Abstract
Importance
The phosphoinositide 3-kinase (PI3K) pathway is among the most frequently activated pathways in human cancers. As the use of PI3K inhibitors for cancer treatment grows, there is increasing need for understanding the cutaneous effects associated with these therapies.
Objective
To systematically review the published literature reporting incidence of cutaneous adverse events with PI3K inhibitors and to provide pooled incidence estimates using meta-analysis.
Data Sources
This systematic review and meta-analysis was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. The literature search concerned entries through September 2021 in the following sources: PubMed, Cochrane registry, ClinicalTrials.gov, and evidence from the NHS UK and Trip medical database. To analyze PI3K inhibitors’ cutaneous adverse events incidence, only randomized clinical trials (RCTs) were considered. The search strategy used the following keywords: (prevalence OR incidence OR epidemiology) and (phosphoinositide 3 kinase inhibitors OR PI3K inhibitors). No language restriction was applied. Analysis was conducted on July 1, 2022.
Study Selection
Studies included phase 2 and phase 3 RCTs that reported incidence of cutaneous adverse events associated with use of PI3K inhibitors.
Data Extraction and Measures
Data extracted included sex, medication name and class, sample size, rash incidence, and grade. The bias risk was assessed by the Cochrane tool for risk of bias assessment in RCTs.
Main Outcomes and Measures
The primary outcome was incidence of PI3K inhibitor cutaneous adverse events (with 95% CIs) among the overall population and among subgroups. Between-study heterogeneity was assessed using the I2 statistic.
Results
The analysis found the incidence of PI3K inhibitor cutaneous events of any grade to be 29.30% in the intervention group, translating to a pooled odds ratio (OR) for incidence of cutaneous adverse events of any grades of 2.55 (95% CI, 1.74-3.75). Incidence of severe grade (grade ≥3) of rash in the intervention group was estimated to be 6.95%, yielding a pooled Peto OR of 4.64 (95% CI, 2.70-7.97). Subgroup analyses revealed that the incidence of severe cutaneous adverse events (grade ≥3) was higher with the use of Pan-class-1 PI3K inhibitors (OR, 6.67; 95% CI, 4.28-10.38) than isoform-selective PI3K inhibitors (OR, 6.37; 95% CI, 3.25-12.48).
Conclusions and Relevance
This systematic review and meta-analysis identified an overall incidence of PI3K inhibitor cutaneous adverse events of any grade to be 29.30% with a pooled OR of 2.55; (95% CI, 1.74-3.75). These findings clarify the risk of cutaneous adverse events associated with this important class of anticancer therapies.
Key Points
Question
What is the incidence of phosphoinositide 3 kinase (PI3K) inhibitor–associated cutaneous adverse events?
Findings
In this systematic review of the overall incidence of PI3K inhibitor–associated cutaneous adverse events of any grade was found to be 29.30%, and an incidence of severe cutaneous adverse events (grade ≥3) estimated at 6.95%.
Meaning
These findings clarify the risk of cutaneous adverse events associated with this important class of anticancer therapy.
This systematic review with meta-analysis examines the incidence of phosphoinositide 3-kinase inhibitor–associated cutaneous adverse events in patients with cancer.
Introduction
The phosphoinositide 3-kinase (PI3K) signaling pathway is key in the regulation of diverse cellular processes including proliferation, adhesion, survival, and motility.1 The PI3K/Akt/mTOR signaling pathway plays a complex role in orchestrating both pro-inflammatory and anti-inflammatory pathways to maintain effective immunity while protecting host tissues, and plays a key signaling role in the regulation of the immune response.
The PI3K pathway is implicated in almost half of all malignant diseases and is among the most frequently activated pathways in human cancers.2 There are 3 classes of PI3K inhibitors based on isoforms, with class 1 isoforms expressed in human cells. This PI3K class 1 family is further subdivided into various isoforms based on the different p110 catalytic subunits, including p110-α, p110-β, which are ubiquitously expressed, and p110-γ and p110-δ, which are exclusively expressed in immune cells.3 There are 3 different groups of PI3K inhibitors based on isoform selectivity for the ATP binding site of PI3Ks and pharmacokinetic properties, including pan, isoform-selective, and dual PI3K/mTOR inhibitors. The PI3K-α/β selective inhibitors are used in treating solid cancers expressing these isoforms.4,5 The PI3K-γ and PI3K-ẟ are restricted to being found on immune cells and are targets in the treatment of hematologic malignant diseases.
There are currently 5 approved PI3K inhibitors: (1) idelalisib, a PI3K-δ inhibitor approved for treating relapsed chronic lymphocytic leukemia (CLL), follicular non-Hodgkin lymphoma (FL), and small lymphocytic lymphoma (SLL); (2) alpelisib, a PI3K-α inhibitor used in combination with fulvestrant for men and postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (ERBB2, formerly HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer; (3) copanlisib, a PI3K pan-p110 inhibitor for relapsed FL; (4) duvelisib, a dual dual PI3K-γ and PI3K-δ inhibitor for refractory or relapsed FL, CLL, and SLL; and (5) umbralisib a dual PI3Kδ and casein kinase-1ε (CK1-ε) inhibitor for FL and marginal zone lymphoma (MZL).3 Other PI3K inhibitors in trials for solid organ malignant diseases include sonolisib, a pan-PI3K inhibitor for metastatic colorectal, head and neck squamous, and non-small cell lung cancers; apitolisib, a pan-PI3K inhibitor for metastatic renal cell carcinoma; and pictilisib, buparlisib, and taselisib for breast cancer and glioblastoma multiforme. Treatment with PI-3065, a PI3K-ẟ inhibitor, has been shown to reduce tumor burden and metastasis in trials for melanoma, thymoma, lung, breast, and pancreatic cancers.6
The use of currently approved PI3K inhibitors as adjuvants in cancer is associated with several serious adverse effects. Many of these are immune-mediated, including pneumonitis, hepatotoxic effects, severe diarrhea with or without colitis, and cutaneous reactions.
The use of PI3K inhibitors has also been associated with mild-to-moderate and with severe or life-threatening (grade ≥3) cutaneous adverse events, including exfoliative dermatitis, erythematous rash, generalized rash, macular rash, maculopapular rash, pruritic rash, and exfoliative rash.7,8 Idelalisib has been reported to be associated with severe rash (grade 3 or 4) in only 2% of cases with most trial participants (10%-22%) having mild (grade <3) eruptions.9,10 Reports of idelalisib being associated with Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) exist in the literature.1,11,12 Copanlisib is associated with grades 3 and 4 cutaneous reactions in 2.8% and 0.6%, respectively, of 317 patients in 1 study.13 The US Food and Drug Administration (FDA) reports the incidence of severe grade cutaneous adverse events resulting in SJS/TEN among approved PI3K inhibitors is 3% to 4%.14 Among different trials of alpelisib for breast cancer, approximately 45% to 64% of patients reported rash anywhere on the body.15,16,17
Despite the frequently reported cutaneous events associated with PI3K inhibitors, to our knowledge, no consolidated meta-analyses have previously been performed. Therefore, this systematic review aims to synthesize evidence pertaining to the adverse cutaneous events associated with the use of PI3K inhibitors in the treatment of cancer.
Methods
This systematic review and meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines for randomized clinical trials (RCTs).18 The literature search included entries through September 2021 in the following sources: PubMed, Cochrane registry, ClinicalTrials.gov, and evidence from the NHS UK and Trip medical database. A search strategy was predefined and adapted for each using the following keywords: (prevalence OR incidence OR epidemiology) and (phosphoinositide 3-kinase inhibitor OR PI3K inhibitor). No search filters pertaining to age, language, or publication year were used at this stage and bibliographic duplicates were removed using Endnote software. Analysis was conducted on July 1, 2022.
Eligibility criteria included phase 2 and 3 RCTs reporting the efficacy of PI3K inhibitors and frequency of cutaneous adverse events among patients with malignant conditions. Two reviewers (A.J. and R.M.) worked independently, first scrutinizing titles for predefined inclusion and exclusion criteria and then full texts of eligible studies, with discrepancies resolved by discussion with a senior investigator (L.G.). They used a predetermined customized extraction form to extract publication, population under study and quantitative data characteristics including oncological disorder and treatment regimen. Differing rash grade intensity after exposure to PI3K inhibitors among patients with cancer was highlighted and graded based on Common Terminology Criteria for Adverse Events (CTCAE) version 5.019 that was adopted in 2018, whereas all included studies used version 4 (versions 4,8,20,21,22,23,24 4.02,25,26,27 4.03,7,12,17,28,29,30 and 1 did not report the version30). The skin and subcutaneous adverse events in the fifth version include more subtypes including eczema, hair color changes, hair texture changes, and subcutaneous emphysema. The Cochrane tool was used for risk of bias assessment in the RCTs.31
All data were analyzed using the Comprehensive meta-analysis software (version 3; Biostat, Inc). Quantitative data were extracted as the frequency of events and sample size in the treatment and control groups, respectively, with outcomes of interest including incidence of rashes of all grades (1-5) and severe grades (3-5). Effect sizes were calculated as ORs, with Peto ORs used to calculate outcome of incidence of severe rashes due to the sparseness of events in both groups.32
Two studies20,25 reported zero events in both arms, so a continuity correction of 0.5 was applied.33 Random effects were employed when statistical heterogeneity was significant, assessed as I2 greater than 60%, and a statistically significant Cochran’s Q.31 Analyses included sensitivity analysis; publication bias using Begg’s funnel plot and Egger’s regression statistic; and subgroup analyses to assess heterogeneity of effects due to different PI3K drugs. A random-effects model was used to combine subgroups and yield the overall effect.31
Results
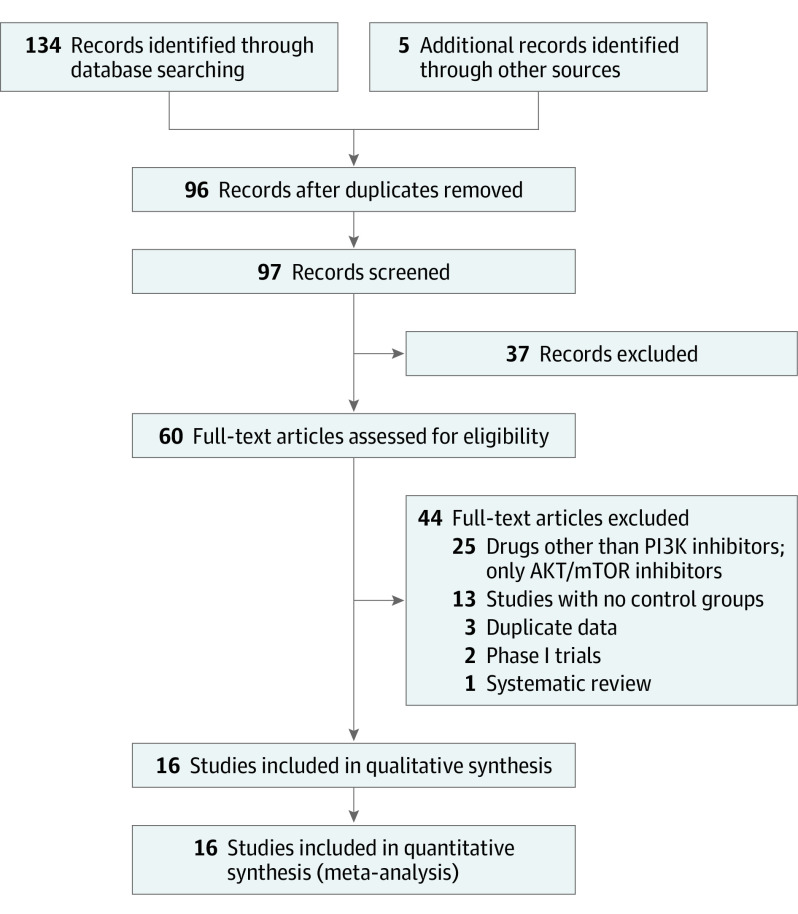
A total of 134 titles and abstracts were identified after electronic database searches, in addition to 5 records manually identified. Using Endnote, 96 nonduplicate titles and abstracts were reviewed, of which 60 full texts were deemed suitable for further scrutiny. After careful screening and manual search, we included 16 studies in quantitative and qualitative synthesis (Figure 1). Characteristics of the included trials are summarized in Table 1.
Figure 1. PRISMA Flowchart for Selection of Studies.
PI3K indicates phosphoinositide 3 kinase.
Table 1. Characteristics of Randomized Controlled Trials Included in the Meta-analysis.
| Source | Phase of trial | Condition | PI3K | Drug dosage | Dose frequency | Duration, days | Cycle | Control drug | |
|---|---|---|---|---|---|---|---|---|---|
| Inhibitor class | Inhibitor | ||||||||
| Bowles et al,26 2016 | 2 | Metastatic colorectal carcinoma | Pan | Sonolisib | 8 mg | NC | 21 | NC | Placebo |
| Jimeno et al,27 2015 | 2 | Metastatic head and neck squamous cell cancer | Pan | Sonolisib and cetuximab | 8 mg | OD | 21 | NC | Cetuximab |
| Jimeno et al,25 2015 | 2 | Metastatic head and neck squamous cell cancer | Pan | Sonolisib and docetaxel | 8 mg | QD | 2 | NC | Docetaxel |
| Krop et al,21 2016a | 2 | Breast cancer | Pan | Pictilisib | 340 mg/d | QD | 28 | 1 cycle = 28 d | Placebo |
| Krop et al,21 2016b | 2 | Breast cancer | Pan | Pictilisib | 260 mg/d | QD | 28 | NC | |
| Levy et al,20 2014 | 2 | Metastatic non–small-cell lung cancer | Pan | Sonolisib and docetaxel | 8 mg | QD | 21 | NC | Docetaxel |
| Martin et al,28 2016 | 2/3 | ERBB2-negative advanced breast cancer | Pan | Buparlisib and paclitaxel | 100 mg/d | NC | 28 | NC | Placebo and paclitaxel |
| Powles et al,8 2016a | 2 | Metastatic renal cell carcinoma | Dual PI3K/mTOR inhibitors | Apitolisib andand everolimus | NC | NC | NC | 1 cycle = 28 d | No control drug |
| Powles et al,8 2016b | 2 | Metastatic renal cell carcinoma | Dual PI3K/mTOR inhibitors | Apitolisib | 40 mg/d | QD | Treatment continued until disease progression, intolerable toxicity, elective patient withdrawal from the study, or study completion/termination | NC | No control drug |
| Saura et al,22 2019 | 2 | Early-stage breast cancer | Isoform-selective α | Taselisib | 4 mg/d 5/d on, 2/d off | QD | 16 wk | NC | Placebo and letrozole at 2 · 5 mg |
| Vuylsteke et al,23 2016 | 2 | Locally recurrent, or metastatic breast cancer | Pan | Pictilisib and paclitaxel | 260 mg/d daily on days 1-5 every week | QD | Treatment was received until progression of disease | NC | Paclitaxel and placebo |
| Soulières et al,29 2017 | 2 | Platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck | Pan | Buparlisib and paclitaxel | 100 mg/d | QD | 28 | 1 cycle = 28 d | Placebo and paclitaxel |
| Dent et al,24 2021 | 3 | Advanced breast cancer | Isoform-selective α | Taselisib and fulvestrant | 4 mg/d | QD | until progressive disease or unacceptable toxic effects | NC | Placebo and fulvestrant |
| Baselga et al,7 2017 | 3 | ERBB2-negative, advanced breast cancer | Pan | Buparlisib and fulvestrant | 100 mg/d starting on day 15 of cycle 1 | QD | NC | 1cycle = 28 d | Placebo and fulvestrant |
| Furman et al,12 2014 | 3 | Chronic lymphocytic leukemia | Isoform-selective δ | Idelalisib and rituximab | 150 mg/d | BID | NC | NC | Placebo and rituximab |
| Loibl et al,30 2017 | 2 | ERBB2-positive primary breast cancer | Pan | Buparlisib and trastuzumab & paclitaxel | 100 mg/d For the first 6 wk and reduced to 80 mg/d when administered along with paclitaxel for 12 wk | QD | 12 wk | NC | Placebo and trastuzumab and paclitaxel |
| Mayer et al,17 2019 | 2 | Receptor 2-negative breast cancer | Isoform-selective α | Alpelisib and letrazole | 300 mg/d | QD | 24 wk | NC | Placebo and letrazole |
| Zelenetz et al, 201744 | 3 | Chronic lymphocytic leukemia | Isoform-selective (δ) | Idelalisib, bendamustine and rituximab | 150 mg | BD | Treatment was received until progression of disease | 6 cycles | Placebo, bendamustine and rituximab |
Abbreviations: BD, twice per day; NC, data not collected or missing from original article; PI3K, phosphoinositide 3 kinase; QD, once per day.
Krop et al21 also included a randomization arm to apitolisib, which was terminated early due to treatment-associated toxic effects.
None of the included trials were rated as having a high risk of bias across random sequence generation, allocation concealment, blinding procedures, selective reporting, and attrition bias. Unclear risk was most frequent in allocation concealment (n = 14), blinding of outcome assessors (n = 10), and random sequence generation (n = 9) (eFigure 1 in the Supplement).
For cutaneous adverse events of any grade, 15 studies with a cumulative sample size of 4200 participants. A total of 647 trial participants (29.30%) reported cutaneous adverse events of any grade in the treatment groups compared with 259 (13%) among the controls, translating to a pooled OR of 2.55 (95% CI, 1.74-3.75) (Figure 2 and Figure 3). There was substantial heterogeneity in reporting of this outcome (I2 = 75.36%, Q = 56.82). Sensitivity analyses did not reveal any changes in pooled results. There was no evidence of publication bias statistically or asymmetry of the funnel plot (Egger regression P = .34; supporting data shown in eFigure 2 in the Supplement). Subgroup analyses according to the classes of PI3K inhibitors revealed statistically nonsignificant differences in effect sizes. However, these were inconclusive since the dual-class of PI3K inhibitors were trialed in only one study.
Figure 2. Forest Plot Showing Pooled Analyses for Incidence of Any Grade (1-5) of Cutaneous Adverse Events With Use of PI3K Inhibitors.
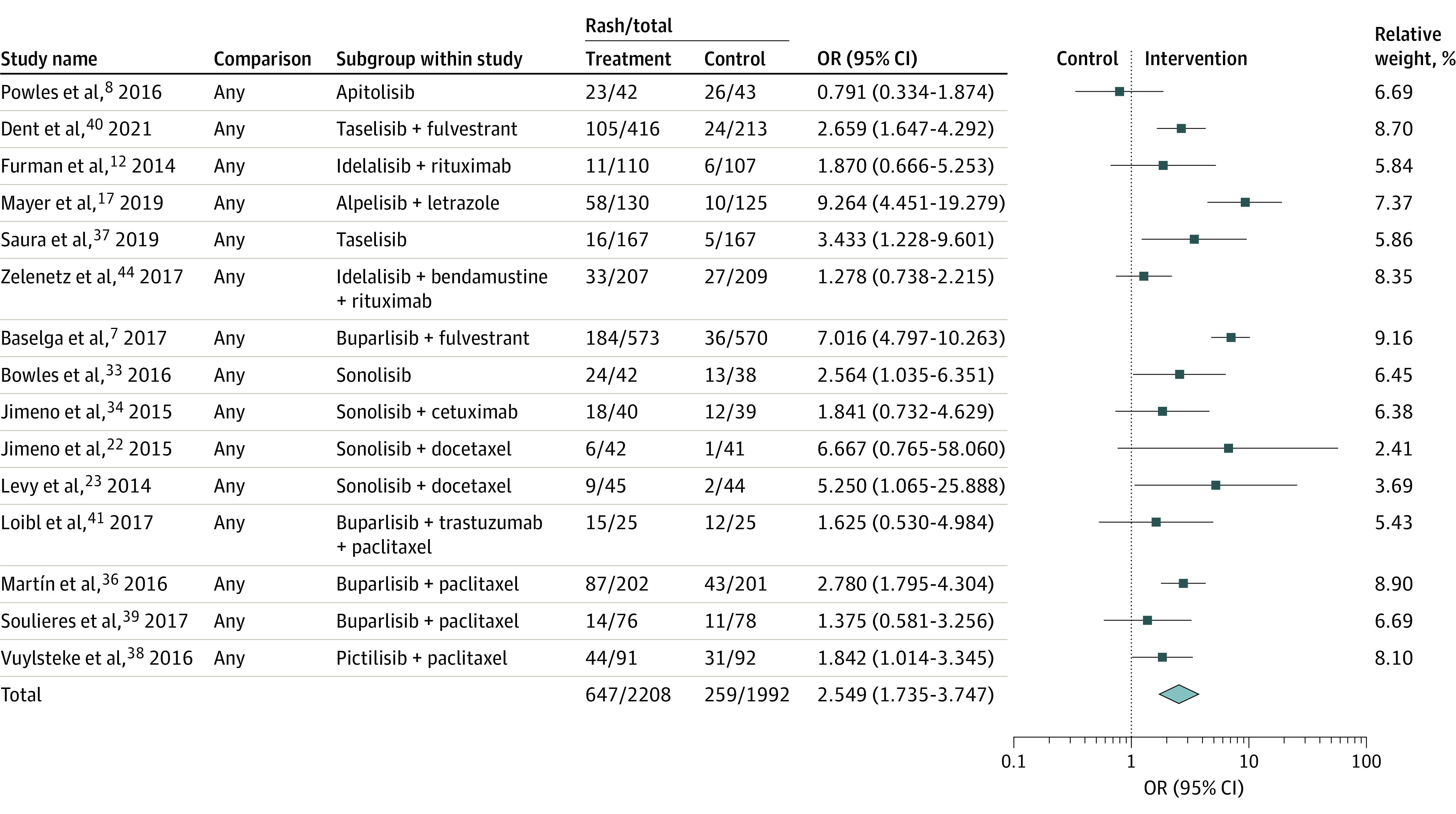
Figure 3. Forest Plot Showing Pooled Analyses for Incidence of Any Grade (1-5) of Cutaneous Adverse Events With Use of PI3K Inhibitors.
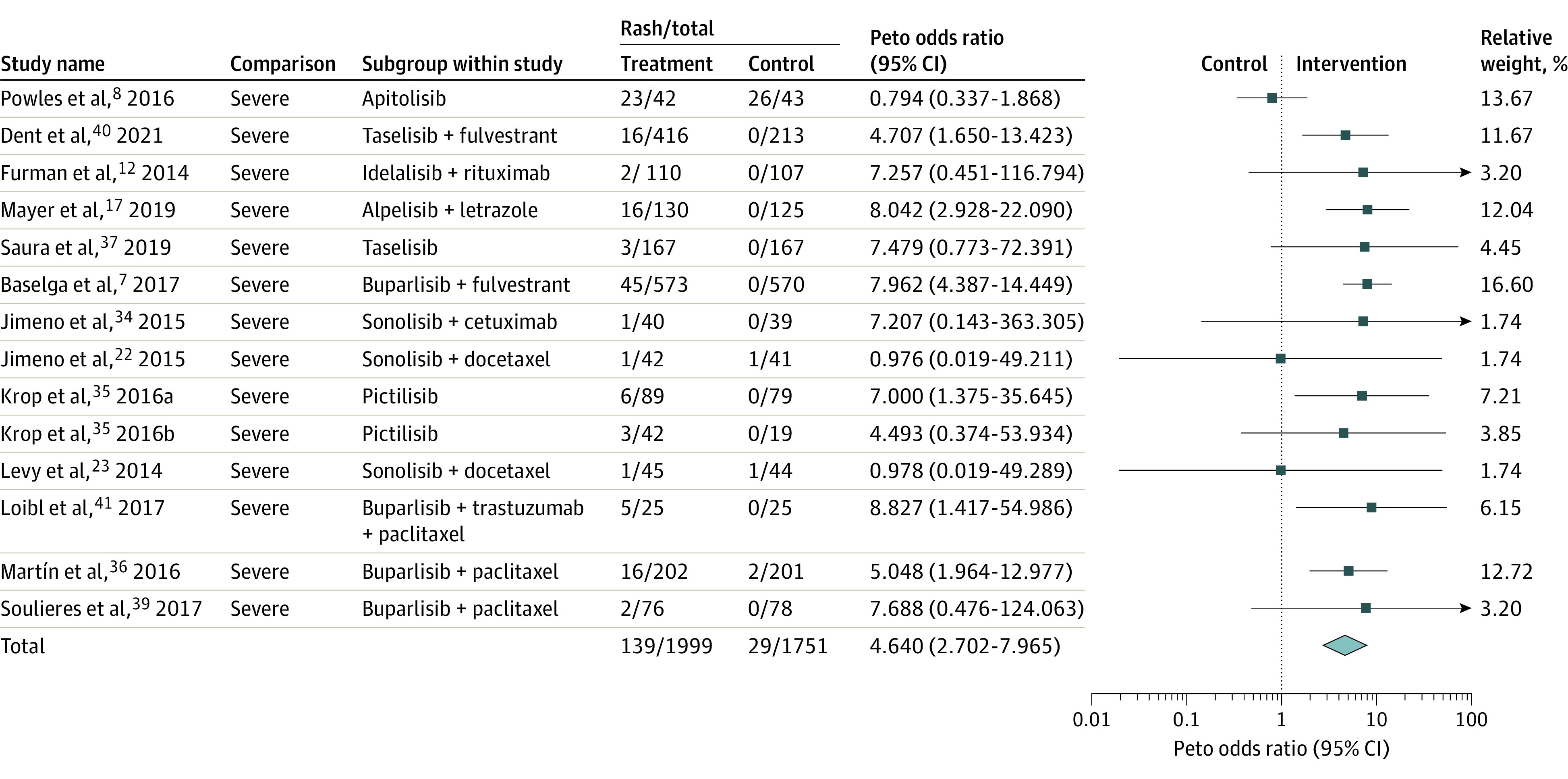
PI3K indicates phosphoinositide 3 kinase.
Incidences of severe cutaneous adverse events (grade 3 or more) were reported in 14 studies with a cumulative sample size of 3750 trial participants. There was significant statistical heterogeneity (I2 = 43.62%, Q = 23.06) in reporting of these outcomes, yielding a pooled Peto OR of 4.64 (95% CI, 2.70-7.97) with an incidence of 6.95%. Sensitivity analyses did not yield changes in the significance of pooled results after removal of studies one by one. The funnel plot was slightly asymmetric; however, Egger’s regression was nonsignificant (P = .89; supporting data shown in eFigure 3 in the Supplement). Duval and Tweedie’s trim and fill method was used to impute 2 studies to the left of the mean leading to a pooled Peto OR of 4.07 (95% CI, 2.50-6.64). Subgroup analyses (Table 2) revealed that the incidence of severe rashes was higher with the use of Pan-class (OR, 6.67; 95% CI, 4.28-10.38) than isoform-selective PI3K inhibitors (OR, 6.37; 95% CI, 3.25-12.48). However, these subgroup analyses were inconclusive. The analyses were also corroborated using full random-effects analyses which revealed comparable pooled ORs (eFigures 4 and 5 in the Supplement).
Table 2. Subgroup Analyses of Cutaneous Adverse Events Incidence as per Classes of PI3K Inhibitors.
| PI3K inhibitors group | Studies, No | OR (95% CI) | Q (df) | P valuea | I2, % |
|---|---|---|---|---|---|
| Rashes of any grade (1-5) | |||||
| Dual PI3K/mTOR inhibitors | 1 | 0.79 (0.18-3.44) | 2.62 (2) | .27 | 0 |
| Isoform selective | 5 | 2.85 (1.52-5.36) | 78.54 | ||
| Pan | 9 | 2.72 (1.65-4.5) | 70.82 | ||
| Rashes of severe grade (3-5) | |||||
| Dual PI3K/mTOR inhibitors | 1 | 0.79 (0.33-1.87) | 19.78 (2) | <.001 | 0 |
| Isoform selective | 4 | 6.37 (3.25-12.48) | 0 | ||
| Pan | 9 | 6.67 (4.28-10.38) | 0 | ||
Abbreviations: OR, odd ratio; PI3K, phosphoinositide 3 kinase.
P value corresponds to Qtotal between statistic testing the hypothesis that the effect size varies by drug class.
Discussion
The findings of this systematic review and meta-analysis elucidate the incidence of adverse cutaneous events associated with use of PI3K inhibitors. We report that PI3K inhibitors used in different types of cancers are significantly associated with a higher risk of mild-to-severe cutaneous adverse events. The higher risk of these events was reported consistently for pan-selective and isoform selective PI3K inhibitors; however, data are lacking for dual PI3K-mTOR inhibitors.
Treatment adherence among patients with cancer is essential to achieving optimum therapeutic benefit. A major challenge in using PI3K inhibitors emerges from toxic events associated with both on-target and off-target effects. The underlying pathogenesis of PI3K inhibitor eruptions are not fully understood, though they are likely multifactorial and influenced by the specific isoform targets of the PI3K inhibitor. The PI3K/Akt/mTOR pathway plays a vital role in keratinocyte differentiation and inhibition of this cycle results in modulation of growth factors leading to epidermal cellular death14; when anticancer agents target molecular pathways of cell proliferation, as with PI3K inhibitors, patients are reported with a wide range of dermatologic adverse reactions ranging from a mild maculopapular rash to life-threatening TEN.34
In terms of cutaneous morphology with PI3K inhibitors, alpelisib, PI3K-α inhibitors showed mostly a maculopapular morphology with peripheral eosinophilia, which may explain the responsiveness to prophylactic antihistamines in this cohort with an OR of 0.39 (P = .09) for rash reduction in those having grade 1 or 2.26 Further, small observational studies aimed at characterizing the rash phenotype included a case series35 of 11 patients receiving PI3K inhibitors and including 4 patients treated with a dual PI3K/mTOR inhibitor (gedatolisib 4, idelalisib 3, tenalisib 3, alpelisib), reporting eczematous morphology as the most common (25%), followed by morbilliform (17%), though only 6 of the 11 patients were evaluated by a dermatologist. Other reported clinical presentations included psoriasiform, pityriasis rubra pilaris-like, granulomatous, exfoliative dermatitis, SJS, and TEN.36 Psoriasiform eruptions were also reported in a case series29 of 4 patients receiving a PI3K inhibitor that targeted p110-δ. The PI3K inhibitors are shown to upregulate IL22 and downregulate anti-inflammatory IL10, important known cytokines in the pathogenesis of psoriasis, which may explain this phenotype.37,38
Comprehensive patient and health care professional education about possible skin toxic effects associated with PI3K inhibitors is needed. There is a paucity of literature exploring the effects of PI3K inhibitor-induced adverse events on quality of life and patient outcomes. There is significant precedent for the role any dAEs have in reducing quality of life.10 Data are lacking regarding the association of cutaneous toxic effects with other organ toxic events and the correlation with rash incidence and grade and tumor response.
A retrospective analysis of 106 patients with alpelisib-induced maculopapular (90%) and acneiform (10%) rash grades 1 and 2 were treated with topical steroids and oral antihistamines (nonsedating loratadine or cetirizine), and if itching interrupted sleep then sedating first-generation antihistamines such as hydroxyzine or diphenhydramine were added. For topical steroids, the most frequently used was clobetasol, and alternatives in certain circumstances were triamcinolone, fluocinonide, and desoximetasone. Recurrence was not observed in 15 of 16 patients who were rechallenged (12 without dose reduction, 3 with dose reduction) after the interruptions of alpelisib due to grade 3 rash.39 One experienced recurrence of skin rash at a lower grade (grade 1) but developed grade 3 mucositis. In case reports, eruptions have recurred after reinitiation.40 Other different morphologies of the rash, such as the psoriasiform or pityriasis-rubra-pilaris like, can be treated with oral retinoids such as acitretin or topical retinoids in addition to topical steroids based on a case series, though high-quality evidence for the beneficial effects of acitretin is lacking.41,42 Further studies should include phenotype-specific information about treatment and prognosis. In summary, lower-grade skin toxic effects (grades 1-2) can be managed with high-potency topical corticosteroids and oral antihistamines, whereas severe (grade ≥3) and persistent toxic effects often require systemic corticosteroids and interruption of PI3K inhibitor therapy. Notably, if therapy is interrupted, rechallenge at a lower dosage can be trialed.
The effects of skin toxic events from PI3K inhibitors can be severe enough to require pausing or terminating treatment. However, in many cases of grade 1 to 2 skin toxic effects, supportive topical anti-inflammatory treatment and oral antihistamines can be used to allow for continued PI3k therapy. Imperative to treating through skin eruptions due to PI3k inhibitors is appropriate education of patients about skin symptoms of severe drug-associated eruptions that necessitate treatment termination including blisters, mucosal lesions, and systemic symptoms associated with eruption such as fever.
It has previously been shown that early identification and prompt treatment of drug-related cutaneous adverse events improves adherence to therapy among patients with cancer and reduces interruption or termination of anticancer treatment.43 Hence, clinicians and researchers investigating novel agents like PI3K inhibitors, with significant incidence of dAES, should be cognizant of potential associated dermatologic reactions. Because low-grade rashes are more frequently observed across targeted therapies, it is vital to ensure appropriate awareness of incidence rate and grade of cutaneous adverse events to counsel patients about these possibilities and to manage them with prophylactic, supportive care and active intervention when required.
Limitations
This systematic review and meta-analysis is the result of rigorous exploration of multiple databases, which yielded a summary of cutaneous adverse effects associated with novel PI3K inhibitors while treating cancer. However, due to substantial heterogeneity in differences in the treatment arms, dosage protocols, duration of follow-up, the combination of different therapeutic protocols among the included trials posed difficult scenarios to pool into clinically meaningful estimates. Although we have explored subgroup analysis to address potential sources of bias, a high proportion of the included trials did not provide information pertaining to methods used for allocation concealment and blinding of outcome assessors. Therefore, these results should be generalized with caution. Even though the review included randomized ckinical trials, most were phase 2 trials with a limited number of participants, variable duration of intervention, heterogeneous methodology, and inconsistent outcome assessment criteria across studies.
Furthermore, it should be noted that most of the RCTs included in the review were of explanatory nature and conducted in controlled settings, often employing a narrow set of inclusion criteria. This limits the evidence presented for use in more pragmatic settings, and in broader populations where adverse cutaneous events may be more or less frequent and severe.
Our study used the CTCAE (version 5), which adds additional subtypes under the skin and subcutaneous tissue adverse events such as bullous dermatitis, acneiform, maculopapular, eczema, erythroderma and erythema multiforme, purpura, urticaria, SJS, TEN, and other adverse events that include hair, nails, sweat glands, and fat. However, this study did not extract data based on rash subtype and the grading in CTCAE version 5 is like version 4 and all studies included used CTCAE version 4.
Conclusions
To our knowledge, this is the first systematic review and meta-analysis conducted to include 16 randomized clinical trials to assess the incidence of cutaneous adverse events of PI3K inhibitors among patients with cancer. We found that most clinical trials focused on breast cancer and other malignant diseases including gastrointestinal cancer, kidney cancer, lung cancer, head and neck squamous cell cancer. The incidence in this study was 29.30% for cutaneous adverse events of any grades with a pooled OR of 2.55 (95% CI, 1.74-3.75) and 6.95%, with a pooled OR of 4.64 (95% CI, 2.70-7.97) for (grade ≥3) cutaneous adverse events among patients with cancer. However, our analyses should be interpreted with caution due to inherent heterogeneity owing to different patient populations investigated in the primary RCTs. Further studies are needed to characterize rash phenotype, to identify risk factors for high-grade rashes and to study the correlation between rash and tumor response, in addition to rash relation to treatment adherence and quality of life.
eTable 1. Risk of bias in eligible clinical trials
eFigure 1. Proportion of risk of bias in included trials
eFigure 2. Funnel plot exhibiting small study effects for studies reporting any grade of cutaneous adverse events
eFigure 3. Funnel plot exhibiting small study effects for studies reporting severe grade of cutaneous adverse events
eFigure 4. Forest plot exhibiting fully random effects subgroup analyses for incidence of any grade of cutaneous adverse events with use of PI3K inhibitors
eFigure 5. Forest plot exhibiting fully random effects subgroup analyses for incidence of severe grade of cutaneous adverse events with use of PI3K inhibitors
References
- 1.Akinleye A, Avvaru P, Furqan M, Song Y, Liu D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J Hematol Oncol. 2013;6(1):88-88. doi: 10.1186/1756-8722-6-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29(33):4452-4461. doi: 10.1200/JCO.2010.34.4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castel P, Toska E, Engelman JA, Scaltriti M. The present and future of PI3K inhibitors for cancer therapy. Nat Cancer. 2021;2(6):587-597. doi: 10.1038/s43018-021-00218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vadas O, Burke JE, Zhang X, Berndt A, Williams RL. Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci Signal. 2011;4(195):re2. doi: 10.1126/scisignal.2002165 [DOI] [PubMed] [Google Scholar]
- 5.Martini M, Ciraolo E, Gulluni F, Hirsch E. Targeting PI3K in Cancer: Any Good News? Front Oncol. 2013;3:108-108. doi: 10.3389/fonc.2013.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali K, Soond DR, Pineiro R, et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510(7505):407-411. doi: 10.1038/nature13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baselga J, Im SA, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(7):904-916. doi: 10.1016/S1470-2045(17)30376-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powles T, Lackner MR, Oudard S, et al. Randomized open-label phase II trial of apitolisib (GDC-0980), a novel inhibitor of the PI3K/mammalian target of rapamycin pathway, versus everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2016;34(14):1660-1668. doi: 10.1200/JCO.2015.64.8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang S, Wu L, Li X, Huang J, Zhong J, Chen X. Crizotinib-associated toxic epidermal necrolysis in an ALK-positive advanced NSCLC patient. Mol Clin Oncol. 2018;8(3):457-459. doi: 10.3892/mco.2018.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S-C, Lin C-C, Lai W-W, et al. Dynamic changes in quality of life after three first-line therapies for EGFR mutation-positive advanced non-small-cell lung cancer. Ther Adv Med Oncol. 2018;10:1758834018755072-1758834018755072. doi: 10.1177/1758834018755072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. NATIONAL LIBRARY OF MEDICINE . DailyMed - ZYDELIG- idelalisib tablet, film coated. Accessed March 3, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=efbdafa9-d18c-4e85-b4a2-1e620fc74e50.
- 12.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997-1007. doi: 10.1056/NEJMoa1315226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curigliano G, Shah RR. Safety and tolerability of phosphatidylinositol-3-kinase (PI3K) inhibitors in oncology. Drug Saf. 2019;42(2):247-262. doi: 10.1007/s40264-018-0778-4 [DOI] [PubMed] [Google Scholar]
- 14.Calautti E, Li J, Saoncella S, Brissette JL, Goetinck PF. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J Biol Chem. 2005;280(38):32856-32865. doi: 10.1074/jbc.M506119200 [DOI] [PubMed] [Google Scholar]
- 15.André F, Ciruelos E, Rubovszky G, et al. ; SOLAR-1 Study Group . Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929-1940. doi: 10.1056/NEJMoa1813904 [DOI] [PubMed] [Google Scholar]
- 16.Juric D, Janku F, Rodón J, et al. Alpelisib plus fulvestrant in PIK3CA-Altered and PIK3CA-wild-type estrogen receptor-positive advanced breast cancer: a phase 1b clinical trial. JAMA Oncol. 2019;5(2):e184475-e184475. doi: 10.1001/jamaoncol.2018.4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer IA, Prat A, Egle D, et al. A phase II randomized study of neoadjuvant letrozole plus alpelisib for hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer (NEO-ORB). Clin Cancer Res. 2019;25(10):2975-2987. doi: 10.1158/1078-0432.CCR-18-3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute NC. Common Terminology Criteria for Adverse Events (CTCAE). U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES; 2017. [Google Scholar]
- 20.Levy B, Spira A, Becker D, et al. A randomized, phase 2 trial of Docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic non-small-cell lung cancer. J Thorac Oncol. 2014;9(7):1031-1035. doi: 10.1097/JTO.0000000000000183 [DOI] [PubMed] [Google Scholar]
- 21.Krop IE, Mayer IA, Ganju V, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17(6):811-821. doi: 10.1016/S1470-2045(16)00106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saura C, Hlauschek D, Oliveira M, et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2019;20(9):1226-1238. doi: 10.1016/S1470-2045(19)30334-1 [DOI] [PubMed] [Google Scholar]
- 23.Vuylsteke P, Huizing M, Petrakova K, et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann Oncol. 2016;27(11):2059-2066. doi: 10.1093/annonc/mdw320 [DOI] [PubMed] [Google Scholar]
- 24.Dent S, Cortés J, Im YH, et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: the SANDPIPER trial. Ann Oncol. 2021;32(2):197-207. doi: 10.1016/j.annonc.2020.10.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimeno A, Bauman JE, Weissman C, et al. A randomized, phase 2 trial of docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Oral Oncol. 2015;51(4):383-388. doi: 10.1016/j.oraloncology.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowles DW, Kochenderfer M, Cohn A, et al. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with metastatic colorectal carcinoma. Clin Colorectal Cancer. 2016;15(4):337-344.e2. doi: 10.1016/j.clcc.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 27.Jimeno A, Shirai K, Choi M, et al. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Ann Oncol. 2015;26(3):556-561. doi: 10.1093/annonc/mdu574 [DOI] [PubMed] [Google Scholar]
- 28.Martín M, Chan A, Dirix L, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann Oncol. 2017;28(2):313-320. doi: 10.1093/annonc/mdw562 [DOI] [PubMed] [Google Scholar]
- 29.Soulières D, Faivre S, Mesía R, et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017;18(3):323-335. doi: 10.1016/S1470-2045(17)30064-5 [DOI] [PubMed] [Google Scholar]
- 30.Loibl S, de la Pena L, Nekljudova V, et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: a randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur J Cancer. 2017;85:133-145. doi: 10.1016/j.ejca.2017.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
- 32.Bradburn MJDJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26(1):53-77. doi: 10.1002/sim.2528 [DOI] [PubMed] [Google Scholar]
- 33.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351-1375. doi: 10.1002/sim.1761 [DOI] [PubMed] [Google Scholar]
- 34.Greenwell IB, Flowers CR, Blum KA, Cohen JB. Clinical use of PI3K inhibitors in B-cell lymphoid malignancies: today and tomorrow. Expert Rev Anticancer Ther. 2017;17(3):271-279. doi: 10.1080/14737140.2017.1285702 [DOI] [PubMed] [Google Scholar]
- 35.Karri PV, Freemyer BD, Pacha O, Patel AB. Characterization of cutaneous adverse events associated with PI3K inhibitors in 11 patients. J Immunother Precis Oncol. 2020;3(4):141-146. doi: 10.36401/JIPO-20-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chadha SA, Shastry J, Sunshine J, Choi J, Guggina L. Cutaneous toxicities of PI3K inhibitors: a series of two cases and review of the literature. SKIN The Journal of Cutaneous Medicine. 2020;4(6):585-590. doi: 10.25251/skin.4.6.16 [DOI] [Google Scholar]
- 37.Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine. 2012;60(1):38-42. doi: 10.1016/j.cyto.2012.06.316 [DOI] [PubMed] [Google Scholar]
- 38.Pesqué D, Sanchez-Gonzalez B, Gallardo F, Segura S, Pujol RM. Psoriasiform eruption secondary to PI3K-delta inhibitor: expanding the spectrum of psoriasiform paradoxical reactions? Acta Derm Venereol. 2021;101(3):adv00418. doi: 10.2340/00015555-3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang DG, Barrios DM, Blinder VS, et al. Dermatologic adverse events related to the PI3Kα inhibitor alpelisib (BYL719) in patients with breast cancer. Breast Cancer Res Treat. 2020;183(1):227-237. doi: 10.1007/s10549-020-05726-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ransohoff JD, Kwong BY. Cutaneous adverse events of targeted therapies for hematolymphoid malignancies. Clin Lymphoma Myeloma Leuk. 2017;17(12):834-851. doi: 10.1016/j.clml.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 41.Dewan AK, Gupta S, Bach DQ, et al. Psoriasiform eruptions secondary to phosphoinositide 3-kinase inhibition. JAAD Case Rep. 2019;5(5):401-405. doi: 10.1016/j.jdcr.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewan AK, Sowerby L, Jadeja S, et al. Pityriasis rubra pilaris-like erythroderma secondary to phosphoinositide 3-kinase inhibition. Clin Exp Dermatol. 2018;43(8):890-894. doi: 10.1111/ced.13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson KR, Chambers CR, Lam N, et al. Medication adherence among adults prescribed imatinib, dasatinib, or nilotinib for the treatment of chronic myeloid leukemia. J Oncol Pharm Pract. 2015;21(1):19-25. doi: 10.1177/1078155213520261 [DOI] [PubMed] [Google Scholar]
- 44.Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18(3):297-311. doi: 10.1016/S1470-2045(16)30671-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Risk of bias in eligible clinical trials
eFigure 1. Proportion of risk of bias in included trials
eFigure 2. Funnel plot exhibiting small study effects for studies reporting any grade of cutaneous adverse events
eFigure 3. Funnel plot exhibiting small study effects for studies reporting severe grade of cutaneous adverse events
eFigure 4. Forest plot exhibiting fully random effects subgroup analyses for incidence of any grade of cutaneous adverse events with use of PI3K inhibitors
eFigure 5. Forest plot exhibiting fully random effects subgroup analyses for incidence of severe grade of cutaneous adverse events with use of PI3K inhibitors