Abstract
Purpose of review
To review the current literature on patient centered goals for the treatment of pelvic floor disorders (PFDs).
Recent findings
Patients have a poor understanding of their PFDs, regardless if they had prior PFD treatments or received counseling, emphasizing the need for improved education from healthcare providers. Understanding the patient perspective provides insights into identifying patient goals, which facilitates communication and allows for tailored counseling, management of expectations, and assessment of treatment response. Functional outcomes are consistently important to patients, often listed as their main treatment goals. The achievement of these goals is fundamental to satisfaction. IMPACT and PROMIS are examples of PCO measures that can be utilized in both research and clinical settings. Finally, telemedicine has emerged as a viable alternative to clinic visits that offers improved access to care with no increase in adverse events or dissatisfaction, in order to aid in monitoring and meeting patient treatment goals.
Summary
Patient involvement is fundamental to providing value-based care. Provider understanding of the patient perspective is necessary to guide counseling and treatment. Patient-centered goals offer a way to engage patients, facilitate communication, and improve patient satisfaction. Although there are multiple validated PCO tools, further development and research involving patient input is needed.
Keywords: Patient-centered goals, Patient reported outcomes (PROs), Patient centered care, Pelvic Floor Disorders (PFD), Value-based care
Introduction
Pelvic floor disorders (PFDs) are highly prevalent conditions that affect approximately 25% of women in the USA [1]. However, with the aging US population, it is projected that the prevalence of PFDs will drastically increase by 2050, with 43.8 million women suffering from at least one PFD [2]. PFDs primarily include urinary incontinence (UI), fecal incontinence (FI) and pelvic organ prolapse (POP). Though these disorders are not life-threatening, they significantly impact the lives of women suffering with PFDs, leading to physical and emotional distress, decreased quality of life, and high economic costs for both the individual and the health care system [2–4].
There is an increasing emphasis on providing value-based healthcare, defined as improvement in patient-centered outcomes (PCOs) at lower healthcare costs [5]. PCOs take into account patient preferences and perspective by allowing them to directly comment on their health status without interpretation of the response by a clinician or others in healthcare [6]. Multiple studies have shown a large discrepancy between what providers and patients deem important [7, 8, 9•], so understanding the patient perspective is critical to the assessment of outcomes among women with PFDs, with PCOs referred to as the fourth dimension of PFD assessment in conjunction with physical findings, symptoms, and impact on quality of life [10–12].
One of the initial assessments when evaluating women with PFDs is the degree of patient bother, and often, this measure is used to determine treatment response. It is logical that the patient’s perception of symptoms and treatment should be used to measure clinical success rather than solely objective data. For patients, perceptions of their PFDs and expectations for treatment are intimately related to their individual goals [13]. In turn, goal attainment is linked with satisfaction, treatment preparedness, and improved quality of life, and correlates with an increased likelihood of continuing treatment [14•, 15]. Hence, establishing patient-centered goals prior to treatment may improve patient satisfaction. Additionally, these same goals can be used to assess treatment response [16•]. Qualitative studies on PFDs have provided further insight into patients’ perception; again, they highlighted the discordance between patient and physician perspectives and identified areas for improvement, such as patient education, counseling, and expectation setting [9•, 17, 18•].
Numerous tools for capturing PCOs have been developed, primarily in the form of questionnaires and surveys. However, these are most often utilized as outcome measures in research and less frequently to guide clinical care [12]. The Pelvic Floor Disorders Consortium, a multidisciplinary group involving providers from colorectal surgery, urogynecology, urology, gynecology, gastroenterology, physical therapy and other advanced practice providers, reviewed the available validated instruments and generated a list of the most accurate and practical tools to use for each condition. The final recommended validated instrument, IMPACT (Initial Measurement of Patient Reported Pelvic Floor Complaints Tool), can be used in any clinical setting, regardless of which specialist saw the patient first [19••].
In this review, the current evidence-based data regarding patient centered outcomes for the treatment of PFDs will be presented and discussed.
Pelvic Floor Disorders — Patient Perspectives
It is crucial for providers to understand a patient’s perspective on their PFDs, and it may highlight areas where patients can benefit from further education and counseling. This understanding also facilitates directed and meaningful conversations regarding expectations and goals for treatment, and serves as a measurement of treatment response. Recent studies provide further—and at times surprising—insight into patient PFD experiences and comprehension.
A main barrier to seeking help is the perception that PFDs are a normal part of aging and that there are no effective treatments [16•,20]. Even when recognized as a problem, many women do not view PFDs as chronic conditions that require long-term management [9•]. For example, patient baseline understanding of UI and POP, as measured by the Prolapse and Incontinence Knowledge Questionnaire (PIKQ), is low, with less than 50% of participants achieving a “passing” score (at least 75% of the questions correctly). There was no difference in understanding by age or history of prior treatment for PFD. Additionally, 23–38% of participant responded incorrectly or with “I don’t know” regarding if UI or POP were treatable conditions [21•]. Current knowledge surveys are limited, but this misconception is likely even higher in women who do not seek care. Compounding this lack of patient understanding, qualitative research by Wieslander et al. found that miscommunication and misunderstanding are significant during the patient-physician interaction in both English and Spanish–speaking women with POP [17].
PFDs have negative impacts on patient quality of life and are associated with depression and anxiety, but may not be well correlated with objective anatomical findings [16•]. The International Urogynecological Association (IUGA) sponsored an International Urogynecology Consultation that reviewed and summarized the available literature on patient perspectives on POP worldwide and found that prolapse-specific body image and genital image are important components of women’s emotional, physical and sexual well-being [16•]. Many women with POP report significant shame and embarrassment, both with their condition and with discussing it with others, including physicians [16•, 22]. More research is needed to understand the impact of these psychological factors on treatment outcomes [23].
Women’s perception of POP symptoms can be highly variable [16•, 24]. The most commonly reported symptom is a vaginal bulge that affects their lifestyle and emotional wellbeing and therefore, the absence of bulge symptoms after treatment has a significant correlation with patient’s perception of overall improvement, while anatomic success alone does not [25]. Hispanic and Native American women reported more bother with Stage 2 POP when compared to non-Hispanic white women. There were no differences in ethnicity/race for higher levels of prolapse, suggesting prolapse severity overcomes any ethnic differences of bother at the more advanced stages of prolapse [26]. Understanding patients’ perspective allows for better tailoring of expectations for treatment and attainment of patient-centered goals. If a patient’s goal is to not feel a bulge, she may be satisfied after surgery if she is no longer aware of the prolapse, regardless of anatomic “success”.
Therefore, patients can identify their own treatment goals and share with their provider, which can facilitate education, shared decision making, and expectations before and after treatment that are individualized for that patient.
Clinic/Non-surgical Treatments
Clinic
PFDs are intimate and sensitive conditions that are difficult for patients to discuss and are associated with depression and anxiety, as noted above. The CAFÉ study by Pham et al. sought to understand the anxiety patients with PFD experienced about their initial clinic visit. They found that anxiety scores did not correlate with PFD distress, or general anxiety disorder, as measured by a variety of condition-specific questionnaires. However, the highest levels of satisfaction after the visit were associated with the largest decrease in anxiety scores. This suggests that directly asking patients to reflect on and consider their anxieties may have a positive effect on their perceptions of anxiety after their initial visit and improve patient satisfaction [27].
Telemedicine
The coronavirus pandemic necessitated a large increase in providing remote access care with telemedicine. The obvious concern is that the quality of care and patient satisfaction would be negatively impacted by telemedicine. Tates et al. compared face-to-face with screen-to-screen consultation on patient-physician communication for POP and SUI and found that virtual visits provide similar satisfaction by building strong therapeutic relationships through education, active listening and shared decision making [28]. Schlittenhardt et al., in an observational survey study comparing patient experience and follow up rates before and after the availability of telemedicine care at a single institution, found that patients with PFD living in rural settings may be more likely to attend follow-up visits if conducted remotely, though limited internet access and technical capabilities for some elderly patients need to be considered [29].
For postoperative visits conducted using telemedicine, including PFD specific surgeries, patients reported high levels of satisfaction with no increase in adverse events (AEs), emergency room visits or primary care visits [30•, 31, 32]. However, the PHONE Study from Italy concluded that telephone follow-up is appropriate for women not reporting urinary incontinence or who had non-mesh prolapse repairs. The authors cited incorrect interpretation of de novo UUI as recurrent SUI and the inability to detect mesh exposure during telephone interviews as justification [31]. Overall, telemedicine appears to be an appropriate tool to provide value-based healthcare by increasing access to care for rural patients, decreasing economic burdens on patients, and delivering quality health care with high patient satisfaction without an increase in AEs.
Patient Handouts
Prior work on the patient perspectives on PFDs has emphasized the need for improved patient education and counseling on PFD diagnoses [9•, 17, 20, 21•]. Patient education handouts can be a useful tool to reinforce verbal counseling and provide further information on their condition. However, this is contingent upon the handouts being understandable to patients. The National Institutes of Health (NIH) and American Medical Association (AMA) recommend that the readability of a patient facing handout should be at the sixth-grade reading level or below [33, 34].
A review of available IUGA and the American Urogynecologic Society (AUGS) patient information handouts, both in English and Spanish, found that only 1 document out of 86 met all criteria for plain language best practice. The English handouts from both IUGA and AUGS exceeded the sixth-grade level, with average readability of 10.5 grade level and 9.9 grade level respectively. Spanish handouts from IUGA were on average at 5.9 grade level readability [35]. This is consistent with findings from Robb et al., who reviewed Spanish language PFD patient handouts from NIH, AUGS, the American Congress of Obstetricians and Gynecologists (ACOG), other online printable handouts, and industry-sponsored brochures for readability. Of the 40 handouts analyzed, none met the sixth-grade level criteria, including government-developed materials [36].
If handouts are not easily understandable, further confusion and miscommunication may occur, highlighting the need for improved patient-centered education materials. Given the low baseline level of patient knowledge, revising patient handouts provides a unique opportunity to involve direct patient input. Including the patient perspective in handout revisions may elicit aspects not previously identified and allow for the creation of more patient-centered education materials.
Pessary
Some PFDs, such as SUI, can be treated effectively with a pessary, improving QoL scores, sexual function and body-image [37–39]. However, women with high symptom bother are more likely to consider surgical intervention despite pessary use [40]. Consistent with other PCO research, Komesu et al. demonstrated that patient goals for pessary use are variable and subjective attainment of self-determined goals is associated with continued pessary use [15]. A systematic review and meta-analysis by Klein et al. in 2022 on the role of pessaries for SUI, did not identify any new studies since 2008, highlighting the need for more updated research [41].
Although there are studies evaluating pessary use for prolapse, few evaluate PCO. Gupta et al. compared the desire for continued pessary use in Hispanic versus non-Hispanic women 3 months following initial fitting, and found no ethnic differences in continued pessary use, despite Hispanic women reporting more pain and increased rates of bacterial vaginosis [42•]. Additionally, information regarding risk factors for pessary dislodgement can help guide expectations before pessary insertion. However, studies offer conflicting information. Coelho et al. found prior pelvic reconstructive surgery and stage 4 apical prolapse was associated with unsuccessful pessary fitting for POP [39]. This contrasts with a study by Fatakia et al., which identified previous hysterectomy, premenopausal status, and increasing genital hiatus and perineal body as predictors for pessary failure in POP [43]. These more recent studies are not consistent with prior literature that identified age, BMI, and weak pelvic floor muscles as risk factors for pessary failure [44]. For SUI, menopause, higher education, no prior UI surgery, and lower incontinence frequency were associated with success and satisfaction with pessary [45]
Pelvic Floor Physical Therapy
There are conflicting results related to additional counseling and education prior to starting pelvic floor muscle training, suggesting method of education is likely significant. Shannon et al. found that the addition of video counseling to a standard handout did not improve patients’ level of preparedness or compliance [46]. An observational study by Blanchard et al. reported significant improvement in both symptoms and QoL scores after four educational physical therapy sessions alone, with continued improvement after completing subsequent visual feedback and personalized training sessions [47]. Though this study has promising results, it is limited due to its lack of comparison to a control.
Surgery
Preoperative Counseling — Are Patients Adequately Informed?
Patient perceptions and concerns with surgery can differ greatly from what surgeons and other healthcare providers expect. Several qualitative studies exploring patient perspectives regarding surgery found that, preoperatively, patients reported a general lack of knowledge about their specific surgery and expressed the need for more information, consistent with the previously mentioned studies on the general lack of patient understanding of PFDs, further emphasizing the need for greater focus on patient education [9•, 18•].
Preoperatively, women reported complications with the surgical procedure, anesthesia, pain, and catheter issues as their greatest concerns and what they would consider AEs. Postoperatively, women listed unsuccessful surgery, continued incontinence, sexual dysfunction, and failure to achieve functional goals as severe AEs. Additionally, despite counseling, participants were not aware that they had been informed of AEs and were unsure if they had undergone an informed consent process [18•]. This aligns with findings from a prior observational study that demonstrated patient satisfaction is strongly associated with knowledge about the planned surgery. The odds of being satisfied increased for every 1 point increase on the Informed Consent Questionnaire (ICQ-20 score), with no association noted for baseline health literacy or anxiety [48]. This may be a practical PCO tool for providers to assess patient understanding to improve the informed consent process.
The role of peer support in preoperative preparedness was examined by Madsen et al. When compared with usual care, more women in the peer support group reported improved preparedness from baseline. However, there was no difference in the proportion of women feeling prepared for surgery between groups [49•]. While peer support may be helpful for some patients, the research supports a greater need for improving physician counseling.
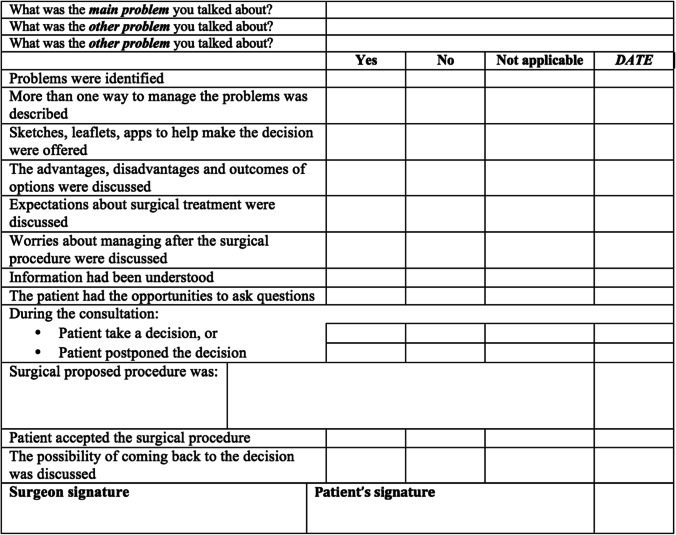
Balzarro et al. described patient centered counseling practices for PFDs. Specifically outlined tasks that surgeons should accomplish through counseling were recommended. They suggest that surgeons should (1) Define the problem, (2) Thoroughly share information about treatment options, (3) Determine whether the patient understands, (4) Explore the patient’s ideas and expectations, (5) Explore the patient’s fears and concerns, (6) Explore the patient’s motivation, (7) Explore the patient’s preferred level of involvement in decisions, and (8) Provide additional time and space, if necessary. They suggest using a counseling checklist to prioritize and complete these eight tasks (Fig. 1) with examples and strategies for each task provided [50•]. Although there may be concern related to the time-intensive process or the practicality of this approach, it is not expected that all counseling is completed in a single visit. Instead, this is viewed as an ongoing discussion with patients over multiple clinic visits, with reinforcement of prior education, assessment of patient understanding, and multiple opportunities for patients to ask questions or raise concerns. This framework could be incorporated into follow up visits to monitor progress and patient-centered goals.
Fig. 1.
Example of counseling checklist [50•]. Used with permission of Springer Nature.
Postop — Are Patient Goals Achieved?
Patients reported they would like to receive written discharge instructions prior to surgery to allow for discussion of immediate and long-term expectations for recovery [18•]. This is particularly important for patients undergoing surgery for PFDs, as patients consistently list improved functional outcomes as their main treatment goals that are fundamental to their recovery [18•,51]. When patients were asked to rank patient- and surgeon-identified AEs in ordered perceived severity, diminished function and QoL (e.g. constipation, UI, sexual dysfunction) were consistently categorized by patients as the same severity as AEs that surgeons consider very severe (e.g. ICU admission, death) [9•].
Awareness of patients’ specific goals allows the surgeon to improve their preoperative counseling and help set reasonable postoperative expectations. When absence of bulge symptoms was the primary identified patient goal, symptomatic POP recurrence after surgical treatment was associated with decreased goal attainment and satisfaction, while asymptomatic POP recurrence has no deterioration on perceived goal achievement, satisfaction, or QoL [51]. Gillingham et al. further demonstrated that goal-achieving women were more satisfied and had less regret with their surgery than goal non-achievers [14•].
PCO related to urinary incontinence after surgery may be more difficult and nuanced to achieve. For example, patients who identified “not needing pads” as a goal, still considered it not achieved even if they did not have UI postoperatively, because they still wore pads “just in case.” Women with UI also had low achievement of body-image, confidence and sexuality goals postoperatively compared to women undergoing surgery for POP, even when they had improvement in UI symptom-based goals. This persisted at two and 10 years [51].
Lack of goal attainment does not only negatively affect a patient’s perception of surgical outcomes and satisfaction, but can also cause significant personal feelings of failure and shame. Responses in a qualitative study revealed that, if surgery failed, patients felt an additional sense of failed obligation to those who cared for them during recovery, ultimately internalizing negative surgical outcomes as personal failures [9•].
Tools
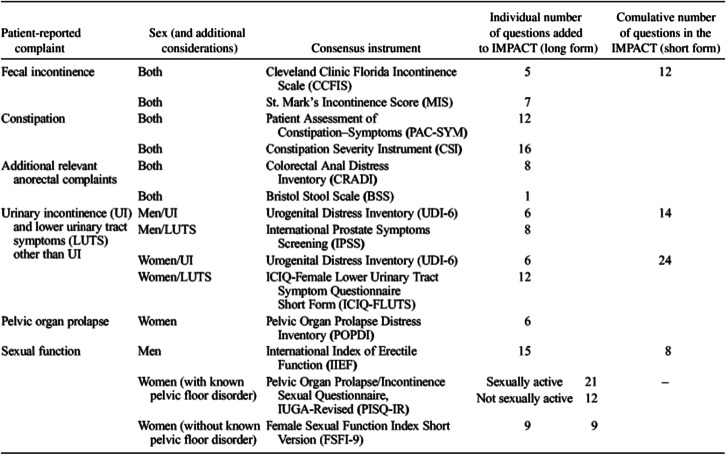
There are multiple tools to assess PCO, symptom bother and QoL. The Pelvic Floor Disorders Consortium, a multidisciplinary group of providers who treat PFDs, reviewed the available validated instruments and generated a list of the most accurate and practical tools for each condition. The final recommended validated instruments are called IMPACT (Initial Measurement of Patient Reported Pelvic Floor Complaints Tool) that can be used in any clinical setting, regardless of which specialist saw the patient initially. IMPACT recommended specific instruments that are already available for urinary incontinence, pelvic organ prolapse, bowel complaints (including fecal incontinence and constipation), disease specific quality of life, and female sexual dysfunction (Table 1) [19••].
Table 1.
Final list of instruments recommended for inclusion in the IMPACT (Initial Measurement of Patient-Reported Pelvic Floor Complaints) tool, long and short forms [19]. Used with permission of Wolters Kluwer Health, Inc.
The NIH Patient-Reported Outcomes Measurement Information System (PROMIS) was developed to establish a national resource for accurate, efficient and flexible measurement of PROs, including symptoms, functioning, and health-related quality of life (HRQL) that can be applied to a wide variety of chronic conditions. PROMIS can be used to evaluate both global and condition-specific mental, physical, and social health of adults and children. It can be used in the general population, those with chronic conditions, and in both clinical and research settings [52, 53].
Future Directions
Though the concept of value-based care and patient-centered outcomes is not novel, there are still many gaps in knowledge about patient preferences and more opportunities to incorporate direct patient input into research, tool development, and their own treatment plans. The Patient-Centered Outcomes Research Institute (PCORI) is striving to close those gaps in research by requiring investigators to engage patient and other stakeholders. Patient serve as consultants and collaborators for study design and outcomes as well as interventions in order to better capture the needs of patients. This patient engagement has resulted in more feasible and relevant studies with improved patient acceptability. The PCORI model exemplifies a patient-centered approach to research that should be use to guide future studies [54].
Many of the existing PCO instruments are not patient-centered and lacking direct input from patients. If the patient perspective is not included in the creation and implementation of tools and materials, it is impossible to know what crucial information is missing and will result in potentially less clinically meaningful outcomes. Future instruments should be developed with patient involvement from the beginning.
Patient-centered outcomes are pervasive in clinical research. However, there is limited and variable use of PCO in routine clinical practice. Existing tools can be integrated into current healthcare delivery models and used to screen, diagnose, decide treatment plans, monitor progress, and assess treatment response for patients with PFD. If questionnaires seem cumbersome, routinely asking patients to identify goals and using stated goals to track progress and measure success is a simple way to integrate PCO into practice.
An innovative way to offer patient-centered treatment is through the use of mobile applications. Though careful review and selection of apps is necessary to avoid inaccurate information [55], there is promising data that apps may improve symptom management, satisfaction, adherence, and reduce costs [56–58], while offering convenience and privacy. PCO data can also be collected through self-reporting on apps. As PFD are more prevalent in older women, there may be concerns that this group will have difficulties with using mobile technology. Lee et al. demonstrated that, although there are age-related differences in the ownership, utilization, and willingness to communicate with providers through mobile technology, the majority of women across all age groups are willing to adopt alternative mobile technology [59]. This, along with the increase in use of mobile technology during the recent pandemic, suggests that mobile apps can be used in an older patient population. Sudol et al. reviewed available mobile apps for PFD and compiled a list of 23 apps with accurate information that can be shared with patients [60].
Conclusions
Patients’ perspectives and input in their own care is of the utmost importance, especially in the field of PFDs. Research has shown that there are significant discrepancies between patient and provider regarding expectations and general understanding, highlighting the areas where improvements are needed. Having women identify goals at the beginning of their care facilitates improved communication and tailored expectations, and can be used as a tool to assess treatment response. Patients significantly value improvement in functional outcomes, often citing this as their primary treatment goal and considering failure to achieve goals as a serious adverse effect. Increasing the integration of PCO in the clinical setting can improve the care provided, placing the patient at the forefront.
Declarations
Conflict of Interest
Angela Dao and Gena Dunivan declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does contain studies with human subjects performed by of the author, Gena Dunivan, all noted with an * in the reference section.
Footnotes
This article is part of the Topical Collection on Patient Engagement, Education, and Literacy for Pelvic Floor Disorders
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Angela Dao, Email: andao@salud.unm.edu.
Gena Dunivan, Email: gcdunivan@uabmc.edu.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, Markland AD. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol. 2014;123:141–148. doi: 10.1097/AOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. women: 2010 to 2050. Obstet Gynecol. 2009;114:1278–1283. doi: 10.1097/AOG.0b013e3181c2ce96. [DOI] [PubMed] [Google Scholar]
- 3.Hu T-W, Wagner TH, Bentkover JD, LeBlanc K, Piancentini A, Stewart WF, Corey R, Zhou SZ, Hunt TL. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123–1128. doi: 10.1016/S0090-4295(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 4.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98:646–651. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell L, Papermaster AE, Halder GE, White AB, Young A, Rogers RG. Evidence-based pelvic floor disorder care pathways optimize shared decision making between patients and surgeons. Int Urogynecology J. 2022 doi: 10.1007/s00192-021-05021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11:163–169. doi: 10.1586/erp.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fayers P, Machin D. Quality of life: the assessment, analysis and interpretation of patient-reported outcomes. 2. Chichester: John Wiley & Sons; 2007. [Google Scholar]
- 8.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, Wexner SD, Bliss D, Lowry AC. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–1531. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 9.•.Dunivan GC, Sussman AL, Jelovsek JE, Sung V, Andy UU, Ballard A, Jakus-Waldman S, Amundsen CL, Chermansky CJ, Bann CM, Mazloomdoost D, Rogers RG. Gaining the patient perspective on pelvic floor disorders’ surgical adverse events. Am J Obstet Gynecol. 2019;220(185):e1–185.e10. doi: 10.1016/j.ajog.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bovbjerg VE, Trowbridge ER, Barber MD, Martirosian TE, Steers WD, Hullfish KL. Patient-centered treatment goals for pelvic floor disorders: association with quality-of-life and patient satisfaction. Am J Obstet Gynecol. 2009;200(568):e1–568.e6. doi: 10.1016/j.ajog.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Lowenstein L, FitzGerald MP, Kenton K, Dooley Y, Templehof M, Mueller ER, Brubaker L. Patient-selected goals: the fourth dimension in assessment of pelvic floor disorders. Int Urogynecology J. 2008;19:81–84. doi: 10.1007/s00192-007-0390-0. [DOI] [PubMed] [Google Scholar]
- 12.Rogers RG. Translating patient-reported outcomes to improve patient care and urogynecologic research. Int Urogynecology J. 2017;28:1765–1766. doi: 10.1007/s00192-017-3524-z. [DOI] [PubMed] [Google Scholar]
- 13.Hullfish KL, Bovbjerg VE, Gibson J, Steers WD. Patient-centered goals for pelvic floor dysfunction surgery: what is success, and is it achieved? Am J Obstet Gynecol. 2002;187:88–92. doi: 10.1067/mob.2002.124838. [DOI] [PubMed] [Google Scholar]
- 14.•.Gillingham A, Collins SA, Kenton K, Bretschneider CE, Lewicky-Gaupp C, Mueller MG, Brown O, Mou T, Geynisman-Tan J. The influence of patients’ goals on surgical satisfaction. Female Pelvic Med Reconstr Surg. 2021;27:170–174. doi: 10.1097/SPV.0000000000001028. [DOI] [PubMed] [Google Scholar]
- 15.Komesu YM, Rogers RG, Rode MA, Craig EC, Schrader RM, Gallegos KA, Villareal B. Patient-selected goal attainment for pessary wearers: what is the clinical relevance? Am J Obstet Gynecol. 2008;198(577):e1–577.e5. doi: 10.1016/j.ajog.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.•.Robinson D, Prodigalidad LT, Chan S, Serati M, Lozo S, Lowder J, Ghetti C, Hullfish K, Hagen S, Dumoulin C. International urogynaecology consultation chapter 1 committee 4: patients’ perception of disease burden of pelvic organ prolapse. Int Urogynecology J. 2022;33:189–210. doi: 10.1007/s00192-021-04997-3. [DOI] [PubMed] [Google Scholar]
- 17.Wieslander CK, Alas A, Dunivan GC, Sevilla C, Cichowski S, Maliski S, Eilber K, Rogers RG, Anger JT. Misconceptions and miscommunication among Spanish-speaking and English-speaking women with pelvic organ prolapse. Int Urogynecology J. 2015;26:597–604. doi: 10.1007/s00192-014-2562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.•.Dunivan GC, McGuire BL, RishelBrakey HA, Komesu YM, Rogers RG, Sussman AL. A longitudinal qualitative evaluation of patient perspectives of adverse events after pelvic reconstructive surgery. Int Urogynecology J. 2019;30:2023–2028. doi: 10.1007/s00192-019-03998-7. [DOI] [PubMed] [Google Scholar]
- 19.••.* Bordeianou LG, Anger JT, Boutros M, Birnbaum E, Carmichael JC, Connell KA, De EJB, Mellgren A, Staller K, Vogler SA, Weinstein MM, Yafi FA, Hull TL. Members of the Pelvic Floor Disorders Consortium Working Groups on Patient-Reported Outcomes, Measuring Pelvic Floor Disorder Symptoms Using Patient-Reported Instruments: Proceedings of the Consensus Meeting of the Pelvic Floor Consortium of the American Society of Colon and Rectal Surgeons, the International Continence Society, the American Urogynecologic Society, and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction. Female Pelvic Med Reconstr Surg. 2020;26 1–15. 10.1097/SPV.0000000000000817. Article provides important information that facilitates clinical practice change with the incorporation of PCOs. [DOI] [PubMed]
- 20.Tinetti A, Weir N, Tangyotkajohn U, Jacques A, Thompson J, Briffa K. Help-seeking behaviour for pelvic floor dysfunction in women over 55: drivers and barriers. Int Urogynecology J. 2018;29:1645–1653. doi: 10.1007/s00192-018-3618-2. [DOI] [PubMed] [Google Scholar]
- 21.•.Davidson ERW, Myers EM, De La Cruz JF, Connolly A. Baseline understanding of urinary incontinence and prolapse in new urogynecology patients. Female Pelvic Med Reconstr Surg. 2019;25:67–71. doi: 10.1097/SPV.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 22.Dunivan GC, Anger JT, Alas A, Wieslander C, Sevilla C, Chu S, Maliski S, Barrera B, Eiber K, Rogers RG. Pelvic organ prolapse: a disease of silence and shame. Female Pelvic Med Reconstr Surg. 2014;20:322–327. doi: 10.1097/SPV.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Touza KK, Rand KL, Carpenter JS, Chen CX, Heit MH. A scoping study of psychosocial factors in women diagnosed with and/or treated for pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2020;26:327–348. doi: 10.1097/SPV.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 24.* Rada MP, Jones S, Falconi G, Milhem Haddad J, Betschart C, Pergialiotis V, Doumouchtsis SK. CHORUS: an international collaboration for harmonising outcomes, research and standards in urogynaecology and women’s health, a systematic review and meta-synthesis of qualitative studies on pelvic organ prolapse for the development of core outcome sets. Neurourol Urodyn. 2020;39:880–889. 10.1002/nau.24297. [DOI] [PubMed]
- 25.Barber MD, Brubaker L, Nygaard I, Wheeler TL, Schaffer J, Chen Z, Spino C. Defining success after surgery for pelvic organ prolapse. Obstet Gynecol. 2009;114:600–609. doi: 10.1097/AOG.0b013e3181b2b1ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunivan GC, Cichowski SB, Komesu YM, Fairchild PS, Anger JT, Rogers RG. Ethnicity and variations of pelvic organ prolapse bother. Int Urogynecology J. 2014;25:53–59. doi: 10.1007/s00192-013-2145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham TT, Chen YB, Adams W, Wolff B, Shannon M, Mueller ER. Characterizing anxiety at the first encounter in women presenting to the clinic: the CAFÉ study. Am J Obstet Gynecol. 2019;221(509):e1–509.e7. doi: 10.1016/j.ajog.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Tates K, Antheunis ML, Kanters S, Nieboer TE, Gerritse MB. The effect of screen-to-screen versus face-to-face consultation on doctor-patient communication: an experimental study with simulated patients. J Med Internet Res. 2017;19:e8033. doi: 10.2196/jmir.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlittenhardt M, Smith SC, Ward-Smith P. Tele-continence care: a novel approach for providers. Urol Nurs. 2016;36:217–223. doi: 10.7257/1053-816X.2016.36.5.217. [DOI] [PubMed] [Google Scholar]
- 30.•.Thompson JC, Cichowski SB, Rogers RG, Qeadan F, Zambrano J, Wenzl C, Jeppson PC, Dunivan GC, Komesu YM. Outpatient visits versus telephone interviews for postoperative care: a randomized controlled trial. Int Urogynecology J. 2019;30:1639–1646. doi: 10.1007/s00192-019-03895-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balzarro M, Rubilotta E, Trabacchin N, Mancini V, Costantini E, Artibani W, Antonelli A. A prospective comparative study of the feasibility and reliability of telephone follow-up in female urology: the Patient Home Office Novel Evaluation (PHONE) study. Urology. 2020;136:82–87. doi: 10.1016/j.urology.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Grimes CL, Balk EM, Crisp CC, Antosh DD, Murphy M, Halder GE, Jeppson PC, Weber LeBrun EE, Raman S, Kim-Fine S, Iglesia C, Dieter AA, Yurteri-Kaplan L, Adam G, Meriwether KV. A guide for urogynecologic patient care utilizing telemedicine during the COVID-19 pandemic: review of existing evidence. Int Urogynecology J. 2020;31:1063–1089. doi: 10.1007/s00192-020-04314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.* Weiss BD. Health literacy: a manual for clinicians. Am Med Assoc Found American Med Assoc. 2006.
- 34.* Clear & Simple, Natl. Inst. Health NIH. (2015). https://www.nih.gov/institutes-nih/nih-office-director/office-communications-public-liaison/clear-communication/clear-simple (accessed May 20, 2022).
- 35.Haller J, Keller Z, Barr S, Hadden K, Oliphant SS. Assessing readability: are urogynecologic patient education materials at an appropriate reading level? Female Pelvic Med Reconstr Surg. 2019;25:139–144. doi: 10.1097/SPV.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 36.Robb J, Mackay A, Rondeau N, Palomino J, Mulla ZD, Montoya TI, Mallett VT. Spanish language pelvic floor disorders patient information handouts: how readable are they? Female Pelvic Med Reconstr Surg. 2019;25:72–75. doi: 10.1097/SPV.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 37.Lamers BHC, Broekman BMW, Milani AL. Pessary treatment for pelvic organ prolapse and health-related quality of life: a review. Int Urogynecology J. 2011;22:637–644. doi: 10.1007/s00192-011-1390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhn A, Bapst D, Stadlmayr W, Vits K, Mueller MD. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914–1918. doi: 10.1016/j.fertnstert.2008.02.142. [DOI] [PubMed] [Google Scholar]
- 39.Coelho SCA, Giraldo PC, de Castro EB, Brito LGO, Juliato CRT. Risk factors for dislodgment of vaginal pessaries in women with pelvic organ prolapse: a cohort study. Female Pelvic Med Reconstr Surg. 2021;27:e247. doi: 10.1097/SPV.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 40.Tenfelde S, Tell D, Thomas TN, Kenton K. Quality of life in women who use pessaries for longer than 12 months. Female Pelvic Med Reconstr Surg. 2015;21:146–149. doi: 10.1097/SPV.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 41.Klein J, Stoddard M, Rardin C, Menefee S, Sedrakyan A, Sansone S, Chughtai B. The role of pessaries in the treatment of women with stress urinary incontinence: a systematic review and meta-analysis. Female Pelvic Med Reconstr Surg. 2022 doi: 10.1097/SPV.0000000000001180.10.1097/SPV.0000000000001180. [DOI] [PubMed] [Google Scholar]
- 42.•.Gupta A, Cox C, Dunivan GC, Gaskins JT, Rogers RG, Iglesia CB, Meriwether KV. Desire for Continued pessary use among women of Hispanic and non-Hispanic ethnic backgrounds for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2019;25:172–177. doi: 10.1097/SPV.0000000000000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.TurelFatakia F, Pixton S, Caudwell Hall J, Dietz HP. Predictors of successful ring pessary use in women with pelvic organ prolapse. Aust N Z J Obstet Gynaecol. 2020;60:579–584. doi: 10.1111/ajo.13152. [DOI] [PubMed] [Google Scholar]
- 44.Panman CMCR, Wiegersma M, Kollen BJ, Burger H, Berger MY, Dekker JH. Predictors of unsuccessful pessary fitting in women with prolapse: a cross-sectional study in general practice. Int Urogynecology J. 2017;28:307–313. doi: 10.1007/s00192-016-3107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaffer J, Nager CW, Xiang F, Borello-France D, Bradley CS, Wu JM, Mueller E, Norton P, Paraiso MFR, Zyczynski H, Richter HE. Predictors of success and satisfaction of nonsurgical therapy for stress urinary incontinence. Obstet Gynecol. 2012;120:91–97. doi: 10.1097/AOG.0b013e31825a6de7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shannon MB, Adams W, Fitzgerald CM, Mueller ER, Brubaker L, Brincat C. Does patient education augment pelvic floor physical therapy preparedness and attendance? A randomized controlled trial. Female Pelvic Med Reconstr Surg. 2018;24:155–160. doi: 10.1097/SPV.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 47.Blanchard V, Nyangoh-Timoh K, Fritel X, Fauconnier A, Pizzoferrato A-C. Importance of a pelvic floor lifestyle program in women with pelvic floor dysfunctions: a pilot study. J Gynecol Obstet Hum Reprod. 2021;50:102032. doi: 10.1016/j.jogoh.2020.102032. [DOI] [PubMed] [Google Scholar]
- 48.Hallock JL, Rios R, Handa VL. Patient satisfaction and informed consent for surgery. Am J Obstet Gynecol. 2017;217(181):e1–181.e7. doi: 10.1016/j.ajog.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 49.•.Madsen AM, Rogers RG, Dunivan GC, Parrillo AM, Raker CA, Sung VW. Perioperative peer support and surgical preparedness in women undergoing reconstructive pelvic surgery. Int Urogynecology J. 2020;31:1123–1132. doi: 10.1007/s00192-019-04105-6. [DOI] [PubMed] [Google Scholar]
- 50.•.Balzarro M, Rubilotta E, Goss C, Costantini E, Artibani W, Sand P. Counseling in urogynecology: a difficult task, or simply good surgeon–patient communication? Int Urogynecology J. 2018;29:943–948. doi: 10.1007/s00192-018-3673-8. [DOI] [PubMed] [Google Scholar]
- 51.Srikrishna S, Robinson D, Cardozo L, Thiagamoorthy G. Patient and surgeon goal achievement 10 years following surgery for pelvic organ prolapse and urinary incontinence. Int Urogynecology J. 2015;26:1679–1686. doi: 10.1007/s00192-015-2760-3. [DOI] [PubMed] [Google Scholar]
- 52.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M. The Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.* Forsythe LP, Carman KL, Szydlowski V, Lauren F, Davidson L, Hickam DH, Hall C, Bhat G, Neu D, Stewart L, Jalowsky M, Aronson N, Anyanwu CU. Patient engagement in research: early findings from the Patient-Centered Outcomes Research Institute. 2019. 10.1377/hlthaff.2018.05067. [DOI] [PubMed]
- 55.Vega M, Mckay ER, Halani PK. Evaluation of mobile applications for patients with fecal incontinence using a modified APPLICATIONS scoring system. Int Urogynecology J. 2021;32:2529–2536. doi: 10.1007/s00192-021-04918-4. [DOI] [PubMed] [Google Scholar]
- 56.* Leme Nagib AB, Riccetto C, Martinho NM, Camargos Pennisi PR, Blumenberg C, Paranhos LR, Botelho S. Use of mobile apps for controlling of the urinary incontinence: a systematic review. Neurourol Urodyn. 2020;39:1036–1048. 10.1002/nau.24335. [DOI] [PubMed]
- 57.Bernard S, Boucher S, McLean L, Moffet H. Mobile technologies for the conservative self-management of urinary incontinence: a systematic scoping review. Int Urogynecology J. 2020;31:1163–1174. doi: 10.1007/s00192-019-04012-w. [DOI] [PubMed] [Google Scholar]
- 58.Whitehead L, Seaton P. The effectiveness of self-management mobile phone and tablet apps in long-term condition management: a systematic review. J Med Internet Res. 2016;18:e4883. doi: 10.2196/jmir.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee DD, Arya LA, Andy UU, Sammel MD, Harvie HS. Willingness of women with pelvic floor disorders to use mobile technology to communicate with their health care providers. Female Pelvic Med Reconstr Surg. 2019;25:134–138. doi: 10.1097/SPV.0000000000000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sudol NT, Adams-Piper E, Perry R, Lane F, Chen KT. In Search of mobile applications for patients with pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2019;25:252–256. doi: 10.1097/SPV.0000000000000527. [DOI] [PubMed] [Google Scholar]