Abstract
Natural products possess structural complexity, diversity and chirality with attractive functions and biological activities that have significantly impacted drug discovery initiatives. Chiral natural products are abundant in nature but rarely occur as racemates. The occurrence of natural products as racemates is very intriguing from a biosynthetic point of view; as enzymes are chiral molecules, enzymatic reactions generating natural products should be stereospecific and lead to single-enantiomer products. Despite several reports in the literature describing racemic mixtures of stereoisomers isolated from natural sources, there has not been a comprehensive review of these intriguing racemic natural products. The discovery of many more natural racemates and their potential enzymatic sources in recent years allows us to describe the distribution and chemical diversity of this ‘class of natural products’ to enrich discussions on biosynthesis. In this Review, we describe the chemical classes, occurrence and distribution of pairs of enantiomers in nature and provide insights about recent advances in analytical methods used for their characterization. Special emphasis is on the biosynthesis, including plausible enzymatic and non-enzymatic formation of natural racemates, and their pharmacological significance.

Subject terms: Structure elucidation, Biosynthesis, Natural products, Pharmacology
Racemic natural products display a wealth of bioactivities and chemical diversity. Their derivation from intriguing racemization processes, through enzymatic or non-enzymatic pathways, are discussed here, as well as their pharmacological properties and the analytical techniques developed for their identification, resolution and characterization.
Introduction
Racemates are equal mixtures of both enantiomers of a chiral molecule. The major chemical building blocks of life, amino acids and sugars, possess a well-defined stereo-configuration making nature a chiral environment1. Nevertheless, the occurrence of racemates has become of great importance for science2,3. Diverse organisms including animals, plants, bacteria and fungi produce racemates as secondary metabolites (that is, naturally occurring small molecules that are intermediates or products of the metabolism — they are evolutionary diversified and optimized to play important roles in organisms, exhibiting diverse biological activities with applications in medicine) with the ratio of each enantiomer varying between different organs of a single organism, for instance roots, leaves, bark, stem, flowers and seeds of plants, or across different species within the same genus or family4–6. As a result, the occurrence of racemates is generally under-appreciated. Most research projects in natural product chemistry focus on isolation, structure elucidation and biological activity evaluation of compounds from natural sources but neglect chiral resolution and absolute configuration determination.
Enantiomers of a molecule share identical physicochemical properties in an achiral environment, such as organic solvents. Therefore, most chiral secondary metabolites reported to date could have been racemic or scalemic (enantiomers in unequal amounts) mixtures as only few works have disclosed the enantiomeric ratio of isolated compounds7. Batista et al. found that only 11% of works describing chiral metabolites (across 268 research papers published in the Journal of Natural Products) contained information about the evaluation of the enantiomeric ratio of isolated compounds8. In the classical chemical structure determination workflow, speculations that a compound is a racemate start with the value of its optical rotation; a pure racemic mixture has an optical rotation value of zero. However, the enantiomeric mixture state of a sample can still be verified with an optical rotation value of up to ±14 (ref.9), posing the question of the optical rotation threshold to consider a compound optically pure.
Numerous other questions are encompassed in the topic of natural racemates. Research is ongoing to understand their biosynthetic origins and functions in nature10,11. In nature, secondary metabolites do not intervene in the primary metabolism functions of cells essential for growth and development but, rather, improve the survival of organisms (for example, competition, defence, inter-communication) in their natural habitats12. Therefore, questions have arisen about the specific reasons for the occurrences of natural product racemates. In addition, this subject requires even greater attention as the enantiomers of a molecule rarely exert a synergistic effect towards a pharmacological response, and in some cases pairs of enantiomers display different activities towards a biological system13. For example, whereas one component of the pair is highly active and produces beneficial effects, its congener is inactive against the same pathogen or is toxic13,14.
This Review focuses on natural products (secondary metabolites) and presents a comprehensive and up-to-date overview of the occurrence, classes and distribution of racemates in nature. Looking at racemates as a ‘class’ of secondary metabolites, important aspects related to their classification, recent advances in analytical developments, biosynthetic discussions and pharmacological relevance are also covered, with extensive referencing of more detailed reviews covering each of these topics when available.
Racemic natural products
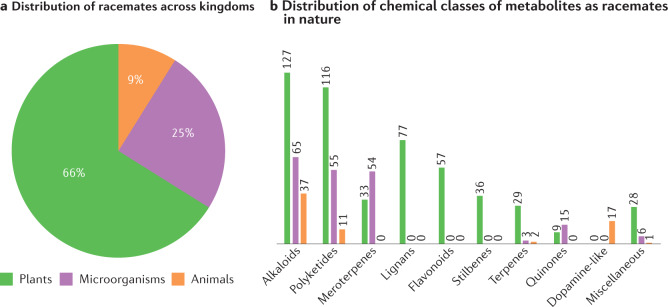
Natural products (secondary metabolites) originate from three main sources: plants, microbiota and animals. Owing to their historical therapeutic significance, natural products have attracted great attention in medicine, chemistry and biology. Research works have focused mainly on higher organisms, such as terrestrial or marine plants and animals, and microorganisms collected from soil, animals and plants. In the frame of this study, we explored 371 research articles (across different journals) describing natural racemates, and found that out of the 778 compounds described, 66% were from plants, 25% from microorganisms and 9% from animals (Fig. 1a,b). This distribution could be explained by the increased number of works on plants compared with other sources. The articles consulted for this comprehensive survey were retrieved from SciFindern, PubMed and Google Scholar when entering the keywords ‘racemic and nature’ or ‘racemic + racemate(s) + natural products’ and refining the search with different concepts/keywords including racemates, compounds, metabolites, nuclear magnetic resonance (NMR), plants, sponges, marines, endophytes, microorganisms, mushrooms, enantiomeric and enantiomers without a limitation of publication date. Some racemates may have been missed in this survey because of the search criteria, references and engines used, but to the best of our knowledge, the largest majority of racemates reported to date from nature has been covered and the present percentages represent the general tendency.
Fig. 1. Distribution and diversity of racemates in nature according to up-to-date published data.
Labels of chemical structures are coloured to highlight the sources in nature: green for plants, purple for microorganisms and orange for animals. a | Distribution of naturally occurring racemates that have been discovered across different kingdoms: 66% in plants, 25% in microorganisms and 9% in animals. b | Distribution of racemic compounds according to their main chemical classes or biosynthetic origins and across different source kingdoms. Alkaloids = nitrogen-containing compounds including amides; polyketides = keto-methylene chains (cyclic or not) including phloroglucinols; terpenes = mainly linear (or monocyclic) sesquiterpenes but also monoterpenes, diterpenes and sesterterpenes; miscellaneous = compounds belonging to other classes not listed in the row. Supplementary Fig. S1 shows the structures of 778 compounds and 248 reference articles.
Throughout our investigation, it was not possible to correlate racemate occurrences with plant families, genera or species. This limitation also goes for the other sources, such as microorganisms and animals, although other reports have suggested that there are classes of natural products in animals, for example dopamine-like compounds, which occur as racemates predominantly15. Racemates can be grouped by how they are generated, either by considering their main carbon scaffolds or by exploiting their key racemization processes. Moreover, the biogenetic precursors of certain secondary metabolites (for example, meroterpenes) are more highly reactive towards racemization, including through non-enzymatic processes, and so are more prone to afford racemates (Fig. 1b). As a result, the classification we chose from the collected data is to group racemates based on their main chemical classes (and biosynthetic origin) of their secondary metabolites. Figure 2 shows the chemical structures of representative examples of racemates selected from each main chemical class. Supplementary Figure S1 shows the structures and classes of all 778 racemates enumerated from the literature. Alkaloids, polyketides and meroterpenes account for more than half of the natural racemates described in the literature (Fig. 1b).
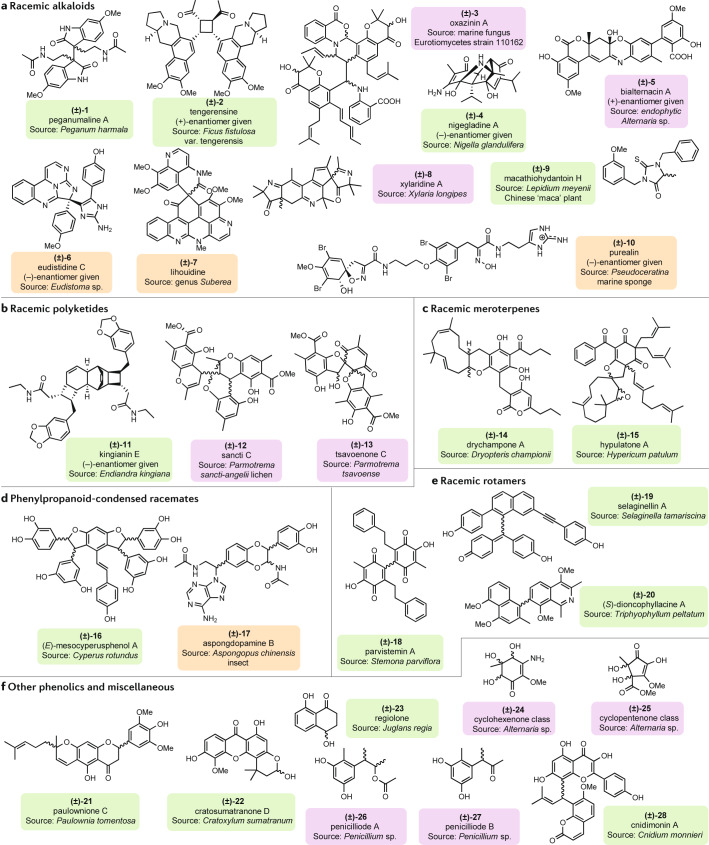
Fig. 2. Main groups and examples of natural racemates.
Labels of chemical structures are coloured to highlight the sources in nature: green for plants, purple for microorganisms and orange for animals. a | Racemic alkaloids peganumaline A (1)174, tengerensine (2)25, oxazinin A (3)27, nigegladine A (4)28, bialternacin A (5)29, eudistidine C (6)30, lihouidine (7)31, xylaridine A (8)32, macathiohydantoin H (9)33 and purealin (10)34. b | Racemic polyketides kingianin E (11)175, sancti C (12)46 and tsavoenone C (13)47. c | Racemic meroterpenes drychampone A (14)54 and hypulatone A (15)56. d | Phenylpropanoid-condensed racemates (E)-mesocyperusphenol A (16)56 and aspongdopamine B (17)70. e | Racemic rotamers parvistemin A (18)76, selaginellin A (19)176 and (S)-dioncophyllacine A (20)78. f | Other phenolics and miscellaneous paulownione C (21)177, cratosumatranone D (22)153, regiolone (23)178, (4R*,5S*,6S*)-3-amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one (24)82, (4S*,5S*)-2,4,5-trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopenten-1-one (25)82, penicilliode A (26)44, penicilliode B (27)44 and cnidimonin A (28)81.
Racemic alkaloids (nitrogen-containing compounds)
Racemic alkaloids are a group of racemates with great chemical diversity and various natural origins16–23 (Fig. 2a). They represent the largest group of naturally occurring racemates encountered to date. Many alkaloids are constituted by indole derivatives or amino acid moieties. The indole-derived alkaloids that have thus far been reported as racemates feature l-tryptophan cores. Racemization occurs either from cyclization, oxidation substitution or dimerization of the amino acid precursor to give, for example, peganumaline A (1). Racemic isoindolinones were also encountered in both plants and ascomycetous fungi. Likewise, alkaloids derived from l-lysine, namely indolizidine, quinolizidine or piperidine-type, yielded racemates22,24–26. In the naphtho-indolizidine series, for instance, Al-Khdhairawi et al. reported one of the unique members of benzopyrroloisoquinoline dimerized (that is, constituted by two identical or similar structural moieties) along a cyclobutane ring25. Tengerensine (2) is one such rare unsymmetrical cyclobutane possessing two alkaloid moieties, mirror images of one another.
In addition to alkaloids deriving from indoles or amino acids such as those listed above, the nitrogen atom(s) in some alkaloids derived from the introduction of other chemical scaffolds, including anthranilate, pyrimidine and imidazole, or from amination. For example, the racemate oxazinin A (3) is a chromene-based polyketide whose nitrogen atom was brought in by an anthranilate derivative followed by subsequent cyclization and dimerization27. Likewise, Tian et al. reported nigegladine A (4), a racemate comprising a unique tricyclo[5.4.0.1]dodecane ring system from the dimerization of two thymoquinones bridged by a nitrogen in the Chinese medicinal plant Nigella glandulifera28. Similarly, bialternacin A (5) is a dimeric polyketide containing a nitrogen probably originating from the amination of one of its biosynthetic precursors29. The animal kingdom has produced unique racemic alkaloids, including eudistidine C (6), featuring a tetracyclic core elaborated with fused pyrimidine and imidazole rings integrating guanidine and amidine structural groups30. A marine sponge of the genus Suberea releases the racemate lihouidine (7) made up of a benzo[de](1,6)naphthyridine fused with a quinoline-derived system and dimerized through a six-membered unit containing a spiro carbon31. Xylaridine A (8) is a racemate characterized by a linear fused 5/6/6 tricyclic ring system with another angular fusion of a special 2-azaspiro[4.4]nonane unit32. Thiohydantoin derivatives were also reported as racemates, as exemplified by macathiohydantoin H (9) from the rhizomes of the Chinese ‘maca’ plant (Lepidium meyenii)33. Purealin (10), a bromotyrosine-derived spiro-isoxazole-type compound isolated from the marine sponge Pseudoceratina sp., is an example of unique spiro-isoxazole derivatives34. Further types of racemic alkaloids feature isoxazole, tetramic acid and pyrrolidone moieties23,35–37.
Racemic polyketides
Polyketides contain at least two carbonyl groups (or the reduced forms) adjacent to the same carbon atom. Compounds in this category are structurally complex and diverse. Racemization in polyketides occurs mainly during polymerization and subsequent cyclization38,39. Oxidized acylphloroglucinols (that is, 1,3,5-trihydroxybenzene derivatives) or polyketides undergo cycloaddition after chain elongation leading to more complex structures40–44. Some examples of polyketides include kingianins, which are 4/6/6/6/4 fused pentacyclic rings arising from the dimerization (cycloaddition) of two bicyclo[4.2.0]octa-2,4-diene monomers45. The chemical structure of kingianin E (11) represents this group of natural products (Fig. 2b). Kingianins constitute one of the largest members of racemic polyketides. Racemic kingianins have been isolated from the bark of Endiandra kingiana. Likewise, sancti analogues, such as sancti C (12) in Fig. 2b, feature a dibenzo-2,8-dioxabicyclo[3.3.1]nonane arrangement originating from two chromene precursors46. Isolated kingianin analogues mentioned in the literature were dimeric and trimeric derivatives. The occurrence of racemic polyketides bearing a quinone unit is scarce owing to their limited stability. Racemates of tsavoenone C (13) and analogues featuring a 1,7-dioxadispiro[4.0.4.4]tetradecane were isolated from the lichen Parmotrema tsavoense47. The other racemic quinone analogues were derived from naphthoquinone48,49.
Racemic meroterpenes
Meroterpenes have a mixed biosynthetic origin (terpenoid/polyketide) as they are produced after the condensation of a terpene (sesquiterpene or monoterpene) with a phenolic compound (quinone, phloroglucinol, guttiferone and so on)50–52. Meroterpenes from the Hypericum, Dryopteris and Psidium genera feature α-humulene fused through its C-6/C-7 double bond to a cis-oriented α,β-unsaturated ketone of acylphloroglucinols, for example drychampone A (14)53–55 (Fig. 2c). Other members of this group include a spiro system from the C-7/C-13 double bond of β-humulene and an oxidated acylphloroglucinol56. Hypulatone A (15), isolated from Hypericum patulum, features a spiro[benzofuran-2,1′-cycloundecan]-4′-ene-4,6(5H)-dione moiety and represents a key example of meroterpenes bearing a spiro system56. Another series in this group consists of rearranged prenylated hydroquinone or resorcinol leading to various ring closure alternatives including 6/6/6/4, 6/6/5/4 and 6/6/5/5 rearranged systems, and others51,57. The latter class of racemic meroterpenoids were isolated from the fruiting bodies of wild fungi belonging to the Ganoderma genus58–61.
Phenylalkenoid-condensed racemates
Compounds in this family are made up of two or more units of phenolic compounds linked to one another due to various oxidation processes, among which cycloaddition is the most common reaction62–65. Racemization in stilbenes, such as (E)-mesocyperusphenol A (16), is observed in dimeric and trimeric molecules64 (Fig. 2d). Condensation of the ortho-positioned hydroxyls in the catechol moiety of stilbenes affords more structurally complex racemic natural products66.The same is true for the 1,2,3-trihydroxydiphenoxylpropanoids group which is mostly found in plants. Upon their C–C or C–O cycloaddition, condensation occurs to generate phenolic compounds which are racemates67–70. Similar behaviours are observed in the formation of some lignan-type racemates71–74. Phenylalkenoid-condensed racemates constitute the most prevalent group of racemates in the animal kingdom, especially in ants through acetylated dopamine dimer analogues, including aspongdopamine B (17). Indeed, dopamine undergoes dimerization to induce dioxane ring closure, from the reaction involving constituent ethylene side chain and catechol hydroxyls15,65,70. In nature, every molecule of the latter group, dopamine-like natural products, exists as a racemate15. Phenylpropanoids are involved in the racemization of some indoles and dihydrobenzophenanthridines, xanthones and coumarins67–69,75.
Racemic rotamers
Three main scaffolds of racemates exhibiting an axis of chirality have been found in the literature. Atropisomers arise when the rotation barrier energy along the σ-stretching bond is high, preventing interconversion of optically active stereoisomers and leading to the isolation of enantiomers for molecules in which that axis is the only stereogenic element; otherwise, diastereomers might be produced. Many factors can cause racemization to occur, including a low steric hindrance and tautomerisms. For instance, the racemization of phenylethylbenzoquinones, such as parvistemins (for example, parvistemin A (18)76), is caused by the lack of steric hindrance and biosynthetic origin. These structures are generated from dimerized and oxidized hydrostylbenes. Selaginellins, the major chemical constituents of the Selaginella genus and naturally occurring pigments from plants, contain a p-quinone methide (QM) and alkynylphenol moieties as shown in the chemical structure of selaginellin A (19)77 (Fig. 2e). Both QM and phenol groups of one side of the σ-bond undergo keto–enol tautomerism, meaning the compounds exist as a mixture of non-separable enantiomers. Alkylation of the phenol fragment prevents the tautomerization occurring but racemization continues from free rotation along the σ-bond axis. Some isolated racemates from Selaginella pulvinata are ethoxyl derivatives of common selaginellins (with free phenol groups and where tautomerization occurs) in which racemization was from the combined actions of axial chirality and molecular rotation. Moreover, a racemic alkaloid called dioncophyllacine A (20), from the leaves of Triphyophyllum peltatum, exhibits axial chirality from the coupling of a naphthalene and an isoquinoline moiety. Racemization occurs because the allowed rotation along the chiral axis causes the formation of equal amounts of both enantiomers of the chiral molecule78.
Other phenolics and miscellaneous
Common secondary metabolites such as flavonoids (for example, paulownione C (21))79,80, benzophenones/xanthones (for example, cratosumatranone D (22))52, chromenes (for example, regiolone (23))81, cycloalkenes (for example, 24, 25)82 and phenolic acids (for example, penicilliodes A and B (26 and 27))83 also occurred as racemates without any oxidative cyclization as is typical for the aforementioned racemates80,84 (Fig. 2f). Several compounds in this group were elaborated from either inter-species or intra-species coupling or following oxidation–cyclization patterns of their substituents (cnidimonin A (28)). Some racemates in this group have a single stereocentre (that is, less complexity) as their core skeletons are benzene and analogues. Racemates with more than one asymmetric carbon arise mainly when the key basic scaffold is from terpenes or cyclohexenes9,85–87. The biosynthesis of such compounds could be of great interest.
Advanced analysis methods
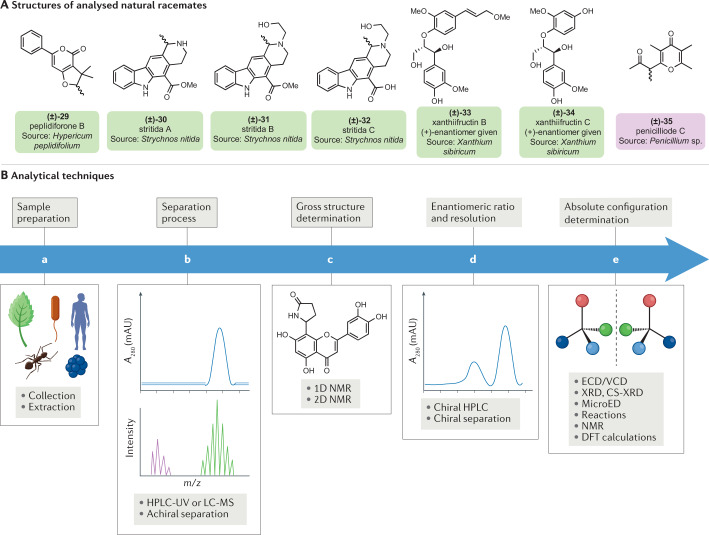
The recognition of a racemic mixture starts with optical rotation determination. In general, optical rotation values of racemates should be close to zero or equal to zero (that is, in cases where the racemate does not contain any impurities). However, racemates containing some minor optically active impurities have been found to have an optical rotation value of up to ±14 (ref.9). Therefore, some racemates with non-zero optical rotation values have been described as enantiopure compounds in the literature when only optical rotation was used without combining it with other analytical methods. As such, emphasis should be placed on the enantiomeric ratio evaluation of chiral compounds, even for those with higher optical rotation values. Electronic and vibrational circular dichroism (ECD and VCD) are oftentimes combined with optical rotation data to identify enantio-enriched mixtures88,89. Peplidiforone B (29) (Fig. 3A), isolated from Hypericum peplidifolium, was confirmed to be a racemate as it exhibited no Cotton effect, the characteristic change in the absorption of a circularly polarized light around the absorption band of a chiral compound, in its ECD spectrum along with an optical rotation value close to zero90. Besides chiroptical methods, well-elaborated chromatographic techniques for racemate recognition (enantiomeric ratio determination), separation and quantification techniques also exist nowadays. Chiral nanoparticles and chiral nanographene-based column materials for high performance liquid chromatography (HPLC), gas chromatography, capillary electrophoresis and capillary electrochromatography have been recently reviewed91,92. In natural products or pharmaceutical research, the separation and quantification of pairs of enantiomers are almost always established on chiral HPLC, gas chromatography or supercritical fluid chromatography (SCFC), from which numerous methods have been developed and reviewed8,93–95. The best of them is the so-called ‘Inverted Chirality Columns Approach’ consisting of the inversion of the elution observed as a response to the change in the chirality of the column — both enantiomeric forms of stationary phases are used in the same column96. Table 1 summarizes the advantages of analytical techniques used for recognition, resolution and quantification of both enantiomers of a chiral compound in natural product research.
Fig. 3. Analysis approaches for natural racemates.
Labels of chemical structures are coloured to highlight the sources in nature: green for plants and purple for microorganisms. A | Structures of analysed racemates peplidiforone B (29)179, stritidas A–C (30–32)20, xanthiifructin B (33)97, xanthiifructin C (34)97 and penicilliode C (35)44. B | Analysis approaches for natural racemates, from extraction and separation to structure elucidation with absolute configuration determination. Detailed reviews on these techniques are published elsewhere67,79,99,103. Ba | Extraction/preparation: during dryness, sun light/energy and high temperature (>24 °C) could induce transformations including racemization. Uses of liquid nitrogen should avoid such transformations leading to artefacts, allowing extraction of genuine natural racemates. Bb | Separation process: basic chromatographic methods such as column chromatography and thin layer chromatography can afford unnatural racemic mixtures (artefacts) from complex extracts. High performance liquid chromatography (HPLC), online liquid chromatography and liquid chromatography–mass spectrometry (LC-MS) improve the isolation avoiding possible racemization due to silica–sample or inter-sample interactions. Bc | Structure determination: the gross structure of a racemate could be achieved by means of nuclear magnetic resonance (NMR), mass spectrometry and LC-MS/tandem, HPLC coupled with ultraviolet photodiode array (HPLC-UV), infrared, nuclear Overhauser effect spectroscopy/rotating frame Overhauser effect spectroscopy and X-ray diffraction (XRD) providing required data to define the relative configuration. Bd | Enantiomeric ratio determination/resolution: the resolution of racemates could be achieved by various techniques, including chiral HPLC (Table 1). Be | Absolute configuration determination: several methods were commonly used including electronic circular dichroism (ECD) and vibrational circular dichroism (VCD), experimental and calculated spectra using density functional theory (DFT), Mosher’s method (chemical reaction), NMR (1H, 13C), quantum mechanics (for example, DP4+ method), X-ray diffraction, microcrystal electron diffraction (MicroED) and crystalline sponge X-ray diffraction (CS-XRD).
Table 1.
Advantages and applications of analytical methods used for natural racemates
| Method/category | Resolution | Identification | Quantification | Absolute configuration | |
|---|---|---|---|---|---|
| HPLC | HPLC-UV90,95 | Enantiomers are separated on a chiral column | Profiles comparison with standards | Quantification and enantiomeric ratio evaluation of ultraviolet absorbing compounds | Not applicable for new compounds; known compounds required chiral HPLC-UV and reference standards for retention time comparison |
| HPLC-ORD160 | Both enantiomers of the mixtures will present oppositely charged specific rotation when separated on a chiral column | Identifies racemate through the evaluation of optical rotation | Chiral columns also help evaluate the yield of each component of the polar mixture | The methods could help absolute configuration determination especially for compounds with standards or fewer stereocentres (one or two) or P/M chiral axis, but will be limited for more complex scaffolds | |
| HPLC-CD88,89 | Can resolve separable enantiomers on a chiral phase | HPLC profiles comparison with known references | Feasible with chiral column HPLC and the method is more accurate as circular dichroism is being used as a detector | The circular dichroism detector helps afford the absolute configuration for compounds which fit into circular dichroism empirical rules or with experimental circular dichroism data of related structures; otherwise, calculated circular dichroism spectra are needed | |
| HPLC/LC-MS8,35,92–96 | Enantiomers are separated on a chiral column | Identification with configurationally known standards | Feasible with chiral column HPLC | Marfey method turns enantiomeric mixture of amino acids in diastereoisomers with the chiral Nα-(5-fluoro-2,4-dinitrophenyl)-L-leucinamide (l-fdla); absolute configuration is then deduced by online LC-ESI-MS matching with standards | |
| SCFC | SCFC-CS-XRD94 | Combine advantages of SCFC to work on thermo-labile compounds and those of CS-XRD to apply on liquid and oil samples | Crystal of racemates formed belongs to specific group space leading to their identification | Feasible with SCFC with lower solvent consumption and shorter time than HPLC | CS-XRD makes it possible to solve the absolute configuration of both enantiomers |
| Gas chromatography | GC-MS161,162 | Enantiomers are separated over a chiral column but the conditions inherent to gas chromatography methods remain | Using chiral column, identification can be made possible by profile comparison with standards | Indicated for low molecular weight and non-heat-sensitive compounds | Chiral GC-MS analysis enables online comparison of retention times of each enantiomer of a chiral substance with reference standards |
| XRD | SC-XRD163 | Racemates crystallize in specific group space but are limited to crystalline solid | Non-centrosymmetric crystal displays the so-called anomalous dispersion effect which breaks down the Friedel law; thus, the absolute configuration could be determined using either the Flack parameter or by comparing the intensities of Bijvoet or Friedel pairs | ||
| CS-SC-XRD8,112 | Each enantiomer of the racemate — solid, oil or liquid — crystallizes in a unique order, allowing their resolution | As in a classical XRD experiment, the nature of the crystal could be assessed | The method is applied with a metal–organic framework (the host) claiming a specific optical activity; thus, leading to the absolute configuration determination of each enantiomer of the racemate | ||
| MicroED110,111 | This method was used for small molecules only recently and has not yet been coupled to chromatography | Can be an alternative to XRD when crystals of suitable quality are not available for gross structure identification | Applicability in the quantification of natural products still has to be demonstrated | Diffraction-based method similar to XRD but does not have associated limitations; crystals of small sizes and even powders not useful for XRD were analysed by MicroED | |
| NMR8,113 | Affordable when hyphenated to a chiral column HPLC system | Best method to assess the gross chemical structure of a racemate; still, the racemic status of a sample is only displayed under optical active species influence during NMR experiments | Affordable when hyphenated to a chiral column HPLC system or after derivatization | Theoretical assessment of the shielding tensor of various stereoisomers of a compound paired with the high compatibility of calculated and analytical results can lead to absolute configuration determination of stereochemical compounds, in large, and could be applied to a racemate when the NMR is recorded in an optical active solvent | |
| Derivatization114,115 | Enantiomers are turned to diastereomers and separated on an achiral HPLC system | Enantiomers are turned to diastereomers and spotted on analytical thin layer chromatography | Feasible on an achiral HPLC system after derivatization | Enantiomers could be turned to esters applying Mosher’s method, for instance, and analysed by NMR, but it is limited to some classes of compounds | |
CS-XRD, crystalline sponge X-ray diffraction; GC-MS, gas chromatography–mass spectrometry; HPLC, high performance liquid chromatography; HPLC-CD, high performance liquid chromatography–circular dichroism; HPLC-UV, high performance liquid chromatography–ultraviolet photodiode array; LC-ESI-MS, liquid chromatography–electrospray ionization–mass spectrometry; LC-MS, liquid chromatography–mass spectrometry; MicroED, microcrystal electron diffraction; NMR, nuclear magnetic resonance; ORD, optical rotation dispersion; P/M chiral axis, plus (P or Ra or right-handed)/minus (M or Sa or left-handed) stereo-descriptors; SCFC, supercritical fluid chromatography; SC-XRD, single crystal X-ray diffraction; XRD, X-ray diffraction.
In most cases, chiral HPLC is the preferred method for the resolution of mixtures of enantiomers. However, in racemic mixtures, some component enantiomers have been reported as non-separable on chiral HPLC. They are exemplified by selaginellin enantiomers, which can be interconverted in solution77. Chiral resolution of the constituents of the pyridocarbazole alkaloids, stritidas A–C (30–32), failed on a Phenomenex Lux cellulose-2 column20. The phenylpropanoid derivatives, xanthiifructins A/B (enantiomeric forms of 33), were separable on a Chiralpak AD-H column and a Phenomenex Lux cellulose-2 column, but not their analogue xanthiifructin C (34) which structurally differs by the lack of the propenyl moiety on its second phenyl group97. Similarly, the enantiomers of the phenylpropanoid derivative penicilliode A (26) were not distinguishable on many chiral columns (Phenomenex Lux celluloses-1, 2, 3, 4 and 5 and FLM Chiral ND(2)), whereas their analogues penicilliode B (27) and penicilliode C (35) were successfully resolved in their respective enantiomers44. In all, available chiral HPLC columns still fail to distinguish between physical properties of components of certain enantiomer mixtures. Alternatively, mixtures of enantiomers can also be resolved by exploiting the difference in the metabolism of both enantiomers by fungi or bacteria, which may recognize only one of the components. Parmaki et al. developed this method for the resolution of racemic mixtures of natural products98. The resolution of natural racemates using chiral reagents has not been mentioned in the natural products literature recently, yet this chromatography method is useful for trace amounts of natural racemates99. The application of chiral mobile phase additives such as cyclodextrins for the resolution of natural racemic mixtures has not been reported in the literature recently. The occurrence of natural products in relatively low quantity renders difficult the use of derivatization and other reaction-mediated separation of pairs of enantiomers. Another resolution approach not found in the literature on natural product enantiomer separation is the mechanical resolution method, based on differentiation of the salts produced from the acid/base reactions of enantiomers and a crystallizing agent99. Enantiomers may not crystallize under the same conditions. Pasteur used this strategy when separating the stereoisomer components of the tartaric acid racemate100 for the first time. Further alternatives to HPLC include the membrane-based chromatography not yet applied to natural occurring racemates but which may be both effective and sustainable99. The quantification of racemates could be achieved by NMR (using chiral solvent and derivatizing agents), ECD, VCD or hyphenated techniques such as high performance liquid chromatography coupled with ultraviolet photodiode array (HPLC-UV), high performance liquid chromatography coupled with circular dichroism (HPLC-CD) and liquid chromatography coupled with mass spectrometry (LC-MS) on chiral columns8,101. Table 1 highlights the advantages of each method.
Absolute configuration determination
After the recognition and resolution of natural racemic mixtures, the gross structure determination by NMR spectroscopy and mass spectrometry is carried out. These are the most common techniques applied in the structure determination of natural products102. Figure 3B summarizes the most common analytical approaches from purification to relative and absolute configuration determination.
Classical NMR and mass spectrometry experiments cannot distinguish between pairs of enantiomers in their mixtures67,103. Both having the same physical properties, only one set of signals are observed in the NMR spectra of a racemic mixture. The presence of two or more sets of NMR signals is a sign of a mixture of either non-related compounds or diastereomers in the sample36,104. Besides NMR, X-ray diffraction (XRD) is also currently used to identify components of a racemic mixture. Diffraction data of the crystalline form of the sample mixture are obtained under a certain light105, most likely under CuKα conditions in conjunction with Friedel values and the Flack parameter of the compound106,107. The key drawbacks of this technique are the difficulty in the crystallization of some sample mixtures and its limitation to solid samples. Recently, the cryogenic electron microscopy method known as microcrystal electron diffraction (MicroED)108–111 was developed as a very promising alternative to XRD, achieving structure elucidation of natural products using crystals of small sizes and poor quality or powders not suitable for XRD. However, its broad use and applicability in the field of natural products has yet to be demonstrated111.
XRD and Fourier transform XRD (FT-XRD or fast FT-XRD), especially in their current modern states, are useful tools for determining the absolute configuration of crystalline and enantiomeric natural products8. Modern workflows combine XRD, circular dichroism calculations and, sometimes, optical rotation dispersion (ORD) spectroscopy. A recent XRD technique proposed the use of metal–organic frameworks to host the mixture of enantiomers and the resultant single crystal of the hybrid material is examined by XRD112. As a result, enantiomers within the mixture adopt a certain order in the porous hole of the metal–organic framework leading to a clear absolute configuration determination. The method is called crystalline sponge XRD (CS-XRD) and has proven to help in the structure elucidation of samples such as oils, liquids or other non-crystalline solids112.
Both XRD and circular dichroism are powerful techniques for absolute configuration determination of chiral molecules. Regardless of the method applied, the calculated circular dichroism spectra of each of the stereoisomers of the gross structure determined from NMR are assessed by density functional theory (DFT) calculations. Absolute configurations are assigned to each of the enantiomers by matching these spectra to the experimental ones. When the gross structure contains more than two stereocentres, the initial XRD structures could help eliminate some stereoisomers prior to the DFT calculations. Where neither VCD nor ECD methods are applicable, NMR coupled to quantum mechanics can still serve to assess the absolute configuration of a compound. Here, enantiomers are derivatized with a chiral reagent, separated into the corresponding diastereomers and their 1D NMR data recorded. Then, conformers of the gross structures are geometry-optimized using density functional methods, for instance B3P86/6-31G(d), B3LYP/6-31G(d), B3LYP/6-311++G(2d,p) and MPW1PW91/6-31G(d,p), and sorted according to the nuclear Overhauser effect data recorded67,103,113. The 13C and 1H NMR shielding tensors of each stereoisomer of retained diastereomers are simulated using quantum mechanics. The DP4+ probability method114 is the best approach to achieve this. However, the method becomes increasingly complex as the number of stereogenic carbons rises.
Chiral HPLC and gas chromatography analyses35 are also absolute configuration determination strategies. Configurations of enantiomers can be achieved by online comparison of retention times of each enantiomer with those of chiral references. The Marfey method, for instance, can be applied to online liquid chromatography–electrospray ionization–mass spectrometry (LC-ESI-MS) to identify the absolute configuration of amino acids using the derivatizing agent Nα-(5-fluoro-2,4-dinitrophenyl)-L-leucinamide (l-fdla)35. Synthetic methods have also been developed to elucidate the absolute configuration of compounds. The most popular of which is the Mosher method for secondary alcohol or amine-bearing stereocentres. The technique assesses the anisotropic effects of the phenyl group of a derivatizing chiral agent, α-methoxy-α-trifluoromethylphenylacetic acid (MTPA), in the 1H NMR of the esterified racemate hybrids115. Moreover, secondary alcohol-containing enantiomers undergo acylation by lipases, such as from Candida or Pseudomonas spp. or cholesterol esterase, under catalysis conditions with distinct kinetics. Under the same conditions, the R-enantiomer of the racemate is most likely resolved faster than the S-congener from the hydrolysis of the ester or acylation of the alcohols, following Kazlauskas’ empirical rules116.
Biosynthetic basis of racemization
The biosynthetic basis of racemization has always intrigued scientists. Most racemates are not a product of a clear enzymatic pathway but, rather, are considered by-products of secondary metabolism. In general, racemization of a given chiral molecule might be a result of its physical properties (for example, solubility) and chemical stability (for example, due to pH or temperature changes)117,118. Racemates could also be intermediates towards the formation of scalemic mixtures or enantiopure compounds. The excellent review by Ballard et al. provides detailed mechanistic and kinetic insights into the racemization and enantiomerization (conversion of one enantiomer into the other) of selected drugs by chemical processes in aqueous solutions (for example, pH variations) and the vapour phase (for example, pyrolysis), or racemization in plasma and serum albumin solutions117. Our focus in this section is natural products produced by living (micro)organisms as racemates, as opposed to artefacts arising from extraction or experimental procedures.
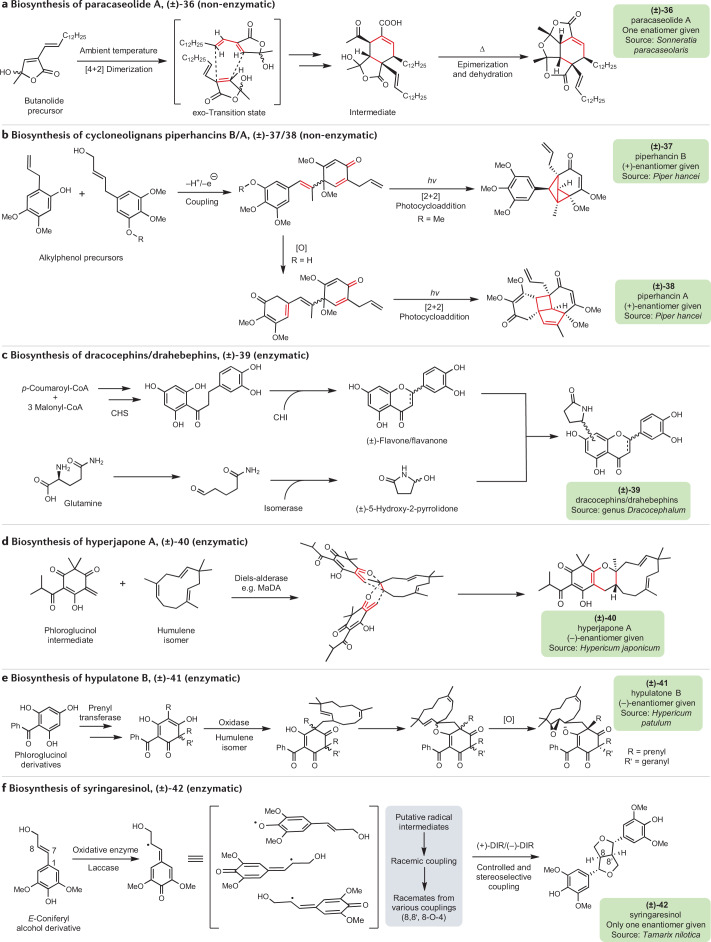
Some plausible mechanisms for the formation of racemic natural products are depicted in Fig. 4. Reactions governing the formation of some groups of racemates in nature are subject to exogenous factors (for example, temperature or light) or high reactivity of biogenic intermediates which can undergo spontaneous non-enzymatic transformations. For instance, increased temperature can trigger a cascade of reactions with successive oxidation, elimination and cyclization transformations yielding new racemic molecular scaffolds119. These non-enzymatic racemization processes resulting in new racemates are exemplified by the proposed biosynthesis of the structurally unique natural product paracaseolide A (36), which is a racemate hypothetically formed through a spontaneous Diels–Alder [4 + 2] cycloaddition (enzyme-free dimerization) starting from two α-dodecenylbutenolide molecules, followed by the epimerization and dehydration of the resulting Diels–Alder product120 (Fig. 4a). Another example features the neolignans piperhancins (37, 38), whose plausible biogenesis involves the oxidative coupling of alkylphenol derivatives and subsequent intramolecular [2 + 2] photocycloaddition74 (Fig. 4b). Biomimetic synthesis as a tool to validate the biosynthetic formation of racemates through non-enzymatic (chemical) racemization has been intensively discussed in the literature and key examples are summarized in Table 2. Still, much is unknown in the biosynthesis of the majority of racemates, as they are not clearly explained by non-enzymatic mechanisms, such as non-stereospecific ring closure or cycloaddition.
Fig. 4. Proposed biosynthetic basis to some racemates.
Labels of chemical structures are coloured to highlight the sources in nature: green for plants. a | Hypothetical biosynthesis of (±)-paracaseolide A (36) proposed by Wang and Hoye120 through biomimetic synthesis. A key step is the non-enzymatic and spontaneous [4 + 2] Diels–Alder dimerization (cycloaddition) of the butanolide precursor at ambient temperature. b | Proposed biosynthesis of cycloneolignans (±)-piperhancins B/A (37/38) by Yang et al.74. Alkylphenol precursors undergo a radical coupling to produce neolignan intermediates. The key non-enzymatic event features the intramolecular [2 + 2] photocycloaddition of the cycloenone with a double bond (red) in both intermediates to yield 37 and 38. c | Biosynthetic basis to racemic pyrrolidinone-containing flavonoids (39) as proposed by Wang et al.180. This bioprocess involves chalcone synthases (CHS) and epimerases as bioengineers. Epimerases such as chalcone isomerases (CHI) have been characterized in the conversion of chalcone to racemic flavanones/flavones. It could also be hypothesized that considering the fermentation process of Wang et al. to produce pyrrolidinone catechin derivatives, an epimerase likely catalyses the cyclization of 4-oxobutanamide to pyrrolidinone. The condensation of flavanone/flavone and pyrrolidinone intermediates afford 39. d | Hypothetical intervention of Diels-alderases in cycloaddition. Intermolecular Diels-alderases similar to the recently discovered Morus alba Diels-alderase (MaDA) could catalyse the formation of racemic natural products such as 40 through [4 + 2] cycloaddition55,128. e | Proposed oxidative coupling to (±)-hypulatone B (41)56. The terpene and phloroglucinol derivatives undergo an enzymatic oxidative coupling (catalysed by an oxidase) followed by subsequent cyclization and oxidation to give 41. f | Proposed enzymatic biosynthesis of racemic lignans in the presence or absence of dirigent proteins (DIRs). Hypothetical intervention of two DIRs of opposite stereoselectivities could yield racemate (±)-syringaresinol (42) preferentially with regioselectivity and stereoselectivity131,133,181.
Table 2.
Some biomimetic syntheses of racemates to explain non-enzymatic racemization pathways
| Main class of racemates | Examples | Proposed biosynthesis routes (creation of racemates when applicable) |
|---|---|---|
| Meroterpenes | Guajadial B135 | Hetero-Diels–Alder cycloaddition for the hybrid condensation and Knoevenagel condensation for the phenyl fixation |
| Alkaloids | Meyeniins A−C164 | Racemization at C-7a during condensation catalysed by pyridine, then epimerization at C-3 by the Edman degradation reaction |
|
Setigerumine I Dactylicapnosinine Dactylicapnosine165 |
Intramolecular 1,3-dipolar cycloaddition | |
| Polyketides | Kingianin A166 | Stereospecific electrocyclization to a bicyclo[4.2.0]octadiene which then undergoes Diels–Alder reaction |
| Epicolactone167 | Racemization arises from a heterodimerization related to [5 + 2] cycloaddition of two o-quinones | |
| Homodimericin A168 | Non-enzyme-catalysed six-electron cascade sequence of oxidation, Michael reaction/aromatization, intramolecular quinone-based Diels–Alder reaction and intramolecular Prins cyclization of two hydroquinone monomers | |
| Santalin Y169 | [2 + 3] cycloaddition of a benzyl styrene and a vinyloxidopyrylium ion from an isoflavylium | |
| Goupiolone B170 | Oxidative condensation via a benzobicyclo[3.2.1]octane intermediate followed by an intermolecular 1,4-addition, then intramolecular 1,2-addition and reduction | |
| Ocellatusones171 | Photo-catalysed isomerization, electrocyclization, [1,3]-sigmatropic shift and Claisen condensation | |
| Miscellaneous | Dracocephins A and B172 | Flavonoid–pyrrolidone racemates arise from a Strecker degradation of the amino acid l-glutaminethrough an alkylating receptor N-acyliminium ion and following the flavonoid formation |
|
Incarviditone Incarvilleatone173 |
Heterochiral and homochiral oxa‐Michael dimerizations |
Enzymes are the metabolism engines of every biological system. They are homochiral ensembles, possessing uniform handedness, and control the carbon framework of each organism cell121. It is quite common for amino acids and resulting derivatives, proteins, peptides and, to some extent, enzymes to exist under the l-stereoisomer in nature122. Interestingly, d-amino acids, such as alanine and serine, were found to occur as components of peptidoglycans, teichoic acids and poly-γ-glutamate capsules in bacteria122. d-Isomeric amino acids have also been found in other living organisms including plants and animals122. d-isomers are far less abundant in nature than l-isomers and may also result from the isomerization of the latter in the living habitat. Enzymes such as racemases and epimerases are responsible for this interconversion of stereochemistry at a given chiral centre122.
In this regard, the occurrence of racemates becomes clear to a certain extent; enantio-enriched species could undergo racemization under the influence of enzymes. Essentially, racemases and epimerases, such as alanine racemase122 and cellobiose 2-epimerase123, catalyse the cleavage and reformation of a bond around a stereocentre, accompanied by an epimerization of the site. This conflicts the consensus emerging from the literature that pairs of enantiomeric compounds may originate from two distinct biosynthetic pathways6. Thus, the number of resulting enantiomer mixtures relies on the number of stereocentres affected by racemases/epimerases. The pyrrolidinone-containing flavonoids such as dracocephins/drahebephins (39) (Fig. 4c) have been isolated as mixtures of pairs of enantiomers for flavones23 and enantiomeric diastereomers for flavanones36,104 from Dracocephalum sp. and Scutellaria moniliorrhiza.
Other phenolic compounds have similar biosynthetic origins70,124, for example longamides and related analogues originate from amino acids. Racemases and epimerases have been revealed for amino acids117,122 and carbohydrates123. However, to fully understand the racemization of secondary metabolites, one should start considering that the traditional enzymes involved in the metabolisms of metabolites — synthases, oxidoreductases, transferases, hydrolases, lyases — are also all potential isomerase enzymes. This assumption is consistent with popular thought, championed by Tanner10, that any enzyme able to induce bond cleavage at a stereogenic centre could potentially catalyse its epimerization. For instance, enzymatic interconversion of configurations is a key argument in the literature to explain racemization and enantiomer occurrence in the benzylisoquinoline alkaloids series125. Conversely, the opposite species, enantioselective enzymes, are found in plants and other organisms6. Therefore, stereoisomers of opposite handedness could be biosynthesized by mirror-image enzymes present in certain organisms6. For example, germacrene D synthases of opposite handedness have been characterized from the plant Solidago canadensis and, interestingly, both enantiomers of this compound occur in the same species126,127.
Few natural enzymes that are dedicated to the transannular cyclohexene ring formation through Diels–Alder cycloaddition reactions have been reported. Most of these cycloaddition-inducing enzymes, or cyclases, intervene in other biological processes with a successive reduction of their catalytic efficacy, whereas few are stand-alone enzymes. Hashimoto and Kuzuyama carried out a comprehensive study to determine the nature of these enzymes11. The discovery of these cyclases changed the understanding of cycloadditions and revolutionized the discussions about the biosynthetic origins of racemates. However, the recognized success of these groups of enzymes was limited to intramolecular cycloaddition. Recently, Gao et al. isolated the first ever reported intermolecular enzyme from Morus alba, named Morus alba Diels-alderase (MaDA) (Fig. 4d), responsible for concerted pericyclization. This is exemplified by the proposed formation of hyperjapone A (40) in Hypericum japonicum, between diene and dienophile entities with exceptional catalytic and stereospecific efficiency128.
Racemization processes in secondary metabolism may include both homodimerization and heterodimerization. Most dimerization events follow oxidative coupling strategies56, with the exception of meroterpenes and polymeric stilbenes, where cycloaddition governs the process. Polyflavonoids are one such example, with a mechanism that involves radical coupling. The meroterpenes, such as hypulatone B (41) for instance, were tentatively assigned to be generated from a radical coupling rather than cycloaddition, as is common in this group56 (Fig. 4e). However, although oxidative coupling may explain dimerization in this case, it cannot support the racemization of these molecules alone, as the mechanism only affects one stereocentre. Most lignans are generated by the oxidative coupling pathway involving oxidative enzymes (for example, peroxidases or laccases) under the influence of dirigent proteins (DIRs), which are proteins that mediate the regioselectivity and stereoselectivity, leading to stereospecific products. If DIRs of opposite handedness are present within the same organism, this would result in the formation of racemates129–131. Indeed, the lack of strict stereospecific/stereoselective enzymatic control can lead to mixtures, including the formation of pairs of enantiomers in equal (racemic) or different ratios (scalemic). In lignan biosynthesis, for example, in vitro experiments revealed that in the absence of DIRs, laccases or peroxidases catalyse the dimerization (phenoxy radical coupling) of two (E)-coniferyl alcohol derivatives leading to a mixture of (±)-8,8′, (±)-8,5′ and (±)-8-O-4-linked racemic products (that is, lack of regioselectivity and stereoselectivity)130,132. Interestingly, in the presence of a DIR, the 8,8′ product is formed preferentially, yielding only the (+)-8,8′-linked (when mediated by (+)-DIR) or (–)-8,8′-linked (when mediated by (–)-DIR) product131. DIRs of opposite stereoselectivities, (+)-DIR and (–)-DIR, were first reported from plants132,133. Thus, 8,8′-linked and predominantly racemic lignans such as (±)-syringaresinol (42) in Tamarix nilotica could hypothetically originate from the intervention of two distinct DIRs, (+)-DIR/(–)-DIR (Fig. 4f). The evidence above establishes that nature may contain many more undiscovered materials, including enzymes and DIRs, and finding their roles in metabolic processes may open new avenues in science.
Pharmacological significance
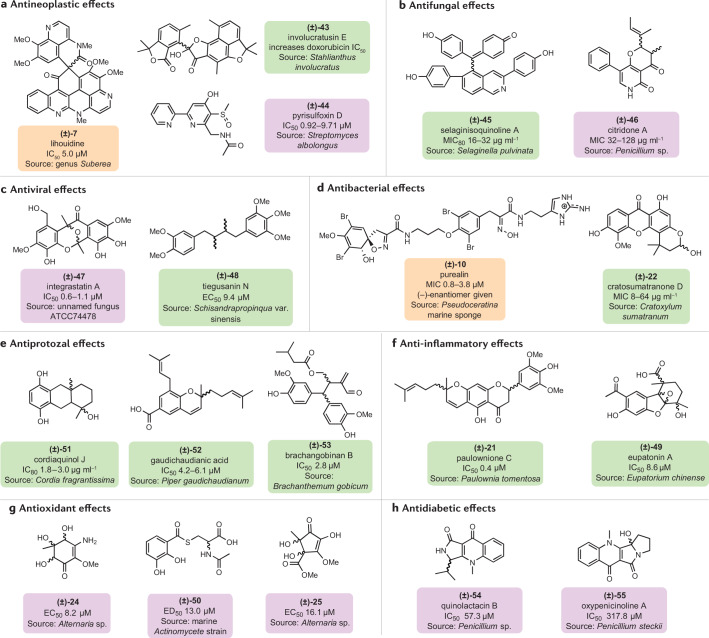
Chiral compounds continue to fuel the pipeline of drug discovery against a panel of diseases and constitute ~50% of marketed drugs, out of which >80% are marketed as racemates134. From a biological standpoint, naturally occurring racemates examined in the course of this survey have shown considerable anticancer (7, 43, 44)135,136, antimicrobial (10, 22, 45, 46)77,137–139, antiviral (47, 48)84,140, anti-inflammatory (21, 49)141,142, antioxidant (24, 25, 50)82,143, antiprotozoal (51, 52)73,144,145, antidiabetic (54, 55)146,147 and other medicinally relevant activities (Fig. 5).
Fig. 5. Chemical structures of racemic natural products with biological activities.
Labels of chemical structures are coloured to highlight the sources in nature: green for plants, purple for microorganisms and orange for animals. a–h | Compounds within the same shape are the most active racemates (evaluated by the values of IC50, EC50, minimum inhibitory concentration (MIC) or MIC80) of the entitled pharmacological activities as reported in the literature for antineoplastic (lihouidine (7)31, involucratusin E (43)86, pyrisulfoxin D (44)136) (part a), antifungal (selaginisoquinoline A (45)77, citridone A (46)84) (part b), antiviral (integrastatin A (47)140, tiegusanin N (48)182) (part c), antibacterial (purealin (10)34, cratosumatranone D (22)153) (part d), antiprotozal (cordiaquinol J (51)144, gaudichaudianic acid (52)159, brachangobinan B (53)73) (part e), anti-inflammatory (paulownione C (21)177, eupatonin A (49)183) (part f), antioxidant ((4R*,5S*,6S*)-3-amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one (24)82, (4S*,5S*)-2,4,5-trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopenten-1-one (25)82, 2-acetamido-3-(2,3-dihydroxybenzoylthio)propanoic acid (50)184) (part g) and antidiabetic (quinolactacin B (54)146, oxypenicinoline A (55)147) (part h) activities.
The majority of the racemates examined with biological activities were based on meroterpene, polyketide and alkaloid scaffolds. Meroterpenoids originate through coupling of terpene with either an acylphloroglucinol derivative148 or 3,5-dimethylorsellinic acid149. Phloroglucinol derivatives are well known for their significant antibacterial effect150,151. However, sesquiterpenoid-based meroterpenoids bearing an acylphloroglucinol skeleton such as drychampone A (14) showed virtually no activity against Gram-positive (Bacillus subtilis, Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli) and a bacterial plant pathogen (Dickeya zeae)54. Several polyketides are clinically important with a broad spectrum of activities mainly against infectious microbes152. Cratosumatranone D (22), a racemic polyketide, shows potent antibacterial activity against Micrococcus luteus, Bacillus cereus, B. subtilis, S. aureus, Staphylococcus epidermidis, E. coli, Salmonella typhimurium and Pseudomonas aeruginosa with minimum inhibitory concentration (MIC) values of 8, 8, 16, 32, 8, 64, 32 and 16 μg ml–1, respectively153. Racemic alkaloids represent a class of secondary metabolites known for their intrinsic and extrinsic mechanism of action against cancer cell lines154,155. Pyrisulfoxin D (44) displays excellent cytotoxicity against 26 out of 27 (96.3%) cancer cells lines tested, with IC50 values ranging from 0.92 to 9.71 µM136.
In general, one enantiomer may be more effective than the other at eliciting a biological response. This results in drug companies having a preference for single-enantiomer clinical compounds because they have potential advantages such as high therapeutic index and no chiral inversion (that is, chiral stability), leading to reduced negative side effects and highly selective pharmacodynamic properties compared with the mixture of both115,156. However, some racemic mixtures display synergetic activities when interacting with drugs. For instance, racemates such as antineoplastic agent involucratusin E (43) show interaction effects on doxorubicin, increasing its cytotoxicity by 2.2 times against a breast cancer cell line and providing a new lead compound for clinical study86.
Considering the concept of ‘one drug one target’ and considering that the human body is incredibly chiral selective, administration of racemates implies treatment of diseases with two drugs with different pharmacokinetic, pharmacological and pharmacodynamic profiles. Moreover, in the pharmacology field, racemic drugs can be classified into three groups. The first group represents racemates containing one active ingredient, or eutomer, and its enantiomer counterpart, or distomer, which produces a less active/toxic or different therapeutic effect. This can be exemplified by the synthetic racemic drug citalopram, with only its (S)-enantiomer carrying the desired therapeutic benefits14. The second group consists of racemic drugs constituted by two enantiomers of equal therapeutic profiles, such as for the drug fluoxetine157. The last group includes drugs with chiral inversion properties, with one or both enantiomer(s) prone to undergo racemization or enantiomerization, such as ketoprofen and thalidomide13.
Besides their effective treatment of numerous diseases, many existing racemic drugs have been associated with chronic side effects. As a result, many racemic drugs have witnessed a strategy known as the chiral switch (that is, replacement of a drug approved as a racemate with a drug containing only one of the two enantiomers in the previous version), increasing the arsenal of enantiomeric drugs. For example, chloroquine and its analogue hydroxychloroquine are well-known antimalarial drugs administered as racemates but result in negative side effects. Switching to a single enantiomer, the dextrorotatory (eutomer) isomer was found to be more effective for the management of COVID-19 (ref.158).
In summary, chirality in drug discovery programmes represents a blueprint for the future generation of drugs. The pharmacological effect of each enantiomer in the racemic mixture often differs in a chiral environment. Thus, the choice to make up a drug of either the racemate or a single enantiomer must take into consideration many clinical assessments, as mixture of enantiomers can induce synergistic, additive, reduced or negative effects. For example, the natural racemate cnidimonin A (28) (IC50 = 1.23 μM) has a greater antiviral potency than its single enantiomers (IC50 ≥ 3.7 μM)81, and gaudichaudianic acid (52) (IC50 = 55.8 μM) has a much increased trypanocidal activity than its single enantiomers (IC50 > 176.0 μM)159.
Conclusion
Overall, racemates occur in every organism on Earth and constitute one of the most intriguing topics in science. Their origin, however, is still deeply discussed. Racemic natural products are most commonly generated from ‘non-enzymatic’ routes. As enzymes typically form single enantiomers, the occurrence of racemates from enzymatic pathways in nature is still difficult to comprehend. The characterization of enzymes which can generate racemic mixtures and the investigation of pathway promiscuity towards racemic intermediates are important areas towards a better understanding of natural racemate occurrence. The discovery of many more racemates or the analysis of intact cells appears to be a good strategy to explore this issue.
Recognizing the presence of racemates upon isolation of natural products is an area for improvement. For instance, there is no optical rotation threshold in the literature, from which an isolated compound can be claimed to be racemic. Moreover, circular dichroism spectroscopy, crucial for the absolute configuration determination, still relies on complex quantum mechanics calculations. This method often requires comparison of both experimental and calculated circular dichroism spectra which is not readily available in some laboratories. The structure elucidation of more racemates including the absolute configuration of both enantiomers must be achieved with a simple technique without DFT methods that are not always affordable. Recently, the crystalline sponge method was developed for X-ray analysis and has already demonstrated several applications in natural product discovery112. Additionally, the MicroED technique has recently been developed as a new promising alternative to XRD for structure determination108–111. The combination of these existent tools and the development of new ones, especially the advanced techniques, for a fast resolution and structure elucidation of small amounts of racemates will significantly advance natural product research and drug discovery. Finally, natural products remain an important source of therapeutically relevant chiral compounds supplying drug discovery pipelines with both enantiomerically pure compounds and racemates. Although natural racemates have historically been underexplored, they have great promise in the future of drug discovery.
Supplementary information
Acknowledgements
The authors are grateful to L. Beerhues (TU Braunschweig) and T. Opatz (University Mainz) for their valuable comments on this manuscript. Work in S.A.F.’s laboratory was supported by the grant for material cost allowances of the Fund der chemischen Industrie and the State of Lower Saxony.
Author contributions
All authors collected data, wrote and edited the manuscript. G.T.M.B. and V.-A.N.-N. drafted the manuscript. D.M. provided substantial content. S.A.F. conceived the content, and edited and finalized the manuscript.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41570-022-00431-4.
References
- 1.Sharma B. Nature of chiral drugs and their occurrence in environment. J. Xenobiotics. 2014;4:14–19. doi: 10.4081/xeno.2014.2272. [DOI] [Google Scholar]
- 2.Zask A, Ellestad GA. Reflections on the intriguing occurrence of some recently isolated natural products as racemates and scalemic mixtures. Chirality. 2021;33:915–930. doi: 10.1002/chir.23360. [DOI] [PubMed] [Google Scholar]
- 3.Devínsky F. Chirality and the origin of life. Symmetry. 2021;13:2277. doi: 10.3390/sym13122277. [DOI] [Google Scholar]
- 4.Kittakoop P. Part 2: occurrence of racemic natural products and their biological activities. J. Chulabhorn R. Acad. 2020;2:27–43. [Google Scholar]
- 5.Lee ST, Gardner DR, Chang CWT, Panter KE, Molyneux RJ. Separation and measurement of plant alkaloid enantiomers by RP-HPLC analysis of their Fmoc-alanine analogs. Phytochem. Anal. 2008;19:395–402. doi: 10.1002/pca.1064. [DOI] [PubMed] [Google Scholar]
- 6.Finefield JM, Sherman DH, Kreitman M, Williams RM. Enantiomeric natural products: occurrence and biogenesis. Angew. Chem. Int. Ed. 2012;51:4802–4836. doi: 10.1002/anie.201107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gawley RE. Do the terms ‘% ee’ and ‘% de’ make sense as expressions of stereoisomer composition or stereoselectivity? J. Org. Chem. 2006;71:2411–2416. doi: 10.1021/jo052554w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batista ANL, dos Santos FM, Batista JM, Cass QB. Enantiomeric mixtures in natural product chemistry: separation and absolute configuration assignment. Molecules. 2018;23:1–18. doi: 10.3390/molecules23020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurimotoa S, Pu J-X, Sun H-D, Takaishi Y, Kashiwada Y. Coleifolides A and B, two new sesterterpenoids from the aerial parts of Scutellaria coleifolia H.Lév. Chem. Biodivers. 2015;12:1200–1207. doi: 10.1002/cbdv.201400248. [DOI] [PubMed] [Google Scholar]
- 10.Tanner ME. Understanding nature’s strategies for enzyme-catalyzed racemization and epimerization. Acc. Chem. Res. 2002;35:237–246. doi: 10.1021/ar000056y. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Kuzuyama T. Mechanistic insights into Diels–Alder reactions in natural product biosynthesis. Curr. Opin. Chem. Biol. 2016;35:117–123. doi: 10.1016/j.cbpa.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Bitchagno, G. T. M. et al. in In Bioactive Compounds in Nutraceutical and Functional Food for Good Human Health 53–77 (IntechOpen, 2020).
- 13.Nguyen LA, He H, Pham-Huy C. Chiral drugs: an overview. Int. J. Biomed. Sci. 2006;2:85–100. [PMC free article] [PubMed] [Google Scholar]
- 14.Lepola U, Wade A, Andersen HF. Do equivalent doses of escitalopram and citalopram have similar efficacy? A pooled analysis of two positive placebo-controlled studies in major depressive disorder. Int. Clin. Psychopharmacol. 2004;19:149–155. doi: 10.1097/00004850-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Yan YM, et al. Compounds from the insect Blaps japanensis with COX-1 and COX-2 inhibitory activities. Bioorg. Med. Chem. Lett. 2015;25:2469–2472. doi: 10.1016/j.bmcl.2015.04.085. [DOI] [PubMed] [Google Scholar]
- 16.Wang KB, et al. (±)-Peharmaline A: a pair of rare β-carboline–vasicinone hybrid alkaloid enantiomers from Peganum harmala. Eur. J. Org. Chem. 2017;2017:1876–1879. doi: 10.1002/ejoc.201700137. [DOI] [Google Scholar]
- 17.Shaker S, Fan RZ, Li HJ, Lan WJ. A pair of novel bisindole alkaloid enantiomers from marine fungus Fusarium sp. XBB-9. Nat. Prod. Res. 2021;35:1497–1503. doi: 10.1080/14786419.2019.1655416. [DOI] [PubMed] [Google Scholar]
- 18.Liu SF, et al. Bioactive spiropyrrolizidine oxindole alkaloid enantiomers from Isatis indigotica Fortune. Org. Biomol. Chem. 2018;16:9430–9439. doi: 10.1039/C8OB02046A. [DOI] [PubMed] [Google Scholar]
- 19.Guo XM, et al. (±)-Quassidine K, a pair of cytotoxic bis-β-carboline alkaloid enantiomers from Picrasma quassioides. Nat. Prod. Res. 2020;34:489–493. doi: 10.1080/14786419.2018.1489388. [DOI] [PubMed] [Google Scholar]
- 20.Li W, et al. New pyridocarbazole alkaloids from Strychnos nitida. Nat. Prod. Res. 2018;32:1532–1536. doi: 10.1080/14786419.2017.1385016. [DOI] [PubMed] [Google Scholar]
- 21.Kusama T, Tanaka N, Kashiwada Y, Kobayashi J. Agelamadin F and tauroacidin E, bromopyrrole alkaloids from an Okinawan marine sponge Agelas sp. Tetrahedron Lett. 2015;56:4502–4504. doi: 10.1016/j.tetlet.2015.05.114. [DOI] [Google Scholar]
- 22.Yang HX, et al. Piperidine alkaloids and xanthone from the roots of Caulophyllum robustum Maxim. Fitoterapia. 2019;132:22–25. doi: 10.1016/j.fitote.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, et al. Phenolic alkaloids from the aerial parts of Dracocephalum heterophyllum. Phytochemistry. 2012;82:166–171. doi: 10.1016/j.phytochem.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Cutillo F, D’Abrosca B, DellaGreca M, Zarrelli A. Chenoalbicin, a novel cinnamic acid amide alkaloid from Chenopodium album. Chem. Biodivers. 2004;1:1579–1583. doi: 10.1002/cbdv.200490118. [DOI] [PubMed] [Google Scholar]
- 25.Al-Khdhairawi AAQ, et al. A bis-benzopyrroloisoquinoline alkaloid incorporating a cyclobutane core and a chlorophenanthroindolizidine alkaloid with cytotoxic activity from Ficus fistulosa var. tengerensis. J. Nat. Prod. 2017;80:2734–2740. doi: 10.1021/acs.jnatprod.7b00500. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, et al. (±)-Homocrepidine A, a pair of anti-inflammatory enantiomeric octahydroindolizine alkaloid dimers from Dendrobium crepidatum. J. Nat. Prod. 2016;79:252–256. doi: 10.1021/acs.jnatprod.5b00801. [DOI] [PubMed] [Google Scholar]
- 27.Lin Z, et al. Oxazinin A, a pseudodimeric natural product of mixed biosynthetic origin from a filamentous fungus. Org. Lett. 2014;16:4774–4777. doi: 10.1021/ol502227x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian J, et al. Nigegladines A–C, three thymoquinone dimers from Nigella glandulifera. Org. Lett. 2017;19:6348–6351. doi: 10.1021/acs.orglett.7b03189. [DOI] [PubMed] [Google Scholar]
- 29.Yang CL, et al. Bialternacins A–F, aromatic polyketide dimers from an endophytic Alternaria sp. J. Nat. Prod. 2019;82:792–797. doi: 10.1021/acs.jnatprod.8b00705. [DOI] [PubMed] [Google Scholar]
- 30.Chan STS, et al. Characterization and synthesis of eudistidine C, a bioactive marine alkaloid with an intriguing molecular scaffold. J. Org. Chem. 2016;81:10631–10640. doi: 10.1021/acs.joc.6b02380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowden BF, McCool BJ, Willis RH. Lihouidine, a novel spiro polycyclic aromatic alkaloid from the marine sponge Suberea n. sp. (Aplysinellidae, Verongida) J. Org. Chem. 2004;69:7791–7793. doi: 10.1021/jo0498819. [DOI] [PubMed] [Google Scholar]
- 32.Li J, et al. (±)-Xylaridines A and B, highly conjugated alkaloids from the fungus Xylaria longipes. Org. Lett. 2019;21:1511–1514. doi: 10.1021/acs.orglett.9b00312. [DOI] [PubMed] [Google Scholar]
- 33.Yu MY, et al. Macathiohydantoins B–K, novel thiohydantoin derivatives from Lepidium meyenii. Tetrahedron. 2017;73:4392–4397. doi: 10.1016/j.tet.2017.05.096. [DOI] [Google Scholar]
- 34.Salim AA, Khalil ZG, Capon RJ. Structural and stereochemical investigations into bromotyrosine-derived metabolites from southern Australian marine sponges, Pseudoceratina spp. Tetrahedron. 2012;68:9802–9807. doi: 10.1016/j.tet.2012.09.008. [DOI] [Google Scholar]
- 35.Xu J, et al. Melophlins P, Q, R, and S: four new tetramic acid derivatives, from two Palauan marine sponges of the genus Melophlus. Chem. Pharm. Bull. 2006;54:852–854. doi: 10.1248/cpb.54.852. [DOI] [PubMed] [Google Scholar]
- 36.Han QT, Ren Y, Li GS, Xiang KL, Dai SJ. Flavonoid alkaloids from Scutellaria moniliorrhiza with anti-inflammatory activities and inhibitory activities against aldose reductase. Phytochemistry. 2018;152:91–96. doi: 10.1016/j.phytochem.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Xia Z, et al. Bioactive sulfur-containing compounds from Xanthium sibiricum, including a revision of the structure of xanthiazinone. Phytochemistry. 2020;173:112293. doi: 10.1016/j.phytochem.2020.112293. [DOI] [PubMed] [Google Scholar]
- 38.Risdian C, Mozef T, Wink J. Biosynthesis of polyketides in Streptomyces. Microorganisms. 2019;7:1–18. doi: 10.3390/microorganisms7050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das A, Khosla C. Biosynthesis of aromatic polyketides in bacteria. Acc. Chem. Res. 2009;42:631–639. doi: 10.1021/ar8002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong W, et al. Euroticins C–E, three pairs of polycyclic salicylaldehyde derivative enantiomers from a marine-derived fungus: Eurotium sp. SCSIO F452. Org. Chem. Front. 2021;8:1466–1473. doi: 10.1039/D0QO01519A. [DOI] [Google Scholar]
- 41.Wang F, et al. Euroticins A and B, two pairs of highly constructed salicylaldehyde derivative enantiomers from a marine-derived fungus Eurotium sp. SCSIO F452. J. Org. Chem. 2020;85:12754–12759. doi: 10.1021/acs.joc.0c01407. [DOI] [PubMed] [Google Scholar]
- 42.Zhao S, et al. Enantiomeric dibenzo-α-pyrone derivatives from Alternaria alternata ZHJG5 and their potential as agrochemicals. J. Agric. Food Chem. 2020;68:15115–15122. doi: 10.1021/acs.jafc.0c04106. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YL, et al. Unprecedented immunosuppressive polyketides from Daldinia eschscholzii, a mantis-associated fungus. Angew. Chem. Int. Ed. 2008;47:5823–5826. doi: 10.1002/anie.200801284. [DOI] [PubMed] [Google Scholar]
- 44.Wei X, et al. Enantiomeric polyketides from the starfish-derived symbiotic fungus Penicillium sp. GGF16-1-2. Chem. Biodivers. 2019;16:e1900052. doi: 10.1002/cbdv.201900052. [DOI] [PubMed] [Google Scholar]
- 45.Azmi MN, et al. Kingianins O–Q: pentacyclic polyketides from Endiandra kingiana as inhibitor of Mcl-1/Bid interaction. Fitoterapia. 2016;109:190–195. doi: 10.1016/j.fitote.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Duong TH, et al. Sanctis A–C: three racemic procyanidin analogues from the lichen Parmotrema sancti-angelii. Eur. J. Org. Chem. 2018;2018:2247–2253. doi: 10.1002/ejoc.201800202. [DOI] [Google Scholar]
- 47.Duong TH, et al. Tsavoenones A–C: unprecedented polyketides with a 1,7-dioxadispiro[4.0.4.4]tetradecane core from the lichen: Parmotrema tsavoense. Org. Biomol. Chem. 2018;16:5913–5919. doi: 10.1039/C8OB01280F. [DOI] [PubMed] [Google Scholar]
- 48.Suyama Y, et al. Stereochemical assignments of rubiaquinones A–C, naphthoquinone derivatives from Rubia yunnanensis. Tetrahedron Lett. 2017;58:4568–4571. doi: 10.1016/j.tetlet.2017.10.051. [DOI] [Google Scholar]
- 49.Tian X, et al. (–) and (+)-Merrilliaquinone, a pair of new quinone enantiomers from Illicium merrillianum and their distinctive effect on human hepatoma and hepatic cells. RSC Adv. 2015;5:75857–75862. doi: 10.1039/C5RA15074D. [DOI] [Google Scholar]
- 50.Hu L, et al. Discovery of acylphloroglucinol-based meroterpenoid enantiomers as KSHV inhibitors from Hypericum japonicum. RSC Adv. 2018;8:24101–24109. doi: 10.1039/C8RA04073G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao HB, et al. Two enantiomeric pairs of meroterpenoids from Rhododendron capitatum. Org. Lett. 2015;17:5040–5043. doi: 10.1021/acs.orglett.5b02515. [DOI] [PubMed] [Google Scholar]
- 52.Wang B, Zhang Z, Guo L, Liu L. New cytotoxic meroterpenoids from the plant endophytic fungus Pestalotiopsis fici. Helv. Chim. Acta. 2016;99:151–156. doi: 10.1002/hlca.201500197. [DOI] [Google Scholar]
- 53.Ryu B, et al. Meroterpenoids from the leaves of Psidium guajava (guava) cultivated in Korea using MS/MS-based molecular networking. Phytochemistry. 2021;186:112723. doi: 10.1016/j.phytochem.2021.112723. [DOI] [PubMed] [Google Scholar]
- 54.Chen NH, et al. Drychampones A–C: three meroterpenoids from Dryopteris championii. J. Org. Chem. 2016;81:9443–9448. doi: 10.1021/acs.joc.6b01720. [DOI] [PubMed] [Google Scholar]
- 55.Yang XW, Li YP, Su J, Ma WG, Xu G. Hyperjapones A–E, terpenoid polymethylated acylphloroglucinols from Hypericum japonicum. Org. Lett. 2016;18:1876–1879. doi: 10.1021/acs.orglett.6b00650. [DOI] [PubMed] [Google Scholar]
- 56.Ye YS, et al. Novel meroterpenoids from Hypericum patulum: highly potent late Nav1.5 sodium current inhibitors. Org. Lett. 2020;22:6339–6343. doi: 10.1021/acs.orglett.0c02170. [DOI] [PubMed] [Google Scholar]
- 57.Liao HB, Huang GH, Yu MH, Lei C, Hou AJ. Five pairs of meroterpenoid enantiomers from Rhododendron capitatum. J. Org. Chem. 2017;82:1632–1637. doi: 10.1021/acs.joc.6b02800. [DOI] [PubMed] [Google Scholar]
- 58.Luo Q, Yang XH, Yang ZL, Tu ZC, Cheng YX. Miscellaneous meroterpenoids from Ganoderma applanatum. Tetrahedron. 2016;72:4564–4574. doi: 10.1016/j.tet.2016.06.019. [DOI] [Google Scholar]
- 59.Luo Q, et al. Isolation and identification of renoprotective substances from the mushroom Ganoderma lucidum. Tetrahedron. 2015;71:840–845. doi: 10.1016/j.tet.2014.12.052. [DOI] [Google Scholar]
- 60.Ding WY, et al. Isolation of lingzhifuran A and lingzhilactones D–F from Ganoderma lucidum as specific Smad3 phosphorylation inhibitors and total synthesis of lingzhifuran A. RSC Adv. 2016;6:77887–77897. doi: 10.1039/C6RA17900B. [DOI] [Google Scholar]
- 61.Wang XL, et al. Renoprotective phenolic meroterpenoids from the mushroom Ganoderma cochlear. Phytochemistry. 2019;162:199–206. doi: 10.1016/j.phytochem.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 62.Zhu Y, et al. Antifungal bromopyrrole alkaloids from the South China Sea sponge Agelas sp. Tetrahedron. 2016;72:2964–2971. doi: 10.1016/j.tet.2016.04.020. [DOI] [Google Scholar]
- 63.Wongsa N, Kanokmedhakul K, Boonmak J, Youngme S, Kanokmedhakul S. Bicyclic lactones and racemic mixtures of dimeric styrylpyrones from the leaves of: Miliusa velutina. RSC Adv. 2017;7:25285–25297. doi: 10.1039/C7RA01609C. [DOI] [Google Scholar]
- 64.Ito T, et al. Occurrence of stilbene oligomers in Cyperus rhizomes. Fitoterapia. 2012;83:1420–1429. doi: 10.1016/j.fitote.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Liao L, Yan YM, Xu T, Xia HL, Cheng YX. A pair of novel sulfonyl-containing N-acetyldopamine dimeric enantiomers from Aspongopus chinensis. Nat. Prod. Commun. 2020;15:4–8. [Google Scholar]
- 66.Zhang M, et al. Bibenzyl derivatives from Dendrobium nobile. Chin. J. Org. Chem. 2019;39:3289–3293. doi: 10.6023/cjoc201903035. [DOI] [Google Scholar]
- 67.Yan ZY, et al. Racemic phenylpropanoids from the root barks of Ailanthus altissima (Mill.) swingle with cytotoxicity against hepatoma cells. Fitoterapia. 2018;130:234–240. doi: 10.1016/j.fitote.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Peng XR, et al. Rare hybrid dimers with anti-acetylcholinesterase activities from a safflower (Carthamus tinctorius L.) seed oil cake. J. Agric. Food Chem. 2017;65:9453–9459. doi: 10.1021/acs.jafc.7b03431. [DOI] [PubMed] [Google Scholar]
- 69.Zhu W, et al. Cytotoxic phenolic constituents from Hypericum japonicum. Phytochemistry. 2019;164:33–40. doi: 10.1016/j.phytochem.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 70.Ding WY, et al. Isolation, total synthesis, and absolute configuration determination of renoprotective dimeric N-acetyldopamine-adenine hybrids from the insect Aspongopus chinensis. Org. Lett. 2020;22:5726–5730. doi: 10.1021/acs.orglett.0c01593. [DOI] [PubMed] [Google Scholar]
- 71.Zhang WY, et al. New lignans attenuating cognitive deterioration of Aβ transgenic flies discovered in Acorus tatarinowii. Bioorg. Med. Chem. Lett. 2018;28:814–819. doi: 10.1016/j.bmcl.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 72.Lai Y, et al. Neolignans with a rare 2-oxaspiro[4.5]deca-6,9-dien-8-one motif from the stem bark of Cinnamomum subavenium. J. Nat. Prod. 2015;78:1740–1744. doi: 10.1021/np5010533. [DOI] [PubMed] [Google Scholar]
- 73.Odonbayar B, et al. Acylated lignans isolated from Brachanthemum gobicum and their trypanocidal activity. J. Nat. Prod. 2019;82:774–784. doi: 10.1021/acs.jnatprod.8b00670. [DOI] [PubMed] [Google Scholar]
- 74.Yang F, et al. Piperhancins A and B, two pairs of antineuroinflammatory cycloneolignane enantiomers from Piper hancei. J. Org. Chem. 2021;86:5284–5291. doi: 10.1021/acs.joc.1c00240. [DOI] [PubMed] [Google Scholar]
- 75.Sai CM, et al. Racemic alkaloids from Macleaya cordata: structural elucidation, chiral resolution, and cytotoxic, antibacterial activities. RSC Adv. 2016;6:41173–41180. doi: 10.1039/C6RA05423D. [DOI] [Google Scholar]
- 76.Yang X, et al. Parvistemins A–D, a new type of dimeric phenylethyl benzoquinones from Stemona parviflora Wright. Tetrahedron. 2007;63:4688–4694. doi: 10.1016/j.tet.2007.03.093. [DOI] [Google Scholar]
- 77.Cao Y, et al. Four new selaginellin derivatives from Selaginella pulvinata: mechanism of racemization process in selaginellins with quinone methide. Tetrahedron. 2015;71:1581–1587. doi: 10.1016/j.tet.2015.01.017. [DOI] [Google Scholar]
- 78.Bringmann G, et al. (±)-Dioncophyllacine A, A naphthylisoquinoline alkaloid with a 4-methoxy substituent from the leaves of Triphyophyllum peltatum. Phytochemistry. 1992;31:4015–4018. doi: 10.1016/S0031-9422(00)97575-7. [DOI] [Google Scholar]
- 79.Xu L, et al. Chiral separation, absolute configuration, and bioactivity of two pairs of flavonoid enantiomers from Morus nigra. Phytochemistry. 2019;163:33–37. doi: 10.1016/j.phytochem.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 80.Allard PM, et al. Alkylated flavanones from the bark of Cryptocarya chartacea as dengue virus NS5 polymerase inhibitors. J. Nat. Prod. 2011;74:2446–2453. doi: 10.1021/np200715v. [DOI] [PubMed] [Google Scholar]
- 81.Su F, et al. Cnidimonins A–C, three types of hybrid dimer from Cnidium monnieri: structural elucidation and semisynthesis. Org. Lett. 2017;19:4920–4923. doi: 10.1021/acs.orglett.7b02290. [DOI] [PubMed] [Google Scholar]
- 82.Wang J, et al. Identification and bioactivity of compounds from the mangrove endophytic fungus Alternaria sp. Mar. Drugs. 2015;13:4492–4504. doi: 10.3390/md13074492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bai Y, et al. The asarone-derived phenylpropanoids from the rhizome of Acorus calamus var. angustatus Besser. Phytochemistry. 2020;170:112212. doi: 10.1016/j.phytochem.2019.112212. [DOI] [PubMed] [Google Scholar]
- 84.Xu Y, et al. New phenylpyridone derivatives from the Penicillium sumatrense GZWMJZ-313, a fungal endophyte of Garcinia multiflora. Chin. Chem. Lett. 2019;30:431–434. doi: 10.1016/j.cclet.2018.08.015. [DOI] [Google Scholar]
- 85.Zou J, et al. Triligustilides A and B: two pairs of phthalide trimers from Angelica sinensis with a complex polycyclic skeleton and their activities. Org. Lett. 2018;20:884–887. doi: 10.1021/acs.orglett.8b00017. [DOI] [PubMed] [Google Scholar]
- 86.Li QM, et al. Involucratusins A–H: unusual cadinane dimers from Stahlianthus involucratus with multidrug resistance reversal activity. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep29744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang HJ, et al. Natural anti-HIV agents. Part 3: litseaverticillols A–H, novel sesquiterpenes from Litsea verticillata. Tetrahedron. 2003;59:141–148. doi: 10.1016/S0040-4020(02)01491-6. [DOI] [Google Scholar]
- 88.Quesada-Moreno MM, et al. A vibrational circular dichroism (VCD) methodology for the measurement of enantiomeric excess in chiral compounds in the solid phase and for the complementary use of NMR and VCD techniques in solution: the camphor case. Analyst. 2018;143:1406–1416. doi: 10.1039/C7AN01855J. [DOI] [PubMed] [Google Scholar]
- 89.Okuom MO, Burks R, Naylor C, Holmes AE. Applied circular dichroism: a facile spectroscopic tool for configurational assignment and determination of enantiopurity. J. Anal. Methods Chem. 2015;2015:1–7. doi: 10.1155/2015/865605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fobofou SAT, et al. Prenylated phenyl polyketides and acylphloroglucinols from Hypericum peplidifolium. Phytochemistry. 2016;124:108–113. doi: 10.1016/j.phytochem.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Aboul-Enein HY, Bounoua N, Rebizi M, Wagdy H. Application of nanoparticles in chiral analysis and chiral separation. Chirality. 2021;33:196–208. doi: 10.1002/chir.23303. [DOI] [PubMed] [Google Scholar]
- 92.Gogoi A, et al. Enantiomeric recognition and separation by chiral nanoparticles. Molecules. 2019;24:1007. doi: 10.3390/molecules24061007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bucar F, Wube A, Schmid M. Natural product isolation — how to get from biological material to pure compounds. Nat. Prod. Rep. 2013;30:525–545. doi: 10.1039/c3np20106f. [DOI] [PubMed] [Google Scholar]
- 94.Speybrouck D, Lipka E. Preparative supercritical fluid chromatography: a powerful tool for chiral separations. J. Chromatogr. A. 2016;1467:33–55. doi: 10.1016/j.chroma.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 95.Cavazzini A, Pasti L, Massi A, Marchetti N, Dondi F. Recent applications in chiral high performance liquid chromatography: a review. Anal. Chim. Acta. 2011;706:205–222. doi: 10.1016/j.aca.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 96.Badaloni E, et al. Combination of HPLC ‘inverted chirality columns approach’ and MS/MS detection for extreme enantiomeric excess determination even in absence of reference samples. Application to camptothecin derivatives. Anal. Chem. 2007;79:6013–6019. doi: 10.1021/ac070776j. [DOI] [PubMed] [Google Scholar]
- 97.Xia Z, et al. New phenylpropanoids from the fruits of Xanthium sibiricum and their anti-inflammatory activity. Nat. Prod. Res. 2022;36:805–813. doi: 10.1080/14786419.2020.1806273. [DOI] [PubMed] [Google Scholar]
- 98.Parmaki S, et al. Resolution of alkaloid racemate: a novel microbial approach for the production of enantiopure lupanine via industrial wastewater valorization. Microb. Cell Fact. 2020;19:1–10. doi: 10.1186/s12934-020-01324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mane S. Racemic drug resolution: a comprehensive guide. Anal. Methods. 2016;8:7567–7586. doi: 10.1039/C6AY02015A. [DOI] [Google Scholar]
- 100.Kauffman GB, Myers RD. Pasteur’s resolution of racemic acid: a sesquicentennial retrospect and a new translation. Chem. Educ. 1998;3:1–4. [Google Scholar]
- 101.Bertucci C, Tedesco D. Advantages of electronic circular dichroism detection for the stereochemical analysis and characterization of drugs and natural products by liquid chromatography. J. Chromatogr. A. 2012;1269:69–81. doi: 10.1016/j.chroma.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 102.Bitchagno, G. T. M. & Fobofou Tanemossu, S. A. in Chemoinformatics of Natural Products (ed. Ntie-Kang, F.) 229–248 (De Gruyter, 2020).
- 103.Lee SR, et al. (±)-Kituramides A and B, pairs of enantiomeric dopamine dimers from the two-spotted cricket Gryllus bimaculatus. Bioorg. Chem. 2020;95:103554. doi: 10.1016/j.bioorg.2019.103554. [DOI] [PubMed] [Google Scholar]
- 104.Ren DM, et al. Stereochemistry of flavonoidal alkaloids from Dracocephalum rupestre. Phytochemistry. 2008;69:1425–1433. doi: 10.1016/j.phytochem.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 105.Evarestov, R. A. Theoretical Modeling of Inorganic Nanostructures: Symmetry and Ab-initio Calculations of Nanolayers, Nanotubes and Nanowires (Springer, 2015).
- 106.Flack HD. The use of X-ray crystallography to determine absolute configuration (II) Acta Chim. Slov. 2008;55:689–691. doi: 10.1002/chir.20473. [DOI] [PubMed] [Google Scholar]
- 107.Flack HD, Bernardinelli G. Reporting and evaluating absolute-structure and absolute-configuration determinations. J. Appl. Crystallogr. 2000;33:1143–1148. doi: 10.1107/S0021889800007184. [DOI] [Google Scholar]
- 108.Shi D, Nannenga BL, Iadanza MG, Gonen T. Three-dimensional electron crystallography of protein microcrystals. eLife. 2013;2:e01345. doi: 10.7554/eLife.01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nannenga BL, Shi D, Leslie AGW, Gonen T. High-resolution structure determination by continuous-rotation data collection in MicroEDED. Nat. Methods. 2014;11:927–930. doi: 10.1038/nmeth.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jones CG, et al. The CryoEM method MicroED as a powerful tool for small molecule structure determination. ACS Cent. Sci. 2018;4:1587–1592. doi: 10.1021/acscentsci.8b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Danelius E, Halaby S, Van Der Donk WA, Gonen T. MicroED in natural product and small molecule research. Nat. Prod. Rep. 2021;38:423–431. doi: 10.1039/D0NP00035C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inokuma Y, et al. X-Ray analysis on the nanogram to microgram scale using porous complexes. Nature. 2013;495:461–466. doi: 10.1038/nature11990. [DOI] [PubMed] [Google Scholar]
- 113.Zhu GY, et al. (±)-Sativamides A and B, two pairs of racemic nor-lignanamide enantiomers from the fruits of Cannabis sativa. J. Org. Chem. 2018;83:2376–2381. doi: 10.1021/acs.joc.7b02765. [DOI] [PubMed] [Google Scholar]
- 114.Grimblat N, Zanardi MM, Sarotti AM. Beyond DP4: an improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015;80:12526–12534. doi: 10.1021/acs.joc.5b02396. [DOI] [PubMed] [Google Scholar]
- 115.Calcaterra A, D’Acquarica I. The market of chiral drugs: chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018;147:323–340. doi: 10.1016/j.jpba.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 116.Kazlauskas RJ, Weissfloch ANE, Rappaport AT, Cuccia LA. A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J. Org. Chem. 1991;56:2656–2665. doi: 10.1021/jo00008a016. [DOI] [Google Scholar]
- 117.Ballard A, et al. Racemisation in chemistry and biology. Chemistry. 2020;26:3661–3687. doi: 10.1002/chem.201903917. [DOI] [PubMed] [Google Scholar]
- 118.Matsuo K, et al. Racemization kinetics of meluadrine tartrate in aqueous solution. Chem. Pharm. Bull. 2001;49:101–104. doi: 10.1248/cpb.49.101. [DOI] [PubMed] [Google Scholar]
- 119.Xu WF, et al. 17-Hydroxybrevianamide N and its N1-methyl derivative, quinazolinones from a soft-coral-derived Aspergillus sp. fungus: 13S enantiomers as the true natural products. J. Nat. Prod. 2021;84:1353–1358. doi: 10.1021/acs.jnatprod.1c00098. [DOI] [PubMed] [Google Scholar]
- 120.Wang T, Hoye TR. Diels-alderase-free, bis-pericyclic, [4+2] dimerization in the biosynthesis of (±)-paracaseolide A. Nat. Chem. 2015;7:641–645. doi: 10.1038/nchem.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Inaki M, Liu J, Matsuno K. Cell chirality: its origin and roles in left–right asymmetric development. Philos. Trans. R. Soc. B Biol. Sci. 2016;371:20150403. doi: 10.1098/rstb.2015.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoshimura T, Esaki N. Amino acid racemases: functions and mechanisms. J. Biosci. Bioeng. 2003;96:103–109. doi: 10.1016/S1389-1723(03)90111-3. [DOI] [PubMed] [Google Scholar]
- 123.Saburi W. Functions, structures, and applications of cellobiose 2-epimerase and glycoside hydrolase family 130 mannoside phosphorylases. Biosci. Biotechnol. Biochem. 2016;80:1294–1305. doi: 10.1080/09168451.2016.1166934. [DOI] [PubMed] [Google Scholar]
- 124.Tori M, Ohara Y, Nakashima K, Sono M. Thymol derivatives from Eupatorium fortunei. J. Nat. Prod. 2001;64:1048–1051. doi: 10.1021/np0101191. [DOI] [PubMed] [Google Scholar]
- 125.Bauer W, Zenk MH. Formation of (R)-configurated tetrahydroprotoberberine alkaloids in vivo and in vitro. Tetrahedron Lett. 1991;32:487–490. doi: 10.1016/S0040-4039(00)79475-5. [DOI] [Google Scholar]
- 126.Prosser I, et al. Enantiospecific (+)- and (–)-germacrene D synthases, cloned from goldenrod, reveal a functionally active variant of the universal isoprenoid-biosynthesis aspartate-rich motif. Arch. Biochem. Biophys. 2004;432:136–144. doi: 10.1016/j.abb.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 127.Schmidt CO, Bouwmeester HJ, Franke S, König WA. Mechanisms of the biosynthesis of sesquiterpene enantiomers (+)- and (–)-germacrene D in Solidago canadensis. Chirality. 1999;11:353–362. doi: 10.1002/(SICI)1520-636X(1999)11:5/6<353::AID-CHIR2>3.0.CO;2-L. [DOI] [Google Scholar]
- 128.Gao L, et al. FAD-dependent enzyme-catalysed intermolecular [4 + 2] cycloaddition in natural product biosynthesis. Nat. Chem. 2020;12:620–628. doi: 10.1038/s41557-020-0467-7. [DOI] [PubMed] [Google Scholar]
- 129.Anarat-Cappillino G, Sattely ES. The chemical logic of plant natural product biosynthesis. Curr. Opin. Plant. Biol. 2014;19:51–58. doi: 10.1016/j.pbi.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davin LB, et al. Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science. 1997;275:362–367. doi: 10.1126/science.275.5298.362. [DOI] [PubMed] [Google Scholar]
- 131.Kim SS, Sattely ES. Dirigent proteins guide asymmetric heterocoupling for the synthesis of complex natural product analogues. J. Am. Chem. Soc. 2021;143:5011–5021. doi: 10.1021/jacs.0c13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paniagua C, et al. Dirigent proteins in plants: modulating cell wall metabolism during abiotic and biotic stress exposure. J. Exp. Bot. 2017;68:3287–3301. doi: 10.1093/jxb/erx141. [DOI] [PubMed] [Google Scholar]
- 133.Kim KW, et al. Opposite stereoselectivities of dirigent proteins in Arabidopsis and Schizandra species. J. Biol. Chem. 2012;287:33957–33972. doi: 10.1074/jbc.M112.387423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang W, et al. β-Cyclodextrin improve the tolerant of freshwater algal spiny Scenedesmus to chiral drugs venlafaxine and its metabolite. J. Hazard. Mater. 2020;399:123076. doi: 10.1016/j.jhazmat.2020.123076. [DOI] [PubMed] [Google Scholar]
- 135.Gao H, et al. Diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Org. Biomol. Chem. 2012;10:9501–9506. doi: 10.1039/c2ob26757h. [DOI] [PubMed] [Google Scholar]
- 136.Du Y, et al. Cytotoxic and optically active pyrisulfoxins from the endophytic Streptomyces albolongus EA12432. Front. Chem. 2020;8:248. doi: 10.3389/fchem.2020.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tanaka N, et al. Nagelamides A and B, dimeric bromopyrrole alkaloids from a marine sponge Agelas sp. Org. Lett. 2013;15:3262–3265. doi: 10.1021/ol401291n. [DOI] [PubMed] [Google Scholar]
- 138.Kusakabe Y, et al. Synthesis, antibacterial and cytotoxic evaluation of flavipucine and its derivatives. Bioorg. Med. Chem. Lett. 2019;29:1390–1394. doi: 10.1016/j.bmcl.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 139.Jia YL, et al. (+)- and (–)-pestaloxazine A, a pair of antiviral enantiomeric alkaloid dimers with a symmetric spiro[oxazinane-piperazinedione] skeleton from Pestalotiopsis sp. Org. Lett. 2015;17:4216–4219. doi: 10.1021/acs.orglett.5b01995. [DOI] [PubMed] [Google Scholar]
- 140.Singh SB, et al. Integrastatins: structure and HIV-1 integrase inhibitory activities of two novel racemic tetracyclic aromatic heterocycles produced by two fungal species. Tetrahedron Lett. 2002;43:2351–2354. doi: 10.1016/S0040-4039(02)00265-4. [DOI] [Google Scholar]
- 141.Wang Q, et al. (+)- and (–)-spiroreticulatine, a pair of unusual spiro bisheterocyclic quinoline-imidazole alkaloids from the South China sea sponge Fascaplysinopsis reticulata. Org. Lett. 2015;17:3458–3461. doi: 10.1021/acs.orglett.5b01503. [DOI] [PubMed] [Google Scholar]
- 142.Zhen B, et al. Hyperterpenoids A and B: two pairs of unprecedented 6/6/4/6/6 polycyclic cyclobutane meroterpenoids with potent neuroprotective and anti-inflammatory activities from Hypericum beanii. Chin. Chem. Lett. 2021;32:2338–2341. doi: 10.1016/j.cclet.2020.10.027. [DOI] [Google Scholar]
- 143.Yang ZN, et al. Isolation, absolute configuration, and biological activities of chebulic acid and brevifolincarboxylic acid derivatives from Euphorbia hirta. J. Nat. Prod. 2020;83:985–995. doi: 10.1021/acs.jnatprod.9b00877. [DOI] [PubMed] [Google Scholar]
- 144.Mori K, et al. Antileishmanial compounds from Cordia fragrantissima collected in Burma (Myanmar) J. Nat. Prod. 2008;71:18–21. doi: 10.1021/np070211i. [DOI] [PubMed] [Google Scholar]
- 145.Ross SA, Al-Azeib MA, Krishnaveni KS, Fronczek FR, Burandt CL. Alkamides from the leaves of Zanthoxylum syncarpum. J. Nat. Prod. 2005;68:1297–1299. doi: 10.1021/np0580558. [DOI] [PubMed] [Google Scholar]
- 146.Del Valle P, Martínez AL, Figueroa M, Raja HA, Mata R. Alkaloids from the fungus Penicillium spathulatum as α-glucosidase inhibitors. Planta Med. 2016;82:1286–1294. doi: 10.1055/s-0042-111393. [DOI] [PubMed] [Google Scholar]
- 147.Chen CM, et al. Pyrrolyl 4-quinolone alkaloids from the mangrove endophytic fungus Penicillium steckii SCSIO 41025: chiral resolution, configurational assignment, and enzyme inhibitory activities. Phytochemistry. 2021;186:112730. doi: 10.1016/j.phytochem.2021.112730. [DOI] [PubMed] [Google Scholar]
- 148.Shen X, Ting CP, Xu G, Maimone TJ. Programmable meroterpene synthesis. Nat. Commun. 2020;11:508. doi: 10.1038/s41467-020-14354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Qi C, et al. New 3,5-dimethylorsellinic acid-based meroterpenoids with BACE1 and AchE inhibitory activities from Aspergillus terreus. Org. Biomol. Chem. 2018;16:9046–9052. doi: 10.1039/C8OB02741B. [DOI] [PubMed] [Google Scholar]
- 150.Coqueiro A, et al. Antistaphylococcal prenylated acylphoroglucinol and xanthones from Kielmeyera variabilis. J. Nat. Prod. 2016;79:470–476. doi: 10.1021/acs.jnatprod.5b00858. [DOI] [PubMed] [Google Scholar]
- 151.Gao C, et al. Investigation of antibacterial activity of aspidin BB against Propionibacterium acnes. Arch. Dermatol. Res. 2016;308:79–86. doi: 10.1007/s00403-015-1603-x. [DOI] [PubMed] [Google Scholar]
- 152.Korman, T. P., Ames, B. & Tsai, S. S. in Polyketides (eds Rimando, A. M. & Baerson, S. R.) 167–184 (American Chemical Society, 2007).
- 153.Tantapakul C, et al. New benzophenones and xanthones from Cratoxylum sumatranum ssp. neriifolium and their antibacterial and antioxidant activities. J. Agric. Food Chem. 2016;64:8755–8762. doi: 10.1021/acs.jafc.6b03643. [DOI] [PubMed] [Google Scholar]
- 154.Varró G, et al. (±)-Trans-dihydronarciclasine and (±)-trans-dihydrolycoricidine analogues modified in their ring A: evaluation of their anticancer activity and a SAR study. Eur. J. Med. Chem. 2019;173:76–89. doi: 10.1016/j.ejmech.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 155.Tillequin F. Sarcomelicope alkaloids as leads for the discovery of new antitumor acronycine derivatives. Phytochem. Rev. 2002;1:355–368. doi: 10.1023/A:1026076825006. [DOI] [Google Scholar]
- 156.Odi R, et al. Synthesis and enantioselective pharmacokinetic/ pharmacodynamic analysis of new CNS-active sulfamoylphenyl carbamate derivatives. Int. J. Mol. Sci. 2021;22:3361. doi: 10.3390/ijms22073361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Davies NM, Wei Teng X. Importance of chirality in drug therapy and pharmacy practice: implications for psychiatry. Adv. Pharm. 2003;1:242–252. [Google Scholar]
- 158.D’Acquarica I, Agranat I. Chiral switches of chloroquine and hydroxychloroquine: potential drugs to treat COVID-19. Drug Discov. Today. 2020;25:1121–1123. doi: 10.1016/j.drudis.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Batista JM, et al. Absolute configuration and selective trypanocidal activity of gaudichaudianic acid enantiomers. J. Nat. Prod. 2011;74:1154–1160. doi: 10.1021/np200085h. [DOI] [PubMed] [Google Scholar]
- 160.Rao RN, Prasad KG, Priya PB, Bijarji S. HPLC-PDA-ORD bioassay of S-(+) and R-(–) clopidogrel on rat dried blood spots. Chirality. 2014;26:102–107. doi: 10.1002/chir.22271. [DOI] [PubMed] [Google Scholar]
- 161.Opekar S, et al. A chiral GC–MS method for analysis of secondary amino acids after heptafluorobutyl chloroformate & methylamine derivatization. Amino Acids. 2021;53:347–358. doi: 10.1007/s00726-021-02949-1. [DOI] [PubMed] [Google Scholar]
- 162.Mifsud J, Sghendo LJ. A novel chiral GC/MS method for the analysis of fluoxetine and norfluoxetine enantiomers in biological fluids. J. Pharm. Bioallied Sci. 2012;4:236–245. doi: 10.4103/0975-7406.99065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bijvoet JM, Peerdeman AF, van Bommel AJ. Determination of the absolute configuration of optically active compounds by means of X-rays. Nature. 1951;168:271–272. doi: 10.1038/168271a0. [DOI] [Google Scholar]
- 164.Zhou M, et al. (+)-Meyeniins A–C, novel hexahydroimidazo[1,5-c]thiazole derivatives from the tubers of Lepidium meyenii: complete structural elucidation by biomimetic synthesis and racemic crystallization. J. Agric. Food Chem. 2017;65:1887–1892. doi: 10.1021/acs.jafc.6b05805. [DOI] [PubMed] [Google Scholar]
- 165.Serna AV, Kürti L, Siitonen JH. Synthesis of (±)-setigerumine I: biosynthetic origins of the elusive racemic Papaveraceae isoxazolidine. Alkaloids Angew. Chem. Int. Ed. 2021;60:27236–27240. doi: 10.1002/anie.202111049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leverrier A, et al. Kingianin A: a new natural pentacyclic compound from Endiandra kingiana. Org. Lett. 2010;12:3638–3641. doi: 10.1021/ol101427m. [DOI] [PubMed] [Google Scholar]
- 167.Ellerbrock P, Armanino N, Ilg MK, Webster R, Trauner D. An eight-step synthesis of epicolactone reveals its biosynthetic origin. Nat. Chem. 2015;7:879–882. doi: 10.1038/nchem.2336. [DOI] [PubMed] [Google Scholar]
- 168.Huang J, et al. Bioinspired total synthesis of homodimericin A. Angew. Chem. 2017;129:7998–8002. doi: 10.1002/ange.201702768. [DOI] [Google Scholar]
- 169.Strych S, et al. Biomimetic total synthesis of santalin Y. Angew. Chem. Int. Ed. 2015;54:5079–5083. doi: 10.1002/anie.201411350. [DOI] [PubMed] [Google Scholar]
- 170.Matsuo Y, Yoshida A, Saito Y, Tanaka T. Structural revision and biomimetic synthesis of goupiolone B. Angew. Chem. Int. Ed. 2017;56:11855–11859. doi: 10.1002/anie.201706532. [DOI] [PubMed] [Google Scholar]
- 171.Wu Q, et al. Complex polypropionates from a South China sea photosynthetic mollusk: isolation and biomimetic synthesis highlighting novel rearrangements. Angew. Chem. Int. Ed. 2020;59:12105–12112. doi: 10.1002/anie.202003643. [DOI] [PubMed] [Google Scholar]
- 172.Ilkei V, et al. Biomimetic synthesis and HPLC-ECD analysis of the isomers of dracocephins A and B. Beilstein J. Org. Chem. 2016;12:2523–2534. doi: 10.3762/bjoc.12.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zask A, Ellestad G. Biomimetic syntheses of racemic natural products. Chirality. 2018;30:157–164. doi: 10.1002/chir.22786. [DOI] [PubMed] [Google Scholar]
- 174.Wang KB, et al. Racemic indole alkaloids from the seeds of Peganum harmala. Fitoterapia. 2018;125:155–160. doi: 10.1016/j.fitote.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 175.Leverrier A, Awang K, Guéritte F, Litaudon M. Pentacyclic polyketides from Endiandra kingiana as inhibitors of the Bcl-xL/Bak interaction. Phytochemistry. 2011;72:1443–1452. doi: 10.1016/j.phytochem.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 176.Zhang L, Liang Y, Wei X, Cheng D. A new unusual natural pigment from Selaginella sinensis and its noticeable physicochemical properties. J. Org. Chem. 2007;72:3921–3924. doi: 10.1021/jo0701177. [DOI] [PubMed] [Google Scholar]
- 177.Hanáková Z, et al. Anti-inflammatory activity of natural geranylated flavonoids: cyclooxygenase and lipoxygenase inhibitory properties and proteomic analysis. J. Nat. Prod. 2017;80:999–1006. doi: 10.1021/acs.jnatprod.6b01011. [DOI] [PubMed] [Google Scholar]
- 178.Liu JX, Di DL, Li C, Huang XY. Regiolone from the pericarps of Juglans regia L. Acta Crystallogr. Sect. E Struct. Rep. Online. 2007;63:2713–2714. doi: 10.1107/S1600536807019976. [DOI] [Google Scholar]
- 179.Li Y, et al. Isopropylpyrone and phenylpyrones from the leaves of Hypericum monogynum. ChemistrySelect. 2020;5:2317–2321. doi: 10.1002/slct.201903815. [DOI] [Google Scholar]
- 180.Wang W, et al. 8-C N-ethyl-2-pyrrolidinone substituted flavan-3-ols as the marker compounds of Chinese dark teas formed in the post-fermentation process provide significant antioxidative activity. Food Chem. 2014;152:539–545. doi: 10.1016/j.foodchem.2013.10.117. [DOI] [PubMed] [Google Scholar]
- 181.Nawwar MAM, Buddrus J, Bauer H. Dimeric phenolic constituents from the roots of Tamarix nilotica. Phytochemistry. 1982;21:1755–1758. doi: 10.1016/S0031-9422(82)85054-1. [DOI] [Google Scholar]
- 182.Li XN, et al. Lignans with anti-HIV activity from Schisandra propinqua var. sinensis. J. Nat. Prod. 2009;72:1133–1141. doi: 10.1021/np900123z. [DOI] [PubMed] [Google Scholar]
- 183.Ke JH, et al. Benzofurans from Eupatorium chinense enhance insulin-stimulated glucose uptake in C2C12 myotubes and suppress inflammatory response in RAW264.7 macrophages. Fitoterapia. 2019;134:346–354. doi: 10.1016/j.fitote.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 184.Sugiyama Y, Hirota A. New potent DPPH radical scavengers from a marine-derived actinomycete strain USF-TC31. Biosci. Biotechnol. Biochem. 2009;73:2731–2734. doi: 10.1271/bbb.90636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.