Abstract
Simple Summary
Melanoma is a major public health issue that claims the lives of thousands of people every year. Furthermore, the outlook for the coming years is not encouraging with increasing morbidity and mortality trends. This review aims to offer an updated overview of various aspects related to cutaneous melanoma, from epidemiology, etiology, clinical presentation, prevention, diagnosis and staging. Moreover, conventional treatments currently available as well as the latest advances in clinical trials regarding new drugs and/or combinations, including nanotechnology-based strategies are also reviewed.
Abstract
Melanoma is the deadliest skin cancer, whose morbidity and mortality indicators show an increasing trend worldwide. In addition to its great heterogeneity, melanoma has a high metastatic potential, resulting in very limited response to therapies currently available, which were restricted to surgery, radiotherapy and chemotherapy for many years. Advances in knowledge about the pathophysiological mechanisms of the disease have allowed the development of new therapeutic classes, such as immune checkpoint and small molecule kinase inhibitors. However, despite the incontestable progress in the quality of life and survival rates of the patients, effectiveness is still far from desired. Some adverse side effects and resistance mechanisms are the main barriers. Thus, the search for better options has resulted in many clinical trials that are now investigating new drugs and/or combinations. The low water solubility of drugs, low stability and rapid metabolism limit the clinical potential and therapeutic use of some compounds. Thus, the research of nanotechnology-based strategies is being explored as the basis for the broad application of different types of nanosystems in the treatment of melanoma. Future development focus on challenges understanding the mechanisms that make these nanosystems more effective.
Keywords: melanoma, epidemiology, etiology, diagnosis, treatment, nanotechnology, clinical trials
1. Introduction
Skin is the largest organ of the human body, covering about 2 m2 of surface and weighing approximately 3.6 kg in adulthood. It has important functions that guarantee protection against physical, chemical and biological agents, regulation of body temperature and production of antimicrobial compounds to prevent infections [1,2]. Skin is divided into three layers, from the outermost to the innermost: epidermis, consisting mainly of keratinocytes (95%) and some dendritic cells such as melanocytes, Merkle and Langerhans cells; dermis, composed of connective tissue, essentially collagen and elastic fibers, as well as blood vessels, nerve endings and glands, such as sebaceous and sweating; and hypodermis, whose function is the connection between the dermis and the underlying organs [3,4].
Skin diseases affect millions of people around the world and cover a wide range of problems going from chronic diseases such as atopic dermatitis and psoriasis to neoplasms. Although potentially treatable, the latter is associated with a high mortality rate [5,6,7]. Regarding skin cancers and according to the International Agency for Research on Cancer (IARC), in 2020, there were around 1.52 million new cases and 121,000 related deaths [8,9]. Every 4 min, one person dies from skin cancer [10]. Moreover, the prospects for the next 20 years by the same agency are not optimistic as increases of 78 and 73% in melanoma’s incidence and mortality, respectively, are expected [8,9].
Neoplastic changes can occur in diverse skin layers, namely, at the epidermal layer. Epidermal cells’ malignancies can be divided into melanoma, which will be the subject of this work, and non-melanoma skin cancers depending on whether the cells of origin are melanocytes or keratinocytes and Merkel cells, respectively [11,12].
Melanoma is a word derived from the Greek melas “dark” and oma “tumor”, having been firstly described by Hippocrates in the 5th century BC [13]. It is an aggressive disease that derives from the malignant transformation and uncontrolled proliferation of melanocytes [14,15]. Melanocytes derive from an existing structure in vertebrate embryos denominated neural crest, and their main function is the production of melanin (melanogenesis) and its storage until transferred to keratinocytes [16,17,18]. Melanin is an important pigment responsible for the color of the skin, hair and eyes. It plays an important role in protecting skin from sun exposure, neutralizing reactive oxygen species (ROS) and storing ions [19]. As already stated, melanocytes are mostly present in the epidermis, more precisely, in the innermost layer out of four layers, stratum basale [20]. The malignant transformation of these epidermal melanocytes comprises cutaneous melanoma, independently of being metastatic or non-metastatic.
Cutaneous melanoma is the focus of this review. This malignancy includes the vast majority of diagnosed cases [21,22] and its principal subtypes are superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma and acral lentiginous melanoma. However, melanocytes can also be present in other tissues, such as hair follicle bulbs, eyes, inner ear, mucosa and central nervous system, comprising in these cases the non-cutaneous form [21,22,23,24]. Furthermore, although melanoma represents the least common form of skin cancer, it is the most aggressive and lethal [15,25,26]. In addition to the considerable social impact, the costs and complexity of health care in advanced stages have attracted attention. In the United States of America, for example, the costs associated with melanoma represent about 40% of the annual budget allocated to skin cancer [14]. Contrarily, despite having 18–20 times higher incidence, non-melanoma skin cancers, whose principal forms are Basal Cell Carcinoma (BCC) and Squamous Cell Carcinoma (SCC), present slow growing and low tendency to metastasize and thus are rarely lethal [27,28,29,30].
In terms of treatment, the management of melanoma has rapidly developed in recent years. The landscape of available therapeutic options was limited to surgery, radiotherapy and chemotherapy until 2011 [31,32]. From then on and leveraged with the approval of the first immune checkpoint inhibitor, ipilimumab, and the first small-molecule kinase inhibitor, vemurafenib, approvals followed one another. However, despite the unquestionable progress, these therapies remain limited in some cases, with resistance and the incidence of adverse side effects [32,33]. Thus, alternative options continue to be investigated either in a clinical or pre-clinical phase [34,35,36,37,38].
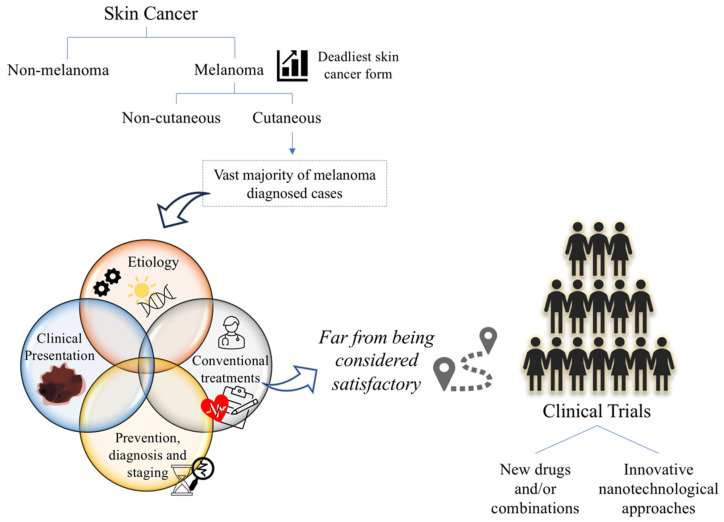
Nanotechnology, a concept created in the middle of the last century, has also proved to be a valuable and promising tool [39], with several lipidic, polymeric, metallic and hybrid nanosystems already approved in various areas, such as cancer [40,41,42]. The improvement of physicochemical properties of the compounds, such as water solubility and stability and consequently the associated pharmacokinetic and pharmacodynamic profiles, as well as the possibility of targeting these nanosystems to the tumor site, either passively or actively, might change the current therapeutic strategy for melanoma [43,44]. Thus, this review aims to provide an updated overview of the various aspects of cutaneous melanoma, starting from epidemiology, etiology, clinical presentation, prevention, diagnosis, staging, available conventional treatments and ending with the latest advances in clinical trials with new drug combinations but also including nanotechnological approaches (Figure 1).
Figure 1.
Melanoma: challenges and opportunities.
2. Cutaneous Melanoma
2.1. Epidemiology and Etiology
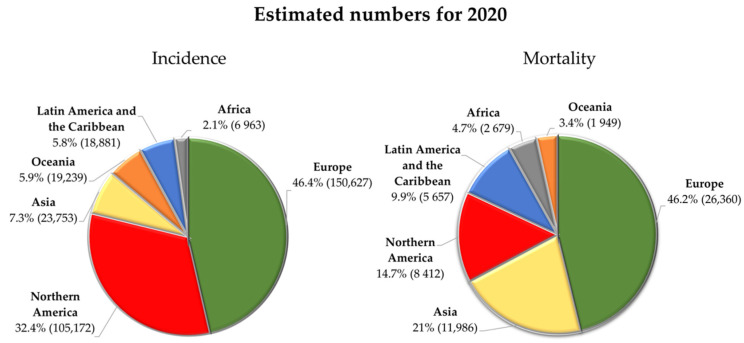
Melanoma is a type of malignancy whose worldwide incidence rate has been increasing very rapidly over the last decades [14,45]. Light-skinned populations such as those in North America, Northern Europe, Australia or New Zealand have seen the annual incidence of melanoma increase by 4–6% [14]. According to IARC estimates, there were around 325,000 new cases associated with cutaneous melanoma in 2020 [46]. Representing 1.7% of all cancer diagnoses [47], melanoma is ranked as one of the most common cancers worldwide, probably reaching 57,000 deaths in the same period [48]. Figure 2 briefly illustrates the incidence and mortality distribution in different countries. There are several factors contributing to this scenario, namely, atmospheric ozone depletion, global warming and air pollution [49,50]. The same agency predicted until 2040 an increase of about 57 and 68% in the number of new cases and related deaths, respectively [51,52].
Figure 2.
Estimated number of new skin melanoma cases and deaths worldwide in 2020 for both sexes and all ages. Data adapted from the Global Cancer Observatory by IARC [46,48].
The transformation of melanocytes into malignant cells is a really complex process that results from the interaction of different modifiable and non-modifiable risk factors (Table 1) [21]. Exposure to UV radiation from sunlight or the use of tanning devices are considered the strongest modifiable risk factor of melanoma, responsible for about 60–70% of all diagnosed cases [53,54]. The IARC even classified them as carcinogens (Group 1), alongside tobacco and asbestos [55,56]. It is not surprising, therefore, that it is in the equatorial regions, where the hours of sunlight exposure are higher, that the highest rates of melanoma incidence are verified [22]. In 2020, the highest incidence rates of melanoma reported by IARC occurred precisely in Australia and New Zealand, with an age-standardized rate (ASR) per 100,000 habitants of 36.6 and 31.6, respectively. In contrast, the following countries with a great incidence of ASR were all located at higher latitudes, as is the case of Denmark, The Netherlands, Norway, Sweden, Switzerland and Germany [57]. A lower incidence was observed in the southern countries of Europe, and this can be explained by the differences in skin pigmentation of the populations of these regions, reflecting the patterns of incidence according to the ethnicity reported further on. The prevalence of darker pigmentation also explains the low values of ASR in other populations living close to the equator, as is the case in many Asian and African countries [14,57]. In addition, altitude has also been suggested as a risk factor, since changes in ozone absorption, less cloud cover as well as an increase in surface reflectance in areas with snowfall, can increase the exposure to UV radiation [14]. Approximately 6.8% of solar radiation belongs to the UV spectrum, which comprises three types of radiation: UVC, UVB and UVA (wavelength range: 200–280, 280–315 and 315–400 nm, respectively) [58]. UVC radiation, as well as almost all UVB radiation, are absorbed by the stratospheric ozone layer, leading that only 5% of UVB radiation and 95% of UVA radiation reaching the earth’s surface [59,60]. Thus, it is UVA, predominantly, and UVB radiation that are responsible for the mutagenic and proliferation effects in melanin-producing cells. UVB radiation, in addition to direct DNA damage, also promotes an inflammatory response that stimulates angiogenesis and the survival, proliferation and metastatic potential of mutated cells. In turn, UVA radiation indirectly triggers DNA strand damage, lipid peroxidation and protein damage through oxidative stress [53,54,58,61].
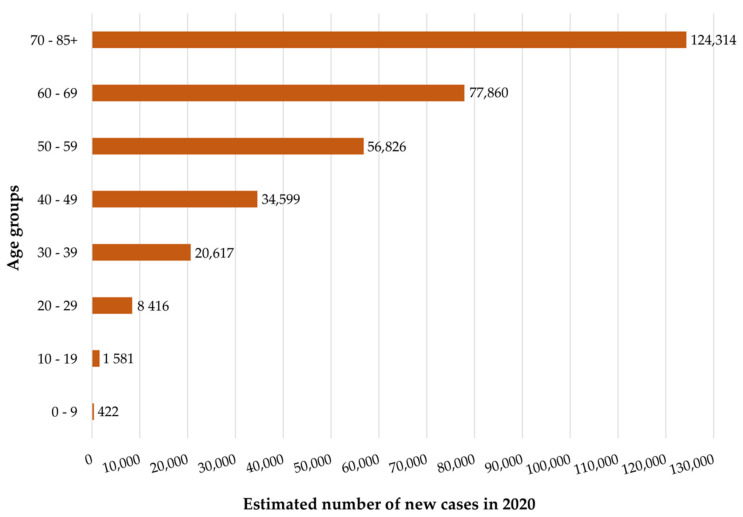
Although melanoma essentially affects an older population, it is clear that there is an increase in prevalence in a younger population (Figure 3) [14]. In this sense, while colon and lung cancer have an average diagnosis of 68 and 70 years old, respectively, melanoma, is only 57 years [21,22].
Figure 3.
Estimated number of new skin melanoma cases worldwide for both sexes by age group. These data correspond to 2020 and were adapted from the Global Cancer Observatory by IARC [62].
Furthermore, sex is also an influential factor. At younger ages, women have the highest incidence rate of melanoma, but as age increases, the trend is reversed, and men are the most predisposed [21,22]. However, age aside, the overall incidence of melanoma is clearly higher in men than in women, with an ASR of 3.8 and 3.0 for 100,000, respectively [47,63,64]. The reason for this sex disparity is still not completely clear. However, there is evidence that in addition to the contribution of sex-specific behavioral factors, there are also intrinsic biological differences. An example is female genetic heterogeneity derived from the epigenetic inactivation of one of the two X chromosomes [64].
Regarding ethnicity, the incidence of melanoma varies considerably. Caucasians are more likely to develop melanoma compared to dark-skinned populations (2.4 vs. 0.1%) [45,65]. Skin color is the reason for this differentiable risk, being influenced by several factors such as the mixture of carotenoids, oxy-/deoxy-hemoglobin, and most outstandingly, different types of melanin. Furthermore, the melanogenic activity as well as the number, size and packaging of melanosomes impact skin color [60,66]. There are two different forms of melanin present in the human body: eumelanin and pheomelanin. Eumelanin is a brownish-black pigment, mainly present in dark-skinned populations, which exerts a protective action on the skin, spreading and absorbing part of the UV radiation as well as eliminating free radicals. In contrast, the own production mechanism of pheomelanin, a reddish-yellow pigment mainly produced by light-skinned people, promotes oxidative stress, leading to greater susceptibility of melanocytes to DNA damage [60,67,68,69,70].
Finally, being melanoma associated with a great genetic component, several genetic alterations (hereditary and somatic) have been reported over the last few years [71]. Germline mutations account for about 5–10% of cases, and among these, the cyclin-dependent kinase inhibitor 2A (CDKN2A) mutation is the most prevalent. However, mutations in several other genes such as melanocortin 1 receptor (MC1R), microphthalmia-associated transcription factor (MITF), cyclin-dependent kinase 4 (CDK4), protection of telomeres 1 (POT1), telomerase reverse transcriptase (TERT), adrenocortical dysplasia (ACD), telomeric repeat-binding factor 2-interacting protein 1 (TERF2IP) and BRCA1-Associated Protein 1 (BAP1) are also present in melanoma-prone families [72,73]. On the other hand, somatic mutations are mostly associated with mitogen-activated protein kinase cascade. They are classified according to the most frequently mutated genes into four principal types: BRAF (serine/threonine protein kinase B-raf), the most frequent, NRAS (neuroblastoma RAS viral oncogene homolog), NF1 (neurofibromin 1) and triple wild type (no mutation in any of these three genes) mutations [71,74,75]. Moreover, also specific genetic conditions such as albinism and xeroderma pigmentosum increase melanoma’s risk [76,77].
Table 1.
| Modifiable risk factors | Exposure to UV radiation (e.g., sunlight or use of tanning devices) |
| History of blistering sunburns at a young age | |
| Medications (e.g., psoralen or immunosuppressive drugs) | |
| Environmental exposure to chemicals (e.g., heavy metals or pesticides) | |
| Non-modifiable risk factors | Age |
| Sex | |
| Ethnicity | |
| Individual phenotypic characteristics (e.g., skin and light eyes, red or blond hair and high density of freckles) | |
| Clinical characteristics of the patient (e.g., increased number of common nevi or presence of atypical nevi) | |
| Personal and family history of skin cancers | |
| Personal history of diseases that compromise the immune system (e.g., hematologic malignancies or infection by HIV) | |
| Genetic alterations | |
| Specific genetic conditions (e.g., albinism or xeroderma pigmentosum) |
2.2. Clinical Presentation
Melanoma is one of the most heterogeneous cancers, both in terms of etiology as previously mentioned and in terms of its clinical characteristics. These characteristics are dependent on several factors such as the origin and anatomical location [79,80].
There are four main types of cutaneous melanoma: superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma and acral lentiginous melanoma, each one of them associated with different epidemiological, dermatological and histopathological factors [24,81].
Briefly, superficial spreading melanoma, the most diagnosed type of melanoma (70–80% of the total cases), is the most prevalent in the population between 30 and 50 years old [82]. Usually, it appears as a macula that tends to evolve into a palpable papule or nodule with irregular margins and a broad range of colors. Histologically, it is common to observe a pagetoid and nested spread of malignant melanocytes in intraepidermal tissues. Intradermal nests of melanocytic proliferation are also observed [24,83]. Although it can arise in any localization, it is more frequently found on the trunk in men and on the lower extremities in women. Intraepidermal horizontal growth can last for a long time and only then become invasive [82,84].
Nodular melanoma is the second type of melanoma more frequent (15–30% of cases). It can also appear in any location; however, it is more common in the trunk, head and neck. This type of cancer is the most aggressive [82]. The brown or black or even blue-black lesions of this type of tumor have a wide range of presentations. It can appear as a polypoid exophytic tumor, a prominent plaque with irregular shapes, or a smoothly surfaced cutaneous nodule. Histologically, intradermal nests and aggregates of tumor cells are observed, while the intraepidermal melanocytic proliferation is minimal and overlying the dermal tumor [83].
Lentigo maligna melanoma represents 5 to 15% of cases. It is more frequent on the sun-damaged faces of elderly people, mainly on the nose and cheeks. This type of tumor has an antecedent skin lesion called lentigo maligna, which can have a horizontal growth period of many years before it becomes invasive [82]. Typically, it might occur as a tan, brown and black flat tumor of irregular contours, presenting flecks with the same colors. Histologically, an extensive melanocytic proliferation is observed in the dermal-epidermal junction that extends through the hair follicle epithelium. Intradermal nested of epithelioid or spindled cells are also observed [83].
Lastly, with only 2–8% of the cases, usually found in people of color, there is acral lentiginous melanoma. It arises mainly on the palms of the hands and soles of the feet as an atypical pigmented macule that develops an elevated plaque or nodule [81,82]. The histological feature is an extensive and poorly circumscribed lentiginous growth of nested tumor cells parallel to the epidermis [85]. Unfortunately, it is detected at advanced stages [86].
Additionally, other variants more rarely reported of cutaneous melanoma comprise the desmoplastic, polypoid, primary dermal, verrucous and amelanotic melanomas [24,87].
2.3. Prevention, Diagnosis and Staging
As an important public health problem, the strategy to tackle melanoma must be based on two essential pillars: prevention and early diagnosis [45,88].
Prevention strategies fundamentally focus on promoting the health literacy of the population, looking for behavioral changes regarding modifiable risk factors as well as raising awareness of the importance of sun protection habits. In addition, there has been increasing interest in the close surveillance of individuals with high-risk characteristics for developing melanoma [54,89,90,91]. On the other hand, an early and accurate diagnosis of the disease is essential to improve the outcomes. The overall survival in 5 years decreases from 99% when detected in the stage IA melanoma to 15–20% in the most advanced stage (IV) [76].
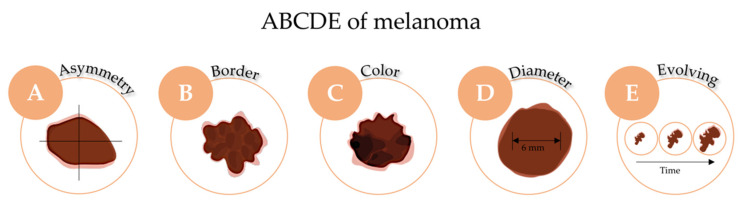
The starting point for clinical diagnosis is the visual screening of suspicious lesions, greatly supported by the ABCDE rule (Asymmetry, Border irregularity, Color heterogeneity, Diameter ≥6 mm and Evolving) as schematized in Figure 4 [92,93]. In the case of nodular melanoma, the mnemonic rule is EFG (Elevated, Firm and Growing). In addition, a comparative dermoscopic analysis must be carried out [26,94]. Moreover, an excisional, whenever possible, punch or shave biopsy with subsequent histopathological analysis should be performed. The histopathological analysis allows the identification of different parameters, such as histotype of melanoma, Breslow thickness, Clark level, mitotic index and the presence or absence of ulceration [95,96,97]. Furthermore, immunohistochemical analysis to identify melanocytic (S-100 protein, Melan-A, HMB-45 or SOX10) and proliferation markers (MIB-1), lymph node biopsy, genetic characterization of targetable somatic mutations and measurement of LDH serum levels can also be performed in specific cases [26,98,99,100]. In addition, many non-invasive pre-biopsy imaging technologies have been developed in recent years. Whole-body 3-D imaging, reflectance confocal microscopy, optical coherence tomography, high-frequency ultrasound and multispectral digital skin lesion tools such as MelaFind, approved by FDA in 2011, are examples [101,102].
Figure 4.
The ABCDE rule for melanoma skin cancer diagnosis.
Furthermore, accurate staging of the tumor is also a crucial step. This will allow the clinician to assess the prognosis of the patient and select the best and most appropriate therapeutic strategy [103]. The most used cutaneous melanoma staging system belongs to the American Joint Committee on Cancer (AJCC). This system, called TNM staging system, is based on an assessment of three aspects, each one with more than one criterium: (T) Breslow tumor thickness of the primary tumor and presence or not of ulceration; (N) number of involved lymph nodes and the presence or not of in-transit, satellite and/or microsatellite metastasis; and (M) anatomic site of distant metastasis and LDH levels. After analysis, patients are included in specific pathological stages groups, which are divided as follows: 0, I (IA and IB), II (IIA, IIB and IIC), III (IIIA, IIIB, IIIC and IIID) and IV. Briefly, while in stage 0 cancer cells are limited to the epidermis, in stages I and II tumors are classified according to their thickness and degree of ulceration. In turn, stages III and IV classification are attributed as soon as the involvement of lymphatic tissues occurs and when there is dissemination to one or more vital organs, respectively [104].
3. Challenges and Opportunities for Cutaneous Melanoma Treatment
As previously mentioned, in the early stages of the disease, the prognosis might be positive, and the patient can be successfully treated surgically [105]. However, as the disease progresses, survival rates significantly decrease [76]. Until 2010, only radiotherapy and chemotherapy were considered alternatives to surgery [31,100,106]. Later on, the growing knowledge about the pathogenesis of the disease, the role of the immune system and the greater capacity for genomic sequencing, allowed the identification of new targets [106,107,108]. Thus, marketing authorization of different drugs and/or combinations has taken place [107,108,109]. However, intra and intertumoral heterogeneity continues to overlap, limiting therapeutic success [107,110,111], but according to the ClinicalTrials.gov database, a large number of clinical trials have been carried out testing new therapeutic strategies, including those based on nanotechnology.
3.1. Current Available Strategies
The currently available strategies for the treatment of melanoma are surgery, radiotherapy, chemotherapy, immunotherapy and targeted therapy (Table 2). The selection of the most suitable therapeutic strategy depends not only on the anatomic location, stage and genetic profile of the tumor but also on the age and general health status of the patient [32]. The different therapeutic alternatives will be discussed in the following Sections.
Table 2.
Melanoma treatment drugs and respective years of approval by Food and Drug Administration (FDA) and European Medicines Agency (EMA).
| Type of Treatment | Mechanism | Drug | FDA Approval Date | EMA Approval Date |
|---|---|---|---|---|
| Chemotherapy | ||||
| Alkylating agent | Dacarbazine | 1975 | 2002 | |
| Immunotherapy | ||||
| Antiviral | Interferon alpha-2b | 1996 | 2000 | |
| Peginterferon alpha-2b | 2011 | - | ||
| Interleukin | Interleukin-2 | 1998 | - | |
| Monoclonal antibody anti-CTLA4 | Ipilimumab | 2011 | 2011 | |
| Monoclonal antibody anti-PD-1 | Pembrolizumab | 2014 | 2015 | |
| Nivolumab | 2014 | 2015 | ||
| Monoclonal antibody anti-PD-L1 | Atezolizumab * | 2020 | - | |
| Monoclonal antibody anti-LAG-3 | Relatlimab-rmbw * | 2022 | - | |
| Oncolytic herpes virus | Talimogene laherparepvec | 2015 | 2015 | |
| Targeted therapy | ||||
| BRAF inhibitor | Vemurafenib | 2011 | 2012 | |
| Dabrafenib | 2013 | 2013 | ||
| Encorafenib * | 2018 | 2018 | ||
| MEK inhibitor | Trametinib | 2013 | 2014 | |
| Cobimetinib * | 2015 | 2015 | ||
| Binimetinib * | 2018 | 2018 | ||
Abbreviations: BRAF: serine/threonine protein kinase B-raf; CTLA-4: T-lymphocyte-associated protein-4; LAG-3: lymphocyte activation gene-3; MEK: mitogen-activated protein kinase; PD-1: programmed cell death protein-1; PD-L1: programmed death-ligand 1. * Only used in combination with other medicines. Data collected from the database of each regulatory agency [112,113].
3.1.1. Surgery Resection
Whenever possible, surgical removal with adequate margins is the first-line treatment of melanoma [105]. Although it is mainly applied in patients up to stage II melanoma, it is also often an option for stage III patients [114,115] or even when the disease has already metastasized to other organs (stage IV) [116,117,118,119,120]. However, especially in some patients at stages II and patients at stages III and IV, surgery alone has limited curative potential. Thus, radiotherapy, chemotherapy, immunotherapy or targeted therapy are often used as adjuvant treatments [116,121,122].
3.1.2. Radiotherapy
Melanoma is a relatively radioresistant tumor as it has the ability to effectively repair DNA damage caused by radiation [123]. Therefore, the choice of radiotherapy as a first-line treatment is applied for exceptional cases, for example, given the impossibility of performing surgery or as a complement to some situations where there is a high risk of recurrence. On the other hand, radiotherapy is widely used as a palliative treatment for metastatic melanoma (stage IV). New techniques such as stereotactic radiosurgery and stereotactic body radiotherapy have commonly been used in the treatment of brain, lung or liver metastases. Compared with whole-brain radiotherapy in the case of treatment of brain metastases, promising results and less severe adverse side effects have been observed [116,124,125]. Nonetheless, the combination of radiotherapy with systemic therapeutic options is currently under study in some clinical trials (NCT02858869 and NCT04902040) [126].
3.1.3. Chemotherapy
Although chemotherapy remains a therapeutic option for melanoma management, especially in palliative or relapsed situations, in metastatic advanced stages of the disease, new therapeutic choices are preferred [127].
The main disadvantages associated with chemotherapy are the lack of specificity for tumor cells and consequent low drug accumulation at the tumor microenvironment. Thus, therapeutic benefits are limited and the incidence of adverse side effects is prominent [43,123,127]. To the best of our knowledge, the DNA alkylating agent dacarbazine (DTIC) remains the only drug approved both by FDA and EMA [128]. Generally, in the various clinical trials conducted, DTIC response rate was around 10 to 20%, with most responses being partial and not sustained over time. In addition, nausea, vomiting and myelosuppression are the most common adverse side effects [127,129].
In addition to DTIC and despite not being officially approved, many other chemotherapeutic agents have been used off-label, namely, temozolomide (TMZ), nitrosoureas, paclitaxel, docetaxel and cis/carboplatin [100,128].
TMZ is a DTIC analog approved for glioblastoma but frequently used in metastatic melanoma [127]. A phase III clinical trial comprising 305 volunteers with advanced disease found similar efficacy between both drugs (13.5 vs. 12.1% objective response rate for TMZ and DTIC, respectively) [130]. Besides this optimistic result, the oral route of administration and the ability of TMZ to cross the blood–brain barrier, relevant in the case of brain metastases, are other advantages [127,131,132].
Like the previous ones, nitrosoureas, such as photomustine, carmustine and lomustine, are alkylating agents, generally used in combination with other drugs [127]. The anticancer activity of these compounds is identical to the gold standard, DTIC. However, they present a much unfavorable toxicity profile [129].
In turn, taxanes, such as paclitaxel and docetaxel, and platinum compounds, namely, carboplatin and cisplatin, have also been clinically tested in the treatment of melanoma as monotherapy. However, because of their moderate anticancer activity, the combination of these and other therapeutic classes continues to be investigated [127,129,131].
3.1.4. Immunotherapy
Immunotherapy has as its primordial objective the stimulation and activation of the immune system. It started with the FDA approval of interleukin 2 (IL-2) and interferon alfa-2b in the 1990s, with the latter being also approved by EMA. Since 2011, there has been an important shift in the role of immunotherapy in melanoma like immunological checkpoint inhibitors (ICIs) [133,134]. Interestingly, melanoma was the first malignancy to take advantage of ICIs [135]. There are four classes of ICIs monoclonal antibodies approved for the treatment of melanoma, namely, ipilimumab, an antagonist of cytotoxic-T lymphocytes antigen 4 (CTLA-4); nivolumab and pembrolizumab, antagonists of programmed cell death protein 1 (PD-1); atezolizumab, an antagonist of programmed cell death ligand 1 (PD-L1) [106]; and more recently, in 2022, relatlimab-rmbw, an antagonist of lymphocyte activation gene-3 (LAG-3) [136]. These last two drugs are already approved for melanoma indication by FDA but not by EMA.
CTLA-4, PD1, PD-L1 and LAG-3 are immune checkpoint proteins expressed on T cells membrane and involved in the signaling pathways that lead to its suppression. T cells are physiologically essential in the maintenance of immune tolerance. However, these immunological checkpoints are often used by cancer cells for immune evasion by down-regulation of their antitumor responses. ICIs, by selectively binding to these proteins, allow overcoming tumor-induced inhibition of T cell functions, re-establishing the anti-tumor immune responses [137,138,139,140,141,142,143].
Nonetheless, there is still a large percentage of patients who present innate and acquired resistance, not responding to this type of therapy [144,145,146,147]. Moreover, due to their mechanism of action that stimulates the immune system, ICIs are associated with an imbalance in immune tolerance leading to numerous immune-related adverse events (irAEs). The most common are dermatological, such as skin rash, pruritus and vitiligo; gastrointestinal such as diarrhea and colitis; hepatic; and endocrine conditions, such as hyperthyroidism and hypothyroidism [138,146,148].
Another relatively recent immunotherapeutic strategy approved for the treatment of advanced melanoma is talimogene laherparepvec (T-VEC or Imlygic®), the first oncolytic virus approved in the cancer treatment field. T-VEC is a herpes simplex virus type 1 genetically modified to reduce pathogenicity and increase immunogenicity. The mechanism of action not only involves the infection and death of melanoma cells but also the local and systemic stimulation of immune response. The main adverse side effects associated are fatigue, chills and fever, which tend to diminish with consecutive administrations [149,150].
3.1.5. Targeted Therapy
Cutaneous melanoma has a high rate of genetic alterations, with 7 out of 10 diagnosed cases having mutations in genes of the main signaling pathways. These oncogenic mutations promote the activation of cell signaling pathways, leading to the proliferation of malignant cells without any type of control [151,152]. The aim of target therapy is precisely to stop this widespread proliferation through the inhibition of the mutated genes [106,138]. The mitogen-activated protein kinase (MAPK) cascade is the most frequently mutated in melanoma, being composed of a receptor tyrosine kinase as well as RAF, RAS, MEK and ERK proteins [153]. Mutations in the BRAF, NRAS and NF1 genes are the most frequent and occur in approximately 50, 25 and 14% of melanoma cases, respectively [154,155]. As previously mentioned, there are already several molecules of this therapeutic class used for clinical management of melanoma, such as vemurafenib, dabrafenib and encorafenib (BRAF inhibitors), and trametinib, cobimetinib and binimetinib (MEK inhibitors). Although they are distinct between different BRAF and MEK inhibitors, arthralgia; fatigue; diarrhea; pyrexia; photosensitivity; and skin, cardiovascular and ocular toxicity are the main adverse effects associated with this type of therapy [155,156]. Finally, the main limitation inherent in this type of treatment is related to the development of resistance [32,154,156,157].
3.1.6. Combination of Therapeutic Approaches
As previously reported, immunotherapy and targeted therapy have shown good results in terms of efficacy; however, they are associated with some limitations. Thus, taking advantage of the targeted and immunotherapies, the American and European guidelines recommend a combination of therapeutic approaches [133,158,159,160]. There are already several combinations approved for clinical use (Table 3). One of the most recently approved combinations was nivolumab with relatlimab-rmbw (Opdualag) on 18 March 2022, by the FDA, for patients with unresectable or metastatic melanoma from 12 years of age [136].
Table 3.
Combination therapies for melanoma and years of approval by FDA and EMA.
| Type of Treatment | Name of Drugs | FDA Approval Date | EMA Approval Date | |
|---|---|---|---|---|
| Combinatorial approaches | Targeted therapy | Trametinib + Dabrafenib | 2014 | 2015 |
| Cobimetinib + Vemurafenib | 2015 | 2015 | ||
| Binimetinib + Encorafenib | 2018 | 2018 | ||
| Immunotherapy | Nivolumab + Ipilimumab | 2015 | 2016 | |
| Nivolumab + Relatlimab-rmbw | 2022 | - | ||
| Targeted therapy + Immunotherapy | Cobimetinib + Vemurafenib + Atezolizumab | 2020 | - | |
3.2. Ongoing Clinical Trials
As described so far, the last few years have been marked by tremendous efforts regarding the development of new therapeutic strategies for the treatment of melanoma [161]. Nevertheless, and as a result of the highly aggressive and heterogeneous nature of this malignancy, existing therapies remain limited [100]. Hereupon, new drugs and/or combinations are being tested to find more and better therapeutic options as described in Table 4. According to the ClinicalTrials.gov database, there are currently 853 clinical trials worldwide focusing on melanoma as an object of study [162]. Our research was restricted to ongoing interventional studies, listed as “not yet recruiting”, “recruiting”, “enrolling by invitation” and “active, not recruiting”.
Table 4.
Examples of melanoma treatments undergoing clinical trials.
| Clinical Trial Phase | Clinical Trial Description | Melanoma Stage | Sponsor | Estimated Starting or Completion Date | Trial ID |
|---|---|---|---|---|---|
| 1 | Safety and efficacy of the combination of ipilimumab and imatinib mesylate. | IV | M.D. Anderson Cancer Center | 2013–2024 | NCT01738139 |
| Safety of the combination of panobinostat (histone deacetylase inhibitor) and ipilimumab. | III/IV | H. Lee Moffitt Cancer Center and Research Institute | 2014–2023 | NCT02032810 | |
| Safety and efficacy of the combination of imiquimod and pembrolizumab. | IIIB-IV | Mayo Clinic | 2017–2023 | NCT03276832 | |
| Efficacy of intermittent dosing in the combination of vemurafenib and cobimetinib in the treatment of advanced BRAF V600 mutant melanoma with elevated levels of LDH. | IIIC-IV | H. Lee Moffitt Cancer Center and Research Institute | 2018–2022 | NCT03543969 | |
| Safety of the administration of neoadjuvant atezolizumab treatment before surgery in non-metastatic resectable melanoma. | I/II | The Methodist Hospital Research Institute | 2020–2025 | NCT04020809 | |
| Safety and efficacy of the combination of PeptiCRAd-1 and pembrolizumab. | Inoperable or metastatic | Valo Therapeutics Oy | 2022–2024 | NCT05492682 | |
| 2 | Efficacy of the combination of T-VEC and pembrolizumab. | III/IV | National Cancer Institute | 2017–2023 | NCT02965716 |
| Safety and effectiveness of the combination of PD-L1 (atezolizumab) and anti-VEGF (bevacizumab) therapies. | III/IV | Elizabeth Buchbinder | 2020–2023 | NCT04356729 | |
| Efficacy of the combination of T-VEC and nivolumab. | IIIB/C/D/IVM1a | The Netherlands Cancer Institute | 2020–2023 | NCT04330430 | |
| Safety and efficacy of the combination of pembrolizumab and infliximab. | III/IV | Massachusetts General Hospital | 2022–2025 | NCT05034536 | |
| Safety and efficacy of the combination of PD-1 antibody tislelizumab and dacarbazine. | III/IV | Henan Cancer Hospital | 2022–2024 | NCT05466474 | |
| 3 | Efficacy of the immunization with natural dendritic cells as adjuvant treatment after complete radical lymph node dissection or sentinel node procedure. | IIIB/C | Radboud University Medical Center | 2016–2024 | NCT02993315 |
| Analysis of the safety, efficacy and pharmacokinetic between the combination of atezolizumab, cobimetinib and vemurafenib or combination of only cobimetinib and vemurafenib in previously untreated BRAF V600 mutant melanoma. | IIIC/IV | Hoffmann-La Roche | 2017–2023 | NCT02908672 | |
| Assessment of fixed-dose combination of relatlimab and nivolumab versus nivolumab monotherapy after complete resection. | III/IV | Bristol-Myers Squibb | 2021–2025 | NCT05002569 | |
| Safety and efficacy of the combination of encorafenib and binimetinib in comparison to placebo in BRAF V600E/K mutant melanoma. | IIB/C | Pierre Fabre Medicament | 2022–2035 | NCT05270044 | |
| 4 | Tolerability and long-term safety of dabrafenib and trametinib, alone or in combination. | Advanced or metastatic | Novartis Pharmaceuticals | 2017–2027 | NCT03340506 |
| Safety of pembrolizumab. | III/IV | Merck Sharp & Dohme LLC | 2019–2026 | NCT03715205 |
Abbreviations: BRAF: serine/threonine protein kinase B-raf; LDH: lactate dehydrogenase; PD-L1: programmed death-ligand 1; PeptiCRAd-1: peptide-coated conditionally replicating adenovirus-1; T-VEC: talimogene laherparepvec; VEGF: vascular endothelial growth factor. Data collected from the ClinicalTrials.gov database [126].
3.3. Completed and Undergoing Innovative Nanotechnological Approaches on Clinical Trials
Nanotechnology-based strategies can play a key role in improving drug efficacy. The controlled delivery and targeting of loaded drugs at the cellular level overcoming the biological barriers is one of the added value of this type of option [43,44]. Currently, several types of nanosystems namely liposomes, solid lipid nanoparticles, and nanostructured lipid carriers and polymeric, metallic, and hybrid nanoparticles are under pre-clinical investigation [163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179]. Despite this, there is still no nanomedicine commercially available for patients with melanoma so far, being limited to volunteers in the different clinical trials. Presently, liposomal formulations and polymeric nanoparticles are the most investigated nanosystems in clinical trials as reported in Table 5.
Table 5.
Examples of completed clinical trials using different types of nanosystems for melanoma treatment.
| Nanosystem | Main Clinical Trial Description | Melanoma Stage | Sponsor | Starting and Completion Date | Trial ID |
|---|---|---|---|---|---|
| Liposomes | Safety, efficacy and pharmacokinetic profile study of vincristine sulfate liposomes (Phase 1). | III/IV | Acrotech Biopharma LLC | 2005–2007 | NCT00145041 |
| Safety and immunogenicity of a dendritic cells targeted-liposomal vaccine (Phase 1). | IV | Lipotek Pty Ltd. | 2009–2012 | NCT01052142 | |
| Polymeric nanoparticles | Safety and efficacy of nanoparticle albumin-bound paclitaxel (Phase 2). | Unresectable or metastatic | Jonsson Comprehensive Cancer Center | 2004–2010 | NCT00081042 |
| Safety and efficacy of the combination of nanoparticle albumin-bound paclitaxel with carboplatin (Phase 2). | IV | Alliance for Clinical Trials in Oncology | 2006–2010 | NCT00404235 | |
| Safety and efficacy of the combination of nanoparticle albumin-bound paclitaxel with avastin (Phase 2). | III/IV | Lynn E. Spitler, MD | 2007–2012 | NCT00462423 | |
| Comparison of the safety and efficacy of the combination of bevacizumab, carboplatin and nanoparticle albumin-bound paclitaxel with the combination of bevacizumab and temozolomide (Phase 2). | IV | Alliance for Clinical Trials in Oncology | 2008–2012 | NCT00626405 | |
| Comparison of the safety and efficacy of nanoparticle albumin-bound paclitaxel versus dacarbazine (Phase 3). | IV | Celgene | 2009–2014 | NCT00864253 | |
| Safety and pharmacokinetic and pharmacodynamic profile of PSMA-targeted PLA/PEG docetaxel nanoparticles (Phase 1). | Advanced or metastatic | BIND Therapeutics | 2011–2016 | NCT01300533 | |
| Comparison of the safety and efficacy of the combination of nanoparticle albumin-bound paclitaxel with bevacizumab versus ipilimumab alone (Phase 2). | IV | Academic and Community Cancer Research United | 2013–2019 | NCT02158520 |
Abbreviations: PEG: polyethylene glycol; PLA: polylactic acid; PSMA: prostate-specific membrane antigen. Data collected from the ClinicalTrials.gov database [126].
In the specific case of melanoma and to overcome the toxicity of paclitaxel in its free form, albumin-bound paclitaxel nanoparticles are one of the most investigated nanosystems in recent years. As an example, Hersh’s team conducted a phase 2 study (NCT00081042) that aimed to assess the safety and efficacy of ABI-007, an albumin-bound paclitaxel nanoparticle formulation, in the treatment of inoperable locally recurrent or metastatic melanoma. The study was divided into 2 cohorts of 37 patients each with or without previous chemotherapy, respectively. Overall, the formulation was well tolerated and demonstrated activity in both cohorts, with the median overall survival (OS) being 12.1 months for the chemotherapy-previously treated versus 9.6 months for the chemotherapy-naïve patients. Regarding this latter group, 8 patients discontinued treatment due to significant adverse effects such as neuropathy and myelosuppression [180].
Phase 2 clinical trial (NCT00404235) sought also to assess the safety and efficacy of albumin-bound paclitaxel nanoparticle but this time in combination with carboplatin. As in the previous clinical test, 76 patients with metastatic melanoma were divided into two cohorts depending on whether they had been previously treated with chemotherapy, a median OS of 10.9 and 11.1 months, respectively, was found. Furthermore, both cohorts observed some serious adverse effects such as neutropenia, leukopenia, fatigue, nausea and vomiting [181]. Later, the same sponsor promoted one more phase 2 study (NCT00626405) to analyze the safety and efficacy of chemotherapy (nanoparticle albumin-bound paclitaxel/carboplatin or temozolomide) and immunotherapy (bevacizumab) combined regimen. Although slightly more toxicity was observed in the bevacizumab-nanoparticle albumin-bound paclitaxel/carboplatin treatment group, efficacy results demonstrated a slight superiority of this, with a median OS of 13.9 months in comparison with the 12.3 months of the other cohort [182].
The comparison of the safety and efficacy of the nanoparticle albumin-bound paclitaxel/bevacizumab combination therapy regimen or the use of ipilimumab alone was investigated in another phase 2 clinical trial (NCT02158520). In the treatment group that integrated the nanoparticles, two complete and one partial response were found and serious adverse effects such as hemolytic uremic syndrome and rectal bleeding were observed. In contrast, only one partial response was achieved with immunotherapy as a single strategy. The majority of toxicities of this cohort were lymphocytopenia, benign or malignant neoplasms and acute kidney injury [183].
Furthermore, in a phase 2 study (NCT00462423), the safety and efficacy of the combination therapy of nanoparticle albumin-bound paclitaxel with avastin in 50 patients with metastatic melanoma were assessed. A median OS of 16.8 months was reached, and about 25% of patients developed some serious adverse effects. Pulmonary embolism, supraventricular tachycardia, macular edema, transient ischemic attack, nephrotic syndrome, renal tubular necrosis and dyspnea are examples [184].
Another example is related to a phase 3 study (NCT00864253) which aimed to compare the safety and efficacy of nanoparticle albumin-bound paclitaxel versus dacarbazine. A total of 529 patients with metastatic disease and chemotherapy-naïve patients were distributed into 2 groups. The nanoparticles demonstrated a clinical benefit compared to the use of dacarbazine (median OS 12.6 months versus 10.5, respectively) and a manageable safety profile. The most common adverse effects were neutropenia, leukopenia, alopecia and neuropathy [185].
One melanoma patient was included in the safety and pharmacokinetic and pharmacodynamic profile phase 1 study (NCT01300533) of another polymeric nanosystem containing docetaxel and targeting prostate-specific membrane antigen (PSMA). In terms of results, the formulation showed a different pharmacokinetic profile than the drug in the free form, with an increase in blood circulation time. Predictably and similarly to conventional docetaxel, the most common adverse effects included neutropenia, anemia, fatigue, alopecia, diarrhea and nausea [186].
In another example, seven volunteers with impaired liver function secondary to metastases participated in the characterization of the pharmacokinetic profile of a liposomal formulation of vincristine sulfate as well as in its safety and efficacy addressed in a single-arm phase 1 clinical trial (NCT00145041). A half-life of approximately 10 h, a clearance of 193 mL/h/m2, and a volume of distribution of 2722 mL/m2 were achieved. Moreover, blood, cardiac, gastrointestinal, respiratory and skin toxicities were detected [187]. The same principal investigator states, in another work [188], that the encapsulation of vincristine sulfate in liposomes resulted in a prolonged plasma half-life in comparison to the drug in its free form. The improved pharmacokinetic profile resulted in a greater accumulation of the compound at the tumor microenvironment, increased anticancer activity devoid of associated toxicity.
Finally, the safety and immunogenicity of escalating doses of a liposomal vaccine targeting dendritic cells, called Lipovaxin-MM, were also clinically evaluated in a phase 1 study (NCT01052142). Although most patients had disease progression, a partial response and disease stabilization in two other cases were found. Furthermore, the toxicological profile was quite favorable with more than 90% of adverse effects classified as grade 1 or 2 [189].
In addition, several other therapeutic strategies are now under clinical investigation and they are listed in Table 6. Others, such as the phase 1 clinical trial that pursues to investigate the safety and feasibility of a tumor mRNA-loaded liposomal vaccine will start in October 2022.
Table 6.
Examples of ongoing or programmed clinical trials using different types of nanosystems for melanoma treatment.
| Nanosystem | Main Clinical Trial Description | Melanoma Stage | Sponsor | Estimated Starting or Completion Date | Trial ID |
|---|---|---|---|---|---|
| Liposomes | Safety and tolerability of a liposomal tetravalent RNA-drug products vaccine (Phase 1). | IIIB/C/IV | BioNTech SE | 2015–2023 | NCT02410733 |
| Safety and feasibility of a tumor mRNA-loaded liposomal vaccine (Phase 1). | IIB-IV | University of Florida | 2022–2027 | NCT05264974 | |
| Lipid nanoparticles | Safety and efficacy of a lipid nanoparticle encapsulating mRNAs encoding a human T-cell co-stimulator and pro-inflammatory cytokines as monotherapy or in combination with durvalumab (Phase 1). | Advanced or metastatic | ModernaTX, Inc. | 2018–2023 | NCT03739931 |
| Polymeric nanoparticles | Safety and efficacy of the combination of nanoparticle albumin-bound paclitaxel with bevacizumab (Phase 1). | IV | Mayo Clinic | 2014–2025 | NCT02020707 |
| Comparison of the safety and efficacy of the combination of nanoparticle albumin-bound paclitaxel and carboplatin with and without endostatin (Phase 2). | Advanced | Peking University Cancer Hospital & Institute | 2019–2022 | NCT03917069 |
Data collected from the ClinicalTrials.gov database [126].
4. Conclusions
Nowadays, melanoma represents a serious public health problem. Due to its complexity and unpredictability, melanoma annually takes the lives of thousands of people around the world. Age, sex, ethnicity and individual phenotypic traits are examples of factors associated with increased risk. Nevertheless, melanoma is a highly preventable cancer since exposure to ultraviolet radiation is the principal risk factor. Thus, investment in the health literacy of the population is extremely important.
Additionally, melanoma is one of the cancers with the most complaints of pathology and dermatological malpractice due to misdiagnosis, which attests to the importance of early and accurate diagnosis and staging.
In terms of treatment, the current therapy for melanoma has reached a limit of clinical responses. Although the emergence of immuno and targeted therapies has resulted in an increase in the life expectancy of melanoma patients, some patients relapse or simply do not respond to these regimens. Therefore, the development of new and better therapies remains a priority for researchers, confirmed by the extensive number of ongoing preclinical and clinical trials. Herein, new drugs, combination therapies and nanotechnology-based strategies have been described which reveal a high potential for melanoma management. Overall, nanotechnological-based strategies show good histocompatibility, enhanced drug targeting, low toxicity and many other excellent characteristics, conferring broad application in the diagnosis and treatment of melanoma, particularly metastatic melanoma.
Author Contributions
Writing—original draft preparation, J.L.; writing—review and editing, J.L., C.M.P.R., M.M.G. and C.P.R.; supervision, C.M.P.R., M.M.G. and C.P.R.; funding acquisition, M.M.G. and C.P.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors acknowledge Fundacão para a Ciência e a Tecnologia (FCT) for financial support through Projects UIDB/00645/2020, UIDB/04138/2020, UIDP/04138/2020, PTDC/BTM-MAT/31794/2017, PTDC/QUI-QIN/0586/2020 and PhD fellowship SFRH/BD/148044/2019.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gilaberte Y., Prieto-Torres L., Pastushenko I., Juarranz Á. Anatomy and Function of the Skin. In: Hamblin M.R., Avci P., Prow T.W., editors. Nanoscience in Dermatology. Elsevier; Amsterdam, The Netherlands: 2016. pp. 1–14. [Google Scholar]
- 2.Park S. Biochemical, structural and physical changes in aging human skin, and their relationship. Biogerontology. 2022;23:275–288. doi: 10.1007/s10522-022-09959-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakur R., Batheja P., Kaushik D., Michniak B. Structural and Biochemical Changes in Aging Skin and Their Impact on Skin Permeability Barrier. In: Dayan N., editor. Skin Aging Handbook. Elsevier; Amsterdam, The Netherlands: 2009. pp. 55–90. [Google Scholar]
- 4.Mota A.H., Rijo P., Molpeceres J., Reis C.P. Broad overview of engineering of functional nanosystems for skin delivery. Int. J. Pharm. 2017;532:710–728. doi: 10.1016/j.ijpharm.2017.07.078. [DOI] [PubMed] [Google Scholar]
- 5.Lim H.W., Collins S.A.B., Resneck J.S., Bolognia J.L., Hodge J.A., Rohrer T.A., Van Beek M.J., Margolis D.J., Sober A.J., Weinstock M.A., et al. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017;76:958–972.e2. doi: 10.1016/j.jaad.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 6.Basra M.K., Shahrukh M. Burden of skin diseases. Expert Rev. Pharmacoecon. Outcomes Res. 2014;9:271–283. doi: 10.1586/erp.09.23. [DOI] [PubMed] [Google Scholar]
- 7.Richard M.A., Paul C., Nijsten T., Gisondi P., Salavastru C., Taieb C., Trakatelli M., Puig L., Stratigos A. Prevalence of most common skin diseases in Europe: A population-based study. J. Eur. Acad. Dermatol. Venereol. 2022;36:1088–1096. doi: 10.1111/jdv.18050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer Cancer Tomorrow Estimated Number of New Cases from 2020 to 2040 of Melanoma of Skin and Non-Melanoma Skin Cancer. [(accessed on 9 August 2022)]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16_17&single_unit=50000&group_cancers=1&multiple_cancers=1.
- 9.International Agency for Research on Cancer Cancer Tomorrow Estimated Number of Deaths from 2020 to 2040 of Melanoma of Skin and Non-Melanoma Skin Cancer. [(accessed on 9 August 2022)]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16_17&single_unit=5000&group_cancers=1&multiple_cancers=1&types=1.
- 10.Euro Melanoma and Global Coaliation for Melanoma Patient Advocacy . 2020 Melanoma Skin Cancer Report: Stemming the Global Epidemic. Global Coalition for Melanoma Patient Advocacy; Oldham, UK: 2020. [Google Scholar]
- 11.Linares M.A., Zakaria A., Nizran P. Skin Cancer. Prim. Care. 2015;42:645–659. doi: 10.1016/j.pop.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Lai V., Cranwell W., Sinclair R. Epidemiology of skin cancer in the mature patient. Clin. Dermatol. 2018;36:167–176. doi: 10.1016/j.clindermatol.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Rebecca V.W., Sondak V.K., Smalley K.S.M. A brief history of melanoma: From mummies to mutations. Melanoma Res. 2012;22:114–122. doi: 10.1097/CMR.0b013e328351fa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews N.H., Li W.-Q., Qureshi A.A., Weinstock M.A., Cho E. Epidemiology of Melanoma. In: Ward W.H., Farma J.M., editors. Cutaneous Melanoma, Etiology and Therapy. Codon Publications; Brisbane, Australia: 2017. pp. 139–149. [PubMed] [Google Scholar]
- 15.Dimitriou F., Krattinger R., Ramelyte E., Barysch M.J., Micaletto S., Dummer R., Goldinger S.M. The World of Melanoma: Epidemiologic, Genetic, and Anatomic Differences of Melanoma Across the Globe. Curr. Oncol. Rep. 2018;20:87. doi: 10.1007/s11912-018-0732-8. [DOI] [PubMed] [Google Scholar]
- 16.Sommer L. Generation of melanocytes from neural crest cells. Pigment. Cell Melanoma Res. 2011;24:411–421. doi: 10.1111/j.1755-148X.2011.00834.x. [DOI] [PubMed] [Google Scholar]
- 17.D’Mello S.A.N., Finlay G.J., Baguley B.C., Askarian-Amiri M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016;17:1144. doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caksa S., Baqai U., Aplin A.E. The future of targeted kinase inhibitors in melanoma. Pharmacol. Ther. 2022;239:108200. doi: 10.1016/j.pharmthera.2022.108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cichorek M., Wachulska M., Stasiewicz A. Heterogeneity of neural crest-derived melanocytes. Cent. Eur. J. Biol. 2013;8:315–330. doi: 10.2478/s11535-013-0141-1. [DOI] [Google Scholar]
- 20.Baroni A., Buommino E., De Gregorio V., Ruocco E., Ruocco V., Wolf R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012;30:257–262. doi: 10.1016/j.clindermatol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Carr S., Smith C., Wernberg J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. N. Am. 2020;100:1–12. doi: 10.1016/j.suc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Davey M.G., Miller N., McInerney N.M. A Review of Epidemiology and Cancer Biology of Malignant Melanoma. Cureus. 2021;13:e15087. doi: 10.7759/cureus.15087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-Guijarro E., Day C.P., Merlino G., Zaidi M.R. Genetically engineered mouse models of melanoma. Cancer. 2017;123:2089–2103. doi: 10.1002/cncr.30684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garbe C., Peris K., Hauschild A., Saiag P., Middleton M., Bastholt L., Grob J.-J., Malvehy J., Newton-Bishop J., Stratigos A.J., et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline—Update 2016. Eur. J. Cancer. 2016;63:201–217. doi: 10.1016/j.ejca.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Millet A., Martin A.R., Ronco C., Rocchi S., Benhida R. Metastatic Melanoma: Insights Into the Evolution of the Treatments and Future Challenges. Med. Res. Rev. 2017;37:98–148. doi: 10.1002/med.21404. [DOI] [PubMed] [Google Scholar]
- 26.Garbe C., Amaral T., Peris K., Hauschild A., Arenberger P., Bastholt L., Bataille V., del Marmol V., Dréno B., Fargnoli M.C., et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics—Update 2019. Eur. J. Cancer. 2020;126:141–158. doi: 10.1016/j.ejca.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Ciążyńska M., Kamińska-Winciorek G., Lange D., Lewandowski B., Reich A., Sławińska M., Pabianek M., Szczepaniak K., Hankiewicz A., Ułańska M., et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021;11:1–10. doi: 10.1038/s41598-021-83502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leiter U., Eigentler T., Garbe C. Epidemiology of Skin Cancer. In: Reichrath J., editor. Sunlight, VitaminD and Skin Cancer. Springer; New York, NY, USA: 2014. pp. 120–140. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A.K., Bharadwaj M., Mehrotra R. Skin Cancer Concerns in People of Color: Risk Factors and Prevention. Asian Pac. J. Cancer Prev. 2016;17:5257. doi: 10.22034/APJCP.2016.17.12.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zambrano-Román M., Padilla-Gutiérrez J.R., Valle Y., Muñoz-Valle J.F., Valdés-Alvarado E. Non-Melanoma Skin Cancer: A Genetic Update and Future Perspectives. Cancers. 2022;14:2371. doi: 10.3390/cancers14102371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mozzillo N., Ascierto P.A. Melanoma: The role of surgery in the era of new therapies. J. Transl. Med. 2014;12:195. doi: 10.1186/1479-5876-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozar I., Margue C., Rothengatter S., Haan C., Kreis S. Many ways to resistance: How melanoma cells evade targeted therapies. Biochim. Biophys. Acta Rev. Cancer. 2019;1871:313–322. doi: 10.1016/j.bbcan.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Ostrowski S.M., Fisher D.E. Biology of Melanoma. Hematol. Oncol. Clin. N. Am. 2021;35:29–56. doi: 10.1016/j.hoc.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Matias M., Pinho J.O., Penetra M.J., Campos G., Reis C.P., Gaspar M.M. The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval. Cells. 2021;10:3088. doi: 10.3390/cells10113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinho J.O., Lopes J., Albino M., Reis C., Matias M., Gaspar M.M. Advances in Nanotechnology-Related Strategies Against Melanoma. In: de Oliveira M.R., editor. Mitochondrial Dysfunction and Nanotherapeutics. Academic Press; Cambridge, MA, USA: 2021. pp. 385–424. [Google Scholar]
- 36.Silva C.O., Pinho J.O., Lopes J.M., Almeida A.J., Gaspar M.M., Reis C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics. 2019;11:22. doi: 10.3390/pharmaceutics11010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halwani A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics. 2022;14:106. doi: 10.3390/pharmaceutics14010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes J., Rodrigues C.M.P., Gaspar M.M., Reis C.P. How to Treat Melanoma? The Current Status of Innovative Nanotechnological Strategies and the Role of Minimally Invasive Approaches like PTT and PDT. Pharmaceutics. 2022;14:1817. doi: 10.3390/pharmaceutics14091817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayda S., Adeel M., Tuccinardi T., Cordani M., Rizzolio F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules. 2020;25:112. doi: 10.3390/molecules25010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodríguez F., Caruana P., la Fuente N.D., Español P., Gámez M., Balart J., Llurba E., Rovira R., Ruiz R., Martín-Lorente C., et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules. 2022;12:784. doi: 10.3390/biom12060784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019;4:e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Lázaro I., Mooney D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021;11:1469–1479. doi: 10.1038/s41563-021-01047-7. [DOI] [PubMed] [Google Scholar]
- 43.Beiu C., Giurcaneanu C., Grumezescu A.M., Holban A.M., Popa L.G., Mihai M.M. Nanosystems for Improved Targeted Therapies in Melanoma. J. Clin. Med. 2020;9:318. doi: 10.3390/jcm9020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shan X., Gong X., Li J., Wen J., Li Y., Zhang Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta. Pharm. Sin. B. 2022;12:3028–3048. doi: 10.1016/j.apsb.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apalla Z., Lallas A., Sotiriou E., Lazaridou E., Ioannides D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017;7:1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.International Agency for Research on Cancer Cancer Today Estimated Number of New Cases Worlwide in 2020, Melanoma of Skin, Both Sexes, All Ages (Global Cancer Observatory) [(accessed on 30 August 2022)]. Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=16&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&g.
- 47.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 48.International Agency for Research on Cancer Cancer Today Estimated Number of Deaths Worlwide in 2020, Melanoma of Skin, Both Sexes, All Ages (Global Cancer Observatory) [(accessed on 30 August 2022)]. Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=16&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&g.
- 49.Silva G.S., Rosenbach M. Climate change and dermatology: An introduction to a special topic, for this special issue. Int. J. Women’s Dermatol. 2021;7:3–7. doi: 10.1016/j.ijwd.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker E.R. The influence of climate change on skin cancer incidence—A review of the evidence. Int. J. Women’s Dermatol. 2021;7:17–27. doi: 10.1016/j.ijwd.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.International Agency for Research on Cancer Cancer Tomorrow Estimated Number of New Cases from 2020 to 2040 of Melanoma of Skin. [(accessed on 9 August 2022)]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16&single_unit=50000&group_cancers=1&multiple_cancers=1.
- 52.International Agency for Research on Cancer Cancer Tomorrow —Estimated Number of Deaths from 2020 to 2040 of Melanoma of Skin. [(accessed on 9 August 2022)]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16&single_unit=5000&group_cancers=1&multiple_cancers=1&types=1.
- 53.Wróbel S., Przybyło M., Stępień E. The Clinical Trial Landscape for Melanoma Therapies. J. Clin. Med. 2019;8:368. doi: 10.3390/jcm8030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dzwierzynski W.W. Melanoma Risk Factors and Prevention. Clin. Plast. Surg. 2021;48:543–550. doi: 10.1016/j.cps.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Le Clair M.Z., Cockburn M.G. Tanning bed use and melanoma: Establishing risk and improving prevention interventions. Prev. Med. Rep. 2016;3:139–144. doi: 10.1016/j.pmedr.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Ghissassi F., Baan R., Straif K., Grosse Y., Secretan B., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., et al. A review of human carcinogens-part D: Radiation. Lancet Oncol. 2009;10:751–752. doi: 10.1016/S1470-2045(09)70213-X. [DOI] [PubMed] [Google Scholar]
- 57.International Agency for Research on Cancer Cancer Today Estimated Number of New Cases Worldwide in 2020, Melanoma of Skin, Both Sexes, Per Countries (Global Cancer Observatory) [(accessed on 2 September 2022)]. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=population&mode_population=countries&population=900&populations=900&key=asr&sex=0&cancer=16&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=
- 58.Emri G., Paragh G., Tósaki Á., Janka E., Kollár S., Hegedűs C., Gellén E., Horkay I., Koncz G., Remenyik É. Ultraviolet radiation-mediated development of cutaneous melanoma: An update. J. Photochem. Photobiol. B. Biol. 2018;185:169–175. doi: 10.1016/j.jphotobiol.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 59.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Solar and Ultraviolet Radiation. In: Galichet L., editor. Radiation—A Review of Human Carcinogens, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 100D. International Agency for Research on Cancer; Lyon, France: 2012. pp. 35–90. [Google Scholar]
- 60.Brenner M., Hearing V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paluncic J., Kovacevic Z., Jansson P.J., Kalinowski D., Merlot A.M., Huang M.L.H., Lok H.C., Sahni S., Lane D.J.R., Richardson D.R. Roads to melanoma: Key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim. Biophys. Acta—Mol. Cell Res. 2016;1863:770–784. doi: 10.1016/j.bbamcr.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 62.International Agency for Research on Cancer Cancer Today Estimated Number of New Skin Melanoma Cases Worldwide in 2020 for Both Sexes (Global Cancer Observatory) [(accessed on 1 September 2022)]. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=16&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer.
- 63.Morgese F., Sampaolesi C., Torniai M., Conti A., Ranallo N., Giacchetti A., Serresi S., Onofri A., Burattini M., Ricotti G., et al. Gender Differences and Outcomes in Melanoma Patients. Oncol. Ther. 2020;8:103–114. doi: 10.1007/s40487-020-00109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bellenghi M., Puglisi R., Pontecorvi G., De Feo A., Carè A., Mattia G. Sex and Gender Disparities in Melanoma. Cancers. 2020;12:1819. doi: 10.3390/cancers12071819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orthaber K., Pristovnik M., Skok K., Perić B., Maver U. Skin Cancer and Its Treatment: Novel Treatment Approaches with Emphasis on Nanotechnology. J. Nanomater. 2018;94:409–420. doi: 10.1155/2017/2606271. [DOI] [Google Scholar]
- 66.Naik P.P., Farrukh S.N. Influence of Ethnicities and Skin Color Variations in Different Populations: A Review. Skin Pharmacol. Physiol. 2021;35:65–76. doi: 10.1159/000518826. [DOI] [PubMed] [Google Scholar]
- 67.Deng L., Xu S. Adaptation of human skin color in various populations. Hereditas. 2017;155:1–12. doi: 10.1186/s41065-017-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hessler M., Jalilian E., Xu Q., Reddy S., Horton L., Elkin K., Manwar R., Tsoukas M., Mehregan D., Avanaki K. Melanoma Biomarkers and Their Potential Application for in Vivo Diagnostic Imaging Modalities. Int. J. Mol. Sci. 2020;21:9583. doi: 10.3390/ijms21249583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nasti T.H., Timares L. MC1R, Eumelanin and Pheomelanin: Their Role in Determining the Susceptibility to Skin Cancer. Photochem. Photobiol. 2015;91:188–200. doi: 10.1111/php.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saginala K., Barsouk A., Aluru J.S., Rawla P., Barsouk A., Sanguedolce F., Murray-Stewart T. Epidemiology of Melanoma. Med. Sci. 2021;9:63. doi: 10.3390/medsci9040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Podlipnik S., Potrony M., Puig S. Genetic markers for characterization and prediction of prognosis of melanoma subtypes: A 2021 update. Ital. J. Dermatol. Venereol. 2021;156:322–330. doi: 10.23736/S2784-8671.21.06957-1. [DOI] [PubMed] [Google Scholar]
- 72.Zocchi L., Lontano A., Merli M., Dika E., Nagore E., Quaglino P., Puig S., Ribero S. Familial Melanoma and Susceptibility Genes: A Review of the Most Common Clinical and Dermoscopic Phenotypic Aspect, Associated Malignancies and Practical Tips for Management. J. Clin. Med. 2021;10:3760. doi: 10.3390/jcm10163760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potrony M., Badenas C., Aguilera P., Puig-Butille J.A., Carrera C., Malvehy J., Puig S. Update in genetic susceptibility in melanoma. Ann. Transl. Med. 2015;3:210. doi: 10.3978/j.issn.2305-5839.2015.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ming Z., Lim S.Y., Kefford R.F., Rizos H. Mitogen-activated protein kinase dependency in BRAF/RAS wild-type melanoma: A rationale for combination inhibitors. Pigment. Cell Melanoma Res. 2020;33:345–357. doi: 10.1111/pcmr.12824. [DOI] [PubMed] [Google Scholar]
- 75.Craig S., Earnshaw C.H., Virós A. Ultraviolet light and melanoma. J. Pathol. 2018;244:578–585. doi: 10.1002/path.5039. [DOI] [PubMed] [Google Scholar]
- 76.O’Neill C.H., Scoggins C.R. Melanoma. J. Surg. Oncol. 2019;120:873–881. doi: 10.1002/jso.25604. [DOI] [PubMed] [Google Scholar]
- 77.Imbernón-Moya A., Podlipnik S., Malvehy J., Puig S. Initial Evaluation of Patients with Pigmented Skin Lesions. Actas Dermosifiliogr. 2016;107:616–618. doi: 10.1016/j.ad.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 78.Horrell E.M.W., Wilson K., D’Orazio J.A. Melanoma—Epidemiology, Risk Factors, and the Role of Adaptive Pigmentation. In: Murph M., editor. Melanoma—Current Clinical Management and Future Therapeutics. IntechOpen; London, UK: 2015. [Google Scholar]
- 79.Coricovac D., Dehelean C., Moaca E.A., Pinzaru I., Bratu T., Navolan D., Boruga O. Cutaneous melanoma-a long road from experimental models to clinical outcome: A review. Int. J. Mol. Sci. 2018;19:1566. doi: 10.3390/ijms19061566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Betancourt L.H., Szasz A.M., Kuras M., Rodriguez Murillo J., Sugihara Y., Pla I., Horvath Z., Pawłowski K., Rezeli M., Miharada K., et al. The Hidden Story of Heterogeneous B-raf V600E Mutation Quantitative Protein Expression in Metastatic Melanoma—Association with Clinical Outcome and Tumor Phenotypes. Cancers. 2019;11:1981. doi: 10.3390/cancers11121981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wagstaff W., Mwamba R.N., Grullon K., Armstrong M., Zhao P., Hendren-Santiago B., Qin K.H., Li A.J., Hu D.A., Youssef A., et al. Melanoma: Molecular genetics, metastasis, targeted therapies, immunotherapies, and therapeutic resistance. Genes Dis. 2022;9:1608–1623. doi: 10.1016/j.gendis.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Šitum M., Buljan M., Kolić M., Vučić M. Melanoma-Clinical, Dermatoscopical, and Histopathological Morphological Characteristics. Acta Dermatovenerol. Croat. 2014;22:1–12. [PubMed] [Google Scholar]
- 83.Duncan L.M.D. The Classification of Cutaneous Melanoma. Hematol. Oncol. Clin. N. Am. 2009;23:501–513. doi: 10.1016/j.hoc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 84.Ammirati C.T., Hruza G.J. Clinical presentations of cutaneous melanoma. Facial Plast. Surg. Clin. N. Am. 2005;13:33–46. doi: 10.1016/j.fsc.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 85.Nakamura Y., Fujisawa Y. Diagnosis and Management of Acral Lentiginous Melanoma. Curr. Treat. Options Oncol. 2018;19:1–11. doi: 10.1007/s11864-018-0560-y. [DOI] [PubMed] [Google Scholar]
- 86.Goydos J.S., Shoen S.L. Acral lentiginous melanoma. In: Kaufman H.L., Mehnert J.M., editors. Cancer Treatment and Research. Volume 167. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2016. pp. 321–329. [DOI] [PubMed] [Google Scholar]
- 87.Cabrera R., Recule F. Unusual Clinical Presentations of Malignant Melanoma: A Review of Clinical and Histologic Features with Special Emphasis on Dermatoscopic Findings. Am. J. Clin. Dermatol. 2018;19:15–23. doi: 10.1007/s40257-018-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cormier J., Voss R., Woods T., Cromwell K., Nelson K. Improving outcomes in patients with melanoma: Strategies to ensure an early diagnosis. Patient Relat. Outcome Meas. 2015;6:229. doi: 10.2147/PROM.S69351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greinert R., Boniol M. Skin cancer—Primary and secondary prevention (information campaigns and screening)—With a focus on children & sunbeds. Prog. Biophys. Mol. Biol. 2011;107:473–476. doi: 10.1016/j.pbiomolbio.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 90.Fontanillas P., Alipanahi B., Furlotte N.A., Johnson M., Wilson C.H., Pitts S.J., Gentleman R., Auton A. Disease risk scores for skin cancers. Nat. Commun. 2021;12:1–13. doi: 10.1038/s41467-020-20246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berwick M., Erdei E., Hay J. Melanoma epidemiology and public health. Dermatol. Clin. 2009;27:205–214. doi: 10.1016/j.det.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abbasi N.R., Shaw H.M., Rigel D.S., Friedman R.J., McCarthy W.H., Osman I., Kopf A.W., Polsky D. Early Diagnosis of Cutaneous Melanoma: Revisiting the ABCD Criteria. JAMA. 2004;292:2771–2776. doi: 10.1001/jama.292.22.2771. [DOI] [PubMed] [Google Scholar]
- 93.Dinnes J., Deeks J.J., Grainge M.J., Chuchu N., Ferrante di Ruffano L., Matin R.N., Thomson D.R., Wong K.Y., Aldridge R.B., Abbott R., et al. Visual inspection for diagnosing cutaneous melanoma in adults. Cochrane Database Syst. Rev. 2018;12:CD013194. doi: 10.1002/14651858.CD013194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Quaglino P., Fava P., Broganelli P., Burzi L., Marra E., Ribero S., Fierro M.T. Epidemiology, Prevention and Clinical Diagnosis of Melanoma. In: Cafiero F., De Cian F., editors. Current Management of Melanoma. Springer; Cham, Switzerland: 2021. pp. 7–16. [Google Scholar]
- 95.Ward W.H., Lambreton F., Goel N., Yu J.Q., Farma J.M. Clinical Presentation and Staging of Melanoma. In: Ward W.H., Farma J.M., editors. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; Brisbane, Australia: 2017. pp. 79–89. [PubMed] [Google Scholar]
- 96.Dorrell D.N., Strowd L.C. Skin Cancer Detection Technology. Dermatol. Clin. 2019;37:527–536. doi: 10.1016/j.det.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 97.Gualco M. Histopathological Examination: The Keystone of Treatment of Melanoma. In: Cafiero F., De Cian F., editors. Current Management of Melanoma. Springer; Cham, Switzerland: 2021. pp. 27–37. [Google Scholar]
- 98.Osella-Abate S., Bertero L., Senetta R., Mariani S., Lisa F., Coppola V., Metovic J., Pasini B., Puig S.S., Fierro M.T., et al. TERT Promoter Mutations are Associated with Visceral Spreading in Melanoma of the Trunk. Cancers. 2019;11:452. doi: 10.3390/cancers11040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dalmasso B., Vanni I., Bruno W., Andreotti V., Pastorino L., Spagnolo F., Ghiorzo P. Molecular Assessment in Patients with Melanoma: When and Why? In: Cafiero F., De Cian F., editors. Current Management of Melanoma. Springer; Cham, Switzerland: 2021. pp. 39–45. [Google Scholar]
- 100.Davis L.E., Shalin S.C., Tackett A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019;20:1366–1379. doi: 10.1080/15384047.2019.1640032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hartman R.I., Lin J.Y. Cutaneous Melanoma—A Review in Detection, Staging, and Management. Hematol. Oncol. Clin. N. Am. 2019;33:25–38. doi: 10.1016/j.hoc.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 102.Meng X., Chen J., Zhang Z., Li K., Li J., Yu Z., Zhang Y. Non-invasive optical methods for melanoma diagnosis. Photodiagnosis Photodyn. Ther. 2021;34:102266. doi: 10.1016/j.pdpdt.2021.102266. [DOI] [PubMed] [Google Scholar]
- 103.Spagnolo F., Boutros A., Croce E., Tanda E., Cecchi F., Queirolo P. New Melanoma Staging: Prognostic Factors. In: Cafiero F., De Cian F., editors. Current Management of Melanoma. Springer; Cham, Switzerland: 2021. pp. 47–53. [Google Scholar]
- 104.Gershenwald J.E., Scolyer R.A., Hess K.R., Sondak V.K., Long G.V., Ross M.I., Lazar A.J., Faries M.B., Kirkwood J.M., McArthur G.A., et al. Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017;67:472. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garbe C., Amaral T., Peris K., Hauschild A., Arenberger P., Basset-Seguin N., Bastholt L., Bataille V., del Marmol V., Dréno B., et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer. 2022;170:256–284. doi: 10.1016/j.ejca.2022.04.018. [DOI] [PubMed] [Google Scholar]
- 106.Eddy K., Chen S. Overcoming Immune Evasion in Melanoma. Int. J. Mol. Sci. 2020;21:8984. doi: 10.3390/ijms21238984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Merlino G., Herlyn M., Fisher D.E., Bastian B.C., Flaherty K.T., Davies M.A., Wargo J.A., Curiel-Lewandrowski C., Weber M.J., Leachman S.A., et al. The state of melanoma: Challenges and opportunities. Pigment. Cell Melanoma Res. 2016;29:404–416. doi: 10.1111/pcmr.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Michielin O., Atkins M.B., Koon H.B., Dummer R., Ascierto P.A. Evolving impact of long-term survival results on metastatic melanoma treatment. J. Immunother. Cancer. 2020;8:e000948. doi: 10.1136/jitc-2020-000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Switzer B., Puzanov I., Skitzki J.J., Hamad L., Ernstoff M.S. Managing Metastatic Melanoma in 2022: A Clinical Review. JCO Oncol. Pract. 2022;18:335–351. doi: 10.1200/OP.21.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grzywa T.M., Paskal W., Włodarski P.K. Intratumor and Intertumor Heterogeneity in Melanoma. Transl. Oncol. 2017;10:956. doi: 10.1016/j.tranon.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ng M.F., Simmons J.L., Boyle G.M. Heterogeneity in Melanoma. Cancers. 2022;14:3030. doi: 10.3390/cancers14123030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Food and Drug Administration Drugs@FDA: FDA-Approved Drugs. [(accessed on 10 August 2022)]; Available online: https://www.accessdata.fda.gov/scripts/cder/daf/
- 113.European Medicines Agency Medicines Database. [(accessed on 10 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines.
- 114.Henriques V., Martins T., Link W., Ferreira B.I. The Emerging Therapeutic Landscape of Advanced Melanoma. Curr. Pharm. Des. 2018;24:549–558. doi: 10.2174/1381612824666180125093357. [DOI] [PubMed] [Google Scholar]
- 115.Kozovska Z., Gabrisova V., Kucerova L. Malignant melanoma: Diagnosis, treatment and cancer stem cells. Neoplasma. 2016;63:510–517. doi: 10.4149/neo_2016_403. [DOI] [PubMed] [Google Scholar]
- 116.Garbe C., Amaral T., Peris K., Hauschild A., Arenberger P., Bastholt L., Bataille V., del Marmol V., Dréno B., Fargnoli M.C., et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2019. Eur. J. Cancer. 2020;126:159–177. doi: 10.1016/j.ejca.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 117.Joyce K.M. Surgical Management of Melanoma. In: Ward W.H., Farma J.M., editors. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; Brisbane, Australia: 2017. pp. 91–100. [PubMed] [Google Scholar]
- 118.Friedman E.B., Thompson J.F. Continuing and new roles for surgery in the management of patients with stage IV melanoma. Melanoma Manag. 2018;5:MMT03. doi: 10.2217/mmt-2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bello D.M. Indications for the surgical resection of stage IV disease. J. Surg. Oncol. 2019;119:249–261. doi: 10.1002/jso.25326. [DOI] [PubMed] [Google Scholar]
- 120.Enomoto L.M., Levine E.A., Shen P., Votanopoulos K.I. Role of Surgery for Metastatic Melanoma. Curr. Manag. Melanoma. 2021;100:127–139. doi: 10.1016/j.suc.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 121.Spagnolo F., Tanda E., Boutros A., Cecchi F., Queirolo P. Medical Treatment of Melanoma: Adjuvant and Neoadjuvant Therapies. In: Cafiero F., De Cian F., editors. Current Management of Melanoma. Springer; Cham, Switzerland: 2021. pp. 157–165. [Google Scholar]
- 122.Spagnolo F., Boutros A., Tanda E., Cecchi F., Queirolo P. Systemic Treatment in Advanced Melanoma. In: Cafiero F., De Cian F., editors. Current Management of Melanoma. Springer; Cham, Switzerland: 2021. pp. 167–174. [Google Scholar]
- 123.Pinho J.O., Matias M., Gaspar M.M. Emergent nanotechnological strategies for systemic chemotherapy against melanoma. Nanomaterials. 2019;9:1455. doi: 10.3390/nano9101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi W. Radiation Therapy for Melanoma. In: Ward W.H., Farma J.M., editors. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; Brisbane, Australia: 2017. pp. 101–120. [PubMed] [Google Scholar]
- 125.Sayan A., Plant R., Eccles B., Davies C., Ilankovan V. Recent advances in the management of cutaneous malignant melanoma: Our case cohort. Br. J. Oral Maxillofac. Surg. 2021;59:534–545. doi: 10.1016/j.bjoms.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 126.United States National Institutes of Health ClinicalTrials.gov Database. [(accessed on 10 August 2022)]; Available online: https://clinicaltrials.gov/
- 127.Wilson M.A., Schuchter L.M. Chemotherapy for Melanoma. In: Kaufman H., Mehnert J., editors. Melanoma. Cancer Treatment and Research. Volume 167. Springer; Cham, Switzerland: 2016. pp. 209–229. [DOI] [PubMed] [Google Scholar]
- 128.Gupta A., Gomes F., Lorigan P. The role for chemotherapy in the modern management of melanoma. Melanoma Manag. 2017;4:125. doi: 10.2217/mmt-2017-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luke J.J., Schwartz G.K. Chemotherapy in the Management of Advanced Cutaneous Malignant Melanoma. Clin. Dermatol. 2013;31:290–297. doi: 10.1016/j.clindermatol.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Middleton M.R., Grob J.J., Aaronson N., Fierlbeck G., Tilgen W., Seiter S., Gore M., Aamdal S., Cebon J., Coates A., et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 131.Gogas H.J., Kirkwood J.M., Sondak V.K. Chemotherapy for metastatic melanoma. Cancer. 2007;109:455–464. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- 132.Yang A.S., Chapman P.B. The History and Future of Chemotherapy for Melanoma. Hematol. Oncol. Clin. N. Am. 2009;23:583. doi: 10.1016/j.hoc.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jenkins R.W., Fisher D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021;141:23–31. doi: 10.1016/j.jid.2020.03.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kuryk L., Bertinato L., Staniszewska M., Pancer K., Wieczorek M., Salmaso S., Caliceti P., Garofalo M. From Conventional Therapies to Immunotherapy: Melanoma Treatment in Review. Cancers. 2020;12:3057. doi: 10.3390/cancers12103057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Queirolo P., Boutros A., Tanda E., Spagnolo F., Quaglino P. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: A model of cancer immunotherapy. Semin. Cancer Biol. 2019;59:290–297. doi: 10.1016/j.semcancer.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 136.Food and Drug Administration FDA Approves Opdualag for Unresectable or Metastatic Melanoma. [(accessed on 11 August 2022)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-opdualag-unresectable-or-metastatic-melanoma.
- 137.Zhang Y., Zhang Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020;17:807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Onitilo A.A., Wittig J.A. Principles of Immunotherapy in Melanoma. Surg. Clin. N. Am. 2020;100:161–173. doi: 10.1016/j.suc.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 139.Kennedy L.B., Salama A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 140.Bagchi S., Yuan R., Engleman E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 141.Chocarro L., Blanco E., Zuazo M., Arasanz H., Bocanegra A., Fernández-Rubio L., Morente P., Fernández-Hinojal G., Echaide M., Garnica M., et al. Understanding LAG-3 Signaling. Int. J. Mol. Sci. 2021;22:5282. doi: 10.3390/ijms22105282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maruhashi T., Sugiura D., Okazaki I.M., Okazaki T. LAG-3: From molecular functions to clinical applications. J. Immunother. Cancer. 2020;8:e001014. doi: 10.1136/jitc-2020-001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tawbi H.A., Schadendorf D., Lipson E.J., Ascierto P.A., Matamala L., Castillo Gutiérrez E., Rutkowski P., Gogas H.J., Lao C.D., De Menezes J.J., et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022;386:24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vukadin S., Khaznadar F., Kizivat T., Vcev A., Smolic M. Molecular Mechanisms of Resistance to Immune Checkpoint Inhibitors in Melanoma Treatment: An Update. Biomedicines. 2021;9:835. doi: 10.3390/biomedicines9070835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ralli M., Botticelli A., Visconti I.C., Angeletti D., Fiore M., Marchetti P., Lambiase A., de Vincentiis M., Greco A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020;2020:9235638. doi: 10.1155/2020/9235638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang A.C., Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: Understanding the molecular basis for immune sensitivity and resistance. Nat. Immunol. 2022;23:660–670. doi: 10.1038/s41590-022-01141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Simeone E., Grimaldi A.M., Festino L., Trojaniello C., Vitale M.G., Vanella V., Palla M., Ascierto P.A. Immunotherapy in metastatic melanoma: A novel scenario of new toxicities and their management. Melanoma Manag. 2019;6:MMT30. doi: 10.2217/mmt-2019-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bommareddy P.K., Patel A., Hossain S., Kaufman H.L. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am. J. Clin. Dermatol. 2017;18:1–15. doi: 10.1007/s40257-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Larocca C.A., LeBoeuf N.R., Silk A.W., Kaufman H.L. An Update on the Role of Talimogene Laherparepvec (T-VEC) in the Treatment of Melanoma: Best Practices and Future Directions. Am. J. Clin. Dermatol. 2020;21:821–832. doi: 10.1007/s40257-020-00554-8. [DOI] [PubMed] [Google Scholar]
- 151.Domingues B., Lopes J.M., Soares P., Pópulo H. Melanoma treatment in review. Immuno Targets Ther. 2018;7:35. doi: 10.2147/ITT.S134842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tanda E.T., Vanni I., Boutros A., Andreotti V., Bruno W., Ghiorzo P., Spagnolo F. Current State of Target Treatment in BRAF Mutated Melanoma. Front. Mol. Biosci. 2020;7:154. doi: 10.3389/fmolb.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Amaral T., Sinnberg T., Meier F., Krepler C., Levesque M., Niessner H., Garbe C. The mitogen-activated protein kinase pathway in melanoma part I—Activation and primary resistance mechanisms to BRAF inhibition. Eur. J. Cancer. 2017;73:85–92. doi: 10.1016/j.ejca.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 154.Czarnecka A.M., Bartnik E., Fiedorowicz M., Rutkowski P. Targeted Therapy in Melanoma and Mechanisms of Resistance. Int. J. Mol. Sci. 2020;21:4576. doi: 10.3390/ijms21134576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mackiewicz J., Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Contemp. Oncol. 2018;22:68. doi: 10.5114/wo.2018.73890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kasakovski D., Skrygan M., Gambichler T., Susok L. Advances in Targeting Cutaneous Melanoma. Cancers. 2021;13:2090. doi: 10.3390/cancers13092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee S., Rauch J., Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020;21:1102. doi: 10.3390/ijms21031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Munhoz R.R., Postow M.A. Combinatorial Approaches to the Treatment of Advanced Melanoma. Hematol. Oncol. Clin. N. Am. 2021;35:145–158. doi: 10.1016/j.hoc.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Haas L., Elewaut A., Gerard C.L., Umkehrer C., Leiendecker L., Pedersen M., Krecioch I., Hoffmann D., Novatchkova M., Kuttke M., et al. Acquired resistance to anti-MAPK targeted therapy confers an immune-evasive tumor microenvironment and cross-resistance to immunotherapy in melanoma. Nat. Cancer. 2021;2:693–708. doi: 10.1038/s43018-021-00221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu C., Liu X., Yang J., Zhang M., Jin H., Ma X., Shi H. Combination of Immunotherapy with Targeted Therapy: Theory and Practice in Metastatic Melanoma. Front. Immunol. 2019;10:990. doi: 10.3389/fimmu.2019.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Villani A., Potestio L., Fabbrocini G., Troncone G., Malapelle U., Scalvenzi M. The Treatment of Advanced Melanoma: Therapeutic Update. Int. J. Mol. Sci. 2022;23:6388. doi: 10.3390/ijms23126388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.U.S. National Institutes of Health Recruiting, Not Yet Recruiting, Active, Not Recruiting, Enrolling by Invitation Studies|Interventional Studies|Melanoma. [(accessed on 4 September 2022)]; Available online: https://clinicaltrials.gov/ct2/results?cond=Melanoma&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=Intr&rslt=&Search=Apply.
- 163.Pinho J.O., Amaral J.D., Castro R.E., Rodrigues C.M., Casini A., Soveral G., Gaspar M.M. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine. 2019;14:835–850. doi: 10.2217/nnm-2018-0388. [DOI] [PubMed] [Google Scholar]
- 164.Nave M., Castro R.E., Rodrigues C.M.P., Casini A., Soveral G., Gaspar M.M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine. 2016;11:1817–1830. doi: 10.2217/nnm-2016-0086. [DOI] [PubMed] [Google Scholar]
- 165.Merino M., Contreras A., Casares N., Troconiz I.F., ten Hagen T.L., Berraondo P., Zalba S., Garrido M.J. A new immune-nanoplatform for promoting adaptive antitumor immune response. Nanomed. Nanotechnol. Biol. Med. 2019;17:13–25. doi: 10.1016/j.nano.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 166.Xu L., Zhang W., Park H.B., Kwak M., Oh J., Lee P.C.W., Jin J.O. Indocyanine green and poly I:C containing thermo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J. Immunother. Cancer. 2019;7:1–14. doi: 10.1186/s40425-019-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goto P.L., Siqueira-Moura M.P., Tedesco A.C. Application of aluminum chloride phthalocyanine-loaded solid lipid nanoparticles for photodynamic inactivation of melanoma cells. Int. J. Pharm. 2017;518:228–241. doi: 10.1016/j.ijpharm.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 168.Clemente N., Ferrara B., Gigliotti C.L., Boggio E., Capucchio M.T., Biasibetti E., Schiffer D., Mellai M., Annovazzi L., Cangemi L., et al. Solid Lipid Nanoparticles Carrying Temozolomide for Melanoma Treatment. Preliminary in Vitro and in Vivo Studies. Int. J. Mol. Sci. 2018;19:255. doi: 10.3390/ijms19020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Malta R., Loureiro J.B., Costa P., Sousa E., Pinto M., Saraiva L., Amaral M.H. Development of lipid nanoparticles containing the xanthone LEM2 for topical treatment of melanoma. J. Drug Deliv. Sci. Technol. 2021;61:102226. doi: 10.1016/j.jddst.2020.102226. [DOI] [Google Scholar]
- 170.Liu D., Liu Z., Wang L., Zhang C., Zhang N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B. Biointerfaces. 2011;85:262–269. doi: 10.1016/j.colsurfb.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 171.Ferraz L.S., Watashi C.M., Colturato-Kido C., Pelegrino M.T., Paredes-Gamero E.J., Weller R.B., Seabra A.B., Rodrigues T. Antitumor Potential of S-Nitrosothiol-Containing Polymeric Nanoparticles against Melanoma. Mol. Pharm. 2018;15:1160–1168. doi: 10.1021/acs.molpharmaceut.7b01001. [DOI] [PubMed] [Google Scholar]
- 172.Zhang J., Liu P., Zhang Z., Han J., Yang X., Wang A., Zhang X. Apatinib-loaded nanoparticles inhibit tumor growth and angiogenesis in a model of melanoma. Biochem. Biophys. Res. Commun. 2020;521:296–302. doi: 10.1016/j.bbrc.2019.10.084. [DOI] [PubMed] [Google Scholar]
- 173.Ledezma D.K., Balakrishnan P.B., Cano-Mejia J., Sweeney E.E., Hadley M., Bollard C.M., Villagra A., Fernandes R. Indocyanine Green-Nexturastat A-PLGA Nanoparticles Combine Photothermal and Epigenetic Therapy for Melanoma. Nanomaterials. 2020;10:161. doi: 10.3390/nano10010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pandesh S., Haghjooy Javanmard S., Shakeri-Zadeh A., Shokrani P. Targeted Photothermal Therapy of Melanoma in C57BL/6 Mice using Fe3O4@Au Core-shell Nanoparticles and Near-infrared Laser. J. Biomed. Phys. Eng. 2021;11:29. doi: 10.31661/jbpe.v0i0.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang X., Teodoro J.G., Nadeau J.L. Intratumoral gold-doxorubicin is effective in treating melanoma in mice. Nanomedicine. 2015;11:1365–1375. doi: 10.1016/j.nano.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 176.Lopes J., Ferreira-Gonçalves T., Figueiredo I.V., Rodrigues C.M.P., Ferreira H., Ferreira D., Viana A.S., Faísca P., Gaspar M.M., Coelho J.M.P., et al. Proof-of-Concept Study of Multifunctional Hybrid Nanoparticle System Combined with NIR Laser Irradiation for the Treatment of Melanoma. Biomolecules. 2021;11:511. doi: 10.3390/biom11040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Matos C.P., Albino M., Lopes J., Viana A.S., Côrte-Real L., Mendes F., Pessoa J.C., Tomaz A.I., Reis C.P., Gaspar M.M., et al. New iron(III) anti-cancer aminobisphenolate/phenanthroline complexes: Enhancing their therapeutic potential using nanoliposomes. Int. J. Pharm. 2022;623:121925. doi: 10.1016/j.ijpharm.2022.121925. [DOI] [PubMed] [Google Scholar]
- 178.Cruz N., Pinho J.O., Soveral G., Ascensão L., Matela N., Reis C., Gaspar M.M. A Novel Hybrid Nanosystem Integrating Cytotoxic and Magnetic Properties as a Tool to Potentiate Melanoma Therapy. Nanomaterials. 2020;10:693. doi: 10.3390/nano10040693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lopes J., Coelho J.M.P., Vieira P.M.C., Viana A.S., Gaspar M.M., Reis C. Preliminary assays towards melanoma cells using phototherapy with gold-based nanomaterials. Nanomaterials. 2020;10:1536. doi: 10.3390/nano10081536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hersh E.M., O’Day S.J., Ribas A., Samlowski W.E., Gordon M.S., Shechter D.E., Clawson A.A., Gonzalez R. A phase 2 clinical trial of nab-paclitaxel in previously treated and chemotherapy-naive patients with metastatic melanoma. Cancer. 2010;116:155–163. doi: 10.1002/cncr.24720. [DOI] [PubMed] [Google Scholar]
- 181.Kottschade L.A., Suman V.J., Amatruda T., McWilliams R.R., Mattar B.I., Nikcevich D.A., Behrens R., Fitch T.R., Jaslowski A.J., Markovic S.N. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma. Cancer. 2011;117:1704–1710. doi: 10.1002/cncr.25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kottschade L.A., Suman V.J., Perez D.G., McWilliams R.R., Kaur J.S., Amatruda T.T., Geoffroy F.J., Gross H.M., Cohen P.A., Jaslowski A.J., et al. A randomized phase 2 study of temozolomide and bevacizumab or nab-paclitaxel, carboplatin, and bevacizumab in patients with unresectable stage IV melanoma. Cancer. 2013;119:586–592. doi: 10.1002/cncr.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.U.S. National Institutes of Health Nab-Paclitaxel and Bevacizumab or Ipilimumab as First-Line Therapy in Treating Patients with Stage IV Melanoma That Cannot Be Removed by Surgery—Study Results. [(accessed on 13 August 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02158520?term=NCT02158520&draw=2&rank=1.
- 184.U.S. National Institutes of Health Abraxane and Avastin As Therapy for Patients with Malignant Melanoma, A Phase II Study—Study Results. [(accessed on 13 August 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT00462423?term=NCT00462423&draw=2&rank=1.
- 185.Hersh E.M., Del Vecchio M., Brown M.P., Kefford R., Loquai C., Testori A., Bhatia S., Gutzmer R., Conry R., Haydon A., et al. A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma. Ann. Oncol. 2015;26:2267. doi: 10.1093/annonc/mdv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Von Hoff D.D., Mita M.M., Ramanathan R.K., Weiss G.J., Mita A.C., Lorusso P.M., Burris H.A., Hart L.L., Low S.C., Parsons D.M., et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res. 2016;22:3157–3163. doi: 10.1158/1078-0432.CCR-15-2548. [DOI] [PubMed] [Google Scholar]
- 187.U.S. National Institutes of Health Pharmacokinetic Study of Liposomal Vincristine in Patients with Malignant Melanoma & Hepatic Dysfunction—Study Results. [(accessed on 13 August 2022)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT00145041?term=NCT00145041&draw=2&rank=1.
- 188.Bedikian A.Y., Silverman J.A., Papadopoulos N.E., Kim K.B., Hagey A.E., Vardeleon A., Hwu W.J., Homsi J., Davies M., Hwu P. Pharmacokinetics and Safety of Marqibo (Vincristine Sulfate Liposomes Injection) in Cancer Patients with Impaired Liver Function. J. Clin. Pharmacol. 2011;51:1205–1212. doi: 10.1177/0091270010381499. [DOI] [PubMed] [Google Scholar]
- 189.Gargett T., Abbas M.N., Rolan P., Price J.D., Gosling K.M., Ferrante A., Ruszkiewicz A., Atmosukarto I.I.C., Altin J., Parish C.R., et al. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol. Immunother. 2018;67:1461–1472. doi: 10.1007/s00262-018-2207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]