, Say I, et al. Deciphering the surgical treatment gap for drug-resistant epilepsy (DRE): A literature review. Epilepsia. 07 2020;61(7):1352–1364. doi: 10.1111/epi.16572 [DOI] [PubMed] [Google Scholar]
- 53.de Tisi J, Bell GS, Peacock JL, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. Oct 15 2011;378(9800):1388–95. doi: 10.1016/S0140-6736(11)60890-8 [DOI] [PubMed] [Google Scholar]
- 54.Jehi LE, O’Dwyer R, Najm I, Alexopoulos A, Bingaman W. A longitudinal study of surgical outcome and its determinants following posterior cortex epilepsy surgery. Epilepsia. Sep 2009;50(9):2040–52. doi: 10.1111/j.1528-1167.2009.02070.x. Epub 2009 Mar 23. [DOI] [PubMed] [Google Scholar]
- 55.Simasathien T, Vadera S, Najm I, Gupta A, Bingaman W, Jehi L. Improved outcomes with earlier surgery for intractable frontal lobe epilepsy. Ann Neurol. May 2013;73(5):646–54. doi: 10.1002/ana.23862. Epub 2013 Mar 11. [DOI] [PubMed] [Google Scholar]
- 56.Englot DJ, Wang DD, Rolston JD, Shih TT, Chang EF. Rates and predictors of long-term seizure freedom after frontal lobe epilepsy surgery: a systematic review and meta-analysis. J Neurosurg. May 2012;116(5):1042–8. doi: 10.3171/2012.1.jns111620. Epub 2012 Feb 3. [DOI] [PubMed] [Google Scholar]
- 57.Sperling MR, Gross RE, Alvarez GE, McKhann GM, Salanova V, Gilmore J. Stereotactic Laser Ablation for Mesial Temporal Lobe Epilepsy: A prospective, multicenter, single-arm study. Epilepsia. 06 2020;61(6):1183–1189. doi: 10.1111/epi.16529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Callaghan B, Schlesinger M, Rodemer W, et al. Remission and relapse in a drug-resistant epilepsy population followed prospectively. Epilepsia. Mar 2011;52(3):619–26. doi: 10.1111/j.1528-1167.2010.02929.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nair DR, Laxer KD, Weber PB, et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 09 January 2020;95(9):e1244–e1256. doi: 10.1212/WNL.0000000000010154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salanova V, Sperling MR, Gross RE, et al. The SANTÉ study at 10 years of follow-up: Effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia. 06 2021;62(6):1306–1317. doi: 10.1111/epi.16895 [DOI] [PubMed] [Google Scholar]
- 61.Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain. Oct 2009;132(Pt 10):2822–30. doi: 10.1093/brain/awp182 [DOI] [PubMed] [Google Scholar]
- 62.Wilson SJ, Bladin PF, Saling MM. The burden of normality: a framework for rehabilitation after epilepsy surgery. Epilepsia. 2007;48 Suppl 9:13–6. doi: 10.1111/j.1528-1167.2007.01393.x [DOI] [PubMed] [Google Scholar]
- 63.Rayner G, Micallef S, Abeywickrama R, Wilson SJ. Pediatric epilepsy surgery patients show normal psychosocial development at long-term follow-up despite dissatisfying family dynamics. Epilepsy Behav. Mar 2019;92:245–252. doi: 10.1016/j.yebeh.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 64.Galovic M, de Tisi J, McEvoy AW, et al. Resective surgery prevents progressive cortical thinning in temporal lobe epilepsy. Brain. 12 May 2020;143(11):3262–3272. doi: 10.1093/brain/awaa284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sherman EM, Wiebe S, Fay-McClymont TB, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. May 2011;52(5):857–69. doi: 10.1111/j.1528-1167.2011.03022.x [DOI] [PubMed] [Google Scholar]
- 66.Baxendale S, Wilson SJ, Baker GA, et al. Indications and expectations for neuropsychological assessment in epilepsy surgery in children and adults: Executive summary of the report of the ILAE Neuropsychology Task Force Diagnostic Methods Commission: 2017–2021. Epilepsia. Sep 2019;60(9):1794–1796. doi: 10.1111/epi.16309 [DOI] [PubMed] [Google Scholar]
- 67.Helmstaedter C, Beeres K, Elger CE, Kuczaty S, Schramm J, Hoppe C. Cognitive outcome of pediatric epilepsy surgery across ages and different types of surgeries: A monocentric 1-year follow-up study in 306 patients of school age. Seizure. Apr 2020;77:86–92. doi: 10.1016/j.seizure.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 68.Burneo JG, Sirven JI, Kiesel LW, et al. Managing common complex symptomatic epilepsies: tumors and trauma: american epilepsy society - 2012 annual course summary. Epilepsy Curr. Sep 2013;13(5):232–5. doi: 10.5698/1535-7597-13.5.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson SJ, Micallef S, Henderson A, et al. Developmental outcomes of childhood-onset temporal lobe epilepsy: a community-based study. Epilepsia. Sep 2012;53(9):1587–96. doi: 10.1111/j.1528-1167.2012.03632.x [DOI] [PubMed] [Google Scholar]
- 70.Hader WJ, Tellez-Zenteno J, Metcalfe A, et al. Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. May 2013;54(5):840–7. doi: 10.1111/epi.12161. Epub 2013 Apr 3. [DOI] [PubMed] [Google Scholar]
- 71.Edelvik A, Rydenhag B, Olsson I, et al. Long-term outcomes of epilepsy surgery in Sweden. Neurology. 2013;81(14):1244. doi: 10.1212/WNL.0b013e3182a6ca7b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pelliccia V, Deleo F, Gozzo F, et al. Early and late epilepsy surgery in focal epilepsies associated with long-term epilepsy-associated tumors. J Neurosurg. Nov 2017;127(5):1147–1152. doi: 10.3171/2016.9.JNS161176 [DOI] [PubMed] [Google Scholar]
- 73.Ramantani G, Kadish NE, Mayer H, et al. Frontal Lobe Epilepsy Surgery in Childhood and Adolescence: Predictors of Long-Term Seizure Freedom, Overall Cognitive and Adaptive Functioning. Neurosurgery. 07 January 2018;83(1):93–103. doi: 10.1093/neuros/nyx340 [DOI] [PubMed] [Google Scholar]
- 74.Bjellvi J, Olsson I, Malmgren K, Wilbe Ramsay K. Epilepsy duration and seizure outcome in epilepsy surgery: A systematic review and meta-analysis. Neurology. July 09 2019;93(2):e159–e166. doi: 10.1212/WNL.0000000000007753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewis AK, Taylor NF, Carney PW, Harding KE. What is the effect of delays in access to specialist epilepsy care on patient outcomes? A systematic review and meta-analysis. Epilepsy Behav. 09 2021;122:108192. doi: 10.1016/j.yebeh.2021.108192 [DOI] [PubMed] [Google Scholar]
- 76.Kapadia M, Walwema M, Smith TR, et al. Seizure outcome in patients with cavernous malformation after early surgery. Epilepsy Behav. 02 2021;115:107662. doi: 10.1016/j.yebeh.2020.107662 [DOI] [PubMed] [Google Scholar]
- 77.Maganti R, Rutecki P, Bell B, et al. Epilepsy surgery outcome among US veterans. Epilepsy Behav. Dec 2003;4(6):723–8. doi: 10.1016/j.yebeh.2003.08.026 [DOI] [PubMed] [Google Scholar]
- 78.Anesthesiology AAoN. Analgesia and Anesthesia for the Substance Use Disorder Patient: Practice Considerations. 2022. https://www.aana.com/docs/default-source/practice-aana-com-web-documents-(all)/professional-practice-manual/analgesia-and-anesthesia-for-the-substance-use-disorder-patient.pdf?sfvrsn=3e6b7548_4
- 79.Belayneh Z, Mekuriaw B. A systematic review and meta-analysis of anti-epileptic medication non-adherence among people with epilepsy in Ethiopia. Arch Public Health. 2020;78:23. doi: 10.1186/s13690-020-00405-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ti L, Ti L. Leaving the Hospital Against Medical Advice Among People Who Use Illicit Drugs: A Systematic Review. Am J Public Health. Dec 2015;105(12):e53–9. doi: 10.2105/AJPH.2015.302885 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.