Abstract
The effect of exogenous transforming growth factor β (TGF-β) on Mycobacterium bovis BCG-induced tumor necrosis factor alpha (TNF-α) production by human mononuclear cells was studied. It was found that TNF-α production by human cells stimulated with BCG was significantly inhibited by TGF-β. The specificity of the observed inhibition was demonstrated, since the addition of an anti-TGF-β neutralizing monoclonal antibody completely reversed the inhibitory effect. Furthermore, the suppressive effect of TGF-β on TNF-α secretion in this system was not due to a direct cytotoxic effect, since cell viability was comparable in the presence or absence of TGF-β. Interestingly, our results demonstrated comparative suppressive effects of TGF-β and interleukin-10 on BCG-induced TNF-α secretion. Together, the data demonstrate, for the first time, that TGF-β inhibits BCG-induced TNF-α secretion by human cells.
Tuberculosis, caused by the intracellular pathogen Mycobacterium tuberculosis, is an important infectious disease that causes 2.9 million deaths and 8 million new active cases annually (14, 19). A host’s initial resistance to M. tuberculosis infection depends on vaccination with Mycobacterium bovis BCG. M. bovis infection of human cells induces the secretion and release of a number of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), by mononuclear phagocytes (15, 23). TNF-α, a 17-kDa polypeptide, is pivotal in the development and maintenance of BCG-induced bactericidal granulomas (1, 13) and activates macrophages to inhibit intracellular mycobacterial growth (9). The balance between this mycobacterial growth-inhibiting cytokine and the release of deactivating macrophage cytokines may be important in regulating cellular effector functions against M. tuberculosis infection. Recently, it has been demonstrated that M. tuberculosis (5, 11) and its purified protein derivative (20) induce the production of the deactivating cytokine transforming growth factor β (TGF-β). TGF-β, a 25-kDa, disulfide-linked, homodimeric protein, is produced by monocytes with active tuberculosis and is present in macrophages of granulomatous lesions of patients with tuberculosis (21). In addition, TGF-β interferes with the TNF-mediated bacteriostatic and bactericidal activities of infected macrophages against mycobacteria and inhibits lipopolysaccharide-induced TNF-α secretion by human and murine cells (2). Therefore, in view of the critical role which TGF-β appears to play in the immune response to M. tuberculosis, we believed that it was important to see whether TGF-β regulates the production of BCG-induced TNF-α by human cells. We found significant suppression of BCG-induced TNF-α secretion in human cells by TGF-β.
Peripheral blood mononuclear cells (PBMC) were separated from heparinized blood of seven BCG-vaccinated, healthy volunteers by centrifugation through Histopaque (Sigma Chemical Co., St. Louis, Mo.). The resultant mononuclear cell suspension was washed three times in RPMI 1640 medium (Sigma Chemical Co.), and its viability was assessed by exclusion of trypan blue. Cells were counted and incubated at a density of 106/ml in complete RPMI 1640 culture medium containing 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 1% heat-inactivated pooled human serum. Cell cultures were incubated at 37°C in 5% CO2–95% air for 18 h in the presence of various concentrations of BCG (1, 5, 10, and 50 μg/ml). As a negative control, cells were incubated without BCG. In some experiments, cells were pretreated with various concentrations of TGF-β (Sigma Chemical Co.) for 2 h prior to stimulation with 10-μg/ml BCG. In addition, the effect of TGF-β was reversed by using a monoclonal antibody to human TGF-β (monoclonal mouse anti-TGF-β; Genzyme, Cambridge, Mass.). Parallel cell cultures were stimulated with BCG in the presence of 10-ng/ml interleukin-10 (IL-10; Sigma Chemical Co.). All culture supernatants were centrifuged to remove cellular debris. The human TNF-α concentration in the culture supernatants was measured by a sandwich enzyme-linked immunosorbent assay (ELISA; Amersham, Aylesbury, United Kingdom) in accordance with the manufacturer’s instructions. Supernatants were tested after dilution to the appropriate concentration. The detection limit of the TNF-α assay was 4.4 pg/ml.
In this study, Student’s t test was used to determine the significance of the differences in TNF-α production between control and experimental groups, and the level of significance was P < 0.01.
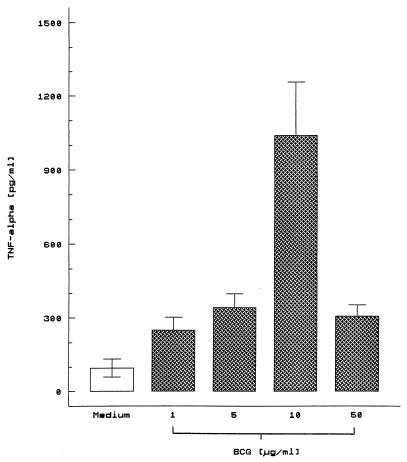
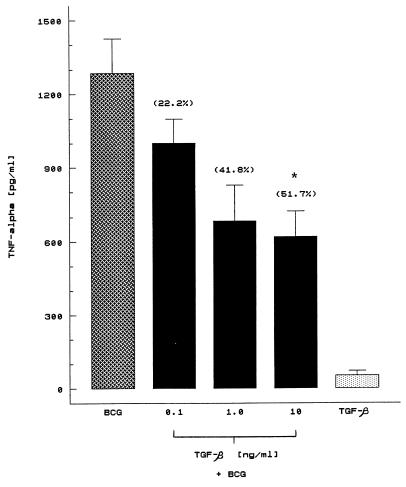
To examine the effect of TGF-β on BCG-induced TNF-α secretion, we first evaluated the induction of TNF-α in human cells activated with different concentrations of BCG. Figure 1 shows a dose-dependent increase of BCG-induced TNF-α secretion by mononuclear cells of seven donors, and the maximal secretion was reached with 10-μg/ml BCG. The effect of exogenous TGF-β on TNF-α secretion by human cells activated with BCG was studied next. D’Andrea et al. (6) have reported that preincubation of PBMC with TGF-β before lipopolysaccharide or Staphylococcus aureus stimulation reduced the production of TNF-α. Therefore, in this study, PBMC were pretreated with different concentrations of TGF-β for 2 h before BCG stimulation. As shown in Fig. 2, BCG at 10 μg/ml induced 1,286.6 ± 138.2-pg/ml TNF-α in the seven donors tested, and preincubation with increasing doses of TGF-β decreased the secretion of this cytokine in a dose-dependent manner. A marked inhibition of TNF-α secretion was seen after treatment of cells with 10-ng/ml TGF-β (51.7% inhibition). This inhibitory effect was significant (P < 0.01). Because pretreatment of human cells with higher concentrations of TGF-β (up to 10 ng/ml) yielded similar results (data not shown), we considered it important to determine whether TGF-β alone may induce TNF-α secretion by human mononuclear cells. Our results indicated that TGF-β in the absence of BCG failed to stimulate human cells to produce significant levels of immunoreactive TNF-α (Fig. 2). In these experiments, the level of TNF-α did not exceed 125 pg/ml.
FIG. 1.
TNF-α secretion by BCG-activated human cells. Mononuclear cells from seven BCG-vaccinated, healthy donors were incubated at 106 cells/ml for 18 h with various concentrations of BCG. Culture supernatants were assayed for TNF-α activity by ELISA. Results are expressed as means ± SEMs.
FIG. 2.
Effect of TGF-β on BCG-induced TNF-α production. Cells at 106/ml were pretreated with various concentrations of TGF-β for 2 h prior to the addition of BCG (10 μg/ml) and incubated for 20 h at 37°C. Simultaneously, cells were incubated for 20 h with TGF-β (10 ng/ml) alone. Cell-free supernatants were assessed for TNF-α activity by ELISA. The results are the means ± the SEMs for seven different individuals. The concentration of TNF-α in cultures containing medium alone was 84 ± 28 pg/ml (mean ± SEM). The values in parentheses indicate percent inhibition by TGF-β with respect to BCG cultures. *, significant difference (P < 0.01) from BCG cultures.
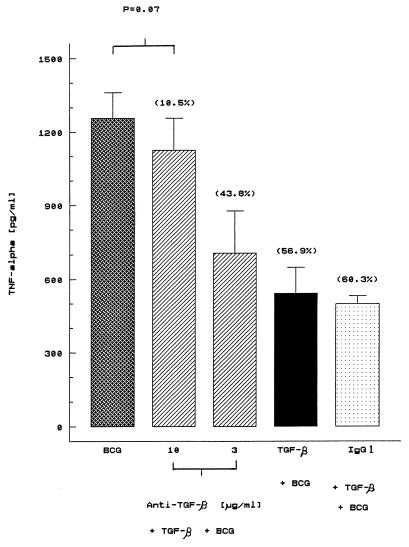
To evaluate further the specificity of the inhibitory effect of TGF-β, a neutralizing monoclonal antibody to TGF-β and an isotype-matched control, mouse immunoglobulin G1 (IgG1; Sigma Chemical Co.) were added to human mononuclear cells that had been cultured with BCG in the presence of TGF-β. As shown in Fig. 3, suppression of BCG-induced TNF-α secretion (56.9% inhibition) was neutralized with 10-μg/ml antibody (10.5% inhibition). It is important to note that BCG-induced TNF-α secretion did not differ significantly between cell cultures that had received BCG alone or in combination with TGF-β in the presence of 10-μg/ml neutralizing TGF-β (P = 0.07) (Fig. 3). In contrast, an isotype-matched control IgG1 antibody was without effect (60.3% inhibition). These results indicate the specificity of the inhibitory effect of TGF-β. In addition, TGF-β alone or in the presence of BCG did not affect the viability of the cells, as determined by their ability to exclude trypan blue (Table 1). Together, these results suggest that the inhibitory effect of TGF-β on BCG-induced TNF-α secretion is not accompanied by a cytotoxic effect.
FIG. 3.
Neutralizing anti-TGF-β monoclonal antibody significantly reverses the inhibitory effect of TGF-β on BCG-induced TNF-α secretion. Cells (106/ml) were incubated with TGF-β in the presence of two different amounts of an anti-TGF-β monoclonal antibody or an isotype-matched control monoclonal antibody (IgG1) for 2 h prior to BCG stimulation for 18 h at 37°C. TNF-α levels were measured by ELISA. The results are the means ± SEMs for seven donors. The values in parentheses indicate percent inhibition by TGF-β with respect to BCG cultures.
TABLE 1.
TGF-β does not alter cell viabilitya
| TGF-β concn (ng/ml) | BCG | Cell viabilityb (%) |
|---|---|---|
| − | + | 90.51 |
| 0.1 | + | 100.00 |
| 1.0 | + | 84.30 |
| 10.0 | + | 97.20 |
| + | − | 95.20 |
Mononuclear cells were pretreated either in the absence of TGF-β or in the presence of increasing concentrations of TGF-β for 2 h and then incubated with BCG (10 μg/ml) for 18 h.
Cell viability was determined by trypan blue exclusion. The results represent the means for seven donors.
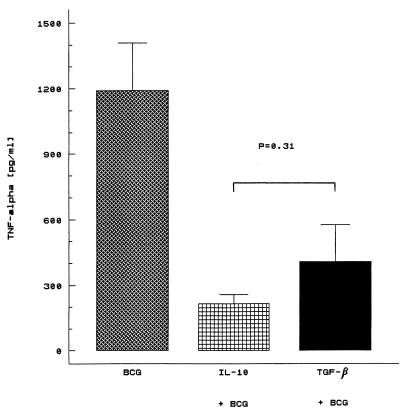
Because IL-10 is a potent downregulator of the immune response implicated in mycobacterial infections (18), we next compared the inhibitory effects of TGF-β and IL-10 on BCG-nduced TNF-α secretion. Human mononuclear cells were stimulated with BCG in the presence of IL-10 (10 ng/ml) as reported previously (16). As shown in Fig. 4, the addition of IL-10 resulted in a significant decrease in BCG-induced TNF-α levels, from 1,190 ± 218.8 (mean ± the standard error of the mean [SEM]) to 217 ± 41.7 pg/ml (P < 0.01 versus BCG alone). The effect of IL-10 was dose dependent (data not shown). Although the inhibition was more pronounced, in cultures incubated with BCG in the presence of IL-10, a significant difference was not achieved (P = 0.31) (Fig. 4). These results demonstrate that TGF-β, like IL-10, is critical for controlling the production of TNF-α by mycobacterium-activated human cells.
FIG. 4.
Comparison of the suppressive effects of TGF-β and IL-10 on TNF-α production by BCG-stimulated human cells. Cell preparations were cultured for 18 h with BCG (10 μg/ml) alone or in the presence of IL-10 (10 ng/ml) or TGF-β (10 ng/ml). The cell-free supernatant fluid was assayed for TNF-α content by ELISA.
Although the efficacy of the BCG vaccine for preventing tuberculosis has been found to vary considerably, BCG is the only currently available vaccine against M. tuberculosis and is still given routinely to millions of children in Mexico. It has been suggested that the lack of an effective means for preventing resistance to M. tuberculosis infection can be due to downregulation of the immune response (3). Previous studies have shown that TGF-β may be an important mechanism by which mycobacteria evade the host’s immune response (2). Since TNF-α can stimulate human cells to inhibit intracellular growth of mycobacteria and is an important cytokine required in the development of BCG-induced bactericidal granulomas, in this study, we examined whether TNF-α secretion induced by BCG is downregulated by TGF-β. Our results demonstrate a suppressor effect of exogenous TGF-β on TNF-α secretion in response to BCG. The suppressor effect of TGF-β on BCG-induced TNF-α secretion was, indeed, due to TGF-β, since a significant reversion was obtained with a neutralizing monoclonal antibody to TGF-β. Such an inhibition is in agreement with the finding that TGF-β inhibits the activation of macrophages (22) and the generation of cytokines, including IL-2, TNF-α, and gamma interferon (7). In contrast, a recent study (17) showed that TGF-β promotes the generation of Th1 cells, probably enhancing gamma interferon production. In the present study, however, the addition of TGF-β resulted in a decrease in cytokine production. These differences may reflect differences in the antigen recognition of a superantigen and mycobacteria.
It is well known that control of tuberculous infection occurs in a granuloma. It is also known that TNF-α is an important immunomodulator required in the development of BCG-induced bactericidal granulomas. Therefore, the effect of TGF-β on endogenous TNF-α production induced by BCG may provide an important mechanism in determining the immune responses of susceptibility or resistance in humans infected with M. tuberculosis.
The mechanism(s) involved in the suppressive effect of TGF-β on BCG-induced TNF-α secretion is not well understood. Recently, Chantry et al. have demonstrated that TGF-β may inhibit translation of the TNF-α mRNA (2). Therefore, it is possible that the suppressive effect of TGF-β on TNF-α secretion by cells stimulated with BCG may result from a direct effect at the level of translation. Alternatively, the anti-TGF-β antibody-mediated downregulation of TNF-α may be the indirect result of interfering IL-2-mediated pathways of signal transduction (8, 10, 12) and/or decreased antigen presentation by modulation of the expression of HLA class II molecules on antigen-presenting cells (4). The present experimental system is being extended to determine the effect of TGF-β on the expression of HLA-DR molecules (by examining expression on monocytes by flow cytometry).
In conclusion, data presented in this study demonstrate the effect of TGF-β on BCG-induced TNF-α secretion and, at the same time, suggest that TGF-β might be an important regulatory cytokine for control of the host’s immune response to mycobacterial infection. Further studies are necessary to determine whether the inhibitory effect of TGF-β, indeed, suppress a human protective immune response in vivo.
Acknowledgments
We thank J. Ruiz-Puente (Instituto Nacional de Higiene, México) for his gifts of BCG.
This research project received financial support from the Dirección de Estudios de Posgrado e Investigación (DEPI).
REFERENCES
- 1.Bermudez L, Wu M, Petrofsky M, Young L. Interleukin-6 antagonizes tumor necrosis factor-mediated mycobacteriostatic and mycobactericidal activities in macrophages. Infect Immun. 1992;60:4245–4248. doi: 10.1128/iai.60.10.4245-4252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chantry D, Turner M, Abney E, Feldman M. Modulation of cytokine production by TGF-β. J Immunol. 1989;142:4295–4300. [PubMed] [Google Scholar]
- 3.Cooper A, Flynn J. The protective immune response to Mycobacterium tuberculosis. Curr Opin Immunol. 1995;7:512–516. doi: 10.1016/0952-7915(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 4.Czarniecki C, Chiu H, Wong G, McCabe S, Palladino M. Transforming growth factor-β1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988;140:4217–4223. [PubMed] [Google Scholar]
- 5.Dahl K E, Shiratsuchi H, Hamilton B D, Ellner J J, Toossi Z. Selective induction of transforming growth factor β in human monocytes by lipoarabinomannan of Mycobacterium tuberculosis. Infect Immun. 1996;64:399–405. doi: 10.1128/iai.64.2.399-405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Andrea A, Ma X, Aste-Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor alpha production. J Exp Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espevik T, Figari S, Shalaby M, Lackides G, Lewis G, Shepard H, Palladino M. Inhibition of cytokine production by cyclosporin A and transforming growth factor β. J Exp Med. 1987;166:571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferris D, Willette-Brown J, Ortaldo J, Farrar W. IL-2 regulation of tyrosine kinase activity is mediated through the p70-75 β-subunit of the IL-2 receptor. J Immunol. 1989;143:870–874. [PubMed] [Google Scholar]
- 9.Flesch I E A, Kaufmann S H E. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect Immun. 1990;58:2675–2677. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatakeyama M, Kono T, Kobayashi N, Kawahara A, Levin S, Perlmutter R, Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56: identification of novel intermolecular association. Science. 1991;252:1523–1524. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch C, Yoneda T, Averill L, Ellner J, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-β1. J Infect Dis. 1994;170:1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 12.Holter W, Kalthoff F, Pickl W, Ebner C, Majdic O, Kraft D, Knapp W. Transforming growth factor-β inhibits IL-4 and IFN-gamma production by stimulated human T cells. Int Immunol. 1994;6:469–475. doi: 10.1093/intimm/6.3.469. [DOI] [PubMed] [Google Scholar]
- 13.Kindler V, Sappino A, Grau G, Piquet P, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 14.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organisation. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 15.Méndez-Samperio P, Hernandez-Garay M, Nuñez-Vazquez A. Cellular activation induced by BCG is a PTK-dependent event. Cell Immunol. 1996;171:147–152. doi: 10.1006/cimm.1996.0185. [DOI] [PubMed] [Google Scholar]
- 16.Méndez-Samperio P, Garcia-Martinez E, Hernandez-Garay M, Solis-Cardona M. Depletion of endogenous interleukin-10 augments interleukin-1β secretion by Mycobacterium bovis BCG-reactive human cells. Clin Diagn Lab Immunol. 1997;4:138–141. doi: 10.1128/cdli.4.2.138-141.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagelkerken L, Gollob K, Tielemans M, Coffman R. Role of transforming growth factor-β in the preferential induction of T helper cells of type 1 by Staphylococcal enterotoxin B. Eur J Immunol. 1993;23:2306–2310. doi: 10.1002/eji.1830230938. [DOI] [PubMed] [Google Scholar]
- 18.Oswald I, Wynn T, Sher A, James S. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor alpha required as a costimulatory factor for interferon gamma-induced activation. Proc Natl Acad Sci USA. 1992;89:8676–8680. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raviglione M, Snider D, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 20.Toossi Z, Young T-G, Averill L E, Hamilton B D, Shiratsuchi H, Ellner J J. Induction of transforming growth factor β1 by purified protein derivative of Mycobacterium tuberculosis. Infect Immun. 1995;63:224–228. doi: 10.1128/iai.63.1.224-228.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner J. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–473. [PubMed] [Google Scholar]
- 22.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-β. Nature (London) 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 23.Valone S E, Rich E A, Wallis R S, Ellner J J. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988;56:3313–3315. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]