Abstract
Purpose
This study aimed to estimate the incidence and prevalence of neovascular age-related macular degeneration (nAMD) in the French population between 2008 and 2018.
Design
This was a retrospective, longitudinal population study using health care consumption data from the Système National des Données de Santé (SNDS; the French National Health Information Database), which covers approximately 99% of the French population.
Participants
We identified individuals treated for nAMD from the French population 50 years of age and older. Identification criteria were nAMD diagnosis or reimbursement of nAMD treatments (anti–vascular endothelial growth factor intravitreal injection or dynamic phototherapy with verteporfin). Exclusion criteria were high myopia, diagnosis of other retinal diseases, and other treatments for macular diseases (dexamethasone implant, laser therapy, etc.).
Methods
We calculated incidence and prevalence based on the age-matched general population in France. Adjustment for age and sex was also performed for incidence.
Main Outcome Measures
Incidence and prevalence of nAMD in the French population between 2008 and 2018.
Results
Between 2008 and 2018, we identified 342 961 patients with nAMD (67.5% women). Mean ± standard deviation age at nAMD diagnosis or first treatment increased from 78.8 ± 8.1 years in 2008 to 81.2 ± 7.9 years in 2018. In 2018, annual incidence was 0.149% and prevalence was 1.062% for the French population 50 years of age or older. Incidence was stable over the 10-year period. Annual incidence increased with age (0.223%, 0.380%, and 0.603% in those 60 years of age or older, 70 years of age or older, and 80 years of age or older, respectively), with similar trends for prevalence. No major differences were observed among the 14 regions of France for incidence or prevalence. Neovascular age-related macular degeneration incidence in 2018 was not impacted by the availability of primary or ophthalmology care in patients’ localities.
Conclusions
The LANDSCAPE study provides exhaustive nationwide data on incidence and prevalence of nAMD in France over a 10-year period.
Keywords: Age-related macular degeneration, France, Incidence, Neovascular, Prevalence
Abbreviations and Acronyms: AMD, age-related macular degeneration; ICD-10, International Classification of Diseases, Tenth Revision; nAMD, neovascular age-related macular degeneration; SNDS, Système National des Données de Santé (National Health Information Database); VEGF, vascular endothelial growth factor
Age-related macular degeneration (AMD) is a leading cause of visual impairment and blindness in people older than 50 years.1 Anti–vascular endothelial growth factor (VEGF) agents such as ranibizumab and aflibercept have revolutionized the treatment of neovascular AMD (nAMD) in Europe since their introduction in 2006 and 2007, significantly reducing visual impairment in patients with nAMD.2,3 Given the increasing life expectancy and aging populations in industrialized countries, it is predicted that the prevalence of age-related diseases such as nAMD will increase markedly over the coming decades.4,5
A 2017 meta-analysis including 42 080 Europeans estimated the prevalence of late AMD (including a mix of nAMD and atrophic AMD) at 0.1% in people 55 to 59 years of age, increasing to 9.8% in those older than 85 years.4 Prevalence decreased from 2006 onward, probably because of anti-VEGF treatments and healthier lifestyles. Other international data are available for selected populations such as adults older than 40 years,6, 7, 8, 9, 10 older than 50 years,11,12 and older than 60 years,13, 14, 15 or based on postal sampling16 or ophthalmic examinations and interviews.17 Recent epidemiologic data about nAMD in France are lacking, with only 5 studies between 1995 and 2013, all limited to specific towns or regions and age ranges and with small sample size populations.18, 19, 20, 21, 22 We aimed to address this gap by estimating the incidence and prevalence of nAMD in the entire French population between 2008 and 2018 using nationwide population data from health records. We also examined incidence related to comorbidities, place of residence, and access to ophthalmology care.
Methods
Study Design and Data Sources
The LANDSCAPE study was a retrospective longitudinal population study that used anonymized data from the Système National des Données de Santé (SNDS; the French National Health Information Database). The SNDS contains exhaustive patient-level data for all individuals in France covered by national health insurance, covering approximately 99% of the French population from birth (or immigration) to death (or emigration).23
The SNDS contains pseudonymized individual-level data on demographics (age, sex, area of residence, date of birth), outpatient management (reimbursed drugs [dispensation date and number of units]), long-term chronic disease diagnosis, dates and descriptions of paramedical interventions, procedures and laboratory tests, private and public hospitalizations (admission date, duration, main and associated diagnoses, medical consultations, etc.), and date and cause of death. Data in the SNDS are coded according to the International Classification of Diseases, Tenth Revision (ICD-10), and medicines (Anatomical Therapeutic Chemical classification), as well as French coding dictionaries for medical acts, clinical procedures, and reimbursed products and services.24,25 We refer readers to Tuppin et al25 for an in-depth description of the SNDS, including further information on coding and data quality control. General population size (including by age and sex) was from the French National Institute of Statistics and Economic Studies.26
Identification of Patients with Neovascular Age-Related Macular Degeneration
We identified patients with nAMD from the SNDS with the following identification criteria: adults older than 50 years and covered by French national health insurance. Individuals were defined as having nAMD if they had at least 1 anti-VEGF treatment reimbursed for nAMD (bevacizumab, pegaptanib, ranibizumab, or aflibercept) administrated by intravitreal injection between 2008 and 2018.
We excluded individuals with other ocular diseases, including other conditions that can be treated with anti-VEGF agents such as diabetic macular edema and retinal vein occlusion. Individuals were excluded if they had any high myopia (high corrective refractive glasses reimbursement in previous years), retinal disease other than AMD (ICD-10 codes H30-H36; e.g., diabetic retinopathy), or treatments for other macular diseases (dexamethasone implant, macular laser therapy, or panretinal photocoagulation) or resided in the French overseas region Mayotte (because of incomplete SNDS data). Despite exclusion criteria, nAMD was confirmed if patient had at least 1 hospital stay or long-term disease with an ICD-10 diagnosis of AMD (code H35.3) or had received phototherapy with verteporfin. Patients were followed up until the end of consumption of health care (regardless of treatment type) or the patient exited the SNDS database (because of death or emigration).
Ethics and Data Protection
The LANDSCAPE study was approved by the French data protection agency (Commission Nationale de l'Informatique et des Libertés) and the French Institute of Health Data, which confirmed that informed consent was not required for access to anonymized data in the SNDS. All research adhered to the tenets of the Declaration of Helsinki.
Statistical Analyses
Annual Incidence and Prevalence of Neovascular Age-Related Macular Degeneration
For each calendar year, nAMD incidence was calculated as the number of patients who received new diagnoses of nAMD over the calendar year as a percentage of the French general population 50 years of age or older in that year. Descriptive subgroups analyses of nAMD incidence were performed by age group (per 5-year age groups), cumulative age group (50 years of age or older, 60 years of age or older, 70 years of age or older, etc.), region in France, density of general practitioners and ophthalmologists in the patient’s residential area, and by classification of the patients’ residential area. Prevalence was calculated as the number of prevalent patients with nAMD in calendar year 2018 as a percentage of the French general population 50 years of age or older in 2018.
Patient Characteristics and Comorbidities
Patient characteristics (age, sex, and comorbidities) were descriptively summarized. Comorbidities between 2008 and 2018 were described only for incident patients in 2018. Cataract surgery was identified via hospitalization over the previous 10 years. Ocular treatments over the previous year such as topical treatment were used to identify dry eye, hypertension, glaucoma, and other ocular comorbidities. Nonocular comorbidities were identified by treatments and ICD-10 codes from hospitalization. Long-term diseases identified via the same method were high blood pressure (treated by antihypertensive drugs), diabetes (identified using a validated algorithm27), myocardial infarction, congestive heart failure, stroke, dementia, kidney disease, and cancer without metastasis. The burden of comorbidities over the previous year was described using the Charlson comorbidity index.28 This score helps clinicians to predict the survival probability in patients with multiple comorbidities.
Statistics
Quantitative variables were described using mean, standard deviation, median, quartiles, and minimum and maximum. Categorical variables were described using counts and percentages. SAS Enterprise Guide software version 7.1 was used for all analyses.
Results
Patient Characteristics
Between 2008 and 2018, we identified 342 961 patients with nAMD. By the end of the study period, 76.6% of patients with nAMD were still being followed up in this study, 9.8% died, and 13.5% had no recorded health care consumption in the previous 12 months. Overall, 67.5% of incident patients with nAMD were women in 2008, decreasing to 64.1% in 2018 (Table 1). Mean ± standard deviation incident age rose from 78.8 ± 8.1 years in 2008 to 81.2 ± 7.9 years in 2018.
Table 1.
Demographics and Characteristics of Patients with Incident Neovascular Age-Related Macular Degeneration in 2008 and 2018
| Variable | 2008 (n = 28 518) | 2018 (n = 38 852) |
|---|---|---|
| Sex, no. (%) | ||
| Male | 9279 (32.5) | 13 960 (35.9) |
| Female | 19 239 (67.5) | 24 892 (64.1) |
| Age, yrs | ||
| No. (%) | 28 518 (100) | 38 852 (100) |
| Mean (SD) | 78.8 (8.1) | 81.2 (7.9) |
| Median (interquartile range) | 80.0 (75.0–84.0) | 82.0 (76.0–87.0) |
| Range | 50.0–106.0 | 50.0–106.0 |
| Comorbidities, Charlson comorbidity index∗ | ||
| No. | 28 518 | 38 852 |
| Mean (SD) | 0.8 (1.2) | 0.9 (1.4) |
| Ocular comorbidities, no., (%) | ||
| Cataract surgery† | — | 18 494 (47.6) |
| Treated dry-eye disease‡ | — | 11 783 (30.3) |
| Treated ocular hypertension§|| | — | 6714 (17.3) |
| Nonocular comorbidities (2018 only), no. (%) | ||
| Hypertension|| | — | 26 986 (69.5) |
| Diabetes | — | 4954 (12.8) |
| Myocardial infarction | — | 3519 (9.1) |
| Congestive heart failure | — | 4022 (10.4) |
| Stroke | — | 4444 (11.4) |
| Dementia | — | 1512 (3.9) |
| Renal disease | — | 2757 (7.1) |
| Nonmetastatic cancer | — | 7925 (20.4) |
SD = standard deviation; — = data not available.
In 2018.
Cataract surgery reported from 2008 through 2018.
Treated in 2018.
Including glaucoma.
Patients treated with antihypertensive medications.
In 2018, the mean ± standard deviation Charlson comorbidity index was 0.9 ± 1.39, meaning that 96% of patients are estimated to survive 10 years or more. Nearly half of patients (47.6%) had undergone cataract surgery, 30.3% had been treated during the last year for dry eye disease, and 17.3% had been treated for increased intraocular pressure, ocular hypertension, or glaucoma. Nonocular comorbidities were most frequently cardiovascular and metabolic: 69.5% of patients were taking antihypertensive medications, 12.8% had a diagnosis of diabetes, 11.4% had undergone a stroke, and 9.1% had experienced a myocardial infarction since 2008 (Table 1).
Neovascular Age-Related Macular Degeneration Incidence 2008–2018
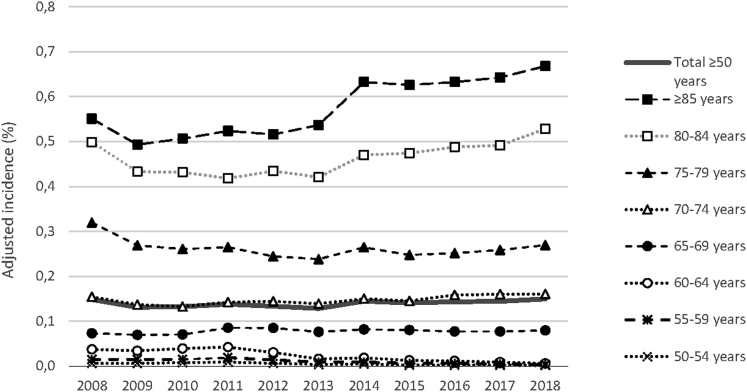
Incident nAMD was identified in 38 852 patients in 2018, yielding an annual incidence of 0.149% for the French population older than 50 years (Table 2). Adjusting incidence to the age and sex distribution of the French population in 2018 did not result in a notable change versus the crude incidence. Annual incidence was relatively stable between 2008 and 2018 (Fig 1), with a slightly increased incidence from 2014, in particular in individuals 85 years of age or older.
Table 2.
Neovascular Age-Related Macular Degeneration Incidence and Prevalence in 2018 by Cumulative Age Group and 5-Year Age Group
| Age Group (yrs) | French Population | No. of Patients with Newly Diagnosed nAMD | Annual Incidence (%) | Total No. of Patients with nAMD per Age Group | Prevalence (%) | |
|---|---|---|---|---|---|---|
| Cumulative age groups, yrs | ||||||
| Total (50 yrs of age and older) | 25 996 150 | 38 852 | 0.149 | 276 187 | 1.062 | |
| ≥ 55 | 21 513 996 | 38 791 | 0.179 | 275 974 | 1.283 | |
| ≥ 60 | 17 233 529 | 38 663 | 0.223 | 274 563 | 1.593 | |
| ≥ 65 | 13 175 132 | 38 422 | 0.290 | 270 935 | 2.056 | |
| ≥ 70 | 9 232 042 | 35 295 | 0.380 | 257 432 | 2.788 | |
| ≥ 75 | 6 196 755 | 30 432 | 0.488 | 230 065 | 3.713 | |
| ≥ 80 | 4 052 125 | 24 636 | 0.603 | 195 271 | 4.819 | |
| ≥ 85 | 2 180 882 | 14 652 | 0.668 | 136 784 | 6.272 | |
| 5-Year age groups | ||||||
| 50–54 | 4 482 154 | 61 | 0.001 | 213 | 0.005 | |
| 55–59 | 4 280 467 | 128 | 0.003 | 1411 | 0.033 | |
| 60–64 | 4 058 397 | 241 | 0.006 | 3628 | 0.089 | |
| 65–69 | 3 943 090 | 3127 | 0.079 | 13 503 | 0.342 | |
| 70–74 | 3 035 287 | 4863 | 0.160 | 27 367 | 0.902 | |
| 75–79 | 2 144 630 | 5796 | 0.270 | 34 794 | 1.622 | |
| 80–84 | 1 871 243 | 9984 | 0.534 | 58 487 | 3.126 | |
| ≥ 85 | 2 180 882 | 14 652 | 0.672 | 136 784 | 6.272 | |
nAMD = neovascular age-related macular degeneration.
Figure 1.
Line graph showing the incidence of neovascular age-related macular degeneration from 2008 through 2018. The incidence is standardized to the corresponding population in France 50 years of age or older by age and sex. Incidence in expressed per 100 inhabitants.
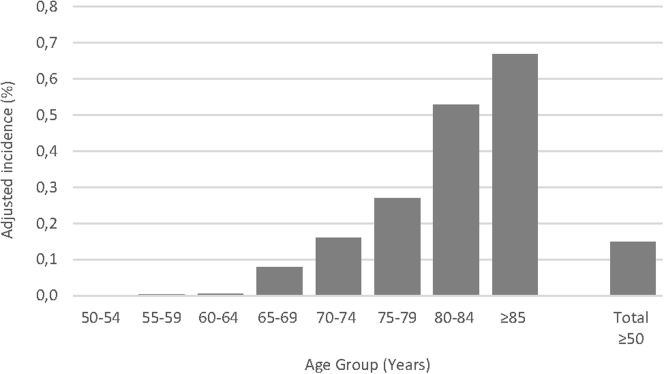
Annual incidence in 2018 was age dependent, rising steadily from 0.001% in people 50 to 54 years of age to 0.679% in people 85 years of age or older (Fig 2). Similarly, incidence rose with cumulative age (0.223%, 0.380%, and 0.603% in people 60 years of age or older, 70 years of age or older, and 80 years of age or older, respectively; Table 2). Incidence seemed to be rather stable in all age categories from 50 to 79 years from 2008 through 2018. Over the 10-year study period, the incidence increased most for the oldest patients 80 to 84 years of age (from 0.498% to 0.528%) and 85 years of age or older (from 0.551% to 0.668%).
Figure 2.
Bar graph showing the incidence of neovascular age-related macular degeneration in 2018 per age category. The incidence is standardized to the corresponding population in France 50 years of age or older by age and sex. Incidence in expressed per 100 inhabitants.
Neovascular Age-Related Macular Degeneration Prevalence in 2018
In 2018, we identified 276 187 individuals living with nAMD out of nearly 26 million French people 50 years of age or older, yielding a prevalence of 1.062% in people older than 50 years (Table 2). As with incidence, prevalence was lowest in younger patients (0.0047% in people 50–54 years of age) and rose steadily to 6.272% in people 85 years of age and older. In 2018, of the patients with prevalent nAMD still followed up in the SNDS, 81.3% were still being followed up by an ophthalmologist and 50.9% were still receiving anti-VEGF treatment for nAMD.
Impact of Geography and Access to Medical Care
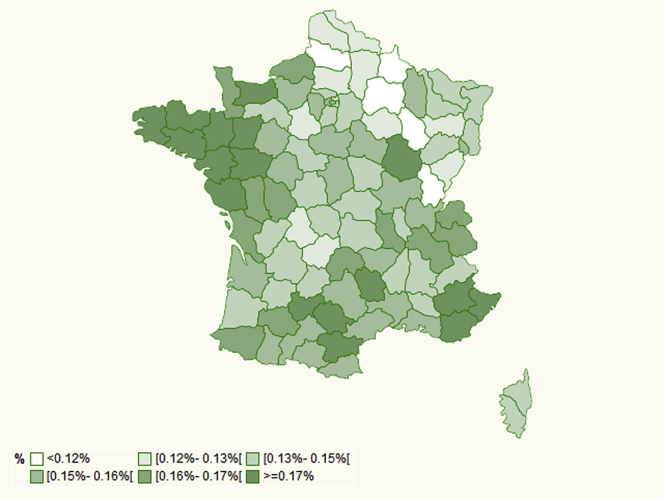
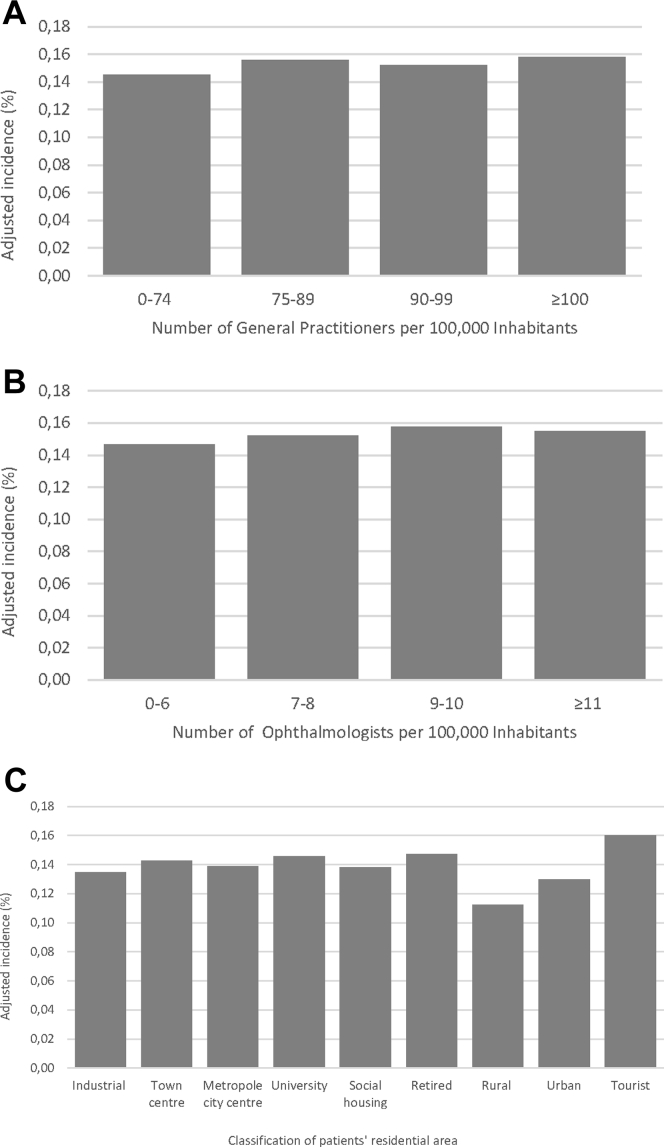
No major geographical differences were observed when nAMD incidence and prevalence were analyzed by French region, although incidence tended to be high in western France and southeastern France and lower in northeastern France (Fig 3). By contrast, rates were one-third lower in overseas regions versus the entire country of France for both incidence (0.058% vs. 0.149%) and prevalence (0.324% vs. 1.062%). Incidence did not vary notably according to the density of general practitioners or ophthalmologists in the patient’s area (Table 3), nor to the classification of the patients’ residential area (Fig 4).
Figure 3.
Map showing the incidence of neovascular age-related macular degeneration in 2018 per region in France. The incidence is standardized to the corresponding population in France 50 years of age or older by age and sex. Incidence in expressed per 100 inhabitants.
Table 3.
Neovascular Age-Related Macular Degeneration Incidence and Prevalence in 2018 by Frequency of Doctors in Residential Area
| Age group (yrs) | No. of Patients with nAMD | nAMD Incidence (%) | nAMD Prevalence (%) |
|---|---|---|---|
| GPs per 100 000 people | |||
| Missing | 386 | ||
| 0–74 | 8919 | 0.145 | 0.987 |
| 75–89 | 9839 | 0.156 | 1.105 |
| 90–99 | 9843 | 0.152 | 1.054 |
| ≥ 100 | 9865 | 0.158 | 1.216 |
| Ophthalmologists per 100 000 people | |||
| Missing | 386 | ||
| 0–6 | 9053 | 0.147 | 1.002 |
| 7–8 | 10 853 | 0.152 | 1.076 |
| 9–10 | 9680 | 0.158 | 1.096 |
| ≥ 11 | 8880 | 0.155 | 1.188 |
GP = general practitioner; nAMD = neovascular age-related macular degeneration.
Figure 4.
Bar graphs showing the incidence of neovascular age-related macular degeneration in 2018 by (A) frequency of general practitioners, (B) frequency of ophthalmologists in residential area, and (C) classification of patients' residential area.
Discussion
The comprehensive SNDS allowed us to report incidence and prevalence for nAMD in the entire French population over a 10-year period. Using the SNDS, which covers approximately 99% of the French population, we created a detailed strategy to identify patients with a diagnosis of or who had been treated for nAMD. Our results found that nAMD incidence was relatively stable from 2008 through 2018. In 2018, annual incidence was 0.149% and prevalence was 1.062% in the French population 50 years of age and older. Both annual incidence and prevalence increased with age. Annual incidence was 0.223%, 0.380%, and 0.603% in those 60 years of age and older, 70 years of age and older, and 80 years of age and older, respectively.
The French system of universal social security and centralized comprehensive reimbursement of medical care underlies the strengths of the SNDS. This comprehensive database has several advantages compared with traditional observational studies based on the sampling of populations to create cohorts.23 First, selection bias was minimized because the population considered all inhabitants of France. Second, the size of the database allows the construction of very large longitudinal cohorts with unprecedented statistical power. Third, the large cohorts allow granular descriptions of variation according to gender, age, geographical location, or access to medical care. Finally, attrition bias is minimal, with few individuals lost to follow-up. The SNDS has been increasingly exploited for epidemiologic studies in various indications, including ophthalmology, for example, to estimate the incidence of cataract surgery29 or to assess risk of endophthalmitis after intravitreal treatment30 and cataract surgery.
The SNDS is an administrative database, rather than a medical database, with several limitations. Consumption of care is recorded, but without information about disease onset, laboratory results, or clinical effectiveness of treatment. Diagnosis is available only for hospitalizations and long-term diseases. Therefore, case identification was based on treatments used and available ICD-10 codes, an approach previously used in diabetes.31 Coding errors are presumed to exist in the SNDS datasets, although the standardized coding dictionaries used, and data quality assurance plan should minimize such errors. The error rate is thought to be very low, because these data determine reimbursement.25 Additionally, information is not captured about undiagnosed and untreated patients. Hence, the LANDSCAPE study probably underestimated nAMD incidence and prevalence, particularly compared with studies relying on systematic eye examinations and imaging. This is offset by high rates of nAMD diagnosis and treatment, assumed by the medical community to cover 95% of patients. Because medical care and treatments are fully reimbursed in France, the economic barrier to treatment is minimized.
Patient Identification
We identified patients with nAMD based on diagnosis and health care consumption data each year. Although detailed, our strategy may have missed or misidentified some patients with nAMD for several reasons. First, some patients may have been treated with an nAMD treatment for another condition such as neovascularization secondary to pathologic myopia. However, we believe the number of such patients to be minimal thanks to subsequent exclusion criteria based on age and other diagnoses (high myopia, retinal diseases other than AMD, treatment of other retina disease such as dexamethasone implant, and panphotocoagulation and laser therapy). Second, we also could have missed untreated patients with late-stage AMD diagnosed too late to be treated. Distinguishing individuals with nAMD from those with diabetic macular edema was challenging. An algorithm was built to distinguish patients with diabetic macular edema treated by dexamethasone implant, macular laser therapy, or panretinal photocoagulation and ICD-10 diagnosis codes and age.
Characteristics of Patients with Incident Neovascular Age-Related Macular Degeneration
In the LANDSCAPE study, two-thirds of patients with nAMD were women. This is consistent with the distribution of the general population older than 80 years.26 The mean age of patients with nAMD at treatment initiation rose from 78.8 years in 2008 to 81.2 years in 2018. This age increase could reflect both the increased life expectancy of elderly French inhabitants (+0.8 and +1.3 years for women and men, respectively, older than 65 from 2008 through 201832) and increased willingness to treat elderly patients with nAMD based on robust literature and meta-analyses confirming efficacy and safety.33,34 Rates of ocular, cardiovascular, metabolic, and other comorbidities were comparable between the LANDSCAPE study’s nAMD population and the age-matched general population.35
Neovascular Age-Related Macular Degeneration Incidence
We estimated an annual incidence of nAMD of 0.149% in 2018 in French people 50 years of age and older. Incidence increased with age group, as per previous reports.10,12,20,36 Incidence adjusted to the age and sex distribution of the French population in 2018 was similar to crude incidence. Our analysis of incidence standardized by age group allowed direct comparison of the LANDSCAPE results with those of previous studies.
The annual incidence in people 80 years of age and older was somewhat lower in the LANDSCAPE study versus the only previous French study (ALIENOR),20 although within the confidence interval (0.603% vs. 0.94% [95% confidence interval, 0.54%–1.61%], respectively). The ALIENOR study used systematic multimodal retinal imaging for accurate diagnosis of nAMD, but selection bias could exist for this study because ALIENOR participants were older (mean age, 79.7 years) and could have been interested in ophthalmic research and follow-up.
Compared with the LANDSCAPE study, other key international studies of nAMD incidence in populations of European descent were much smaller (439–4029 participants) and shorter (1–8 years; Supplemental Table 1). Annual incidence in matched age groups in the LANDSCAPE study was similar to that in an Icelandic study of 439 participants older than 60 years (0.22% vs. 0.29%, respectively) and was higher than the Portuguese Coimbra study in people older than 55 years (0.179% vs. 0.067%). Incidence in the LANDSCAPE study was slightly lower than in the Australian Blue Mountains Eye Study, including 1149 people older than 49 years (0.149% vs. 0.29%). Other risk factors such as genetic background or lifestyle could have contributed to these differences. However, our estimates were generally within the confidence intervals of the estimates from these studies.
The incidence of nAMD was relatively stable over time, with a slight upward trend after 2014 that could be the result of the launch of aflibercept or of inclusion of data from extra categories of French workers, that is, other insurance plans with older workers (including farmers) integrated into the SNDS in 2014. Several studies predicted an increase in AMD cases as global populations age4,5; however, this was not observed in the 2008 through 2018 period in the LANDSCAPE study. The stable incidence over time and the increasing mean age of patients with nAMD could reflect better ageing, with improved lifestyle, medical care, and overall health, as observed in other age-related diseases, in particular dementia.37, 38, 39
Prevalence of Neovascular Age-Related Macular Degeneration Total and by Age Group
In 2018, we estimated nAMD prevalence to be 1.062% in the French population 50 years of age or older. As expected, prevalence increased with age.40 National nAMD prevalence has not been estimated previously in France, and only 1 regional French study estimated prevalence of nAMD (Supplemental Table 2). Prevalence was similar in individuals 80 years of age or older in the LANDSCAPE study compared with the ALIENOR study of 963 residents of Bordeaux from 2006 through 2008 (4.8 vs. 4.9).21
The European meta-analysis by Colijn et al4 included all cases of late AMD without focusing on nAMD. Nonetheless, their estimate of late AMD prevalence in people 85 years of age or older (9.8%) is consistent with the nAMD prevalence of 6.3% for the same age population in the LANDSCAPE study, because nAMD is known to represent approximately half the cases of late AMD. The prevalence of late AMD in the Wong et al5 global meta-analysis of people 80 years of age or older of European ancestry (4.56%) was similar to the LANDSCAPE study estimate for nAMD in the same age group (4.819%).
Prevalence in the LANDSCAPE study varied slightly from other European reports, probably because of methodologic or geographical differences, or both. Prevalence in people older than 65 years was similar in the LANDSCAPE study versus studies from Norway13 and Spain41 (2.06%, 2.5%, and 1.9%, respectively). It was higher than in reports from Ireland16 (1.06% vs. 0.3% in people older than 50 years) and Portugal42 (1.28% vs. 0.55% in people older than 55 years), but lower than in an Icelandic study13 of patients with cardiovascular pathologic features who were older than 66 years (2.06% vs. 3.3%). In the German study by Korb et al,7 nAMD prevalence was 0.1%, but the younger age of the studied population (35–74 years) prevents comparison with the LANDSCAPE study, which included people older than 50 years. The prevalence of nAMD in the LANDSCAPE study was also comparable to a United States study in White people older than 60 years6 (1.59% vs. 1.0%, respectively).
Prevalence clearly increased with age, rising to 6.272% in people 85 years of age and older. Similar age dependency was demonstrated in the European meta-analysis by Colijn et al4 and in other European and United States studies.6,13,14,16,17,21,42
Impact of Geography and Access to Care
Geographic variations in AMD treatment have been demonstrated in the United Kingdom.43 In contrast, in this French study, we did not observe any major trends in incidence or prevalence by region or by the characteristics of the patient’s place of residence. The only notable difference was lower incidence and prevalence in overseas French regions. This could be the result of ethnic variability, differing genetics, environmental factors, or difficulty in accessing health care. Access to care did not seem to influence nAMD diagnosis in France in 2018. No trends for incidence were observed by the density of general practitioners or ophthalmologists in the patient’s locality in 2018, nor by the classification of the patients’ residential area.
In conclusion, the LANDSCAPE study estimated nAMD prevalence and incidence exhaustively in the entire population of a country over the last decade. This comprehensive longitudinal study of the entire French population revealed an nAMD prevalence of 1.062% and an incidence of 0.149% in the French population 50 years of age or older, with markedly higher incidence and prevalence in older age groups. Incidence increased slightly over the 10-year study period. Neovascular AMD epidemiologic factors did not depend on geography, socioeconomic index, or access to care within France.
Manuscript no. D-21-00155.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): C.P.C.G.: Consultant – Thea; Board member – Novartis, Allergan AbbVie, Bayer, Bausch & Lomb
F.B.: Financial support – Novartis, Théa
V.D.: Financial support – Novartis, Bayer, Thea Pharma
C.D.: Consultant – AbbVie, Bayer, Novartis, Horus
S.N.-B.: Consultant – Novartis, Allergan, Bayer
J.-F.G.: Board member – Novartis, Bayer; Financial support – Tilak Healthcare
A.P.: Employee – Novartis Pharma SAS
C.D.: Financial support – Novartis Pharma, Allergan, Bausch & Lomb, Laboratoires Théa
Supported by NovartisSAS, France. The sponsor or funding organization participated in design of the study, conducting the study, data collection, data management, data analysis, interpretation of the data, and preparation, review and approval of the manuscript.
HUMAN SUBJECTS: Human subjects were included in this study. This study was approved by the French data protection agency (CNIL) and the French Institute of Health Data (INDS), who confirmed that informed consent was not required for access to anonymized data in the SNDS. All research adhered to the tenets of the Declaration of Helsinki.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Creuzot Garcher, Srour, Baudin, Daien, Dot, Nghiem-Buffet, Girmens, Coulombel, Ponthieux, Delcourt
Analysis and interpretation: Creuzot Garcher, Srour, Baudin, Daien, Dot, Nghiem-Buffet, Girmens, Coulombel, Ponthieux, Delcourt
Data collection: Coulombel
Obtained funding: N/A; Study was performed as part of regular employment duties at Novartis Pharma S.A.S. (Ponthieux) and IQVIA (Coulombel). No additional funding was provided.
Overall responsibility: Creuzot Garcher, Srour, Baudin, Daien, Dot, Nghiem-Buffet, Girmens, Coulombel, Ponthieux, Delcourt
Supplementary Data
References
- 1.GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144–e160. doi: 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holz F.G., Schmitz-Valckenberg S., Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest. 2014;124(4):1430–1438. doi: 10.1172/JCI71029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell P., Liew G., Gopinath B., Wong T.Y. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 4.Colijn J.M., Buitendijk G.H.S., Prokofyeva E., et al. Prevalence of age-related macular degeneration in Europe: the past and the future. Ophthalmology. 2017;124(12):1753–1763. doi: 10.1016/j.ophtha.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 6.Klein R., Chou C.F., Klein B.E., et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 7.Korb C.A., Kottler U.B., Wolfram C., et al. Prevalence of age-related macular degeneration in a large European cohort: results from the population-based Gutenberg Health Study. Graefes Arch Clin Exp Ophthalmol. 2014;252(9):1403–1411. doi: 10.1007/s00417-014-2591-9. [DOI] [PubMed] [Google Scholar]
- 8.Vanderbeek B.L., Zacks D.N., Talwar N., et al. Racial differences in age-related macular degeneration rates in the United States: a longitudinal analysis of a managed care network. Am J Ophthalmol. 2011;152(2):273–282.e3. doi: 10.1016/j.ajo.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma R., Torres M. Prevalence of lens opacities in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111(8):1449–1456. doi: 10.1016/j.ophtha.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Fisher D.E., Jonasson F., Eiriksdottir G., et al. Age-related macular degeneration and mortality in community-dwelling elders: the age, gene/environment susceptibility Reykjavik study. Ophthalmology. 2015;122(2):382–390. doi: 10.1016/j.ophtha.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Björnsson O.M., Syrdalen P., Bird A.C., et al. The prevalence of age-related maculopathy (ARM) in an urban Norwegian population: the Oslo Macular study. Acta Ophthalmol Scand. 2006;84(5):636–641. doi: 10.1111/j.1600-0420.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 12.Joachim N., Mitchell P., Burlutsky G., et al. The incidence and progression of age-related macular degeneration over 15 years: the Blue Mountains Eye Study. Ophthalmology. 2015;122(12):2482–2489. doi: 10.1016/j.ophtha.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Erke M.G., Bertelsen G., Peto T., et al. Prevalence of age-related macular degeneration in elderly Caucasians: the Tromsø Eye Study. Ophthalmology. 2012;119(9):1737–1743. doi: 10.1016/j.ophtha.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Jonasson F., Arnarsson A., Eiríksdottir G., et al. Prevalence of age-related macular degeneration in old persons: Age, Gene/environment Susceptibility Reykjavik Study. Ophthalmology. 2011;118(5):825–830. doi: 10.1016/j.ophtha.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geirsdottir A., Jonsson O., Thorisdottir S., et al. Population-based incidence of exudative age-related macular degeneration and ranibizumab treatment load. Br J Ophthalmol. 2012;96(3):444–447. doi: 10.1136/bjophthalmol-2011-300304. [DOI] [PubMed] [Google Scholar]
- 16.Akuffo K.O., Nolan J., Stack J., et al. Prevalence of age-related macular degeneration in the Republic of Ireland. Br J Ophthalmol. 2015;99(8):1037–1044. doi: 10.1136/bjophthalmol-2014-305768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farinha C.V.L., Cachulo M.L., Alves D., et al. Incidence of age-related macular degeneration in the central region of Portugal: the Coimbra Eye Study—report 5. Ophthalmic Res. 2019;61(4):226–235. doi: 10.1159/000496393. [DOI] [PubMed] [Google Scholar]
- 18.Delcourt C., Diaz J.L., Ponton-Sanchez A., Papoz L. Smoking and age-related macular degeneration. The POLA Study. Pathologies Oculaires Liées à l’Age. Arch Ophthalmol. 1998;116(8):1031–1035. doi: 10.1001/archopht.116.8.1031. [DOI] [PubMed] [Google Scholar]
- 19.Delcourt C., Lacroux A., Carrière I. The three-year incidence of age-related macular degeneration: the “Pathologies Oculaires Liées à l’Age” (POLA) prospective study. Am J Ophthalmol. 2005;140(5):924–926. doi: 10.1016/j.ajo.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Saunier V., Merle B.M.J., Delyfer M.N., et al. Incidence of and risk factors associated with age-related macular degeneration: four-year follow-up from the ALIENOR Study. JAMA Ophthalmol. 2018;136(5):473–481. doi: 10.1001/jamaophthalmol.2018.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creuzot-Garcher C., Binquet C., Daniel S., et al. The Montrachet Study: study design, methodology and analysis of visual acuity and refractive errors in an elderly population. Acta Ophthalmol. 2016;94(2):e90–e97. doi: 10.1111/aos.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delcourt C., Korobelnik J.F., Barberger-Gateau P., et al. Nutrition and age-related eye diseases: the Alienor (Antioxydants, Lipides Essentiels, Nutrition et maladies OculaiRes) Study. J Nutr Health Aging. 2010;14(10):854–861. doi: 10.1007/s12603-010-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scailteux L.M., Droitcourt C., Balusson F., et al. French administrative health care database (SNDS): the value of its enrichment. Therapie. 2019;74(2):215–223. doi: 10.1016/j.therap.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 24.Bezin J., Duong M., Lassalle R., et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954–962. doi: 10.1002/pds.4233. [DOI] [PubMed] [Google Scholar]
- 25.Tuppin P., Rudant J., Constantinou P., et al. Value of a national administrative database to guide public decisions: from the systeme national d’information interregimes de l’Assurance Maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(suppl 4):S149–S167. doi: 10.1016/j.respe.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Institut national de la statistique et des études économiques, the French Offical Statistic Authority (INSEE) FNIoSaES. Demographics bulletin. 2019. INSEE.fr2019. Available at: https://www.insee.fr/fr/statistiques/4281618
- 27.Fuentes S., Cosson E., Mandereau-Bruno L., et al. Identifying diabetes cases in health administrative databases: a validation study based on a large French cohort. Int J Public Health. 2019;64(3):441–450. doi: 10.1007/s00038-018-1186-3. [DOI] [PubMed] [Google Scholar]
- 28.Quan H., Li B., Couris C.M., et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 29.Daien V., Le Pape A., Heve D., et al. Incidence and characteristics of cataract surgery in France from 2009 to 2012: a national population study. Ophthalmology. 2015;122(8):1633–1638. doi: 10.1016/j.ophtha.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Baudin F., Benzenine E., Mariet A.S., et al. Association of acute endophthalmitis with intravitreal injections of corticosteroids or anti-vascular growth factor agents in a nationwide study in France. JAMA Ophthalmol. 2018;136(12):1352–1358. doi: 10.1001/jamaophthalmol.2018.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuentes S., Mandereau-Bruno L., Regnault N., et al. Is the type 2 diabetes epidemic plateauing in France? A nationwide population-based study. Diabetes Metab. 2020;46(6):472–479. doi: 10.1016/j.diabet.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Institut national de la statistique et des études économiques, the French Offical Statistic Authority (INSEE). Demographics of French population in 2020. Available at: https://www.insee.fr/fr/statistiques/5347620
- 33.Thulliez M., Angoulvant D., Le Lez M.L., et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta-analysis. JAMA Ophthalmol. 2014;132(11):1317–1326. doi: 10.1001/jamaophthalmol.2014.2333. [DOI] [PubMed] [Google Scholar]
- 34.Pham B., Thomas S.M., Lillie E., et al. Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis. BMJ Open. 2019;9(5) doi: 10.1136/bmjopen-2018-022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Direction de la Recherche de L’évaluation et des Statistiques (DREES). French Health Statistic Department, Health of the French population [L’état de santé de la population en France]. 2015. Available at: https://drees.solidarites-sante.gouv.fr/publications/rapports/letat-de-sante-de-la-population-en-france-rapport-2015
- 36.Varma R., Choudhury F., Klein R., et al. Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5) doi: 10.1016/j.ajo.2009.11.014. 752–761.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Research Council (US) Panel on a Research Agenda and New Data for an Aging World. The Health of Aging Populations. In: (US) NAP, ed. Preparing for an Aging World: The Case for Cross-National Research. 2001. Washington, DC: The National Academies Press. Available at: 10.17226/10120. [DOI] [PubMed]
- 38.Grasset L., Brayne C., Joly P., et al. Trends in dementia incidence: evolution over a 10-year period in France. Alzheimers Dement. 2016;12(3):272–280. doi: 10.1016/j.jalz.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Wolters F.J., Chibnik L.B., Waziry R., et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: the Alzheimer Cohorts Consortium. Neurology. 2020;95(5):e519–e531. doi: 10.1212/WNL.0000000000010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delcourt C., Michel F., Colvez A., et al. Associations of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA Study. Ophthalmic Epidemiol. 2001;8(4):237–249. doi: 10.1076/opep.8.4.237.1613. [DOI] [PubMed] [Google Scholar]
- 41.Spanish Eyes Epidemiological (SEE) Study Group Prevalence of age-related macular degeneration in Spain. Br J Ophthalmol. 2011;95(7):931–936. doi: 10.1136/bjo.2010.187773. [DOI] [PubMed] [Google Scholar]
- 42.Cachulo Mda L., Laíns I., Lobo C., et al. Age-related macular degeneration in Portugal: prevalence and risk factors in a coastal and an inland town. The Coimbra Eye Study—report 2. Acta Ophthalmol. 2016;94(6):e442–e453. doi: 10.1111/aos.12950. [DOI] [PubMed] [Google Scholar]
- 43.Keenan T.D., Wotton C.J., Goldacre M.J. Trends over time and geographical variation in rates of intravitreal injections in England. Br J Ophthalmol. 2012;96(3):413–418. doi: 10.1136/bjophthalmol-2011-300338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.