Abstract
The woodrats or packrats of the genus Neotoma have been the subject of a wide array of research including paleoecology, physiology, morphological evolution, systematics, speciation, and hybridization. In recent years, much work has been done to elucidate evolutionary relationships within and between closely related species of the genus; in particular the addition of newly collected specimens from critical geographic regions has provided new opportunities for taxonomic assessment. Given these new data and their potential, parsimony (PARS), maximum likelihood (ML), and Bayesian inference (BI) analyses were conducted on DNA sequences obtained from nine individual genes (four mitochondrial loci: 12S, 16S, CoII, and Cytb; five nuclear loci: AdhI2, BfibI7, En2, Mlr, and Myh6) to estimate the phylogenetic relationships among 23 species of Neotoma. Results of these analyses depicted a wide array of phylogenetic relationships among taxa; with substantial nodal support recovered in both the ML and PARS analyses at some mid-level and terminal positions. Several individual genes, particularly 12S, AdhI2, BfibI7, CoII, and Cytb, provided support at several basal positions; however, phylogenetic resolution was limited in the other genes. A final BI analysis where the nine genes were concatenated into a single data set produced several supported clades that corresponded to previously recognized species groups (floridana, micropus, mexicana, and lepida) and the subgenus Homodontomys. Levels of genetic divergence for within-species comparisons (estimated from the Cytb data set) ranged from 0.88% (N. magister) to 6.82% (N. fuscipes); for between sister species comparisons ranged from 4.68% (N. devia and N. lepida) to 12.70% (N. angustapalata and N. nelsoni); and for members within closely related clades ranged from 8.70% (N. bryanti and N. lepida) to 12.57% (N. goldmani and N. magister). Evaluations of generic, subgeneric, and species group boundaries were explored using phylogenetic principles on the DNA sequence data presented herein, as well as morphological findings from previous studies. Results obtained suggest that the most conservative taxonomic interpretation involves the abandonment of subgeneric delineations and relies on the recognition of eight species groups (cinerea, floridana, fuscipes, lepida, mexicana, micropus, phenax, and stephensi) as the backbone of the woodrat classification.
Keywords: genetic species, mitochondrial genes, Neotoma, nuclear genes, phylogenetics, systematics
Abstract
Las ratas cambalacheras del género Neotoma han sido estudiadas en varios tipos de investigaciones incluyendo paleoecología, fisiología, evolución morfológica, sistemática, especiación e hibridación. Recientemente, se han realizado numerosos estudios para elucidar las relaciones evolutivas dentro del género y entre especies cercanamente relacionadas al mismo; en particular la inclusión de nuevos especímenes provenientes de regiones geográficas críticas han brindado nuevas oportunidades para evaluaciones taxonómicas. A partir de estos nuevos datos se realizaron análisis de parsimonia (PARS), Máxima Verosimilitud (MV), e Inferencia Bayesiana (IB) en secuencias de ADN provenientes de nueve genes individuales (cuatro loci mitocondriales: 12S, 16S, CoII, y Cytb; cinco loci nucleares: Adh-I2, Bfib-I7, En2, Mlr, and Myh6) para determinar la relación filogenética de 23 especies de Neotoma. Los resultados de estos análisis presentan una amplia gama de relaciones filogenéticas entre taxa con un soporte nodal importante en los análisis de MV y PARS en algunas posiciones terminales de nivel medio. Varios genes individuales, en particular 12S, Adh-I2, Bfib-I7, CoII, and Cytb, ofrecieron soporte en varias posiciones basales; sin embargo, la resolución filogenética fue reducida en los demás genes. El último análisis de IB, en donde nueve genes se concatenaron en un solo conjunto de datos, produjo soporte en varios clados que correspondieron a especies de grupos previamente reconocidos (floridana, micropus, mexicana, y lepida) y el sub-género Homodontomys. Los niveles de divergencia genética para comparaciones intraespecíficas fluctuaron entre 0.88% (N. magister) y 6.82% (N. fuscipes); para especies hermanas (4.68%—N. devia y N. lepida hasta 12.70%—N. angustapalata y N. nelsoni); y para los miembros de clados cercanos (8.70%—N. bryanti y N. lepida hasta 12.57%—N. goldmani y N. magister). Las evaluaciones de los limites genéricos, subgenéricos y de grupos de especies fueron explorados usando principios filogenéticos en las secuencias de ADN de este trabajo, y también se basaron en las conclusiones morfológicas de estudios previos. Los resultados obtenidos sugieren que la interpretación taxonómica más conservadora incluye el abandono de las delineaciones subgenéricas y se depende en el reconocimiento de ocho grupos de especies (cinerea, floridana, fuscipes, lepida, mexicana, micropus, phenax, y stephensi) como el pilar central de la clasificación de las ratas cambalacheras.
Woodrats or packrats (genus Neotoma) are members of the New World subfamily Neotominae (Musser and Carleton 2005). Their relatively large body size (for North American rodents), propensity for constructing middens (stick houses), and tendency to collect shiny objects for decoration of middens have made woodrats one of the most easily recognizable rodents in North America. Woodrats are adapted to a variety of habitats and ecological regions and occur throughout southern Canada, most of the continental United States, Mexico, and northern portions of Central America (see Hall 1981). Twenty-three extant species currently are recognized (Musser and Carleton 2005; Patton et al. 2007; Ordóñez-Garza et al. 2014; Bradley and Mauldin 2016; Pardiñas et al. 2017) and three species are presumed to have been extirpated in recent years (Mellink 1992; Smith et al. 1993; Cortés-Calva et al. 2001).
Early alpha-level taxonomic studies (Merriam 1892, 1894; Goldman 1904, 1905, 1909, 1915, 1932; Hall and Genoways 1970; and many others) utilizing morphological data resulted in the description of several species and subspecies. Goldman (1910), in his revision of Neotoma, provided not only a comprehensive synopsis of the taxonomy and systematics for the genus, but formulated a rudimentary organization of relationships through his recognition of subgenera and species groups. Following several decades of morphometric, allozymic, and chromosomal investigations (see citations in Edwards and Bradley 2002b) the first DNA sequence-based phylogeny was developed for the genus Neotoma (Edwards and Bradley 2002b). Although the phylogenetic and taxonomic results presented in Edwards and Bradley (2002b) resulted solely from DNA sequences obtained from the mitochondrial cytochrome-b gene (Cytb) and was limited to 13 species that were available for study at that time, they were able to develop phylogenetic hypotheses concerning the relationships of species within Neotoma, determine the validity and composition of species groups, status of subgenera, and to ascertain the taxonomic status of Hodomys and Xenomys relative to Neotoma.
In an attempt to expand phylogeny reconstruction beyond mitochondrial DNA data, Longhofer and Bradley (2006) used DNA sequences obtained from intron 2 of the nuclear gene alcohol dehydrogenase (Adh1-I2) and a subset of the individuals reported in Edwards and Bradley (2002b). Their findings supported the phylogenetic relationships and topology based on examination and analysis of Cytb sequences (Edwards and Bradley 2002b). Although not a primary focus of their research, phylogenetic analysis of a multigene data set (and morphologic data) by Matocq et al. (2007) further enhanced our knowledge of the phylogenetic relationships of woodrats by: (i) expanding the data set of Edwards and Bradley (2002b) to include additional individuals per taxon, (ii) increasing the number of sequenced genes (three mitochondrial and four nuclear), (iii) expanded the taxonomic coverage by including a newly recognized taxon, N. macrotis (Matocq 2002), and (iv) adding morphologic characters associated with the male genitalia. The topology presented in Matocq et al. (2007), for the most part, was in agreement with the findings of the earlier studies by Edwards and Bradley (2002b) and Longhofer and Bradley (2006) and provided support (posterior probabilities) at additional nodes. In addition, Matocq et al. (2007) provided increased resolution for: (i) composition and interrelationships among species groups and (ii) interpretation and recognition of subgenera.
Several recent taxonomic changes warrant a reevaluation of phylogenetic relationships within Neotoma as well as a reassessment of the overall classification of Neotoma and its allies (Hodomys and Xenomys). First, Patton et al. (2007) provided a much-needed revision of the N. lepida complex. Their findings revealed that four species should be recognized (N. bryanti, N. devia, N. insularis, and N. lepida) and that N. anthonyi, N. bunkeri, and N. martinensis should be subsumed into N. bryanti. Second, Rogers et al. (2011) suggested that although N. angustapalata genetically was indistinguishable from N. leucodon, sufficient morphological differentiation existed to retain N. angustapalata as a species. Third, Edwards and Bradley (2002a) and Ordóñez-Garza et al. (2014) reevaluated the taxonomy of the N. mexicana complex and applied a senior synonym (N. ferrunginea) to woodrats in southern Mexico and portions of northern Central America; resulting in the placement of N. isthmica into synonymy with N. ferrunginea. In addition, Hernández-Canchola et al. (2021) subsumed two additional subspecies of N. mexicana (tropicalis and solitaria) into N. ferrunginea further solidifying the status of that taxon. Fourth, Fernández (2014) reeval- uated the status of N. nelsoni and suggested it was no more than a subspecies of N. leucodon. Fifth, Bradley and Mauldin (2016) recognized N. melanura as a species distinct from N. albigula. Together, these revisions indicate that 23 species reside in Neotoma (see Pardiñas et al. (2017) for a list).
Therefore, our goals were to: (i) develop a molecular phylogeny based on DNA sequences that encompasses numerous individuals per species and that includes a reasonable subsampling of the geographic distribution of each species to determine an approximation of within- and between-species genetic variation, (ii) augment the multigene data set of Matocq et al. (2007) based on recent taxonomic changes, newly available samples, and additional genetic data to examine the phylogenetic relationships within Neotoma, and (iii) produce a classification that addresses the validity of previously described species groups (Goldman 1910; Burt and Barkalow 1942; Birney 1976; Planz et al. 1996; Edwards and Bradley 2002b) and subgenera (Gray 1843; Merriam 1894, 1903; Goldman 1910; Burt and Barkalow 1942; Edwards and Bradley 2002b). To accomplish this task, DNA sequences from five mitochondrial and four nuclear genes were examined as described below.
Materials and Methods
Samples
The sampling scheme involved two stages relative to the goals outlined herein. First, DNA sequences from the entire mitochondrial cytochrome-b gene (Cytb—1,143 bp) were obtained from 691 individuals representing 21 of the 23 recognized species of Neotoma (see Appendix I) and examined to investigate intraspecific variation and to establish broad hypothesized species delineations. Second, DNA sequences were obtained from three additional mitochondrial (small subunit rRNA, 12S—531 bp; large subunit rRNA, 16S—566 bp; and cytochrome-c oxidase subunit II, CoII—633 bp) and five nuclear loci (intron 2 of alcohol dehydrogenase, Adh1-I2—577 bp; intron 7 of B-fibrinogen, BfibI7—668 bp; exon 3 of engrailed 2, En2—146 bp; exon 3 of mineralocorticoid receptor, Mlr—205 bp; and exon 35 and intron 35 of myosin heavy polypeptide 6α, Myh6—238 bp) as presented in Matocq et al. (2007) and Longhofer and Bradley (2006). For the second data set, one to two individuals per species were examined for the 21 Neotoma species. Following Edwards and Bradley (2002b), samples of Xenomys nelsoni and Hodomys alleni were included to establish a genetic benchmark for evaluating generic (Xenomys and Hodomys) and subgeneric (Homodontomys, Neotoma, Teonoma, and Teonopus) level relationships relative to Neotoma.
DNA isolation, polymerase chain reaction, and sequence methods
For most specimens, genomic DNA was isolated from approximately 0.1 g of frozen liver tissue using the DNeasy kit (Qiagen, Valencia, California). The four mitochondrial loci (12S, 16S, CoII, and Cytb) and five nuclear loci (AdhI2, BfibI7, En2, Mlr, and Myh6) were amplified using the polymerase chain reaction (PCR) method (Saiki et al. 1988) following Matocq et al. (2007) and Longhofer and Bradley (2006); specific primers and thermal profiles are listed in Table 1. PCR products were purified with a QIAquick PCR Purification Kit or QIAquick Gel Extraction Kit (Qiagen, Valencia, California). Purified products were sequenced with an ABI 3100-Avant automated sequencer and ABI Prism Big Dye version 3.1 terminator technology (Applied Biosystems, Foster City, California). Resulting sequences were aligned and checked by eye using Sequencher 4.10 software (Gene Codes, Ann Arbor, Michigan); chromatograms were examined to verify all base changes. All sequences obtained in this study were deposited in GenBank and museum specimen catalog numbers are listed in Table 2.
Table 1.
Information for specific loci examined in this study: locus being investigated, size of polymerase chain reaction (PCR) amplicons, primers used in PCR and sequencing methods, and thermal profiles for PCR reactions (all profiles included an initial 2–10 min denaturation cycle). Abbreviations for genes are as follows: small subunit rRNA, 12S; large subunit rRNA, 16S; intron 2 of alcohol dehydrogenase, Adh1-I2; intron 7 of B-fibrinogen, Bfib; cytochrome-c oxidase subunit II, CoII; cytochrome-b, Cytb; exon 3 of engrailed 2, En2; exon 3 of mineralocorticoid receptor, Mlr; and exon 35 and intron 35 of myosin heavy polypeptide 6α, Myh6. References for methods are listed in parentheses. The annealing temperature for the Adh1-I2 reaction was ramped from 53°C to 48°C to −53°C to 73°C at a rate of 0.6°C per second.
| Locus | Size | Primers | Thermal profile | |||
|---|---|---|---|---|---|---|
| Cycles | Denaturation | Annealing | Extension | |||
| 12S | 531 bp | 12Sa (Matocq et al. 2007) | 33 | 94°C, 1 min | 62°C, 1 min | 72°C, 1 min |
| 12Sb (Matocq et al. 2007) | ||||||
| 16S | 571 bp | 16Sa (Matocq et al. 2007) | 33 | 94°C, 1 min | 45°C, 1 min | 72°C, 1 min |
| 16Sb (Matocq et al. 2007) |
||||||
| Adh1-I2 | 583 bp | EXONII-F (Amman et al. 2006) | 30 | 95°C, 30 s | 53°C to 73°C* | 73°C, 4 min |
| EXONIII-R (Amman et al. 2006) | ||||||
| Bfib | 777 bp | β17-mammL (Matocq et al. 2007) | 33 | 94°C, 1 min | 60°C, 1 min | 72°C, 1 min |
| βfib-mammU (Matocq et al. 2007) | ||||||
| CoII | 633 bp | COIIa (Atkins and Honeycutt 1994) | 33 | 94°C, 1 min | 51°C, 1 min | 72°C, 1 min |
| COIIb (Atkins and Honeycutt 1994) | ||||||
| Cytb | 1,143 bp | LGL765 (Bickham et al. 1995) | 33 | 94°C, 40 s | 51°C, 45 s | 73°C, 1 min 20 s |
| LGL766 (Bickham et al. 2004) | ||||||
| 700H (Peppers and Bradley 2000) | ||||||
| 400F (Edwards et al. 2001) | ||||||
| En2 | 146 bp | EN2f (Lyons et al. 1997) | 33 | 94°C, 1 min | 57°C, 1 min | 72°C, 1 min |
| EN2r (Lyons et al. 1997) | ||||||
| Mlr | 205 bp | MLRf (Lyons et al. 1997) | 33 | 94°C, 1 min | 56°C, 1 min | 72°C, 1 min |
| MLRr (Lyons et al. 1997) | ||||||
| Myh6 | 236 bp | MYH2f (Lyons et al. 1997) | 33 | 94°C, 1 min | 62°C, 1 min | 72°C, 1 min |
| MYH2r (Lyons et al. 1997) | ||||||
Table 2.
List of museum catalog and GenBank accession numbers for taxa examined in this study. No sequence data were obtained for N. chrysomelas and N. palatina. Abbreviations for genes are as follows: small subunit rRNA, 12S; large subunit rRNA, 16S; cytochrome-c oxidase subunit II, CoII; cytochrome-b, Cytb; exon 3 of mineralocorticoid receptor, Mlr; exon 35 and intron 35 of myosin heavy polypeptide 6α, Myh6; exon 3 of engrailed 2, En2; intron 7 of B-fibrinogen, Bfib; and intron 2 of alcohol dehydrogenase, Adh1-I2. An N/A indicates that no sequence data were available. Abbreviations for museum catalog numbers follow Hafner et al. (1997) are: University of Kansas, Natural History Museum and Biodiversity Research Center (KU), Louisiana State University Museum of Natural Science (LSUMZ), Brigham Young University, Monte L. Bean Life Science Museum (BYU), University of New Mexico, Museum of Southwestern Biology (MSB), Museum of Texas Tech University (TTU), University of California-Berkeley, Museum of Vertebrate Zoology (MVZ), and University of California, Los Angeles, Dickey Collection (UCLA). A TK number (reference number used by the TTU Robert J. Baker Genetic Resources Collection) was used when museum catalog numbers were unavailable.
To expand taxonomic coverage, skin clips were obtained from N. phenax. Small pieces of skin, approximately 2 mm × 2 mm in size, were obtained from the ventral suture of museum voucher specimens. Skin clips were rinsed, and DNA extracted following protocols outlined in Fulton et al. (2012) and Campos and Gilbert (2012). PCRs for skin clips used Phire II Hot Start DNA polymerase (Finnzymes Thermo Scientific, Rockford, Illinois), and the thermal profiles listed in Table 1. PCR products were purified, sequenced, aligned, and proofed as above. All DNA extractions and PCR methods were performed in a separate laboratory from the general PCR laboratory to minimize the risk of contamination; standard positive and negative controls were included at all stages to further reduce the possibility of contamination.
Phylogenetic and genetic divergence analyses
DNA sequence data were analyzed using four approaches. First, the 691 Cytb sequences were used to construct a robust phylogeny that could be used to (i) establish a global view of nucleotide diversity within Neotoma and its allies, (ii) denote genetically divergent taxa and delineate species boundaries, and (iii) based on the rapid genetic divergence associated with this gene, serve as the primary data set for identifying nodal support for more terminal taxa. Second, each of the nine individual genes (four mitochondrial loci—12S, 16S, CoII, and Cytb; five nuclear loci—AdhI2, BfibI7, En2, Mlr, and Myh6) were analyzed independently so that the contribution of each gene could be visualized relative to the overall phylogeny. Third, the DNA sequences from the nine genes were concatenated into a single data set and analyzed jointly, with each gene analyzed under its own priors. Fourth, genetic distances were estimated for DNA sequences obtained from the Cytb gene and used to examine the magnitude of genetic divergence between taxa.
In two of the analytical approaches (nine individual genes and concatenated), sequence data were analyzed using Bayesian inference (BI; MrBayes v3.2.6; Huelsenbeck and Ronquist 2001; Ronquist et al. 2012), maximum likelihood (ML; RAxML Version 8.2.12, Stamatakis 2014), and parsimony methods (PARS, PAUP* Version 4.0a167; Swofford 2002); only the BI and ML methods were used to analyze the Cytb data set. In all analyses, Ototylomys phyllotis and Tylomys nudicaudus were used as outgroups to set character polarity.
For the BI and ML analyses, 88 likelihood models were evaluated using jModelTest-2.1.10 (Guindon and Gascuel 2003; Darriba et al. 2012) and the Akaike information criterion with a correction for finite sample sizes (AICc; Hurvich and Tsai 1989; Burnham and Anderson 2004) was used to identify the most appropriate model of evolution for each data set. The “best-fit models” predicted for each gene are shown (Table 3); however, the GTR model of evolution (and +I +G when appropriate) was used because RAxML only uses the GTR model. The most appropriate nucleotide substitution model and the following parameters were used: two independent runs with four Markov chains (one cold and three heated; MCMCMC), 106 generations, and sample frequency of every 1,000 generations from the last 9 million generated. A visual inspection of likelihood scores resulted in the first 2,000,000 trees being discarded (10% burn-in) and a consensus tree (50% majority rule) constructed from the remaining trees. Clade probability values (CPV) ≥ 0.95 were used to designate nodal support (Huelsenbeck et al. 2002) in BI analyses and bootstrap values (BS; ≥ 70) based on 1,000 iterations (Felsenstein 1985) were used to designate nodal support in the ML analyses.
Table 3.
Summary of best-fit evolutionary models as determined by the programs jModelTest-2.1.10 (Guindon and Gascuel 2003; Darriba et al. 2012) and the Akaike information criterion with a correction for finite sample sizes (AICc; Hurvich and Tsai 1989; Burnham and Anderson 2004). Columns depict: locus name for each gene examined; best-fit evolutionary model; and appropriate GTR model used in the program RAxML for maximum likelihood analysis.
| Locus | Best-fit evolutionary model | RAxML model |
|---|---|---|
| 12S | GTR+I+G | GTR+I+G |
| 16S | TIM2+I+G | GTR+I+G |
| Adh | HKY+G | GTR+G |
| Bfib | TIM2+G | GTR+G |
| CoII | TIM2+I+G | GTR+I+G |
| Cytb | GTR+I+G | GTR+I+G |
| En2 | HKY | GTR |
| Mlr | HKY+G | GTR+G |
| Myh6 | TrN+I+G | GTR+I+G |
In the parsimony analysis (PAUP* Version 4.0a167; Swofford 2002), characters were assigned equal weight and variable nucleotide positions were treated as unordered, discrete characters with four possible states; A, C, G, and T. Phylogenetically uninformative characters were removed from the analysis. The most parsimonious trees were estimated using the heuristic search and tree bisection–reconnection option. A strict consensus tree from the resulting pool of most parsimonious trees and bootstrap values (BS; ≥ 70) based on 1,000 iterations (Felsenstein 1985) were used to designate nodal support.
The Kimura 2-parameter model of evolution (Kimura 1980) was used to estimate genetic distances (Table 4). Comparisons were based on sequences generated herein, as well as sequences obtained from other studies (Edwards et al. 2001) and Edwards and Bradley (2002b), Ordóñez-Garza et al. (2014), and GenBank. These values were then used to assess levels of genetic divergence among species of Neotoma following the criteria for the Genetic Species Concept as outlined in Bradley and Baker (2001) and Baker and Bradley (2006).
Table 4.
Genetic distances, estimated from mitochondrial cytochrome-b sequences using the Kimura 2-parameter model (Kimura 1980), for sister taxa and closely related (supported) species. Within-species distance values are in parentheses.
| Pairwise comparison | Genetic distance |
|---|---|
| N. albigula (2.57%) vs. N. melanura (2.19%) | 7.51% |
| N. albigula (2.57%) vs. N. floridana (2.26%) | 9.98% |
| N. albigula (2.57%) vs. N. magister (0.88%) | 11.61% |
| N. albigula (2.57%) vs. N. goldmani (1.06%) | 10.73% |
| N. floridana (2.26%) vs. N. magister (0.88%) | 8.01% |
| N. floridana (2.26%) vs. N. melanura (2.19%) | 9.61% |
| N. floridana (2.26%) vs. N. goldmani (1.06%) | 11.06% |
| N. magister (0.88%) vs. N. melanura (2.19%) | 10.38% |
| N. magister (0.88%) vs. N. goldmani (1.06%) | 12.57% |
| N. melanura (0.88%) vs. N. goldmani (1.06%) | 10.14% |
| N. leucodon (4.20%) vs. N. nelsoni (NA) | 13.00% |
| N. leucodon (4.20%) vs. N. angustapalata (NA) | 3.78% |
| N. leucodon (4.20%) vs. N. micropus (0.83%) | 9.87% |
| N. nelsoni (NA) vs. N. angustapalata (NA) | 12.70% |
| N. nelsoni (NA) vs. N. micropus (0.83%) | 16.89% |
| N. angustapalata (NA) vs. N. micropus (0.83%) | 9.42% |
| N. ferruginea (2.21%) vs. N. picta (1.04%) | 7.84% |
| N. ferruginea (2.21%) vs. N. mexicana (2.98%) | 9.52% |
| N. picta (1.04%) vs. N. mexicana (2.98%) | 9.86% |
| N. bryanti (2.88%) vs. N. devia (2.11%) | 8.34% |
| N. bryanti (2.88%) vs. N. lepida (2.71%) | 8.70% |
| N. bryanti (2.88%) vs. N. insularis (NA) | 6.28% |
| N. devia (2.11%) vs. N. lepida (2.71%) | 4.68% |
| N. devia (2.11%) vs. N. insularis (NA) | 8.31% |
| N. lepida (2.71%) vs. N. insularis (NA) | 8.68% |
| N. fuscipes (6.82%) vs. N. macrotis (2.74%) | 8.52% |
Results
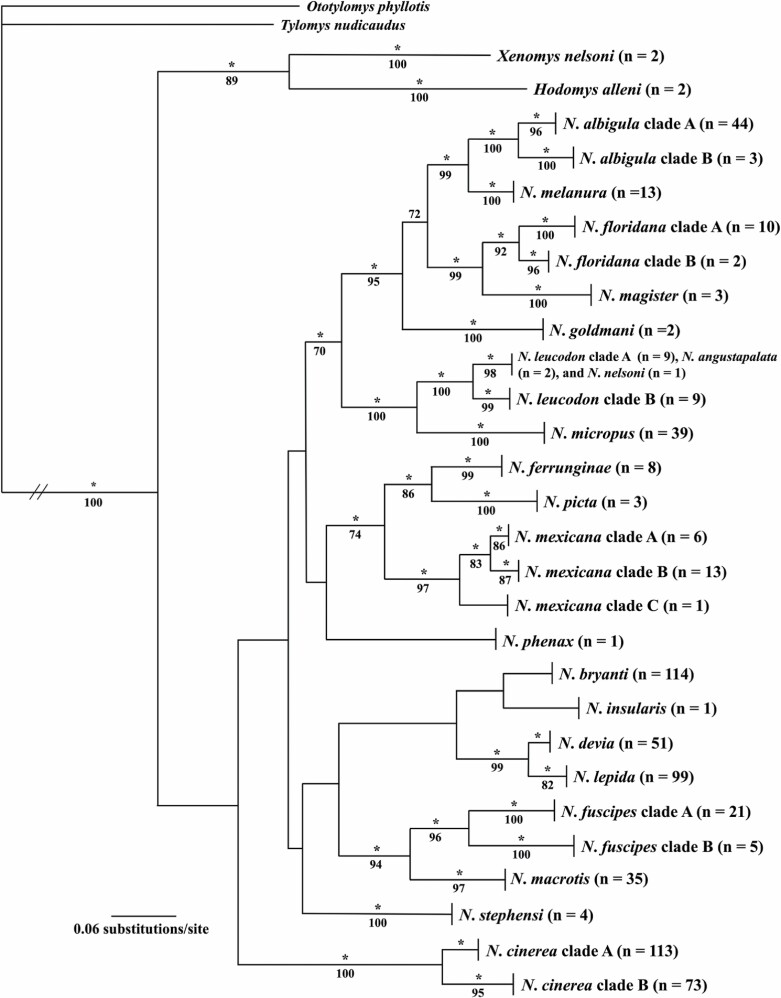
A total of 691 DNA sequences, obtained from the mitochondrial Cytb gene, were examined under ML and BI frameworks in order to ascertain the phylogenetic relationships, delineate species boundaries, and identify genetically diverse taxa (Fig. 1) between and within the 21 species of Neotoma included in this study. For all species, a monophyletic arrangement was recovered with the exceptions of a clade containing samples of N. leucodon, N. angustapalata, and N. nelsoni. In most cases, sister species and other closely related taxa exhibited substantial nodal support (CPV ≥ 0.95 and BS ≥ 70) at the terminal positions of the topology; however, little support was recovered for clades located at middle-level positions (Fig. 1) and no support was recovered for clades located at basal positions.
Fig. 1.
Phylogenetic tree generated using Bayesian methods (MrBayes; Huelsenbeck and Ronquist 2001), the GTR+I+G model of evolution, and 691 DNA sequences obtained from the mitochondrial Cytb gene for the 23 species of Neotoma and allies examined in this study. Clade probability values (≥ 0.95) are indicated by an asterisk and are depicted above branches and bootstrap support values (≥ 70) obtained from the maximum likelihood analysis (RaxML Version 8.2.12, Stamatakis 2014) of the same data set are depicted below the branches.
Results of the PARS, ML, and BI analyses of the nine individual genes (four mitochondrial loci: 12S, 16S, CoII, and Cytb; five nuclear loci: AdhI2, BfibI7, En2, Mlr, and Myh6) depicted a wide array of phylogenetic relationships among taxa. A synthesis of each analysis is provided in Fig. 2. Not only was substantial nodal support recovered in both the ML and PARS analyses at the mid-level and terminal position; several individual genes, particularly A, C–E, and I, provided support at the basal positions; however, phylogenetic resolution was limited in most genes.
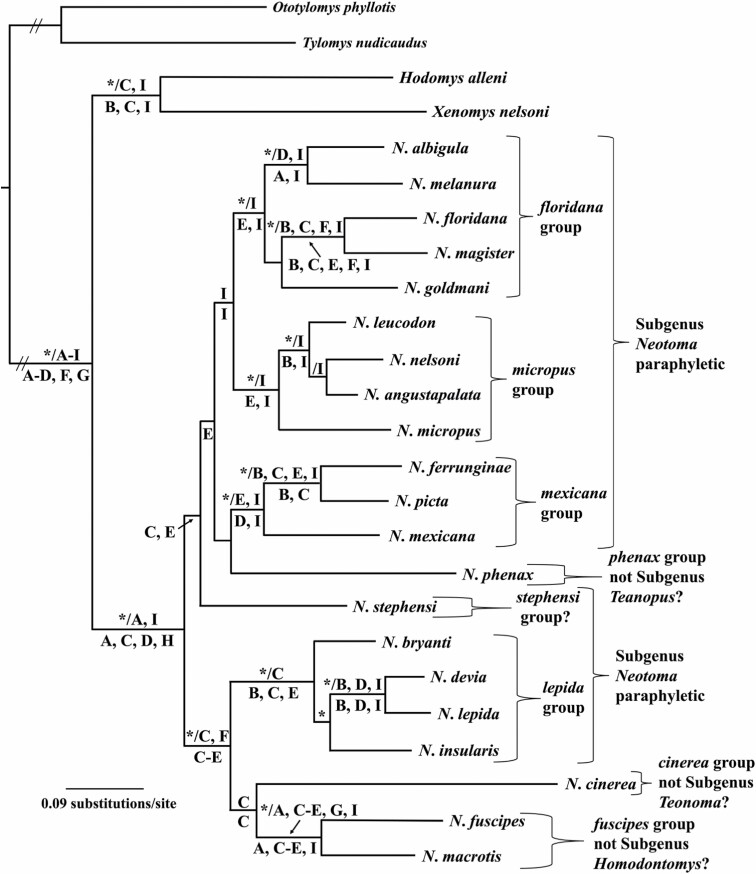
Fig. 2.
Phylogenetic tree generated using Bayesian methods (MrBayes; Huelsenbeck and Ronquist 2001), the GTR+I+G model of evolution, and a single data set represented by concatenated DNA sequences obtained from nine individual genes (four mitochondrial loci: 12S, 16S, CoII, and Cytb - denoted by A, B, E, and I, respectively; five nuclear loci: AdhI2, BfibI7, En2, Mlr, and Myh6 - denoted by C, D, F, G, and H, respectively) for the 23 species of Neotoma examined in this study. Clade probability values (≥ 0.95) are placed above branches and depicted by an asterisk (*) to the left of the slash (/). Results of the maximum likelihood (ML; RaxML Version 8.2.12, Stamatakis 2014) and parsimony analysis (PARS, PAUP* Version 4.0a167; Swofford 2002) of individual genes (A–I) were superimposed on the Bayesian inference topology. Bootstrap support values (≥ 70) obtained from the ML analysis of individual genes (A–I) were placed above branches and to the right of the slash (/). Bootstrap support values (≥ 70) obtained from the PARS analysis of individual genes (A–I) were placed below branches.
The final BI analysis was based on concatenation of the nine genes into a single data set (Fig. 2). Several supported clades (CPV ≥ 0.95) were evident and are shown by an asterisk (*) above the node and to the left of the slash (/). Most of the supported clades corresponded to previously recognized species groups (floridana, micropus, mexicana, and lepida) and the subgenus Homodontomys. In general, CPV obtained from the concatenated analysis depicted support at the terminal and basal positions of the topology; however, little support was recovered for clades located at middle-level positions (i.e., species group relationships).
Genetic distances (Kimura 2-parameter; Kimura 1980) were estimated for DNA sequences obtained from the Cytb gene (Table 4) and used to examine the magnitude of genetic divergence between sister taxa and selected members of closely related clades. Values for within-species variation ranged from 0.88% (N. magister) to 6.82% (N. fuscipes). Between sister species values ranged from 4.68% (N. devia and N. lepida) to 12.70% (N. angustapalata and N. nelsoni); whereas members within closely related clades ranged from 8.70% (N. bryanti and N. lepida) to 12.57% (N. goldmani and N. magister).
Discussion
The first goal of this study was to use DNA sequence data from the Cytb gene (obtained from 691 individuals) to determine the magnitude of genetic variation within and between species and to develop a molecular phylogeny that could serve as a test of monophyly for taxonomic boundaries (Fig. 1). Within-species variation was relatively low compared to other species of rodents with most values < 3% and ranging from 0.88% for individuals of N. magister to 6.82% for individuals of N. fuscipes (Table 4). Given that sister species values obtained herein ranged from 4.68% (N. devia and N. lepida) to 12.70% (N. angustapalata and N. nelsoni) only N. magister seemed to approach those observed for other sister species of woodrats (see Hayes and Harrison 1992; Hayes and Richmond 1993; Edwards and Bradley 2001 for a synopsis of the relationship of N. magister to N. floridana). The intraspecific genetic variation identified within N. fuscipes (6.82%) primarily was due to the two genetically divergent groups (8.81% divergent at the Cytb locus) of N. fuscipes first noted by Matocq (2002). These groups exhibit divergence on the higher end of the between-species average for rodents (see Bradley and Baker 2001; Baker and Bradley 2006). Matocq (2002) referred to these groups as the “northern” and “west central” groups and estimated they may have diverged 1.8 mya. Recent genome-wide, single-nucleotide polymorphism data coupled with demographic modeling support this estimate of the timing and magnitude of divergence between the major lineages of N. fuscipes (Boria et al. 2021), and work is ongoing to reevaluate morphological variation across the group.
The second goal of this study was to determine if the phylogenetic relationships obtained from the expanded multigene data set added resolution to previous topologies generated for Neotoma and its allies. The topology obtained from the phylogenetic analysis of the 691 Cytb sequences (Fig. 1) was similar to that obtained in the concatenated analyses (Fig. 2, ML and BI), especially for terminal and mid-level clades. In no cases were clades based solely on Cytb sequences incongruent with supported clades (CPV ≥ 0.90; BS ≥ 70) obtained from the concatenated analyses, although the concatenated analyses provided superior support for basal and some mid-level arrangements. In addition, topologies and support values obtained from the ML, BI, and PARS were similar across most nodes.
Although the three molecular-based studies by Edwards and Bradley (2002b), Longhofer and Bradley (2006), and Matocq et al. (2007) supported similar interpretations of the phylogenetic relationships of Neotoma and its allies; Matocq et al. (2007) was the most inclusive in terms of taxonomic and character coverage. Consequently, the topology presented in Matocq et al. (2007) served as the primary comparison and point of discussion for the topology obtained from the concatenated analyses (Fig. 2). Given that the current study contained additional taxa not examined by Matocq et al. (2007), there were instances where the topologies could not be compared directly. To simplify the comparison of topologies (current study and Matocq et al. 2007) and to initiate a discussion of phylogenetic relationships that was used as the basis of developing a classification (Table 5), clades were described in relation to species-group assignment (Fig. 2) following that presented by Edwards and Bradley (2002b), Matocq et al. (2007), and modified herein.
Table 5.
Classification of Neotoma and allies (following Edwards and Bradley 2002; Musser and Carleton 2005; Matocq et al 2007). Neotoma anthonyi, N. bunkeri, and N. martinensis are included as either subspecies of, or synonyms of, N. bryanti following Patton et al. (2007). Neotoma palatina and N. chrysomelas were not examined in this study; however, they were placed into the classification based on the most current synopsis of the literature. Letter representations are as follows: X = no sequences available for examination, did not evaluate, or taxon was not recognized at time of study; A = agreement of previous taxonomy with that proposed herein; D = disagreement of previous taxonomy with proposed herein; Y = agreement of previous classifications (Goldman 1910; Hall 1981; Edwards and Bradley 2002; Musser and Carleton 2005) with that proposed herein; N = disagreement of previous classification with proposed herein; and NSG = no species-group assignment. Explanations relative to disagreements in taxonomy or classification between previous and current studies are placed in parentheses.
| Proposed classification | Goldman (1910) | Hall (1981) | Edwards and Bradley (2002) | Musser and Carleton (2005) |
|---|---|---|---|---|
| Genus Hodomys | ||||
| H. alleni Merriam, 1894 | A, Y | D (N. alleni), N (subgenus Hodomys) | A, Y | A, Y |
| Genus Xenomys | ||||
| X. nelsoni Merriam, 1892 | A, Y | A, Y | A, Y | A, Y |
| Genus Neotoma | A, N (subgenus Neotoma) | A, N (subgenus Neotoma) | A, N (subgenus Neotoma) | A, N (subgenus Neotoma) |
| Species group floridana | ||||
| N. albigula Hartley, 1894 | A, N (albigula group) | A, NSG | A, Y | A, Y |
| N. floridana (Ord, 1818) | A, Y | A, NSG | A, Y | A, Y |
| N. magister Baird, 1858 | A, N (pennsylvanica group) | D (N. fl. magister), NSG | A, Y | A, Y |
| N. melanura Merriam, 1894 | D (N. a. melanura), N | D (N. a. melanura), NSG | X | D (N. a. melanura), Y (floridana group) |
| N. goldmani Merriam, 1903 | A, N (desertorum group) | A, NSG | A, Y | A, Y |
| Species group lepida | ||||
| N. bryanti Merriam, 1887 | A, N (intermedia group) | A, NSG | X, X | A, Y |
| N. devia Goldman, 1927 | X, X | D (N. l. devia), NSG | A, Y | A, Y |
| N. insularis Townsend, 1912 | X, X | D (N. l. insularis), NSG | X, X | D (N. l. insularis), Y |
| N. lepida Thomas, 1893 | A, N (desertorum group) | A, NSG | A, Y | A, Y |
| Species group mexicana | ||||
| N. chrysomelas J. A. Allen, 1908 | A, Y | A, NSG | X, X | A, Y |
| N. ferruginea Tomes, 1862 | D (N. fe. ferruginea), Y | D (N. fe. ferruginea), NSG | X, X | D (N. me. ferruginea), Y |
| N. mexicana Baird, 1855 | A, Y | A, NSG | A, Y | A, Y |
| N. picta Goldman, 1904 | D (N. fe. picta), Y | D (N. fe. picta), NSG | A, Y | D (N. me. picta), Y |
| Species group micropus | ||||
| N. angustapalata Baker, 1951 | X, X | A, NSG | X, X | A, N (mexicana group) |
| N. leucodon Merriam, 1894 | D (N. a. leucodon), N (albigula group) | D (N. a. leucodon), NSG | A, Y | A, Y |
| N. micropus Baird, 1855 | A, Y | A, NSG | A, Y | A, Y |
| N. nelsoni Goldman, 1905 | A, N (albigula group) | A, NSG | X, X | A, Y |
| N. palatina Goldman, 1905 | A, N (albigula group) | A, NSG | X, X | A, Y |
| Species group stephensi | ||||
| N. stephensi Goldman, 1905 | D (N. l. stephensi), N (desertorum group) | A, NSG | A, N (lepida group) | A, N (lepida group) |
| Species group fuscipes | ||||
| N. fuscipes Baird, 1858 | A, N (Homodontomys) | A, NSG | A, N (lepida group) | A, N (lepida group) |
| N. macrotis Thomas, 1893 | D (N. fu. macrotis), N (fuscipes group) | D, (N. fu. macrotis), NSG | X, X | A, N (lepida group) |
| Species group phenax | ||||
| N. phenax (Merriam, 1903) | D (T. phenax), N (genus Teanopus) | A, N (subgenus Teanopus) | X, X | A, N (subgenus Teanopus) |
| Species group Teonoma | ||||
| N. cinerea (Ord, 1815) | A, N (subgenus Teanoma) | A, N (subgenus Teanoma) | A, NSG | A, N (subgenus Teanoma) |
A sister relationship between Xenomys and Hodomys was recovered (to the exclusion of a monophyletic Neotoma—remaining 21 species examined); this relationship agreed with that discussed by Matocq et al. (2007). This finding supports: (i) the interpretation that Hodomys should be recognized as a separate genus (Goldman 1910; Carleton 1980; Edwards and Bradley 2002b; Musser and Carleton 2005) and not as a subgenus within Neotoma (Burt and Barkalow 1942; Hall 1981) and (ii) Neotoma is monophyletic (Edwards and Bradley 2002b; Musser and Carleton 2005).
Four well-supported clades (Fig. 2) were recovered that corresponded to the floridana (albigula, floridana, goldmani, magister, and melanura), lepida (bryanti, devia, insularis, and lepida), mexicana (ferrunginae, mexicana, and picta), and micropus (angustapalata, leucodon, micropus, and nelsoni) species groups as described in Edwards and Bradley (2002b) and Matocq et al. (2007). These species groups were identified based on support as defined by BS (> 70) and CPV (> 0.95) and are indicated by letters A–I (corresponding to the nine mitochondrial and nuclear markers, respectively) and superimposed on Fig. 2, where appropriate.
Two species (phenax and stephensi) were not associated within any species group because of lack of nodal support values (Fig. 2). However, both species were included within the traditional subgenus Neotoma (Fig. 2) as defined by data from AdhI2 and CoII markers. Three additional species (macrotis, fuscipes, and cinerea) formed a clade based on data from the AdhI2 gene. Within this clade, macrotis and fuscipes were sister taxa as defined by the 12S, AdhI2, BfibI7, CoII, Mlr, and Cytb genes; the taxonomic ramifications of this clade are discussed in the classification section.
The four mitochondrial genes (12S, 16S, CoII, and Cytb) provided support at 7, 11, 10, and 23 nodes, respectively, whereas the five nuclear markers (AdhI2, BfibI7, En2, Mlr, and Mlr) provided support at 18, 10, 5, 3, and 2 nodes, respectively. The distribution and contribution of nodal support for each marker is provided (Fig. 2).
The final goal of this study was to produce a classification for Neotoma and its allies based on DNA sequences obtained from single-gene analyses and from analyses of nine concatenated mitochondrial and nuclear genes. To accomplish this task, monophyletic groups obtained from analyses were evaluated for: (i) levels of support (BS > 70 and CPV > 0.95) and (ii) congruence to previous classifications and taxonomic arrangements. For this exercise, we used the arrangements presented in Goldman (1910), Hall (1981), Edwards and Bradley (2002b), and Musser and Carleton (2005) knowing that these studies differed in the types of data sets used and number of taxa recognized. In several cases, we drew upon information presented in more narrowly focused taxonomic studies (Patton et al. 2007; Rogers et al. 2011; Fernández 2014; Ordóñez-Garza et al. 2014; Bradley and Mauldin 2016). The proposed classification (Table 5) and below we provide a synopsis highlighting the differences and similarities between the five classification schemes.
Recognition of genera
Essentially all analyses (single or concatenated) depicted a sister relationship between Xenomys and Hodomys and all remaining ingroup taxa contained within a monophyletic Neotoma. The recognition of Xenomys as a separate genus is supported by all other classifications discussed herein. Support for the recognition of Hodomys as a separate genus is supported in all other classifications except that of Hall (1981) where Hodomys was treated as a subgenus of Neotoma and, by extension H. alleni was assigned to N. alleni. Recognition of a traditional Neotoma assemblage (Musser and Carleton 2005) was supported by the concatenated data set and five single-gene data sets (Fig. 2). Recognition of Teanopus (T. phenax, Goldman 1910) was not supported by the sequence data analyzed herein because N. phenax was included in a clade containing 13 other species of Neotoma (although this support was weak; Fig. 2). To recognize Teanopus as a genus would require a major reorganization of the genus Neotoma and given the limited data we were able to assemble for N. phenax, any restructuring at that level would be premature.
Recognition of subgenera
Historically, five subgenera (Hodomys, Homodontomys, Neotoma, Teanopus, and Teonoma) have been recognized (see Edwards and Bradley 2002b; Matocq et al. 2007; Table 5 for a discussion of the historical perspectives). In short, the subgenus Neotoma contained all recognized species except: alleni which previously was assigned to subgenus Hodomys; macrotis and fuscipes were assigned to subgenus Homodontomys; cinerea assigned to subgenus Teonoma; and phenax was assigned to subgenus Teanopus. Recognition of subgenera historically was based on sound morphological interpretations; and one could argue for the use of subgenera based on those data; however, given the lack of statistical support provided by the phylogenetic analysis of the DNA sequence data presented herein, there appears to be no justification for the continued recognition of subgenera. First, although Hodomys represents a monophyletic assemblage, it was statistically supported as sister to Xenomys and outside of Neotoma; consequently, Hodomys was so different from other species of Neotoma that we follow Goldman (1910), Edwards and Bradley (2002b), Musser and Carleton (2005), and Matocq et al. (2007) in considering it distinct at the generic level.
Second, recognition of the remaining four subgenera (Homodontomys, Neotoma, Teanopus, and Teonoma) would require several major reorganizations of the remaining 22 species. For example: (A) the subgenus Neotoma is not monophyletic because bryanti, devia, insularis, and lepida are sister to a clade containing cinerea (subgenus Teonoma), fuscipes and macrotis (subgenus Homodontomys) resulting in a need to reorganize the subgenus Neotoma by splitting it into at least two clades (one poorly supported clade representing 14 species formerly comprising the subgenus Neotoma and the second being a strongly supported but unnamed subgenus containing (bryanti, devia, insularis, and lepida)); (B) Teonoma and Homodontomys are each monophyletic and represent distinct clades that could be recognized at the subgeneric level, but doing so would require a reevaluation of many species groups within the former subgenus Neotoma which appears to have evolved prior to the divergence of the Teonoma and Homodontomys clades; (C) alternatively, given that Teonoma and Homodontomys are sister groups they could be combined into a single subgenus, with Teonoma having taxonomic priority; and (D) similarly, to recognize Teanopus as a subgenus would require a major reorganization of the subgenus Neotoma and as discussed above, is premature. Based on the data presented herein, it seems prudent to abandon the use of subgenera.
Recognition of species groups
Edwards and Bradley (2002b), Musser and Carleton (2005), Matocq et al. (2007), and Patton et al. (2007) placed most Neotoma species into four basic species groups (Table 5; floridana, lepida, mexicana, and micropus). Herein, we use information obtained from the inclusion of additional sequence data and increased taxon sampling to expand on these earlier premises, by further refining the species groups; as well as suggesting the formation of four new species groups. This refinement necessitates five taxonomic interpretations: (i) the lepida species group should be restricted to bryanti, devia, insularis, and lepida (recently extinct taxa from the Baja California region could be added following the synopsis provided by Patton et al. 2007); (ii) stephensi should be removed from the lepida species group as tentatively suggested by Edwards and Bradley (2002b) and recognized as its own species group (stephensi species group); (iii) given its level of genetic and morphologic distinction (summarized by Matocq et al. 2007) cinerea should be recognized as its own species group (cinerea species group) unless it is combined into a larger species group with fuscipes and macrotis with cinerea species group having priority; (iv) fuscipes and macrotis should be recognized in a separate species group (fuscipes species group) unless it is combined into a larger species group with cinerea as mentioned above; and (v) although little DNA sequence data were available for analyses, it appears that phenax should be recognized as its own species group (phenax species group) unless it is combined into a larger species group with members of the mexicana species group with the mexicana species group having priority. Conservatively, this interpretation results in the recognition of eight species groups (cinerea, floridana, fuscipes, lepida, mexicana, micropus, phenax, and stephensi).
At this juncture, the use of additional species groups to partition and define monophyletic groupings over the historical implementation of subgenera and a limited number of species groups seems to be a reasonable and superior approach given that: (i) six of the eight species (as defined herein) are monophyletic and are defined by statistical support; only phenax and stephensi received no support as species groups but given their exclusion from any species group predicates their independent evolutionary trajectory compared with other species groups that were defined by statistical support, and (ii) this simplifies the controversies in trying to invoke the subgenera concepts onto the phylogenetic results.
Recognition of species
Description of woodrat species has taken place over two major time intervals, loosely defined here as the morphology/natural history era (circa pre-1970) and the molecular data era (circa post-1970). Our intent is not to justify one method over the other but simply to reflect the efforts of alpha- and beta-taxonomists as different research methods were employed; however, it is interesting that although several subspecific names have been elevated to species rank, no novel species name has been proposed since N. angustapalata (Baker 1951) and no new subspecies name has been proposed since N. albigula subsolana (Alvarez 1962; now N. leucodon subsolana - Edwards et al. 2001). A detailed list of species names and the authorities is provided in Table 5.
For species described prior to the utilization of molecular data in taxonomic decision-making, data presented herein unequivocally support the initial species recognition of Hartley (albigula, 1894), Merriam (bryanti, 1887), Ord (cinerea, 1818), J. A. Allen (chrysomelas, 1908), Ord (floridana,1815), Baird (fuscipes, 1858), Merriam (goldmani, 1903), Thomas (lepida, 1893), Baird (mexicana and micropus, 1855), Goldman (palatina, 1905), Merriam (phenax, 1903), and Goldman (stephensi, 1905). Recognition of two additional species (angustapalata - Baker 1951 and nelsoniGoldman 1905), described during the morphology/natural history era, is less certain; as these taxa were either embedded within the N. leucodon clade (Cytb data set) or were poorly supported as distinct taxa (concatenated data set). The only Cytb sequence available to this study (GenBank accession number KC758854) differs from other Neotoma sequences near the 3′ end. Elimination of the last ~230 bp of the sequence reduces the genetic divergence estimate between angustapalata and nelsoni from 12.7% to 7.6% and between angustapalata and leucodon from 12.7% to 8.8%, respectively; indicating a closer genetic relationship than originally implied. However, until additional samples of these two taxa can be examined, we take a conservative approach and retain both as species—agreeing with the findings of Rogers et al. (2011) but disagreeing with Fernández (2014). In defense of Fernández (2014), whose position was based on a single specimen, the leucodon clade is more complex than depicted herein and additional studies are needed to determine the taxonomic status of populations in Mexico relative to those in the United States. During the molecular data era, two taxa originally described as subspecies were elevated to species status (devia - Goldman 1927, see Patton et al. 2007 and melanura - Merriam 1894, see Bradley and Mauldin 2016) and six taxa originally described as species but eventually relegated to subspecies, were reelevated to species status (ferruginea - Tomes 1862, see Ordóñez-Garza et al. 2014; insularis - Townsend 1912, see Patton et al. 2007; leucodon - Merriam 1894, see Edwards et al. 2002; macrotis - Thomas 1893, see Matocq 2002; magister - Baird 1858, see Edwards et al. 2001; and picta - Goldman 1904, see Edwards and Bradley 2002b). The elevation of these eight species was strongly supported by the data presented herein.
Epilogue
Clearly, a more complete sequence data set and multiple samples would enhance the analyses and ultimately the interpretation of this taxonomic group. However, the combination of data (sequence and morphometric) and concepts discussed in Edwards and Bradley (2002b), Musser and Carleton (2005), Matocq et al. (2007), Patton et al. (2007), and this study act as a hypothesis for now. Samples of N. chrysomelas and N. palatina are needed to complete the complement of species. In addition, our understanding of phylogenetic relationships within Neotoma will be enhanced by examination of whole genomes; for example, the genomes of N. lepida, N. bryanti, N. fuscipes, and N. macrotis currently are being completed (M.D. Matocq and M.D. Dearing, pers. comm.). Genome-wide data coupled with morphological analyses and field collections targeted to represent the full scope of within- and between-species variation will be needed to further elucidate the evolutionary history of Neotoma and its allies.
Acknowledgments
This manuscript is dedicated to the late Dr. Charles (Chuck) F. Fulhorst who talked us into working on woodrat systematics in 1997. Inclusion of Dr. Fulhorst as an author is warranted, as Chuck was involved in data acquisition, conceptual design, funding, and overall direction of the manuscript until his untimely death. We thank E. K. Roberts, E. A. Wright, S. C. Vrla, M. A. Krishnamoorthy, S. Castañeda-Rico, and M. Becker for comments on earlier versions of the manuscript. We thank H. Garner and K. MacDonald (Museum of Texas Tech University) for assisting tissue loans from the Natural Science Research Laboratory. Portions of this project were funded by grants from the National Institutes of Health (DHHS A141435-01 to RDB) and by the State of Texas line-item through the Biological Database Project.
Appendix I
Specimens for which cytochrome-b sequences were either generated herein or obtained from GenBank. For each specimen, museum catalog numbers (abbreviations for museum acronyms follow Hafner et al. 1997) are provided to the left of the slash (/) and GenBank accession numbers are provided to the right of the slash. Abbreviations are as follows: Centro de Investigaciones Biológicas del Noroeste (CIB), Cornell University Museum of Vertebrates (CUMV), Denver Museum of Nature and Science (DMNS), Louisiana State University, Museum of Natural Science (LSUMZ), Midwestern State University (MWSU), Monte L. Bean Life Science Museum (BYU), New Mexico Museum of Natural History (NMMNH); Recent Collection of Mammals, Museum of Texas Tech University (TTU), Royal Alberta Museum (RAM), Royal British Columbia Museum (RBCM), Royal Ontario Museum (ROM), United States National Museum of Natural History (USNM), Universidad Autónoma de Morelos (CMC), University of Alaska Museum (UAM), University of California, Berkeley, Museum of Vertebrate Zoology (MVZ), University of California Los Angeles, Dickey Collection (UCLA), University of Colorado, Museum of Natural History (UCM), University of Kansas, Natural History Museum and Biodiversity Research Center (KU), University of New Mexico, Museum of Southwestern Biology (MSB), University of Utah, Natural History Museum of Utah (UMNH), and University of Washington, Thomas Burke Memorial Washington State Museum (UWBM). If museum catalog numbers were unavailable, specimens were referenced with a corresponding collector or other special number (CR, Camp Roberts collection by Marjorie D. Matocq; JLP, James L. Patton; JO, James G. Owen, collector number; MDM, Majorie D. Matocq collector number; MDMNCN, Majorie D. Matocq no collector number; OK, Scott J. Steppan, collector number; OSR, Clinton Epps, collector number; SP, special number of the Carnegie Museum of Natural History; and TK, number special number of the Museum of Texas Tech University).
Ototylomys phyllotis.—TTU84371/AY009789.
Tylomys nudicaudus.—TTU62082/AF307839.
Xenomys nelsoni.—TTU37790/AF307838; TTU28546/KY754179.
Hodomys alleni.—TK45042/AF186801; TK45043/AF186802.
Neotoma albigula.—MSB77708/AF186811; MSB77331/AF186810; NMMNH3795/KM488335; NMMNH4891/KM488336; MSB82999/EU141962; TTU99846/EU141960; TTU99895/EU141964; MSB60812/AF186804; MSB60818/KF267873; TTU78448/AF186816; TTU106657/EU141959; TTU89870/KM488337; TTU97573/KF733991; TTU106915/KM488340; TTU99958/KM488341; TTU97577/KM488339; TTU97812/KF733995; TTU97811/KF733994; TTU97692/KF733990; TTU97776/KF733993; TTU106768/KM488338; TTU106619/KM488347; TTU97139/KF733989; TTU116582/KC250463; TTU76476/AF376472; TTU97148/EU141961; TTU88387/EU141963; TTU97657/KM488342; TTU88153/MZ064559; TTU99878/MZ064560; TTU97156/KF733996; TTU76474/AF186803; MSB77977/AF186807; MSB77371/AF186808; MSB83932/KM488343; MSB41715/AF186814; MSB83827/KM488346; MSB42599/KM488345; NMMNH5325/KM488344; TTU78447/AF186817; TTU78450/AF376476; TTU78451/AF376477; CIB14472/HQ328520; CIB1572/HQ328519; CIB15474/HQ328518; CIB4551/HQ328516; CIB14458/HQ328515; CIB14445/HQ328514; CIB4546/HQ328513; CIB14444/HQ328512; CIB14443/HQ328511; CIB4542/HQ328510; CIB4539/HQ328509; CIB14432/HQ328508; CIB4558/HQ328507; CIB4536/HQ328506; CIB14467/HQ328517.
Neotoma angustapalata.—KU37062/HM989965; BYU27733/HM989966.
Neotoma bryanti.—MSB42979/AF307832; TTU41898/AF376467; TTU83326/KF267875; TTU79131/AF307835; TTU119510/MZ064560; TTU119509/MZ064561; TTU119508/KF267874; MVZ186296/DQ781160; MVZ197380/DQ781135; MVZ195972/KY754056; MVZ186295/DQ781159; MVZ195981/DQ781158; MVZ198589/DQ781157; MVZ198588/DQ781156; MVZ198585/DQ781155; MVZ198584/DQ781154; MVZ195968/DQ781153; MVZ195967/DQ781152; MVZ195963/DQ781151; MVZ195962/DQ781150; MVZ196769/DQ781149; MVZ196098/DQ781148; MVZ196097/DQ781147; MVZ195977/DQ781146; MVZ195976/DQ781145; MVZ198580/DQ781144; MVZ198583/DQ781143; MVZ198582/DQ781142; MVZ196756/DQ781141; MVZ196755/DQ781140; USNM137201/DQ781138; USNM137173/DQ781137; MVZ197381/DQ781136; MVZ197376/DQ781134; MVZ197375/DQ781133; MVZ197175/DQ781132; MVZ197174/DQ781131; MVZ195244/DQ781130; MVZ195243/DQ781129; MVZ196146/DQ781128; MVZ196145/DQ781127; MVZ196144/DQ781126; MVZ196140/DQ781125; MVZ196138/DQ781124; MVZ202544/DQ781123; MVZ202543/DQ781122; MVZ198658/DQ781121; MVZ198657/DQ781120; MVZ196103/DQ781119; MVZ196101/DQ781118; MVZ202539/DQ781117; MVZ199807/DQ781116; MVZ199806/DQ781115; MVZ195325/DQ781114; MVZ195323/DQ781113; MVZ198577/DQ781112; MVZ196120/DQ781111; MVZ196119/DQ781110; MVZ196133/DQ781109; MVZ196132/DQ781108; MVZ198678/DQ781107; CIB7577/DQ781106; CIB7576/DQ781105; CIB7580/DQ781104; CIB7579/DQ781103; CIB7584/DQ781102; CIB7583/DQ781101; CIB2781/DQ781100; CIB2785/DQ781099; UCLA19720/DQ781098; CIB11575/DQ781097; CIB11574/DQ781096; CIB11572/DQ781095; CIB11579/DQ781094; CIB11595/DQ781093; CIB9840/DQ781092; CIB9844/DQ781091; CIB9842/DQ781090; CIB7590/DQ781089; CIB9835/DQ781088; CIB9246/DQ781087; CIB10908/DQ781086; CIB10907/DQ781085; CIB10909/DQ781084; CIB8659/DQ781083; CIB8657/DQ781082; CIB865/DQ781081; CIB8654/DQ781080; CIB7594/DQ781079; CIB2798/DQ781078; CIB7707/DQ781077; CIB7706/DQ781076; CIB7595/DQ781075; CIB5158/DQ781074; CIB7591/DQ781073; CIB7589/DQ781072; CIB8651/DQ781071; CIB7586/DQ781070; CIB8650/DQ781069; CIB7585/DQ781068; CIB3396/DQ781067; CIB2788/DQ781066; CIB7709/DQ781065; CIB7708/DQ781064; USNM139030/DQ781139.
Neotoma cinerea.—MDM148/AF337751; MSB121427/AF186799; MSB121427/AF186799; MSB74610/AF186800; BYU17790/DQ179859; MSB149469/JN593138; RAM04121/JQ241228; UWBM78604/JQ241243; RBCM0207/JN593210; UAM35116/JN593213; MVZ223394/JN593165; MVZ201915/JN593152; MVZ223440/JN593189; MVZ223425/JN593181; MVZ223445/JN593193; MVZ223443/JN593192; MVZ223435/JN593187; MSB140843/JN593136; MVZ207659/KY754057; UWBM76808/JQ241242; UWBM72137/JQ241241; UWBM72108/JQ241240; UWBM72106/JQ241239; UMNH32654/JQ241238; UMNH32653/JQ241237; UMNH32187/JQ241236; UMNH31874/JQ241235; UMNH3187/JQ241234; UAM50132/JQ241233; UAM35118/JQ241232; UAM35117/JQ241231; UAM24567/JQ241230; UAM2456/JQ241229; RAM0183/JQ241227; MVZ223462/JQ241226; MVZ223461/JQ241225; MVZ223460/JQ241224; MVZ223459/JQ241223; MVZ223458/JQ241222; MVZ223455/JQ241221; MVZ223448/JQ241220; MVZ223447/JQ241219; MVZ223446/JQ241218; MVZ223444/JQ241217; MVZ223439/JQ241216; MVZ223437/JQ241215; MVZ223436/JQ241214; MVZ223432/JQ241213; MVZ223429/JQ241212; MVZ223427/JQ241211; MVZ223426/JQ241210; MVZ223421/JQ241209; MVZ223420/JQ241208; MVZ223419/JQ241207; MVZ223418/JQ241206; MVZ223417/JQ241205; MVZ223415/JQ241204; MVZ223412/JQ241203; MVZ223411/JQ241202; MVZ223410/JQ241201; MVZ223409/JQ241200; MVZ223408/JQ241199; MVZ223402/JQ241198; MVZ223400/JQ241197; MVZ223399/JQ241196; MVZ223396/JQ241195; MVZ220747/JQ241194; MVZ218379/JQ241193; MVZ201916/JQ241192; MSB92136/JQ241191; MSB76967/JQ241190; MSB76527/JQ241189; MSB76526/JQ241188; MSB74646/JQ241187; MSB74611/JQ241186; MSB74609/JQ241185; MSB152693/JQ241184; MSB152633/JQ241183; DMNS11517/JQ241182; UWBM79658/JN593237; UWBM79495/JN593236; UWBM78852/JN593235; UWBM78606/JN593234; UWBM78139/JN593233; UWBM78001/JN593232; UWBM76813/JN593231; UWBM76811/JN593230; UWBM76799/JN593229; UWBM76697/JN593228; UWBM75217/JN593227; UWBM73810/JN593226; UWBM49066/JN593225; UMNH32655/JN593224; UMNH32468/JN593223; UMNH32202/JN593222; UMNH31875/JN593221; UMNH31172/JN593220; UMNH29794/JN593219; UCM18902/JN593218; UAM71646/JN593217; UAM54423/JN593216; UAM49980/JN593215; UAM35134/JN593214; UAM35063/JN593212; UAM35061/JN593211; RBCM20000/JN593209; RAM953034/JN593208; RAM03132/JN593207; RAM03131/JN593206; OSU/JN593205; NMMNH614/JN593204; NMMNH1006/JN593203; MVZ223463/JN593202; MVZ223457/JN593201; MVZ223456/JN593200; MVZ223454/JN593199; MVZ223453/JN593198; MVZ223452/JN593197; MVZ223451/JN593196; MVZ223450/JN593195; MVZ223449/JN593194; MVZ223442/JN593191; MVZ223441/JN593190; MVZ223438/JN593188; MVZ223434/JN593186; MVZ223433/JN593185; MVZ223431/JN593184; MVZ223430/JN593183; MVZ223428/JN593182; MVZ223424/JN593180; MVZ223423/JN593179; MVZ223422/JN593178; MVZ223416/JN593177; MVZ223414/JN593176; MVZ223413/JN593175; MVZ223407/JN593174; MVZ223406/JN593173; MVZ223405/JN593172; MVZ223404/JN593171; MVZ223403/JN593170; MVZ223401/JN593169; MVZ223398/JN593168; MVZ223397/JN593167; MVZ223395/JN593166; MVZ222572/JN593164; MVZ220748/JN593163; MVZ220746/JN593162; MVZ220623/JN593161; MVZ220570/JN593160; MVZ220134/JN593159; MVZ219951/JN593158; MVZ219950/JN593157; MVZ218378/JN593156; MVZ216409/JN593155; MVZ215473/JN593154; MVZ206431/JN593153; MVZ199348/JN593151; MVZ197092/JN593150; MSB99135/JN593149; MSB92141/JN593148; MSB92140/JN593147; MSB92137/JN593146; MSB90783/JN593145; MSB86004/JN593144; MSB76968/JN593143; MSB74612/JN593142; MSB73684/JN593141; MSB70026/JN593140; MSB152695/JN593139; MSB141113/JN593137; MDM148/JN593135; LSUMZ452/JN593134; DMNS11518/JN593133; DMNS11383/JN593132; CUMV20269/JN593131; BYU18998/JN593130; BYU18997/JN593129; BYU16618/JN593128; BYU16617/JN593127; BYU14868/JN593126; BYU13888/JN593125; BYU13887/JN593124; BYU13886/JN593123; MDMNCN 182/JN593122; MDMNCN 137/JN593121; DMNS11178/JN593120.
Neotoma devia.—MSB41675/AF307829; MSB41678/AF307830; MVZ200714/DQ781302; MVZ195239/DQ781288; MVZ197123/DQ781283; BYU18948/DQ781290; MVZ197117/KY754058; MVZ200713/DQ781301; MVZ199819/DQ781300; MVZ200712/DQ781299; MVZ200711/DQ781298; MVZ200710/DQ781297; MVZ200709/DQ781296; MVZ202447/DQ781295; MVZ200708/DQ781294; MVZ200707/DQ781293; MVZ200706/DQ781292; MVZ200705/DQ781291; MVZ195240/DQ781289; MVZ195238/DQ781287; MVZ195237/DQ781286; MVZ195236/DQ781285; MVZ199818/DQ781284; MVZ197122/DQ781282; MVZ197121/DQ781281; MVZ197120/DQ781280; MVZ197119/DQ781279; MVZ197118/DQ781278; MVZ197117/DQ781277; MVZ197116/DQ781276; MVZ197115/DQ781275; MVZ197097/DQ781274; MVZ197096/DQ781273; MVZ197095/DQ781272; MVZ197093/DQ781271; BYU18947/DQ781270; MVZ199820/DQ781269; MVZ199384/DQ781268; MVZ199381/DQ781267; MVZ199380/DQ781266; MVZ199378/DQ781265; MVZ197112/DQ781264; MVZ197107/DQ781263;l MVZ197106/DQ781262; MVZ197105/DQ781261; MVZ197104/DQ781260; MVZ197103/DQ781259; MVZ197114/DQ781258; MVZ197113/DQ781257.
Neotoma ferrunginae.—TTU104897/MZ064574; JO9027/KF772873; TTU36179/AF298840; TTU82666/AF305567; TTU82665/AF329079; USNM569657/KF772874; USNM569672/KF772875; USNM569553/KF772876.
Neotoma floridana.—TTU75413/AF186819; TTU71587/AF294343; TTU75402/AF294344; MSB74955/AF294335; TK28244/AF186818; TK27751/AF294341; TTU54731/AF294340; MWSU15766/AF294342; MSB84770/AF294333; TTU54717/AF294339; MSB81532/AF294334; OK107/KY754059.
Neotoma fuscipes.—MVZ195212/DQ781303; MVZ196356/DQ179826; MVZ196405/DQ179822; CRS697/KP129298; CR3356/KP129297; CRS514/KP129296; CRS492/KP129295; CRS374/KP129294; CR758/KP129293; CR3696/KP129292; CR3600/KP129291; CR3560/KP129290; CRS129/KP129289; CRS358/KP129288; CRS573/KP129287; CRS521/KP129286; MDM662/AF337767; MDM495/AF337766; MDM656/AF337765; MDM314/AF337764; MDM764/AF337763; MDM433/AF337762; MDM299/AF337761; MDM356/AF337760; MDM273/AF337759; MDM1824/AF337758; MDM415/AF337757; MDM1912/AF337756; MDM259/AF337755; MDM351/AF337754; JLP17478/AF337753; MDM313/AF337752; MVZ196371/DQ179825; MVZ196394/DQ179824; MVZ196386/DQ179823.
Neotoma goldmani.—TTU45227/AF186829; TTU45228/AF186830.
Neotoma insularis.—UCLA19911/DQ781161.
Neotoma lepida.—JLP16824/AF337749; BYU18153/DQ781256; BYU18300/DQ781254; BYU15035/DQ781234; MSB77775/AF307833; MSB56401/AF307831; TTU137769/KC250464; BYU18154/DQ781255; MVZ199397/DQ781253; MVZ199396/DQ781252; MVZ199395/DQ781251; MVZ199393/DQ781250; MVZ199392/DQ781249; MVZ199391/DQ781248; MVZ199404/DQ781247; MVZ199400/DQ781246; MVZ199399/DQ781245; MVZ199389/DQ781244; MVZ199373/DQ781243; MVZ199370/DQ781242; MVZ199369/DQ781241; MVZ197154/DQ781240; MVZ199376/DQ781239; MVZ197152/DQ781238; MVZ197151/DQ781237; MVZ197148/DQ781236; BYU15034/DQ781233; MVZ197158/DQ781232; MVZ197157/DQ781231; MVZ197156/DQ781230; MVZ197155/DQ781229; MVZ199359/DQ781228; MVZ199358/DQ781227; MVZ199356/DQ781226; MVZ199355/DQ781225; MVZ199354/DQ781224; MVZ199353/DQ781223; MVZ199352/DQ781222; MVZ202485/DQ781221; MVZ197167/DQ781220; MVZ197130/DQ781219; MVZ197165/DQ781218; MVZ197126/DQ781217; MVZ199366/DQ781216; MVZ199364/DQ781215; MVZ199362/DQ781214; MVZ199361/DQ781213; MVZ195261/DQ781212; MVZ195311/DQ781211; MVZ198670/DQ781210; MVZ198914/DQ781209; MVZ195912/DQ781208; MVZ198668/DQ781207; MVZ198662/DQ781206; MVZ195923/DQ781205; MVZ202461/DQ781204; MVZ202459/DQ781203; MVZ195282/DQ781202; MVZ195277/DQ781201; MVZ195266/DQ781200; MVZ197161/DQ781199; MVZ197159/DQ781198; MVZ195934/DQ781197; MVZ198578/DQ781196; MVZ195931/DQ781195; MVZ195930/DQ781194; MVZ195919/DQ781193; MVZ195917/DQ781192; MVZ199803/DQ781191; MVZ199817/DQ781190; MVZ199772/DQ781189; MVZ199816/DQ781188; MVZ199349/DQ781187; MVZ199776/DQ781186; MVZ202511/DQ781185; MVZ195926/DQ781184; MVZ202497/DQ781183; MVZ202495/DQ781182; MVZ192240/DQ781181; MVZ199787/DQ781180; MVZ199786/DQ781179; MVZ199798/DQ781178; MVZ202486/DQ781177; MVZ202540/DQ781176; MVZ198665/DQ781175; MVZ202527/DQ781174; MVZ195319/DQ781173; MVZ202524/DQ781172; MVZ199809/DQ781171; MVZ199808/DQ781170; MVZ199805/DQ781169; MVZ199804/DQ781168; MVZ202502/DQ781167; MVZ199800/DQ781166; MVZ199814/DQ781165; MVZ199813/DQ781164; MVZ199812/DQ781163; MVZ195324/DQ781162; TTU119266/KY754060; MVZ197094/DQ179836; MVZ159790/DQ179835; MVZ195223/DQ179834; MVZ197379/DQ179833; MVZ202447/DQ179832; MVZ197142/DQ179831; TTU79131/DQ179830.
Neotoma leucodon.—TTU71198/AF186806’ TTU77530/AF186828; TTU75440/AF186809; MSB48164/AF186812; TTU44923/AF186805; TTU35381/AF186813; TTU43294/AF186815; TTU111780/KM488349; TTU111790/KM488350; TTU111791/MZ099558; TTU109269/GU220381; TTU83378/KF733992; MSB58295/KM488348; TTU119729/MK253559; TTU138867/MK253561; TTU135171/MK253560; TTU120435/MK253564; TTU138862/MK253565.
Neotoma macrotis.—TTU81391/AF376479; MDM800/DQ179841; TTU79134/AF307836; TTU79132/AF307837; TTU79133/AF376475; TTU83037/FJ744107; MVZ198597/DQ781304; TTU83010/KC250457; TTU83358/KC250456; TTU83629/KC250459; TTU83033/KC250458; TTU83026/KC250460; TTU107307/KC250461; TTU115841/KC250462; TTU83017/KF267876; TTU107272/KF860897; TTU83019/KF860898; MDM1824/AF337758; CRS367/KP129305; CRS67/KP129304; CRS266/KP129302; CRS439/KP129301; CRS344/KP129300; CRS477/KP129299; MVZ196550/KY754061; MVZ196585/DQ179842.
Neotoma magister.—MSB74952/AF294336; SP799/AF294338; SP798/AF294337.
Neotoma melanura.—MVZ147661/AF337750; MVZ147667/AF108704; MSB55524/KM488355; MSB140889/KM488352; NMMNH2750/KM488360; NMMNH5132/KM488354; TTU110072/KM488358; TTU110074/KM488359; TTU110070/KM488357; NMMNH4947/KM488351; NMMNH4934/KM488361; MSB53951/KM488362; MSB55037/KM488353.
Neotoma mexicana.—TTU104970/KF801364; TTU104969/KF801365; MSB121363/AF298841; TTU101643/AF294346; TK45631/AF298842; TTU100791/FJ716222; DMNS8577AF186821; TTU79129/AF294345; TTU79128/AF298849; MSB74280/AF298848; MSB82309/AF298846; MSB74277/AF298847; TTU81714/AF376478; TTU107426/FJ716223; TTU110066/KF772877; TK47774/AF298843; TTU110064/KM488363; TTU122944/KY754062; CMC1204/MK803421; TTU137443/MZ064563.
Neotoma micropus.—TK31643/AF186822; TTU79086/AF376473; TTU79096/AF376474; TTU79078/KC153474; TTU79095/KC153473; TTU78977/KC153475; TTU79069/KC153472; TTU77529/AF298844; TTU136394/AF298845; TTU81033AF186825; TTU80855/AF186826; TTU80856/AF186827; TTU75113/AF376469; TTU81029/FJ716221; TTU80957/KC153488; TTU80915/FJ716220; TTU100185/KC153485; TTU115428/KC153482; TTU115413/KC153483; TTU115412/KC153484; TTU115427/KC153481; TTU115424/KC153480; TTU115410/KC153486; TTU115411/KC153487; TTU119505/KC153477; TTU109272/KC153476; TTU130662/KC153479; TTU115759/KC153478; TTU43296/FJ716217; TTU43297/KC812730; TTU35383/AF186824; TTU70894/DQ179818; ROM114902/EF989952; ROM114903/EF989953; TTU116487/MK253562; TTU116475/MK253563; TTU138864/MK253566; TTU42833/EU286808; TTU116316/KY754063.
Neotoma nelsoni.—LSUMZ36663/KC758854.
Neotoma phenax.—TTU6947/MK202784.
Neotoma picta.—TTU82667/AF305568; TK93390/AF305569; 1118.27/AB618728.
Neotoma stephensi.—MSB72986/AF307834; TTU78505/AF308867; MVZ197170/DQ781305; MVZ197173/KY754064.
Contributor Information
Robert D Bradley, Department of Biological Sciences, Texas Tech University, Lubbock, Texas 79409-3131, USA; Natural Science Research Laboratory, Museum of Texas Tech University, Lubbock, Texas 79409-3191, USA.
Cody W Edwards, Smithsonian-Mason School of Conservation (SMSC), George Mason University, Front Royal, Virginia 22030, USA.
Laramie L Lindsey, Department of Biological Sciences, Texas Tech University, Lubbock, Texas 79409-3131, USA.
Joanna R Bateman, Department of Biological Sciences, Texas Tech University, Lubbock, Texas 79409-3131, USA.
Maria N B Cajimat, Department of Pathology, University of Texas Medical Branch, Galveston, Texas 77555-0609, USA.
Mary L Milazzo, Department of Pathology, University of Texas Medical Branch, Galveston, Texas 77555-0609, USA.
Charles F Fulhorst, Department of Pathology, University of Texas Medical Branch, Galveston, Texas 77555-0609, USA.
Marjorie D Matocq, Department of Natural Resources and Environmental Science, University of Nevada, Reno, Nevada 89557, USA.
Matthew R Mauldin, Department of Biological Sciences, Texas Tech University, Lubbock, Texas 79409-3131, USA; Natural Science Research Laboratory, Museum of Texas Tech University, Lubbock, Texas 79409-3191, USA.
Literature Cited
- Adkins R.M., Honeycutt R.L. 1994. Evolution of the primate cytochrome c oxidase subunit II gene. Journal of Molecular Evolution 38:215–231. [DOI] [PubMed] [Google Scholar]
- Alvarez T. 1962. A new subspecies of wood rat (Neotoma) from northeastern Mexico. University of Kansas Publications, Museum of Natural History; 14:139–143. [Google Scholar]
- Amman B.R., Hanson J.D., Longhofer L.K., Hoofer S.R., Bradley R.D. 2006. Intron 2 of the alcohol dehydrogenase gene (ADH1-I2): a nuclear DNA marker for mammalian systematics. Occasional Papers, Museum of Texas Tech University 256:1–16. [Google Scholar]
- Baker R.J., Bradley R.D. 2006. Speciation in mammals and the genetic species concept. Journal of Mammalogy 87:643–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickham J.W., Patton J.C., Schlitter D.A., Rautenbach I.L., Honeycutt R.L. 2004. Molecular phylogenetics, karyotypic diversity, and partition of the genus Myotis (Chiroptera: Vespertilionidae). Molecular Phylogenetics and Evolution 33:333–338. [DOI] [PubMed] [Google Scholar]
- Bickham J.W., Wood C.C., Patton J.C. 1995. Biogeographic implications of cytochrome b sequences and allozymes in sockeye (Oncorhynchus nerka). Journal of Heredity 86:140–144. [DOI] [PubMed] [Google Scholar]
- Birney E.C. 1976. An assessment of relationships and effects of interbreeding among woodrats of the Neotoma floridana species-group. Journal of Mammalogy 57:103–132. [Google Scholar]
- Boria R.A., Brown S.K., Matocq M.D., Blois J.L. 2021. Genome-wide genetic variation coupled with demographic and ecological niche modeling of the dusky-footed woodrat (Neotoma fuscipes) reveal patterns of deep divergence and widespread Holocene expansion across northern California. Heredity 126:521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.D., Baker R.J. 2001. A test of the genetic species concept: cytochrome-b sequences and mammals. Journal of Mammalogy 82:960–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.D., Mauldin M.R. 2016. Molecular data indicate a cryptic species in Neotoma albigula (Cricetidae: Neotominae) from northwestern México. Journal of Mammalogy 97:187–199. [Google Scholar]
- Burnham K.P., Anderson D.R. 2004. Multimodel inference: understanding AIC and BIC in model selection. Sociological Methods and Research 33:261–304. [Google Scholar]
- Burt W.H., BarkalowF.S., Jr. 1942. A comparative study of the baculum of woodrats (subfamily Neotominae). Journal of Mammalogy 23:287–297. [Google Scholar]
- Campos P.F., Gilbert T.M. 2012. DNA extraction from keratin and chitin. In: Shapiro B., Hofreiter M., editors. Ancient DNA: methods and protocols. Humana Press, New York, USA; p. 43–49. [Google Scholar]
- Carleton M.D. 1980. Phylogenetic relationships neotomine-peromyscine rodents (Muroidea) and a reappraisal of dichotomy within New World Cricetinae. Miscellaneous Publications Museum of Zoology University of Michigan 157:1–146. [Google Scholar]
- Cortès-Calva P., Alvarez-Castañeda S.T, Yenssen E. 2001. Neotoma anthonyi. Mammalian Species 663:1–3. [Google Scholar]
- Darriba D., Taboada L.G.L., Doallo R., Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C.W., Bradley R.D. 2001. Molecular phylogenetics of the Neotoma floridana species group. Journal of Mammalogy 82:791–798. [Google Scholar]
- Edwards C.W., Bradley R.D. 2002a. Molecular systematics and the historical phylobiogeography of the Neotoma mexicana species group. Journal of Mammalogy 83:20–30. [Google Scholar]
- Edwards C.W., Bradley R.D. 2002b. Molecular systematics of the genus Neotoma. Molecular Phylogenetics and Evolution 25:489–500. [DOI] [PubMed] [Google Scholar]
- Edwards C.W., Fulhorst C.F, Bradley R.D. 2001. Molecular phylogenetics of the Neotoma albigula species group: further evidence of a paraphyletic assemblage. Journal of Mammalogy 82:267–279. [Google Scholar]
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. [DOI] [PubMed] [Google Scholar]
- Fernàndez J.A. 2014. Mitochondrial phylogenetics of a rare Mexican endemic: Nelson’s woodrat, Neotoma nelsoni (Rodentia: Cricetidae), with comments on its biogeographic history. The Southwestern Naturalists 59:81–90. [Google Scholar]
- Fulton T.L., Wagner S.M, Shapiro B. 2012. Case study: recovery of ancient nuclear DNA from toe pads of the extinct passenger pigeon. Methods in Molecular Biology 840:29–35. [DOI] [PubMed] [Google Scholar]
- Goldman E.A. 1904. Description of five new mammals from Mexico. Proceedings of the Biological Survey of Washington 17:79–82. [Google Scholar]
- Goldman E.A. 1905. Twelve new wood rats of the genus Neotoma. Proceedings of the Biological Survey of Washington 18:27–34. [Google Scholar]
- Goldman E.A. 1909. Five new woodrats of the genus Neotoma. Proceedings of the Biological Survey of Washington 22:139–142. [Google Scholar]
- Goldman E.A. 1910. Revision of the wood rats of the genus Neotoma. North American Fauna 31:1–124. [Google Scholar]
- Goldman E.A. 1915. Five new mammals from México and Arizona. Proceedings of the Biological Survey of Washington 31:133–137. [Google Scholar]
- Goldman E.A. 1932. Review of the woodrats of Neotoma lepida group. Journal of Mammalogy 13:59–67. [Google Scholar]
- Gray J.E. 1843. List of the specimens of Mammalia in the collection of the British Museum. British Museum of Natural History Publication, London, United Kingdom. [Google Scholar]
- Guindon S., Gascuel O. 2003. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52:696–704. [DOI] [PubMed] [Google Scholar]
- Hafner M.S., Gannon W.L., Salazar-Bravo J., Alvarez-Castañeda S.T. 1997. Mammal collections in the western hemisphere: a survey and directory of existing collections. Allen Press, Lawrence, Kansas, USA. [Google Scholar]
- Hall E.R. 1981. The mammals of North America. 2nd ed. John Wiley & Sons, Inc., New York, USA. [Google Scholar]
- Hall E.R., Genoways H.H. 1970. Taxonomy of the Neotoma albigula-group of woodrats in central México. Journal of Mammalogy 51:504–516. [Google Scholar]
- Hayes J.P., Harrison R.G. 1992. Variation in mitochondrial DNA and the biogeographic history of woodrats (Neotoma) in the eastern United States. Systematic Biology 41:331–344. [Google Scholar]
- Hayes J.P., Richmond M.E. 1993. Clinal variation and the morphology of woodrats (Neotoma) of the eastern United States. Journal of Mammalogy 74:204–216. [Google Scholar]
- Hernández-Canchola G., Léon-Panagua L., Esselstyn J. 2021. Mitochondrial DNA indicates paraphyletic relationships of disjunct populations in the Neotoma mexicana species group. Therya 12:163–169. [Google Scholar]
- Huelsenbeck J.P., Larget B., Miller R.E., Ronquist F.R. 2002. Potential applications and pitfalls of Bayesian inference of phylogeny. Systematic Biology 51:673–688. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquist F.R. 2001. MrBayes: Bayesian inference for phylogeny. Biometrics 17:754–756. [DOI] [PubMed] [Google Scholar]
- Hurvich C.M., Tsai C.-L. 1989. Regression and time series model selection in small samples. Biometrika 76:297–307. [Google Scholar]
- Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16:111–120. [DOI] [PubMed] [Google Scholar]
- Longhofer L.K., Bradley R.D. 2006. Molecular systematics of the genus Neotoma based on DNA sequences from intron 2 of the alcohol dehydrogenase gene. Journal of Mammalogy 87:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons L.A., Laughlin T.F., Copeland N.G., Jenkins N.A., Womack J.E., O’Brien S.J. 1997. Comparative anchor tagged sequences (CATS) for integrative mapping of mammalian genomes. Nature Genetics 15:47–56. [DOI] [PubMed] [Google Scholar]
- Matocq M.D. 2002. Morphological and molecular analysis of a contact zone in the Neotoma fuscipes species complex. Journal of Mammalogy 83:866–883. [Google Scholar]
- Matocq M.D., Shurtliff Q.R., Feldman C.R. 2007. Phylogenetics of the woodrat genus Neotoma (Rodentia: Muridae): implications for the evolution of phenotypic variation in male external genitalia. Molecular Phylogenetics and Evolution 42:637–652. [DOI] [PubMed] [Google Scholar]
- Mellink E. 1992. The status of Neotoma anthonyi (Rodentia; Muridae, Cricetinae) of Todos Santos Islands, Baja California, Mexico. Bulletin of the California Academy of Sciences 9:137–140. [Google Scholar]
- Merriam C.H. 1892. Description of nine new, Mexico mammals collected by E. W. Nelson in the states of Colima and Jalisco. Proceedings of the Biological Society of Washington 7:164–174. [Google Scholar]
- Merriam C.H. 1894. Abstract of a study of the American wood rats, with descriptions of fourteen new species and subspecies of the genus Neotoma. Proceedings of the Biological Survey of Washington 9:117–128. [Google Scholar]
- Merriam C.H. 1903. Two new wood rats (genus Neotoma) from the state of Coahuila, Mexico. Proceedings of the Biological Survey of Washington 16:47–48. [Google Scholar]
- Musser G.G., Carleton M. D. 2005. Superfamily Muroidea. In: Wilson D.E., Reeder D.M., editors. Mammal species of the World: a taxonomic and geographic reference. 3rd ed. Johns Hopkins University Press, Baltimore, Maryland, USA; p. 894–1531. [Google Scholar]
- Ordóñez-Garza N., Thompson C.W., Unkefer M., Edwards C.W., Owen J.G., Bradley R.D. 2014. Systematics of the Neotoma mexicana species group (Mammalia: Rodentia: Cricetidae) in Mesoamerica: new molecular evidence on the status and relationships of N. ferruginea Tomes, 1862. Proceedings of the Biological Society of Washington 127:518–532. [Google Scholar]
- Pardiñas U., Myers P., León-Paniagua L., Ordóñez Garza N., Cook J.A., Kryštufek B., Haslauer R., Bradley R.D., Shenbrot G., Patton J.L. 2017. Cricetidae (true hamsters, voles, lemmings, and New World rats and mice). In: Wilson D.E., LacherT.E., Jr., Mittermeier R.A., editors. Handbook of the mammals of the world, volume 7. Rodents II. Lynx Editions in association with Conservation International and ICUN, Barcelona, Spain; p. 355–396. [Google Scholar]
- Patton J.L., Huckaby D.G., Alvarez-Castañeda S.T. 2007. The evolutionary history and a systematic revision of the Neotoma lepida group. University of California Publications in Zoology 135:1–411. [Google Scholar]
- Peppers L.L., Bradley R.D. 2000. Cryptic species in Sigmodon hispidus: evidence from DNA sequences. Journal of Mammalogy 81:332–343. [Google Scholar]
- Planz J.V., Zimmermann E.G., Spradling T.A., Akins D.R. 1996. Molecular phylogeny of the Neotoma floridana species group. Journal of Mammalogy 77:519–535. [Google Scholar]
- Rogers D.S., Leite R.N., Reed R.J. 2011. Molecular phylogenetics of an endangered species: the Tamaulipan woodrat (Neotoma angustapalata). Conservation Genetics 12:1035–1048. [Google Scholar]
- Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M., Huelsenbeck J.P. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61:539–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R.K., Gelfand D.H., Stoffel S., Scharf S.J., Horn G.T., Mullis K.B., Erlich H.A. 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491. [DOI] [PubMed] [Google Scholar]
- Smith F.A., Bestelmeyer B.T., Strong M. 1993. Anthrogenic extinction of the endemic woodrat Neotoma bunkeri Burt. Biodiversity Letters 1:149–155. [Google Scholar]
- Stamatakis A. 2014. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. 2002. PAUP* phylogenetic analysis using parsimony (*and other methods). Version 4.0a (build 169). Sinauer Associates, Sunderland, Massachusetts, USA. [Google Scholar]