Abstract
Background
Stroke patients have to face a high risk of recurrence, especially for those with comorbid T2DM, which usually lead to much more serious neurologic damage and an increased likelihood of death. This study aimed to explore determinants of stroke relapse among patients with comorbid T2DM.
Materials and methods
We conducted this case-control study nested a prospective cohort of ischemic stroke (IS) with comorbid T2DM. During 36-month follow-up, the second stroke occurred in 84 diabetic IS patients who were allocated into the case group, while 613 patients without recurrence were the controls. We collected the demographic data, behaviors and habits, therapies, and family history at baseline, and measured the variables during follow-up. LASSO and Logistic regression analyses were carried out to develop a prediction model of stroke recurrence. The receiver operator characteristic (ROC) curve was employed to evaluate the performance of the prediction model.
Results
Compared to participants without recurrence, the higher levels of pulse rate (78.29 ± 12.79 vs. 74.88 ± 10.93) and hypertension (72.6 vs. 61.2%) were recorded at baseline. Moreover, a lower level of physical activity (77.4 vs. 90.4%), as well as a higher proportion of hypoglycemic therapy (36.9 vs. 23.3%) was also observed during 36-month follow-up. Multivariate logistic regression revealed that higher pulse rate at admission (OR = 1.027, 95 %CI = 1.005–1.049), lacking physical activity (OR = 2.838, 95% CI = 1.418–5.620) and not receiving hypoglycemic therapy (OR = 1.697, 95% CI = 1.013–2.843) during follow-up increased the risk of stroke recurrence. We developed a prediction model using baseline pulse rate, hypoglycemic therapy, and physical activity, which produced an area under ROC curve (AUC) of 0.689.
Conclusion
Physical activity and hypoglycemic therapy play a protective role for IS patients with comorbid diabetes. In addition to targeted therapeutics, the improvement of daily-life habit contributes to slowing the progress of the IS.
Keywords: ischemic stroke, diabetes mellitus, recurrence, risk factors, nested case-control study
Background
Globally, stroke has ranked as the second leading cause of death (Wu Y. et al., 2019; Zheng et al., 2022) and the third leading cause of disability (Cao et al., 2019; Collaborators, 2021). Ischemic stroke (IS), characterized by temporary or permanent cerebrovascular occlusion (Liu et al., 2018), accounts for 82% of acute stroke events (Mehra et al., 2019; Feske, 2021). The incidence and mortality of stroke are significantly higher in China than in developed countries: a study on the Global Burden of Disease in 2019 reported stroke as the leading cause of death in this country (Wu S. et al., 2019).
Stroke patients face a high likelihood of recurrence after the initial onset of IS, ranging from 5.4% at 1 year to 11.3% at 5 years (Khanevski et al., 2019). Patients with stroke recurrence have a higher likelihood to endure more serious brain damage and neurologic dysfunction, leading to a worse functional status and/or higher case fatality rate vs. initial stroke (Lin B. et al., 2021; Wang et al., 2021). Physical activity, antihypertensive treatment, and high fiber intake (i.e., fruits and vegetables) have preventive effects on IS recurrence (Gillman et al., 1995; Zonneveld et al., 2018; Lin Y. et al., 2021). Other modifiable lifestyle characteristics, including obesity, hypertension, diabetes, depression and smoking, usually serve as risk factors (Black et al., 2015; Chen et al., 2019; Huang et al., 2019; Zheng and Yao, 2019; Cao et al., 2020; Szlachetka et al., 2020; Kumral et al., 2021). Despite recent progress in the management and prevention of recurrent stroke, enhanced approaches are needed to further reduce relapse risk.
Diabetes mellitus (DM), a complex disease featuring a deficiency or resistance to insulin, exposes individuals to hyperglycemia (Tang et al., 2014). This state causes pathologic changes in the blood vessels of various organs, including cerebral vessels (Li et al., 2020). The incidence of cardiovascular diseases, such as IS, is apparently higher in patients with DM than in persons without. As one of established risk factors for stroke, the prevalence of DM-induced IS has been increasing in all age groups (Benjamin et al., 2019; Venkat et al., 2021). Acute stroke with comorbid DM has significantly higher risks of aggravated pathology, disability, and death. These consequences mostly arise from extensive unbalance of metabolism, severe vascular damage, deteriorated white matter, and specific inflammatory milieu (Ergul et al., 2016; Venkat et al., 2017, 2021). As understood, diabetic stroke patients face a higher probability of stroke recurrence. Controlling glucose levels and other risk factors has been considered an effective means of preventing subsequent strokes (Lau et al., 2019). However, few studies have focused on precise prevention strategies for stroke recurrence in Chinese patients with comorbid DM. Identifying determinants of stroke recurrence in DM patients will help to alleviate stroke-related deterioration. Findings will also advance personalized healthcare strategies.
Materials and methods
Study participants
This nested case–control study was conducted with a Chinese Han stroke cohort established at the Brain Hospital of Liaocheng People’s Hospital (BHLPH) in Shandong Province, China. This cohort enrolled 1,793 patients with acute IS who were admitted to BHLPH between January 1, 2017, and September 30, 2018. Among these patients, 717 had T2DM. After 36 months of follow-up, patients with recurrent stroke were recruited in the case group; those without recurrence constituted the control group.
Study inclusion criteria were as follows: (1) First-ever stroke; (2) with comorbid T2DM; (3) a diagnosis of acute IS, confirmed via magnetic resonance imaging with reference to the Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke (Neurology and Society, 2018); (4) age ≥ 18 years; and (5) complete clinical data were available. The exclusion criteria covered (1) patients with severe mental disorders; (2) pregnant or breastfeeding women; (3) patients with incomplete clinical characteristics; (4) patients with severe somatic diseases (e.g., heart diseases, cancers, and infectious diseases); and (5) patients enrolled in other clinical trials.
The study protocol was reviewed and approved by the ethics committee of BHLPH (No. BHLPH085). All participants provided their written informed consent.
Data collection
Participants’ demographic data were obtained through face-to-face interviews. Clinical features were collected by trained physicians. According to body mass index (BMI), the participants were classified were classified as (1) underweight (BMI < 18.5), (2) normal weight (BMI of 18.5–23.9), (3) overweight (BMI of 24.0–27.9), or (4) obese (BMI ≥ 28). The National Institutes of Health Stroke Scale (NIHSS), a 15-item neurologic-impairment scale with scores ranging from 0 (no deficit) to 42 (quadriplegia and coma) was used to assess acute stroke deficits. NIHSS frequencies were classified into 5 groups: no measured stroke symptoms (0); minor stroke (1–4); moderate stroke (5–15); moderate to severe stroke (16–20); and severe stroke (21–42) (Saber and Saver, 2020). The Modified Rankin Scale (mRS) is a disability scale ranging from 0 (no symptoms) to 6 (death), by which patients were classified into two groups: good outcome (0–2), and worse outcome (3–6) (Armangue et al., 2018). A fasting venous blood sample was collected for determination of hematologic and biochemical parameters, including high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), total cholesterol (TC), fasting blood glucose (FBG), and total homocysteine (tHcy), were quantified with an automatic analyzer (Hitachi, Tokyo, Japan). Pulse rate was automatically recorded by a finger pulse oximetric device (Yuwell, Jiangsu, China). Blood pressure (BP) was measured by a trained nurse in a sitting position with at least 5 min rest before the first measurement, and the mean of two measurements was recorded.
The 36-month follow-up was performed by a trained clinical neurologist after patients were discharged from the hospital. Parameters including stroke recurrence, age, gender, smoking, alcohol use, systolic blood pressure (SBP), diastolic blood pressure (DBP), FBG, physical activity (often: ≥ 3 sessions per week and ≥ 30 min per session, or moderate-intensity exercise), family history, and medications were obtained when visited annually. The mean of each variable was calculated during the follow-up period.
Patients who take the medicines as prescribed are regarded as receiving the relevant treatment: (1) Antiplatelet therapy (e.g., aspirin, clopidogrel, or ticagrelor), (2) antihypertensive therapy [e.g., angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blocker (ARB)], (3) hypoglycemic therapy (e.g., insulin, pioglitazone), (4) antithrombotic therapy (e.g., aspirin, ozagrel), and (5) lipid-lowering drug therapy (e.g., statins, niacin and its derivatives).
Statistical analysis
For the purposes of reporting results, continuous variables are expressed herein as means and standard deviations; categorical variables are presented as frequencies and/or percentages. The Kolmogorov–Smirnov test was used to examine the normal distribution of continuous data. If normality was assumed, a Student’s t-test was performed to analyze the difference between groups; otherwise, the data were analyzed using a Mann–Whitney U-test. Meanwhile, between-group differences in categorical data were tested via a chi-square test. To alleviate collinearity between multiple variables, a least absolute shrinkage and selection operator (LASSO) regression was carried out to select variables potentially associated with IS recurrence in patients with comorbid T2DM. A multivariate logistic regression was performed to screen statistically significant variables: The odds ratio (OR) and 95% confidence interval (CI) were calculated for each variable. Then, a multivariate prediction model was developed using these significant variables. To evaluate the model’s performance in predicting stroke recurrence, a receiver operator characteristic (ROC) curve was constructed, and the area under the ROC curve (AUC) was determined. In addition, a nomogram was designed on the basis of the multivariate logistic regression model; the concordance index (C-index) was used to assess the nomogram’s prediction accuracy.
Statistical analyses were performed in SPSS 26.0 (IBM, NY) and R packages 4.1.0 (R Core Team). A two-tailed P-value of less than 0.05 was considered statistically significant.
Results
Characteristics of study participants
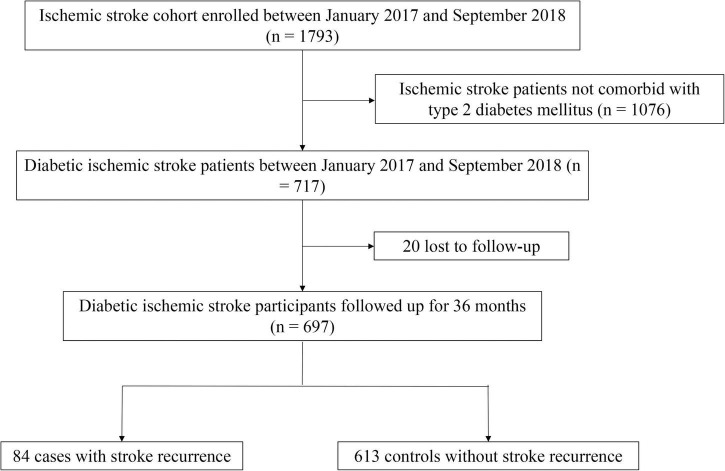
Twenty individuals were lost to follow-up after 36 months (2.8%, 20/717). Stroke recurred in 84 (12.05%) of participants in the case group, while 613 patients without recurrence were allocated into the control group. Study participants were aged 65.2 ± 11.28 years, of whom 61.4% (428/697) were men. Figure 1 presents a flowchart describing study population selection.
FIGURE 1.
Flow chart for study participant selection.
Participants’ baseline data are summarized in Table 1 and Supplementary Table 1. Differences in age, gender, BMI, smoking, alcohol consumption, FBG, triglycerides, TC, LDL-C, HDL-C, tHcy, neck vascular stenosis, Family history of stroke, coronary heart disease and hypertension, mRS score on admission, and NIHSS score on admission were not statistically significant between the case and control groups. Patients with stroke recurrence had a higher prevalence of hypertension at admission than the control group (P < 0.05). In addition, the pulse rate of recurrent cases was significantly higher than the controls (P < 0.05). The results of biochemical tests are shown in Figure 2.
TABLE 1.
Baseline data of participants.
| Variables | Cases of recurrence (N = 84) |
Cases of non-recurrence (N = 613) |
t/χ2 | P |
| Age group (n, %) | ||||
| < 60 | 23 (27.4) | 197 (32.1) | 1.493 | 0.474 |
| 60− | 25 (29.8) | 194 (31.6) | ||
| ≥ 70 | 36 (42.9) | 222 (36.2) | ||
| Gender (n, %) | ||||
| Male | 52 (61.9) | 376 (61.3) | 0.010 | 0.920 |
| Female | 32 (38.1) | 237 (38.7) | ||
| BMI classification (n, %) | ||||
| Normal (18.5–23.9) | 34 (40.5) | 232 (37.8) | 0.370 | 0.831 |
| Overweight (24–27.9) | 38 (45.2) | 299 (48.8) | ||
| Obese (≥ 28) | 12 (14.3) | 82 (13.4) | ||
| Smoking (n, %) | ||||
| Yes | 69 (82.1) | 463 (75.5) | 1.788 | 0.181 |
| No | 15 (17.9) | 150 (24.5) | ||
| Alcohol consumption (n, %) | ||||
| Yes | 62 (73.8) | 430 (70.1) | 0.477 | 0.490 |
| No | 22 (26.2) | 183 (29.9) | ||
| Hypertension (n, %) | ||||
| Yes | 61 (72.6) | 375 (61.2) | 4.131 | 0.042 |
| No | 23 (27.4) | 238 (38.8) | ||
| Neck vascular stenosis (n, %) | ||||
| Yes | 20 (23.8) | 205 (33.4) | 3.136 | 0.077 |
| No | 64 (76.2) | 408 (66.6) | ||
| Family history of stroke (n, %) | ||||
| Yes | 3 (3.6) | 59 (9.6) | 3.340 | 0.068 |
| No | 81 (96.4) | 554 (90.4) | ||
| Family history of CHD (n, %) | ||||
| Yes | 1 (1.2) | 29 (4.7) | 1.471 | 0.225 |
| No | 83 (98.8) | 584 (95.3) | ||
| Family history of hypertension (n, %) | ||||
| Yes | 5 (6.0) | 45 (7.3) | 0.214 | 0.644 |
| No | 79 (94.0) | 568 (92.7) | ||
| mRS score at admission (n, %) | ||||
| Low (0–2) | 497 (81.1) | 72 (85.7) | 1.060 | 0.303 |
| High (3–5) | 116 (18.9) | 12 (14.3) | ||
| NIHSS score on admission (n, %) | ||||
| Normal (0) | 20 (23.8) | 128 (20.9) | 3.932 | 0.415 |
| Minor stroke (1–4) | 26 (31.0) | 186 (30.3) | ||
| Moderate stroke (5–15) | 22 (26.2) | 132 (21.5) | ||
| Moderate to severe stroke (16–20) | 16 (19.0) | 13 (2.1) | ||
| Severe stroke (21–42) | 0 (0.0) | 154 (25.1) | ||
| Pulse rate (times/min) | 78.29 ± 12.79 | 74.88 ± 10.93 | 2.619 | 0.009 |
BMI, body mass index; CHD, coronary heart disease; NIHSS, National Institutes of Health Stroke Scale; mRS, Modified Rankin Scale.
FIGURE 2.
Biochemical tests. (A) The level of Homocysteine; (B) the level of HDL-C; (C) the level of LDL-Cs; (D) the level of Cholesterol; (E) the level of Triglyceride; (F) the level of FBG; the data shown in the graphs represent the mean ± SD. FBG, fasting blood-glucose; LDL-C, low density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; SD, standard deviations.
Follow-up
As shown in Table 2 and Supplementary Table 1, no significant between-group differences were detected for variables including smoking; FBG; mRS score, rehabilitation treatment; and antihypertension, antiplatelet, antithrombosis, and lipid-lowering therapies. The proportions of physical activity and hypoglycemic therapy were statistically different between groups (P < 0.05).
TABLE 2.
Parameters during follow-up.
| Characteristics | Cases of recurrence (N = 84) |
Cases of non-recurrence (N = 613) |
χ2 | P |
| Smoking (n, %) | ||||
| Yes | 74 (88.1) | 513 (83.7) | 1.08 | 0.299 |
| No | 10 (11.9) | 100 (16.3) | ||
| Physical activity (n, %) | ||||
| Often* | 65 (77.4) | 554 (90.4) | 12.551 | < 0.001 |
| Lacking | 19 (22.6) | 59 (9.6) | ||
| mRS score (n, %) | ||||
| Low (0–2) | 76 (90.5) | 568 (92.7) | 0.501 | 0.479 |
| High (3–5) | 8 (9.5) | 45 (7.3) | ||
| FBG | ||||
| <7.0 mmol/L | 75 (89.3) | 572 (93.3) | 1.798 | 0.180 |
| ≥7.1 mmol/L | 9 (10.7) | 41 (6.7) | ||
| Antiplatelet therapy (n, %) | ||||
| Yes | 65 (77.4) | 487 (79.4) | 0.191 | 0.662 |
| No | 19 (22.6) | 126 (20.6) | ||
| Antihypertensive therapy (n, %) | ||||
| Yes | 46 (54.8) | 312 (50.9) | 0.442 | 0.506 |
| No | 38 (45.2) | 301 (49.1) | ||
| Hypoglycemic therapy (n, %) | ||||
| Yes | 31 (36.9) | 143 (23.3) | 7.270 | 0.007 |
| No | 53 (63.1) | 470 (76.7) | ||
| Rehabilitation therapy (n, %) | ||||
| Yes | 58 (69.0) | 444 (72.4) | 0.420 | 0.517 |
| No | 26 (31.0) | 169 (27.6) | ||
| Antithrombotic therapy (n, %) | ||||
| Yes | 45 (53.6) | 317 (48.3) | 0.102 | 0.749 |
| No | 39 (46.4) | 296 (51.7) | ||
| Lipid-lowering drug (n, %) | ||||
| Yes | 48 (57.1) | 298 (48.6) | 2.150 | 0.143 |
| No | 36 (42.9) | 315 (51.4) |
mRS, Modified Rankin Scale; FBG, fasting blood glucose.
* ≥ 3 sessions per week and ≥ 30 min per session, or moderate-intensity exercise.
Development of prediction model of stroke recurrence
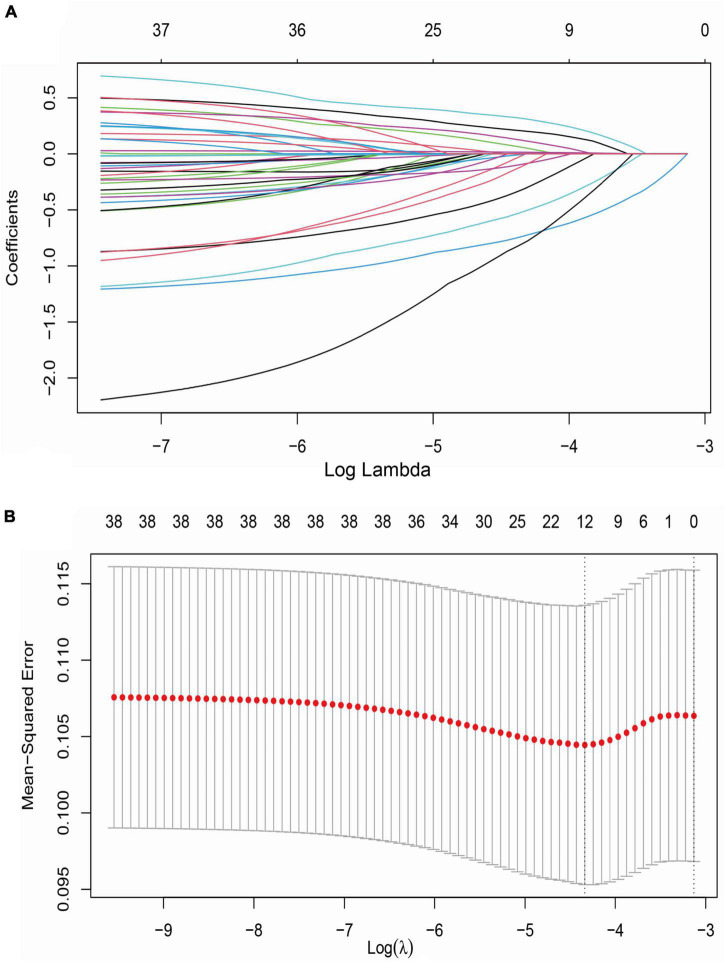
A LASSO model was used to screen potential determinants associated with stroke recurrence in T2DM patients. In Figure 3, red dots denote the target parameter to which each λ corresponds; the two dotted lines refer to two special λ values. Each curve matched the track of a single covariate coefficient. Finally, 38 covariates were selected for this model, with an optimal λ of 0.000584.
FIGURE 3.
LASSO regression analysis for variable selection. (A) LASSO coefficient of 38 variables; (B) optimal penalty coefficient (λ = 0.000584) in LASSO regression identified with the minimum criterion; LASSO, least absolute shrinkage and selection operator; SE, standard error.
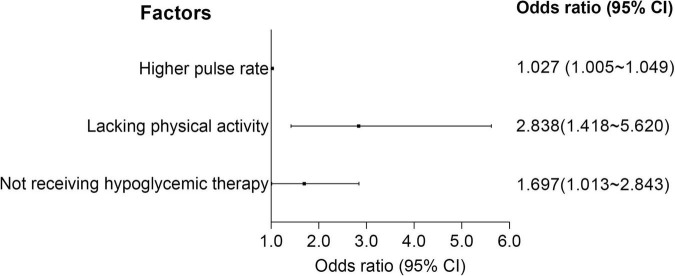
A logistic regression model, along with the LASSO regression, was established to screen the determinants of stroke recurrence. After adjusting for age, sex, BMI, tHcy, LDL-C, HDL-C, cholesterol, smoking and family history of stroke, a higher pulse rate at admission (OR = 1.027, 95% CI = 1.005–1.049) was significantly associated with increased risk for recurrence of stroke. Not receiving Hypoglycemic therapy (OR = 1.697, 95% CI = 1.013–2.843) and lacking physical activity during follow-up (OR = 2.838, 95% CI = 1.418–5.620) correlated with a higher risk for recurrence (Figure 4 and Supplementary Table 2).
FIGURE 4.
Forest plots of logistic regression. CI, confidence interval.
Performance of prediction model
The ROC curve analysis (Figure 5) showed a high performance of differentiation between high and low risk for stroke recurrence (AUC = 0.689, 95% CI = 0.628–0.750). According to the ROC curve, our prediction model exerted a relatively high accuracy, with a sensitivity of 61.9% and a specificity of 70.8% at a cut-off point of 0.130.
FIGURE 5.
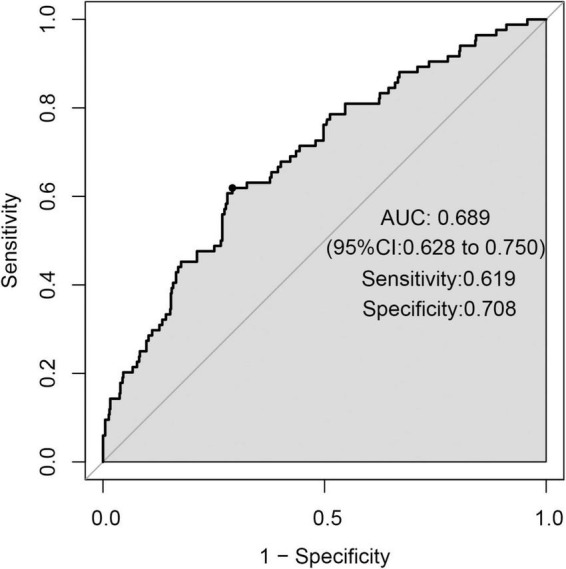
Receiver operator characteristic (ROC) curve.
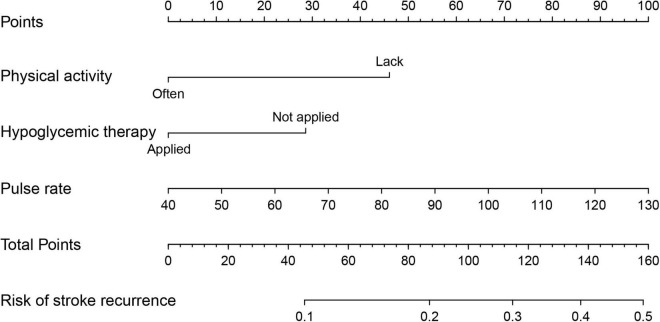
A nomogram was constructed with the above three predictors to forecast the 36-month risk of stroke recurrence (Figure 6). Internal validation was based on the random 70:30 partitioning of study participants data into training data: Test data sets. The nomogram’s prediction accuracy was evaluated by the C-index: 0.624 (95% CI = 0.553–0.695) for the training set and 0.653 (95% CI = 0.513–0.793) for the test set. As such, the nomogram displayed relatively good performance.
FIGURE 6.
Nomogram to predict 36-month risk of stroke recurrence. Draw a line perpendicular from the corresponding axis of each factor until it reaches the top line labeled “Points”. Sum up the number of points for all factors, then draw a line descending from the axis labeled “Total Points” until it intercepts each of the axes to predict 36-month risk of stroke recurrence.
Discussion
The recurrence rate of stroke was 12.05% over 36 months of follow-up in this diabetic IS cohort. Higher pulse rate at admission, physical inactivity and not receiving hypoglycemic therapy during follow-up increased the risk of stroke recurrence among patients with comorbid T2DM. Our prediction model based on the three risk factors appeared capable of identifying recurrence risk.
Accurately identifying modifiable risk factors for stroke recurrence is crucial for developing strategies to lower stroke-related mortality and morbidity. Compared with first-ever stroke, patients with recurrence have markedly more severe functional disability and higher mortality (Andreasen et al., 2018). One randomized controlled trial found that DM is associated with a higher risk of new stroke in patients with minor stroke (Chen et al., 2017). Although prevention measures targeting stroke have lessened relapse rates, patients with recurrence account for nearly 30% of all stroke cases (He et al., 2020). Several determinants correlated with stroke recurrence have been acknowledged (Kolmos et al., 2021). However, few studies have been conducted on IS with comorbid T2DM in China. Within the Chinese Han population profiled in this research, we observed 12.05% stroke recurrence during 36 months of follow-up, consistent with previous studies (Andersen et al., 2015; He et al., 2015; Khanevski et al., 2019).
IS patients with comorbid T2DM have poorer clinical outcomes and long-term prognoses than those with normal blood glucose levels (Kang and Park, 2017). Hyperglycemia increases stroke patients’ risk of death by 87% at 30 days, 75% at 1 year, and 41% at 6 years after stroke, respectively (Johnston and Beckman, 2019). In addition to being an independent predictor of stroke-induced death (Geary et al., 2019), hyperglycemia is a determinant of stroke recurrence (Hotter et al., 2019). Hyperglycemia augments brain injury through multiple potential mechanisms, including endothelial dysfunction, oxidative stress, increment of inflammatory response, and impaired fibrinolysis (Garg et al., 2006; MacDougall and Muir, 2011; Nogueira-Machado et al., 2011; Krinock and Singhal, 2021). Compared with patients who have normal blood glucose levels, hyperglycemia leads to a 2.5-fold increase in the risk of 90-day stroke recurrence (Guo et al., 2021). Our between-group comparisons revealed the proportion of hypoglycemic therapy to be lower in patients with recurrent stroke. Multivariate logistic regression contrarily demonstrated that not receiving hypoglycemic therapy was associated with a higher risk of recurrence after adjusting for age, sex, BMI, tHcy, LDL-C, HDL-C, cholesterol, family history, smoking, and pulse rate. This finding corroborates clinical trials and a systematic review (Lee et al., 2017; Spence et al., 2019). Hypoglycemic agents such as pioglitazone reduce the risk of stroke recurrence by activating PPARγ signaling in adipocytes, immune and endothelial cells, and other tissues (Kernan et al., 2016; Yaghi et al., 2018). This process contributes to a lower likelihood of stroke recurrence among patients with insulin resistance, prediabetes, or DM (Yang et al., 2018).
Physical activity can lower venous pressure, elevate venous flow, and reduce thrombosis (Johansson et al., 2019). Physical activity also helps reduce the risk of atherosclerosis by boosting lipid metabolism and raising blood levels of HDL-C (Sun et al., 2021). These effects can prevent relapse in atherosclerotic stroke patients (Waters et al., 2016; Sun et al., 2018; Gardener et al., 2019). Clinical studies have documented that physical activity and exercise enhance neuroplasticity and change brain activity patterns in post-stroke survivors, reducing the risk of recurrence (Kramer et al., 2019). A dose–response relationship has been identified between physical activity duration and stroke recurrence as well (Hou et al., 2021). Regular exercise results in genome-wide epigenetic modifications in human skeletal muscle and adipose tissue, which could affect metabolic phenotypes associated with stroke (Ling and Rönn, 2014). Our study revealed a significant relationship between physical inactivity and stroke relapse, similar to relevant studies (Oza et al., 2017; Turan et al., 2017).
In terms of pulse waves, scholars have taken pulse wave velocity and heart rate as major parameters when assessing arteriosclerosis development (Munakata, 2014; Townsend et al., 2015; Ueki et al., 2017). Increased pulse pressure may expose cerebral small vessels to high pulsatile pressure and flow, which damage the cerebral microvasculature and hinder the restoration of stroke-impaired neurological function (O’Rourke and Safar, 2005; Ishizuka et al., 2016). Pulse rate has been proposed as a surrogate for heart rate (Chang and Chang, 2009). Although the direct connection between stroke and pulse rate has yet to be clearly explained, epidemiological evidence implies that an elevated resting heart rate is associated with cardiovascular morbidity and mortality (Hjalmarson et al., 1990). Experimental and clinical findings suggest that sustained heart rate elevation, independent of the underlying trigger, is associated with the pathogenesis of cardiovascular diseases; pharmacological or interventional heart rate reduction can help to mitigate cardiovascular outcomes (Custodis et al., 2010). Animal studies have illustrated that a high heart rate contributes to upregulation of vascular oxidative stress, endothelial dysfunction, and accelerated atherogenesis (Custodis et al., 2008). Clinical studies point to an association between increased resting heart rate and inflammatory markers (i.e., C-reactive protein [CRP], white blood cell count, and fibrinogen) (Sajadieh et al., 2004; Rogowski et al., 2007). In this study, we illustrated that pulse rate is associated with increased risk of stroke recurrence.
Several limitations of our research require attention. First, this study was conducted with a single cohort from one medical center in northern China. This factor might cause Berkson’s bias, limiting the generalizability of findings. Second, because this study entailed a long-term follow-up among stroke patients, loss to follow-up might underestimate recurrence. Third, potentially significant variables may have been overlooked at baseline due to this study’s limited aims. Information bias could arise as a result.
Conclusion
Our findings indicate that higher pulse rate at admission, physical inactivity and not receiving hypoglycemic therapy during follow-up expose the individuals with comorbid T2DM to higher risk of stroke recurrence. This result underlines the importance of healthy lifestyle behaviors and targeted therapeutics in alleviating the adverse outcomes of stroke.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: All data and methods supporting the findings of this study are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed to YBZ, bbnnbn@163.com.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of BHLPH (No. BHLPH085). The participants provided their written informed consent to participate in this study.
Author contributions
HH, HXL, LZ, and YBZ designed the study and took responsibility for the integrity of the data and the accuracy of the data analysis. LW, HYL, JH, CL, JW, JF, ZG, and YLZ contributed to data collection. LW, HYL, and JH contributed to statistical analysis and manuscript writing. HXL, LZ, and YBZ revised the manuscript. All authors made a significant intellectual contribution and approved the final version.
Acknowledgments
We thank Jun Wen at Edith Cowan University for English language editing and proofreading to help improve the quality of this manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81773527) and European Commission Horizon 2020 Framework Programme (PRODEMOS-779238).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.999568/full#supplementary-material
References
- Andersen S. D., Gorst-Rasmussen A., Lip G. Y., Bach F. W., Larsen T. B. (2015). Recurrent stroke: The value of the CHA2DS2VASc score and the essen stroke risk score in a nationwide stroke cohort. Stroke 46 2491–2497. 10.1161/strokeaha.115.009912 [DOI] [PubMed] [Google Scholar]
- Andreasen C., Jørgensen M. E., Gislason G. H., Martinsson A., Sanders R. D., Abdulla J., et al. (2018). Association of timing of aortic valve replacement surgery after stroke with risk of recurrent stroke and mortality. JAMA Cardiol. 3 506–513. 10.1001/jamacardio.2018.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armangue T., Spatola M., Vlagea A., Mattozzi S., Cárceles-Cordon M., Martinez-Heras E., et al. (2018). Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: A prospective observational study and retrospective analysis. Lancet Neurol. 17 760–772. 10.1016/s1474-4422(18)30244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin E. J., Muntner P., Alonso A., Bittencourt M. S., Callaway C. W., Carson A. P., et al. (2019). Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation 139:e56–e528. 10.1161/cir.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Black M., Wang W., Wang W. (2015). Ischemic stroke: From next generation sequencing and GWAS to community genomics? OMICS 19 451–460. 10.1089/omi.2015.0083 [DOI] [PubMed] [Google Scholar]
- Cao W., Li X., Zhang X., Zhang J., Sun Q., Xu X., et al. (2019). No causal effect of telomere length on ischemic stroke and its subtypes: A Mendelian randomization study. Cells 8:159. 10.3390/cells8020159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Zheng D., Zhang J., Wang A., Liu D., Zhang J., et al. (2020). Association between telomere length in peripheral blood leukocytes and risk of ischemic stroke in a Han Chinese population: A linear and non-linear Mendelian randomization analysis. J. Transl. Med. 18:385. 10.1186/s12967-020-02551-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.-M., Chang K.-M. (2009). Pulse rate derivation and its correlation with heart rate. J. Med. Biol. Eng. 29 132–137. [Google Scholar]
- Chen J., Li S., Zheng K., Wang H., Xie Y., Xu P., et al. (2019). Impact of smoking status on stroke recurrence. J. Am. Heart Assoc. 8:e011696. 10.1161/jaha.118.011696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Pan Y., Jing J., Zhao X., Liu L., Meng X., et al. (2017). Recurrent stroke in minor ischemic stroke or transient ischemic attack with metabolic syndrome and/or diabetes mellitus. J. Am. Heart Assoc. 6:e005446. 10.1161/jaha.116.005446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators G. B. D. S. (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol. 20 795–820. 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custodis F., Baumhäkel M., Schlimmer N., List F., Gensch C., Böhm M., et al. (2008). Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation 117 2377–2387. 10.1161/circulationaha.107.746537 [DOI] [PubMed] [Google Scholar]
- Custodis F., Schirmer S. H., Baumhäkel M., Heusch G., Böhm M., Laufs U. (2010). Vascular pathophysiology in response to increased heart rate. J. Am. Coll. Cardiol. 56 1973–1983. 10.1016/j.jacc.2010.09.014 [DOI] [PubMed] [Google Scholar]
- Ergul A., Hafez S., Fouda A., Fagan S. C. (2016). Impact of comorbidities on acute injury and recovery in preclinical stroke research: Focus on hypertension and diabetes. Transl. Stroke Res. 7 248–260. 10.1007/s12975-016-0464-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S. K. (2021). Ischemic stroke. Am. J. Med. 134 1457–1464. 10.1016/j.amjmed.2021.07.027 [DOI] [PubMed] [Google Scholar]
- Gardener H., Leifheit E. C., Lichtman J. H., Wang Y., Wang K., Gutierrez C. M., et al. (2019). Racial/ethnic disparities in mortality among medicare beneficiaries in the FL–PR CR eSD study. J. Am. Heart Assoc. 8:e009649. 10.1161/jaha.118.009649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R., Chaudhuri A., Munschauer F., Dandona P. (2006). Hyperglycemia, insulin, and acute ischemic stroke: A mechanistic justification for a trial of insulin infusion therapy. Stroke 37 267–273. 10.1161/01.Str.0000195175.29487.30 [DOI] [PubMed] [Google Scholar]
- Geary L., Hasselström J., Carlsson A. C., Eriksson I., von Euler M. (2019). Secondary prevention after stroke/transient ischemic attack: A randomized audit and feedback trial. Acta Neurol. Scand. 140 107–115. 10.1111/ane.13109 [DOI] [PubMed] [Google Scholar]
- Gillman M. W., Cupples L. A., Gagnon D., Posner B. M., Wolf P. A. (1995). Protective effect of fruits and vegetables on development of stroke in men. JAMA 273 1113–1117. [DOI] [PubMed] [Google Scholar]
- Guo Y., Wang G., Jing J., Wang A., Zhang X., Meng X., et al. (2021). Stress hyperglycemia may have higher risk of stroke recurrence than previously diagnosed diabetes mellitus. Aging 13 9108–9118. 10.18632/aging.202797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He V. Y., Condon J. R., You J., Zhao Y., Burrow J. N. (2015). Adverse outcome after incident stroke hospitalization for Indigenous and non-Indigenous Australians in the Northern Territory. Int. J. Stroke 10 89–95. 10.1111/ijs.12600 [DOI] [PubMed] [Google Scholar]
- He X. F., Zeng Y. X., Li G., Feng Y. K., Wu C., Liang F. Y., et al. (2020). Extracellular ASC exacerbated the recurrent ischemic stroke in an NLRP3-dependent manner. J. Cereb. Blood Flow Metab. 40 1048–1060. 10.1177/0271678x19856226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarson A., Gilpin E. A., Kjekshus J., Schieman G., Nicod P., Henning H., et al. (1990). Influence of heart rate on mortality after acute myocardial infarction. Am. J. Cardiol. 65 547–553. 10.1016/0002-9149(90)91029-6 [DOI] [PubMed] [Google Scholar]
- Hotter B., Galinovic I., Kunze C., Brunecker P., Jungehulsing G. J., Villringer A., et al. (2019). High-resolution diffusion-weighted imaging identifies ischemic lesions in a majority of transient ischemic attack patients. Ann. Neurol. 86 452–457. 10.1002/ana.25551 [DOI] [PubMed] [Google Scholar]
- Hou L., Li M., Wang J., Li Y., Zheng Q., Zhang L., et al. (2021). Association between physical exercise and stroke recurrence among first-ever ischemic stroke survivors. Sci. Rep. 11:13372. 10.1038/s41598-021-92736-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z. X., Lin X. L., Lu H. K., Liang X. Y., Fan L. J., Liu X. T. (2019). Lifestyles correlate with stroke recurrence in Chinese inpatients with first-ever acute ischemic stroke. J. Neurol. 266 1194–1202. 10.1007/s00415-019-09249-5 [DOI] [PubMed] [Google Scholar]
- Ishizuka K., Hoshino T., Shimizu S., Shirai Y., Mizuno S., Toi S., et al. (2016). Brachial-ankle pulse wave velocity is associated with 3-month functional prognosis after ischemic stroke. Atherosclerosis 255 1–5. 10.1016/j.atherosclerosis.2016.08.027 [DOI] [PubMed] [Google Scholar]
- Johansson M., Johansson L., Wennberg P., Lind M. (2019). Physical activity and risk of first-time venous thromboembolism. Eur. J. Prev. Cardiol. 26 1181–1187. 10.1177/2047487319829310 [DOI] [PubMed] [Google Scholar]
- Johnston F. M., Beckman M. (2019). Updates on management of gastric cancer. Curr. Oncol. Rep. 21:67. 10.1007/s11912-019-0820-4 [DOI] [PubMed] [Google Scholar]
- Kang D. H., Park J. (2017). Endovascular stroke therapy focused on stent retriever thrombectomy and direct clot aspiration: Historical review and modern application. J. Korean Neurosurg. Soc. 60 335–347. 10.3340/jkns.2016.0809.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan W. N., Viscoli C. M., Furie K. L., Young L. H., Inzucchi S. E., Gorman M., et al. (2016). Pioglitazone after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 374 1321–1331. 10.1056/NEJMoa1506930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanevski A. N., Bjerkreim A. T., Novotny V., Naess H., Thomassen L., Logallo N., et al. (2019). Recurrent ischemic stroke: Incidence, predictors, and impact on mortality. Acta Neurol. Scand. 140 3–8. 10.1111/ane.13093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmos M., Christoffersen L., Kruuse C. (2021). Recurrent Ischemic Stroke–a systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 30:105935. 10.1016/j.jstrokecerebrovasdis.2021.105935 [DOI] [PubMed] [Google Scholar]
- Kramer S. F., Hung S. H., Brodtmann A. (2019). The impact of physical activity before and after stroke on stroke risk and recovery: A narrative review. Curr. Neurol. Neurosci. Rep. 19:28. 10.1007/s11910-019-0949-4 [DOI] [PubMed] [Google Scholar]
- Krinock M. J., Singhal N. S. (2021). Diabetes, stroke, and neuroresilience: Looking beyond hyperglycemia. Ann. N.Y.Acad. Sci. 1495 78–98. 10.1111/nyas.14583 [DOI] [PubMed] [Google Scholar]
- Kumral E., Erdoğan C. E., Arı A., Bayam F. E., Saruhan G. (2021). Association of obesity with recurrent stroke and cardiovascular events. Rev. Neurol. 177 414–421. 10.1016/j.neurol.2020.06.019 [DOI] [PubMed] [Google Scholar]
- Lau L. H., Lew J., Borschmann K., Thijs V., Ekinci E. I. (2019). Prevalence of diabetes and its effects on stroke outcomes: A meta-analysis and literature review. J. Diabetes Investig. 10 780–792. 10.1111/jdi.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Saver J. L., Liao H. W., Lin C. H., Ovbiagele B. (2017). Pioglitazone for secondary stroke prevention: A systematic review and meta-analysis. Stroke 48 388–393. 10.1161/strokeaha.116.013977 [DOI] [PubMed] [Google Scholar]
- Li R. Z., Ding X. W., Geetha T., Al-Nakkash L., Broderick T. L., Babu J. R. (2020). Beneficial effect of genistein on diabetes-induced brain damage in the ob/ob mouse model. Drug Des. Dev. Ther. 14 3325–3336. 10.2147/dddt.S249608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B., Zhang Z., Mei Y., Wang C., Xu H., Liu L., et al. (2021). Cumulative risk of stroke recurrence over the last 10 years: A systematic review and meta-analysis. Neurol. Sci. 42 61–71. 10.1007/s10072-020-04797-5 [DOI] [PubMed] [Google Scholar]
- Lin Y., Zhang B., Hu M., Xu M., Qin C., Zhu C. (2021). [Causal relationship between physical exercise and risk of ischemic stroke recurrence based on the potential outcome theory]. Nan Fang Yi Ke Da Xue Xue Bao 41 1191–1197. 10.12122/j.issn.1673-4254.2021.08.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C., Rönn T. (2014). Epigenetic adaptation to regular exercise in humans. Drug Discov. Today 19 1015–1018. 10.1016/j.drudis.2014.03.006 [DOI] [PubMed] [Google Scholar]
- Liu D., Zhao Z., Wang A., Ge S., Wang H., Zhang X., et al. (2018). Ischemic stroke is associated with the pro-inflammatory potential of N-glycosylated immunoglobulin G. J. Neuroinflammation 15 123. 10.1186/s12974-018-1161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall N. J., Muir K. W. (2011). Hyperglycaemia and infarct size in animal models of middle cerebral artery occlusion: Systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 31 807–818. 10.1038/jcbfm.2010.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra M. R., Vaduganathan M., Fu M., Ferreira J. P., Anker S. D., Cleland J. G. F., et al. (2019). A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: The COMMANDER HF trial. Eur. Heart J. 40 3593–3602. 10.1093/eurheartj/ehz427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata M. (2014). Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: Recent evidence and clinical applications. Curr. Hypertens. Rev. 10 49–57. 10.2174/157340211001141111160957 [DOI] [PubMed] [Google Scholar]
- Neurology C., Society C. S. (2018). Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin. J. Neurol. 51 666–682. [Google Scholar]
- Nogueira-Machado J. A., Volpe C. M., Veloso C. A., Chaves M. M. (2011). HMGB1, TLR and RAGE: A functional tripod that leads to diabetic inflammation. Expert Opin. Ther. Targets 15 1023–1035. 10.1517/14728222.2011.575360 [DOI] [PubMed] [Google Scholar]
- O’Rourke M. F., Safar M. E. (2005). Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 46 200–204. 10.1161/01.Hyp.0000168052.00426.65 [DOI] [PubMed] [Google Scholar]
- Oza R., Rundell K., Garcellano M. (2017). Recurrent ischemic stroke: Strategies for prevention. Am. Fam. Physician 96 436–440. [PubMed] [Google Scholar]
- Rogowski O., Shapira I., Shirom A., Melamed S., Toker S., Berliner S. (2007). Heart rate and microinflammation in men: A relevant atherothrombotic link. Heart 93 940–944. 10.1136/hrt.2006.101949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber H., Saver J. L. (2020). Distributional validity and prognostic power of the national institutes of health stroke scale in US administrative claims data. JAMA Neurol. 77 606–612. 10.1001/jamaneurol.2019.5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadieh A., Nielsen O. W., Rasmussen V., Hein H. O., Abedini S., Hansen J. F. (2004). Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur. Heart J. 25 363–370. 10.1016/j.ehj.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Spence J. D., Viscoli C. M., Inzucchi S. E., Dearborn-Tomazos J., Ford G. A., Gorman M., et al. (2019). Pioglitazone therapy in patients with stroke and prediabetes: A post hoc analysis of the iris randomized clinical trial. JAMA Neurol. 76 526–535. 10.1001/jamaneurol.2019.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Wang L., Li X., Zhang J., Zhang J., Liu X., et al. (2021). Intracranial atherosclerotic plaque characteristics and burden associated with recurrent acute stroke: A 3D quantitative vessel wall MRI study. Front. Aging Neurosci. 13:706544. 10.3389/fnagi.2021.706544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Liu L., Pan Y., Wang X., Mi D., Pu Y., et al. (2018). Intracranial atherosclerosis burden and stroke recurrence for symptomatic intracranial artery stenosis (sICAS). Aging Dis. 9 1096–1102. 10.14336/ad.2018.0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szlachetka W. A., Pana T. A., Tiamkao S., Clark A. B., Kongbunkiat K., Sawanyawisuth K., et al. (2020). Impact of diabetes on complications, long term mortality and recurrence in 608,890 hospitalised patients with stroke. Glob. Heart 15:2. 10.5334/gh.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. H., Stitham J., Jin Y., Liu R., Lee S. H., Du J., et al. (2014). Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation 129 1598–1609. 10.1161/circulationaha.113.005224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R. R., Wilkinson I. B., Schiffrin E. L., Avolio A. P., Chirinos J. A., Cockcroft J. R., et al. (2015). Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the american heart association. Hypertension 66 698–722. 10.1161/hyp.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan T. N., Nizam A., Lynn M. J., Egan B. M., Le N. A., Lopes-Virella M. F., et al. (2017). Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology 88 379–385. 10.1212/wnl.0000000000003534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y., Miura T., Minamisawa M., Abe N., Nishimura H., Hashizume N., et al. (2017). The usefulness of brachial-ankle pulse wave velocity in predicting long-term cardiovascular events in younger patients. Heart Vessels 32 660–667. 10.1007/s00380-016-0919-6 [DOI] [PubMed] [Google Scholar]
- Venkat P., Chopp M., Chen J. (2017). Blood-brain barrier disruption, vascular impairment, and ischemia/reperfusion damage in diabetic stroke. J. Am. Heart Assoc. 6:e005819. 10.1161/jaha.117.005819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat P., Ning R., Zacharek A., Culmone L., Liang L., Landschoot-Ward J., et al. (2021). Treatment with an Angiopoietin-1 mimetic peptide promotes neurological recovery after stroke in diabetic rats. CNS Neurosci. Ther. 27 48–59. 10.1111/cns.13541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. A., Wu T. H., Pan S. L., Chen H. H., Chiu S. Y. (2021). Impacts of treatments on recurrence and 28-year survival of ischemic stroke patients. Sci. Rep. 11:15258. 10.1038/s41598-021-94757-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. F., Hoh B. L., Lynn M. J., Kwon H. M., Turan T. N., Derdeyn C. P., et al. (2016). Factors associated with recurrent ischemic stroke in the medical group of the SAMMPRIS Trial. JAMA Neurol. 73 308–315. 10.1001/jamaneurol.2015.4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Wu B., Liu M., Chen Z., Wang W., Anderson C. S., et al. (2019). Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 18 394–405. 10.1016/s1474-4422(18)30500-3 [DOI] [PubMed] [Google Scholar]
- Wu Y., Fan Z., Chen Y., Ni J., Liu J., Han J., et al. (2019). Determinants of developing stroke among low-income, rural residents: A 27-year population-based, prospective cohort study in Northern China. Front. Neurol. 10:57. 10.3389/fneur.2019.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi S., Furie K. L., Viscoli C. M., Kamel H., Gorman M., Dearborn J., et al. (2018). Pioglitazone prevents stroke in patients with a recent transient ischemic attack or ischemic stroke: A planned secondary analysis of the IRIS Trial (Insulin Resistance Intervention After Stroke). Circulation 137 455–463. 10.1161/circulationaha.117.030458 [DOI] [PubMed] [Google Scholar]
- Yang J. L., Mukda S., Chen S. D. (2018). Diverse roles of mitochondria in ischemic stroke. Redox Biol. 16 263–275. 10.1016/j.redox.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Yao B. (2019). Impact of risk factors for recurrence after the first ischemic stroke in adults: A systematic review and meta-analysis. J. Clin. Neurosci. 60 24–30. 10.1016/j.jocn.2018.10.026 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Guo Z., Zhang Y., Shang J., Yu L., Fu P., et al. (2022). Rapid triage for ischemic stroke: A machine learning-driven approach in the context of predictive, preventive and personalised medicine. EPMA J. 13 285–298. 10.1007/s13167-022-00283-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld T. P., Edo R., Mervyn D. V., Nederkoorn P. J., Rob D. H., Bwem R. Y., et al. (2018). Blood pressure-lowering treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of stroke or transient ischaemic attack. Cochrane Database Syst. Rev. 7:CD007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: All data and methods supporting the findings of this study are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed to YBZ, bbnnbn@163.com.