Abstract
Purpose
To evaluate PAX8 expression by immunohistochemistry in the normal pediatric and adult crystalline lens and to assess the usefulness of PAX8 immunohistochemical stain in the diagnosis of morphologically challenging lesions of lenticular origin.
Design
Retrospective, observational case series.
Participants
Fourteen congenital and acquired lens-derived lesions and 10 control crystalline lenses.
Methods
Hematoxylin–eosin and periodic acid–Schiff stains and an immunohistochemical panel of PAX8, vimentin, S100, smooth muscle actin, AE1/AE3, cytokeratin 7, and cytokeratin 5/6 antibodies were performed on all tissues.
Main Outcome Measures
Distribution of PAX8 expression in normal crystalline lens and in lens-derived lesions.
Results
Records search identified 10 normal pediatric and adult crystalline lenses, 1 phakomatous choristoma, 1 Peters anomaly with adherent leukoma, 1 lens capsule with congenital pyramidal cataract formation, 2 lenses with anterior and posterior subcapsular cataract formation, 3 postsurgical cataractous lenses (Soemmerring ring cataract and capsular fibrosis), and 6 retrocorneal membranes that incorporated various components of metaplastic corneal endothelium, metaplastic lens epithelium, corneal stroma, and epithelial downgrowth. Strong nuclear PAX8 expression was observed in the lens epithelium and in the equatorial lens bow of normal pediatric and adult lenses. Nuclear PAX8 expression also was observed in the lesions that retained some of the epithelial morphologic features, such as phakomatous choristoma, adherent leukoma, congenital pyramidal cataract, and components of intraocular membranes with lens epithelial differentiation. PAX8 expression was lost in lens epithelial lesions that had undergone mesenchymal transition, such as anterior subcapsular cataract and capsular fibrosis.
Conclusions
PAX8 antibody may be a useful adjunct to the immunohistochemical panels in morphologically challenging lens epithelial-derived lesions that retain epithelial morphologic features. PAX8 is not useful in the diagnosis of lens-derived lesions that feature epithelial–mesenchymal transition.
Keywords: PAX8 immunohistochemistry eye, PAX8 immunohistochemistry lens, PAX8 lens, PAX8 lens epithelium, PAX8 phakomatous choristoma
Abbreviations and Acronyms: CK, cytokeratin; PAS, periodic acid–Schiff; SMA, smooth muscle actin
The paired box (PAX) gene family encodes tissue-specific transcription factors. The PAX2/5/8 gene subfamily and PAX6 gene play an important role in ocular development, maintenance, and pathologic processes.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 We recently demonstrated nuclear PAX8 expression in normal ocular tissues, including the lens epithelium, iris pigment epithelium, and ciliary body epithelium, and in iris and ciliary body epithelial–derived neoplasms.12 These observations suggest that PAX8 can be a useful diagnostic marker of a select group of intraocular tumors and that the widely available PAX8 antibody should be considered in diagnostic immunohistochemical panels for morphologically challenging intraocular neoplasms.12 In this study, we explored PAX8 expression by immunohistochemistry in the normal human crystalline lens and lens-derived lesions and assessed the usefulness of the PAX8 immunohistochemical stain in the diagnosis of morphologically challenging lesions of lenticular origin.
Methods
The Wills Eye Hospital Institutional Review Board approved this retrospective, observational case series and deemed that informed consent was not required for this study. The study was performed in compliance with Health Insurance Portability and Accountability Act guidelines and with the tenets of the Declaration of Helsinki.
Patient and Tissue Selection
Records at the Department of Pathology at Wills Eye Hospital, Philadelphia, Pennsylvania, and the Histopathology Department at Royal Hallamshire Hospital, Sheffield, United Kingdom, were searched for representative cases of normal pediatric and adult enucleated eyes and crystalline lenses, congenital lens-derived lesions (Peters anomaly with adherent leukoma, phakomatous choristoma, and congenital cataract), and degenerative lens-derived lesions (age-related cataracts, postsurgical cataractous changes, and retrocorneal membranes incorporating lens-derived tissue). Data collected included patient age, sex, specimen type, and diagnosis.
Histopathologic and Immunohistochemistry Analysis
Routine sections stained with hematoxylin–eosin and periodic acid–Schiff (PAS) were prepared from paraffin-embedded, formalin-fixed tissues. Immunostaining was performed with the following primary antibodies: PAX8 (mouse monoclonal immunoglobulin G, clone MRQ-50, raised against N-terminal part of PAX8, prediluted; Cell Marque, Rocklin, CA); S100 (prediluted; DAKO); smooth muscle actin (SMA; 1:600; DAKO); cytokeratin (CK) 5/6–antigen CK5, major partner of CK14 (prediluted; DAKO); CK7 (prediluted; DAKO); pancytokeratin AE1/AE3 (1:400; Leica Biosystems); and vimentin (prediluted; Leica Biosystems). Peroxidase activity was visualized by applying diaminobenzidine solution containing 0.05% H2O2. Sections were counterstained with a modified Mayer’s hematoxylin, dehydrated, cleared, and mounted. Appropriate positive and negative controls were run with each batch. Additionally, PAX8 expression in normal lymphocytes served as an internal positive control. All immunohistochemical stains were prepared on Leica autostainer BOND III (Leica Biosystems) in accordance with the manufacturer’s instructions. Immunohistochemical stains were scored semiquantitatively based on the strength of cytoplasmic (S100, SMA, CK5/6, CK7, and AE1/AE3) and nuclear (PAX8 and S100) expression as follows: 0 = no staining, 1+ = weak staining, and 2+ = moderate to strong staining. The tissue distribution of the reaction product also was recorded.
Results
Patients and Tissues
A pathology record search identified 10 normal pediatric and adult crystalline lenses, 1 phakomatous choristoma, 1 Peters anomaly with adherent leukoma, 1 lens capsule with congenital pyramidal cataract formation, 2 lenses with anterior and posterior subcapsular cataract formation, 3 postsurgical cataractous lenses (Soemmerring ring cataract and capsular fibrosis), and 6 retrocorneal membranes that incorporated various components of metaplastic corneal endothelium, metaplastic lens epithelium, corneal stroma, and epithelial downgrowth. The patient demographics, histopathologic diagnosis, and tissue submitted for pathologic evaluation are summarized in Supplemental Table 1.
Histopathologic and Immunohistochemistry Analysis
In pathologic processes, lens epithelial-derived tissues were recognized by the presence of cuboidal epithelial cells with eosinophilic cytoplasm associated with thick PAS-positive basement material (compatible with lens epithelium and lens capsule), cells with centrally or slightly eccentrically located small ovoid nuclei and abundant dense eosinophilic cytoplasm (bladder cells of Wedl), and fibroblast-like cells individually surrounded by a delicate layer of PAS-positive material frequently associated with a segment of lens capsule (fibrous metaplasia of the lens epithelium, capsular fibrosis). These findings are illustrated in Figures 1, 2, 3, and 4.
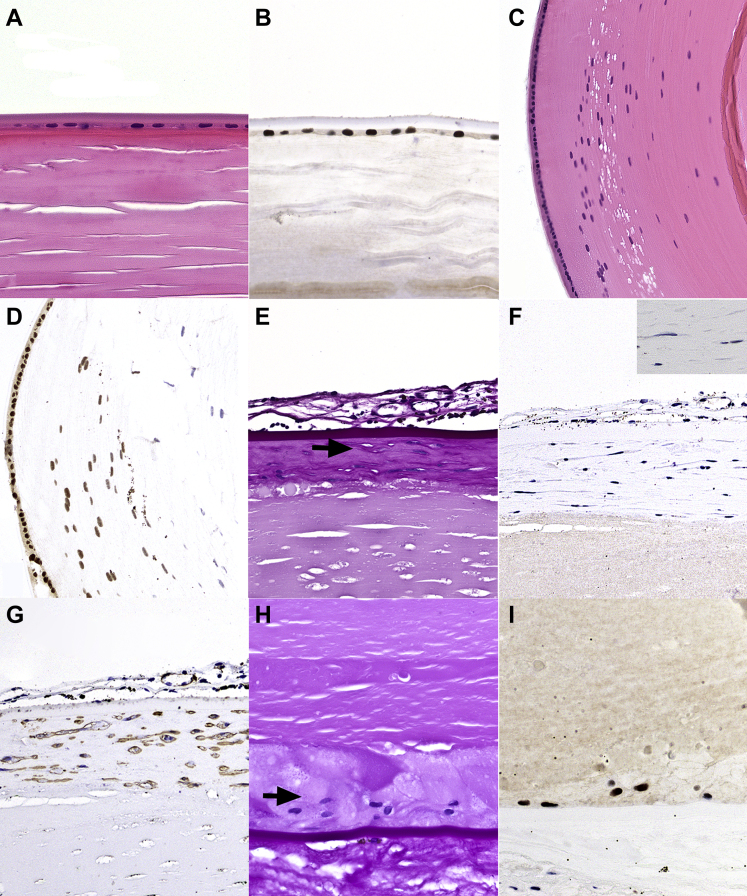
Figure 1.
Photomicrographs showing PAX8 expression in normal crystalline lens and in cataract lens. A, B, Normal adult crystalline lens: (A) normal adult crystalline lens demonstrating (B) strong nuclear PAX8 staining in the lens epithelium. C, D, Normal pediatric crystalline lens: (C) normal equatorial lens bow in an infant crystalline lens demonstrating (D) strong nuclear PAX8 staining in the lens epithelium and the adjacent lens epithelial cells transitioning into lens fibers. E–G, Anterior subcapsular cataract. E, Anterior subcapsular cataract demonstrating fibroblast-like cells surrounded by a delicate periodic acid–Schiff (PAS)-positive material (arrow). The PAS stain also highlights the thick overlying anterior lens capsule and a fibrovascular pupillary membrane. F, PAX8 results are negative in the nuclei of fibroblast-like cells of the anterior subcapsular cataract, magnified in the inset. The granular black pigmentation noted in the pupillary membrane is iris melanin pigment. G, Smooth muscle actin (SMA) staining of the anterior subcapsular cataract, compatible with myofibroblast-like metaplasia of the lens epithelium. H, I, Posterior subcapsular cataract: (H) bladder cells in posterior subcapsular cataract (arrow) demonstrating (I) strong nuclear staining results for PAX8. Stains, hematoxylin–eosin (A, C), PAX8 (B, D, F, I), PAS (E, H), and SMA (G); original magnifications, ×400 (A, B, E, G, H, I) and ×200 (C, D).
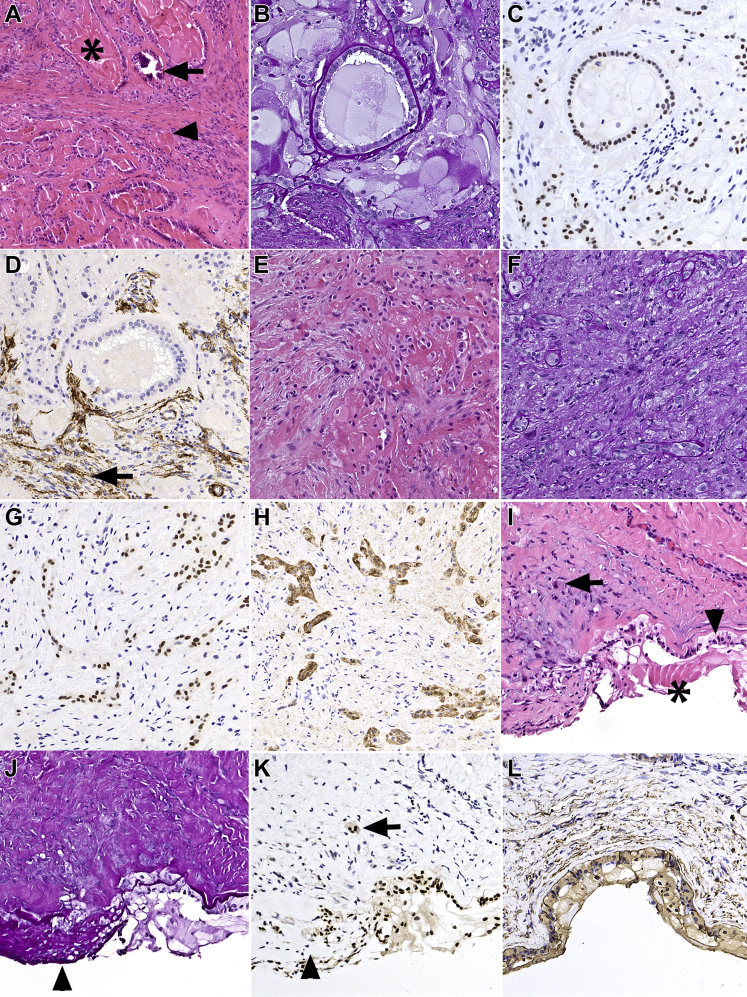
Figure 2.
Photomicrographs showing PAX8 expression in phakomatous choristoma and Peters anomaly with keratolenticular adhesion. A–H, Phakomatous choristoma. A, Phakomatous choristoma demonstrating characteristic islands composed of cataractous bladder cells (asterisk) and foci of dystrophic calcification (arrow) surrounded by cuboidal lens epithelium with thick lens capsule-like basement membrane material in a background of fibrotic stroma (arrowhead). B, Periodic acid–Schiff (PAS) stain highlighting the thick basement membrane material reminiscent of lens capsule surrounding the cuboidal lens epithelium. C, Corresponding section showing a strong nuclear PAX8 expression in lens epithelium and bladder cells. The fibrotic stroma does not show positive stain results with PAX8. D, Focal positivity (arrow) of stromal component for smooth muscle actin (SMA), compatible with myofibroblastic differentiation reminiscent of anterior subcapsular cataract and capsular fibrosis. E, F, A region in phakomatous choristoma that lacks typical morphologic features and may present a diagnostic challenge with hematoxylin–eosin and PAS stains alone. G, Corresponding section stained with PAX8 highlighting bands and strands of lens epithelial cells that (H) coexpress S100. I–L, Peters anomaly with keratolenticular adhesion. I, Peters anomaly with keratolenticular adhesion demonstrating lens epithelium adherent to the posterior corneal surface (arrowhead) and focally entrapped in the fibrotic corneal stroma (arrow). Bladder cells also are present (asterisk). J, Corresponding PAS-stained preparation highlighting thick PAS-positive basement membrane material (lens capsule) overlying the cuboidal lens epithelium and PAS-positive material surrounding individual spindle cells (arrowhead), compatible with capsular fibrosis. K, Corresponding section stained with PAX8 highlighting the strong nuclear expression in the lens epithelium on the posterior corneal surface and entrapped in the corneal stroma (arrow). Nuclear staining also shows positive results in bladder cells, but is lost in the fibrous metaplasia of the lens epithelium (arrowhead). L, Vimentin shows positive results in the lens tissue and in the corneal stroma. Stains, hematoxylin–eosin (A, E, I), PAS (B, F, J), PAX8 (C, G, K), SMA (D), S100 (H), and vimentin (L); original magnifications, ×100 (A) and ×200 (B–L).
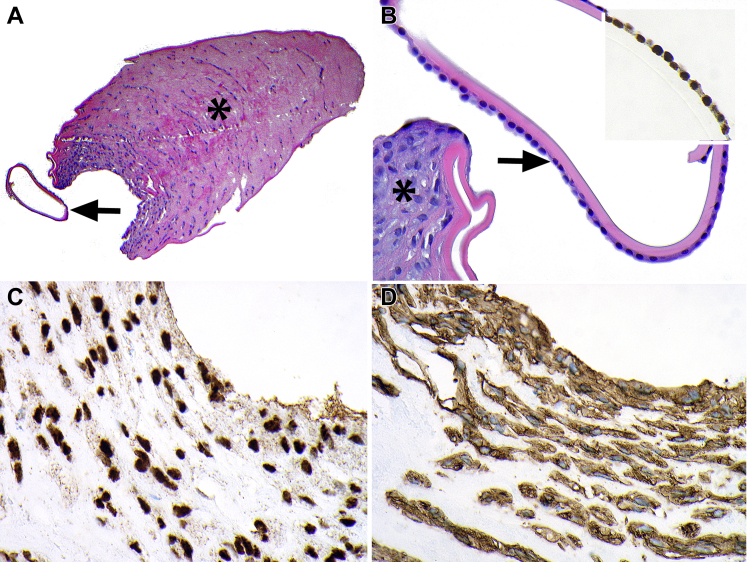
Figure 3.
Photomicrographs showing PAX8 expression in congenital anterior polar pyramidal cataract. A, Pyramidal cataract (asterisk) with an adjacent anterior lens capsule (arrow). B, Higher magnification of anterior lens capsule with morphologically normal lens epithelium (arrow) demonstrating strong nuclear PAX8 expression (inset). The adjacent anterior pyramidal cataract (asterisk) features a proliferation of plump cells with morphologic features intermediate between epithelium and fibroblasts. C, Strong nuclear PAX8 expression in pyramidal cataract. D, Strong cytoplasmic smooth muscle actin (SMA) expression in pyramidal cataract. Stains, hematoxylin–eosin (A, B), PAX8 (C), and SMA (D); original magnifications, ×100 (A) and ×400 (B–D).
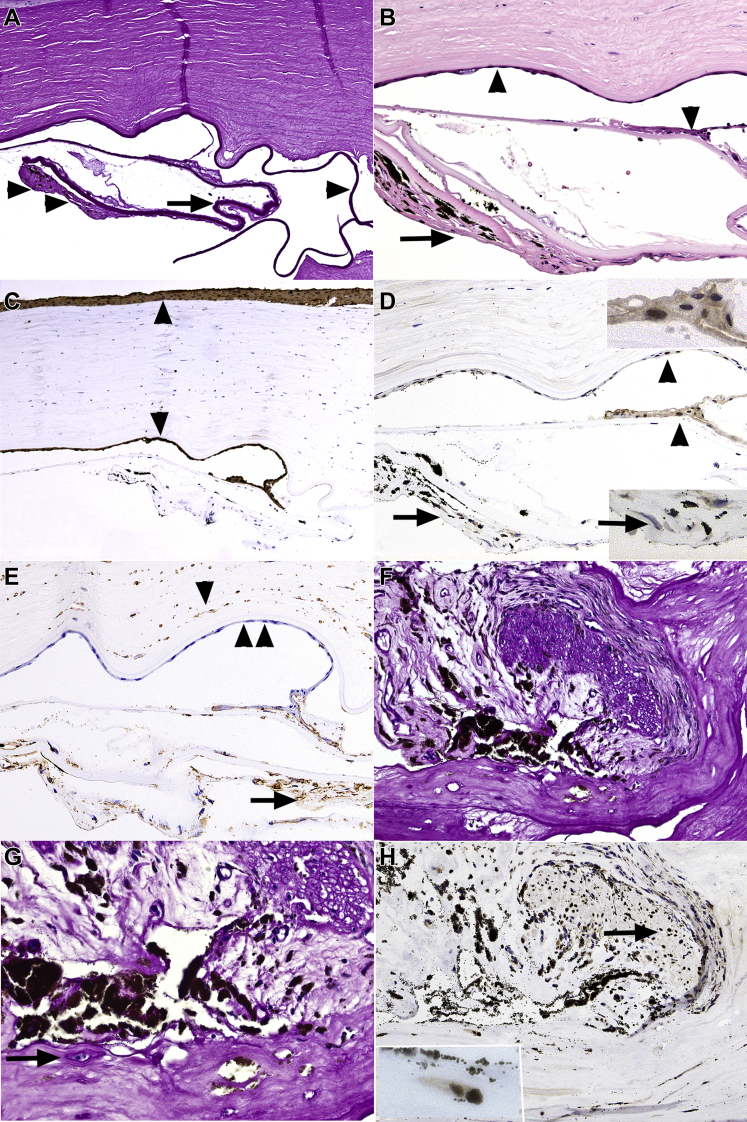
Figure 4.
Photomicrographs showing PAX8 expression in retrocorneal and keratoprosthesis membranes. A–E, Epithelial downgrowth and capsular fibrosis. A, Variably thick basement membrane material, compatible with lens capsule (arrow) and adjacent capsular fibrosis (double arrowheads). Also note the overlying cornea with uniformly thick Descemet membrane (single arrowhead). B, Higher magnification highlighting surface epithelial membrane on the posterior corneal surface and focally on the surface of lens capsule (arrowheads). Capsular fibrosis with black iris melanin pigment (arrow) is present beneath the thick anterior lens capsule. C, cytokeratin (CK5/6) results are strongly positive in the corneal surface epithelium and in the epithelial membrane (arrowheads), compatible with epithelial downgrowth. D, PAX8 results are positive focally in the nuclei of epithelial downgrowth (arrowheads and upper inset). The capsular nuclei of fibroblast-like cells in capsular fibrosis (arrow) and lower inset (arrow) show negative results for PAX8. E, Vimentin shows positive results in the capsular fibrosis (arrow) and in the corneal stromal fibroblasts (single arrowhead) and negative results in the epithelial downgrowth (double arrowheads). F–H, Lens epithelial fibrous metaplasia in keratoprosthesis membrane. F, Spindle cell proliferation, associated with periodic acid–Schiff (PAS)-positive basement membrane material surrounding a segment of distorted and degenerated iris. G, Higher magnification highlighting a few cuboidal lens epithelial cells surrounded by PAS-positive material (arrow). H, PAX8-stained preparation corresponding to (F) highlighting the positive staining results in the nuclei of iris sphincter muscle (arrow). The lens epithelial cells seen in (G) also show positive staining results with PAX8 (inset). The fibrous component of the capsular fibrosis does not show positive staining results with PAX8. Stains, PAS (A, F, G), hematoxylin–eosin (B), CK5/6 (C), PAX8 (D, H), and vimentin (E); original magnifications, ×100 (A, C, E, F), ×200 (B, D, E, F), ×400 (G, H), and ×600 (all insets).
The immunohistochemical profile of normal pediatric and adult anterior segment tissues is summarized in Table 1 and Supplemental Table 1. Specifically, PAX8 was expressed with strong intensity in the basal and suprabasal corneal epithelium and focally in the conjunctival epithelium, in nearly all nuclei of the lens epithelium, peripheral cells of the equatorial lens bow, ciliary body epithelium, iris pigment epithelium, and iris sphincter and dilator muscle. Corneal stroma and corneal endothelium showed negative results for PAX8 (Figure 1, Figure 2, Figure 3, Figure 4).
Table 1.
Immunohistochemical Profile of Normal Pediatric and Adult Anterior Segment Structures
| Tissue | PAX8 | Vimentin | Smooth Muscle Actin | S100 | AE1/AE3 | Cytokeratin 5/6 | Cytokeratin 7 |
|---|---|---|---|---|---|---|---|
| Corneal epithelium | 2+ B, SB | 0 | 0 | 0 | 2+, diffuse | 2+, diffuse | 0 |
| Corneal stroma (keratocytes) | 0 | 1+ | 0 | 0 | 0 | 0 | 0 |
| Corneal endothelium | 0 | 1+, diffuse | 0 | 0 | 0 | 0 | 0 |
| Conjunctival epithelium | 2+ B, SB (focal) | 0 | 0 | 0 | 2+, diffuse | 2+, diffuse | 2+, diffuse |
| Lens epithelium | 2+, most cells | 1+, diffuse | 0 | 1+ to 2+, diffuse | 0 | 0 | 0 |
| Equatorial lens bow | 1-2+, peripheral | 1+, diffuse | 0 | 0 | 0 | 0 | 0 |
| Iris pigment epithelium | 2+, most cells | 1+, diffuse | 0 | 1+, diffuse | 0 | 0 | 1+, diffuse |
| Iris sphincter/dilator muscles | 2+, most cells | 1+, diffuse | 2+, diffuse | 0 | 0 | 0 | 0 |
| Iris stroma | 0 | 1+, diffuse | 0 | 2+ M | 0 | 0 | 0 |
| Ciliary body epithelium (bilayer) | 2+, most cells | 1+, diffuse | 0 | 1+, diffuse | 0 | 0 | 1+, diffuse |
| Ciliary body muscle | 2+, most cells | 1+, diffuse | 2+, diffuse | 0 | 0 | 0 | 0 |
| Ciliary body stroma | 0 | 1+, diffuse | 0 | 2+ M | 0 | 0 | 0 |
B = basal epithelium; M = melanocytes; SB = suprabasal epithelium; 0 = no staining; 1+ = weak staining; 2+ = moderate to strong staining.
The immunohistochemical profile of congenital and degenerative lens-derived lesions is summarized in Supplemental Table 1. Specifically, PAX8 results were moderately to strongly positive in the nuclei of most lens epithelial and bladder cells of phakomatous choristoma, in congenital anterior polar pyramidal cataract, in the area of keratolenticular adhesion in Peters anomaly, in the cuboidal lens epithelial cells incorporated into retrocorneal fibrous membranes, and in the occasional bladder cells of posterior subcapsular and Soemmerring ring cataracts (Figure 1, Figure 2, Figure 3, Figure 4). PAX8 finding also were moderately to strongly positive in both membranes featuring corneal epithelial downgrowth (Fig 4) and in 1 of 2 membranes featuring conjunctival epithelial downgrowth. PAX8 stain results were either positive for rare nuclei or were entirely negative in the tissues demonstrating fibroblast-like/myofibroblast-like metaplasia of the lens epithelium, including the fibrous component of phakomatous choristoma, anterior subcapsular cataract, lens capsular fibrosis, and retrocorneal fibrous membranes of lens epithelial derivation. Conversely, these tissues showed positive stain results with SMA, consistent with myofibroblastic differentiation. Interestingly, the anterior polar pyramidal cataract coexpressed diffusely both PAX8 and SMA, possibly reflecting an intermediate stage in epithelial–mesenchymal transition and correlating with histopathologic findings featuring plump cells that have hybrid epithelial–myofibroblast features (Fig 3). S100 expression paralleled PAX8 expression, although the staining was, for the most part, weak to moderate (Figure 1, Figure 2, Figure 3).
Similar to prior studies, phakomatous choristoma and adherent leukoma showed positive results for vimentin, S100, and CK7 and showed negative results for CK5/6 and AE1/AE3 (Fig 2).13,14 Similar to prior studies, reactive corneal endothelium showed positive results for CK7, and intraocular membranes of corneal endothelial derivation showed positive results for vimentin, SMA, and CK7 and showed negative results for CK5/6 and AE1/AE3. The intraocular membranes of fibrocytic derivation showed positive results for vimentin and SMA and showed negative results for CK7, CK5/6, and AE1/AE3. The intraocular membranes derived from the surface epithelium showed negative results for vimentin and showed positive results for CK5/6 and AE1/AE3 (Fig 4).15, 16, 17
Discussion
The paired box transcription factor PAX8 is critical for development of the eye, thyroid gland, and urogenital tract.1, 2, 3,18 In adults, PAX8 overexpression is seen in renal, ovarian, and thyroid neoplasms and has emerged as a specific marker for these cancers.18 As a result, the PAX8 antibody is widely used diagnostically and is readily available in most pathology laboratories. Recently, we demonstrated PAX8 expression by immunohistochemistry analysis in normal iris and ciliary body epithelium and in the neoplasms originating from these tissues, suggesting that PAX8 antibody can be a useful adjunct in diagnostic immunohistochemical panels for morphologically challenging intraocular tumors.12 In addition, we noted PAX8 expression in the normal lens epithelium leading to the conception of the current study, designed to evaluate in further detail PAX8 expression in the normal pediatric and adult crystalline lenses and in various congenital and degenerative pathologic processes that involve the lens.12
This study confirms PAX8 nuclear staining in the normal lens epithelium and in the superficial equatorial lens bow in pediatric and adult crystalline lens. PAX8 expression also was noted in lens epithelial-derived lesions that retained some lens epithelial morphologic features, such as the bladder cells of Wedl, epithelial ribbons and islands in phakomatous choristoma, anterior polar pyramidal cataract, and occasional lens epithelial cells entrapped in capsular fibrosis. These findings suggest that PAX8 may be a useful immunohistochemical marker in morphologically challenging cases of phakomatous choristoma and keratolenticular adhesion and in cases when lens epithelium may need to be distinguished from corneal endothelium. However, these diagnoses generally are based on morphologic assessment of the tissue with hematoxylin–eosin and PAS stains and rarely require ancillary immunohistochemical studies.
PAX8 expression is lost in lesions where lens epithelium undergoes mesenchymal transition, such as lens capsular fibrosis, anterior subcapsular cataract, and the myofibroblastic component of phakomatous choristoma. Importantly, PAX8 cannot distinguish between epithelial downgrowth and lens epithelium. Thus, PAX8 is not a useful diagnostic marker for these tissues, limiting its usefulness in the assessment of intraocular membranes.
Observation of PAX8 expression in the corneal, lens, iris, and ciliary body epithelia; the iris sphincter and dilator muscles; and in retinal glial cells and a subset of retinal neurons suggests that PAX8 may be involved not only in embryologic development of the eye, but also in the homeostasis and regeneration of these tissues.2,12 However, it has not escaped our attention that the distribution of PAX8 expression is similar to PAX2 and PAX6, which are known to play a crucial role in eye development and disease.4, 5, 6, 7, 8, 9, 10, 11 Specifically, PAX6 has been shown to be expressed in the ocular surface and crystalline lens epithelia, ciliary body and iris pigment epithelia, iris muscle, and retina.5,6,7,9,10 PAX2, PAX5, PAX6, and PAX8 proteins all share conserved regions, and it is possible that PAX8 antibody may cross-react with the epitopes of other PAX family members.18 Although the cross-reactivity of PAX proteins can be decreased with the use of monoclonal antibodies, which were used in this study, we cannot completely exclude this possibility.18 Thus, additional investigations, such as mRNA expression studies, would be required to evaluate the role of PAX8 in the postnatal human eye conclusively.
In summary, we documented PAX8 expression in the lens epithelium and in a subset of lesions that retain lens epithelial morphologic features. PAX8 expression is absent in lens epithelium that has undergone mesenchymal transition. These findings suggest that the PAX8 antibody may be a useful adjunct to immunohistochemical panels for assessing morphologically challenging lenticular lesions that retain epithelial morphologic features, although such morphologic challenges admittedly are rare.
Manuscript no. D-21-00019.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Supported by the Filkins Family Foundation, Council Bluffs, Iowa; and by the Pennsylvania Lions Sight Conservation and Eye Research Foundation, Inc., Beaver Falls, Pennsylvania.
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at Wills Eye Hospital approved the study. All research complied with the Health Insurance Portability and Accountability Act (HIPAA) of 1996 and adhered to the tenets of the Declaration of Helsinki. The human ethics committee deemed that informed consent was not required for this study.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Milman, Singh Mudhar, Eagle
Analysis and interpretation: Milman, Singh Mudhar, Eagle
Data collection: Milman, Singh Mudhar, Eagle
Obtained funding: N/A
Overall responsibility: Milman, Singh Mudhar, Eagle
Supplementary Data
References
- 1.Kozmik Z. Pax genes in eye development and evolution. Curr Opin Genet Dev. 2005;15:430–438. doi: 10.1016/j.gde.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Goode D.K., Elgar G. The PAX258 gene subfamily: a comparative perspective. Dev Dyn. 2009;238:2951–2974. doi: 10.1002/dvdy.22146. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer P.L., Gerster T., Lun K., Brand M., Busslinger M. Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development. 1998;125:3063–3074. doi: 10.1242/dev.125.16.3063. [DOI] [PubMed] [Google Scholar]
- 4.Ma X., Ma Z., Jiao X., Hejtmancik J.F. Functional non-coding polymorphism in an EPHA2 promoter PAX2 binding site modifies expression and alters the MAPK and AKT pathways. Sci Rep. 2017;7:9992. doi: 10.1038/s41598-017-10117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis N., Yoffe C., Raviv S., et al. Pax6 dosage requirements in iris and ciliary body differentiation. Dev Biol. 2009;333:132–142. doi: 10.1016/j.ydbio.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Koroma B.M., Yang J.M., Sundin O.H. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1997;38:108–120. [PubMed] [Google Scholar]
- 7.Stanescu D., Iseli H.P., Schwerdtfeger K., et al. Continuous expression of the homeobox gene Pax6 in the ageing human retina. Eye (Lond) 2007;21:90–93. doi: 10.1038/sj.eye.6702166. [DOI] [PubMed] [Google Scholar]
- 8.Dziedzic K., Heaphy J., Prescott H., Kavaler J. The transcription factor D-Pax2 regulates crystallin production during eye development in Drosophila melanogaster. Dev Dyn. 2009;238:2530–2539. doi: 10.1002/dvdy.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan M.K., Xie L., David L.L., et al. Ectopic Pax6 expression disturbs lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2004;45:3589–3598. doi: 10.1167/iovs.04-0151. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen H.R., Baraas R.C., Landsend E.C.S., et al. PAX6 genotypic and retinal phenotypic characterization in congenital aniridia. Invest Ophthalmol Vis Sci. 2020;61:14. doi: 10.1167/iovs.61.5.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sels L., Dirven W., Devriendt K., Leys A. Severe case of renal coloboma syndrome in long-term follow-up. Retin Cases Brief Rep. 2020;14:77–81. doi: 10.1097/ICB.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 12.Mudhar H.S., Milman T., Eagle R.C., Jr., et al. Usefulness of PAX8 immunohistochemistry in adult intraocular tumor diagnosis. Ophthalmology. 2021;128(5):765–778. doi: 10.1016/j.ophtha.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Ellis F.J., Eagle R.C., Jr., Shields J.A., et al. Phakomatous choristoma (Zimmerman’s tumor). Immunohistochemical confirmation of lens-specific proteins. Ophthalmology. 1993;100:955–960. doi: 10.1016/s0161-6420(93)31573-3. [DOI] [PubMed] [Google Scholar]
- 14.Seregard S. Phakomatous choristoma may be located in the eyelid or orbit or both. Acta Ophthalmol Scand. 1999;77:343–346. doi: 10.1034/j.1600-0420.1999.770320.x. [DOI] [PubMed] [Google Scholar]
- 15.Stacy R.C., Jakobiec F.A., Michaud N.A., et al. Characterization of retrokeratoprosthetic membranes in the Boston type 1 keratoprosthesis. Arch Ophthalmol. 2011;129:310–316. doi: 10.1001/archophthalmol.2011.26. [DOI] [PubMed] [Google Scholar]
- 16.Hou J.H., Sivaraman K.R., de la Cruz J., et al. Histopathological and immunohistochemical analysis of melt-associated retroprosthetic membranes in the Boston type 1 keratoprosthesis. JAMA Ophthalmol. 2014;132:1133–1136. doi: 10.1001/jamaophthalmol.2014.1959. [DOI] [PubMed] [Google Scholar]
- 17.Jakobiec F.A., Bhat P. Retrocorneal membranes: a comparative immunohistochemical analysis of keratocytic, endothelial, and epithelial origins. Am J Ophthalmol. 2010;150:230–242.e2. doi: 10.1016/j.ajo.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzo P.I., Jimenez Moreno C.M., Delgado I., et al. Immunohistochemical assessment of Pax8 expression during pancreatic islet development and in human neuroendocrine tumors. Histochem Cell Biol. 2011;136:595–607. doi: 10.1007/s00418-011-0866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.