Abstract
Introduction
The aim of this randomised controlled trial (RCT) is to investigate whether prolonged consumption of sweeteners and sweetness enhancers (S&SEs) within a healthy diet will improve weight loss maintenance and obesity-related risk factors and affect safety markers compared with sugar.
Methods and analysis
SWEET (S&SEs: prolonged effects on health, obesity and safety) is a 1-year multicentre RCT including at least 330 adults with overweight (18–65 years, body mass index (BMI) >25 kg/m2) and 40 children (6–12 years, BMI-for-age >85th percentile). In an initial 2-month period, adults will consume a low-energy diet with the aim to achieve ≥5% weight loss. Children are advised to consume a generally healthy diet to maintain body weight, thus reducing their BMI-for-age z-score. In the following 10 months, participants will be randomised to follow a healthy ad libitum diet with or without S&SE products. Clinical investigations are scheduled at baseline, after 2, 6 and 12 months. The primary outcomes are body weight for efficacy and gut microbiota composition (in relation to metabolic health) for safety, both in adults. Secondary outcomes include anthropometry, risk markers for type-2 diabetes and cardiovascular diseases, questionnaires including, for example, food preferences, craving and appetite and tests for allergenicity.
Ethics and dissemination
The trial protocol has been approved by the following national ethical committees; The research ethics committees of the capital region (Denmark), approval code: H-19040679, The medical ethics committee of the University Hospital Maastricht and Maastricht University (the Netherlands), approval code: NL70977.068.19/METC19-056s, Research Ethics Committee of the University of Navarra (Spain), approval code: 2019.146 mod1, Research Ethics Committee of Harokopio University (Greece), approval code: 1810/18-06-2019. The trial will be conducted in accordance with the Declaration of Helsinki. Results will be published in international peer-reviewed scientific journals regardless of whether the findings are positive, negative or inconclusive.
Trial registration number
NCT04226911 (Clinicaltrials.gov)
Keywords: Nutrition, DIABETES & ENDOCRINOLOGY, Microbiology, MICROBIOLOGY, Allergy
Strengths and limitations of this trial
The trial investigates long-term effects of sweeteners and sweetness enhancers (S&SEs) compared with sugar in the contexts of an ad libitum healthy diet including both foods and drinks.
Weight maintenance after weight loss is difficult to achieve, and in this trial, the effects of consuming S&SE foods and drinks compared with sugar during 10-month weight maintenance after 2-month weight loss are investigated.
A broad range of measurements related to health and safety, appetite and food preferences are included to address concerns raised in relation to consumption of S&SEs.
This is a multicentre trial covering Northern, Central and Southern Europe, thereby reflecting different geographic distributions of adult and childhood obesity in Europe.
A potential limitation is that the number of included children was reduced through the recruitment period, but this will not affect the two primary outcomes as sample size determination was done exclusively for adults.
Introduction
Obesity is a major global health problem giving rise to increased risk of non-communicable diseases such as type-2-diabetes (T2D) and cardiovascular diseases (CVD).1 Sustaining energy balance is critical to maintain body weight. However, sugar contributes to the energy density of most diets and may, therefore, promote a positive energy balance.2 In 2015, the WHO strongly recommended that free sugar intake should be <10 energy percentage (E%) and preferably <5 E% as a conditional recommendation.2 The latter is still not fulfilled by large parts of the population, including Denmark,3 4 Greece,5 Spain6 and the Netherlands.7
One often-recommended approach to reduce sugar intake is to replace sugar with sweeteners and sweetness enhancers (S&SEs). The use of S&SEs allows products to retain their palatability without the associated calories, creating a perception of a ‘healthier’ product.8 Although drinks often constitute the largest part of S&SE products consumed, S&SEs products also include foods. In the US and worldwide, the consumption of S&SEs products such as desserts, gums and breakfast foods has increased.9 However, foods with S&SEs have been less extensively investigated. S&SEs comprise a variety of compounds with proposed negative effects on health parameters;10 however, evidence is conflicting. For example, S&SEs have been claimed to result in detrimental effects on appetite, body weight, glucose metabolism and gut microbiota.8 In contrast, several systematic reviews and meta-analysis found no detrimental effects on appetite and body weight—rather on the contrary11–13 and interestingly, a large 1-year study found S&SEs to be superior to water for weight loss and weight maintenance.14 Not all reviews have come to the same conclusion, but selective citation of the different studies could be the cause.15 In relation to postprandial glycaemia and insulinemia, no differences were observed between S&SEs and controls in recent systematic reviews and meta-analysis.16 17 Following consumption of S&SEs changes in the gut microbiota composition and functionality have been debated as a food safety issue because some changes in specific bacteria have been associated with diseases and risk markers of diseases.18 19 As an example, a change in microbial composition after 7 days consumption of saccharin has been associated with impaired glucose homeostasis.20 However, that study included only seven human participants, there was no control group, and conclusions were based on a post hoc division of responders and non-responders.20 In general, safety concerns have derived from animal studies using S&SE doses far above habitual intake in humans. Long-term controlled human intervention studies are, therefore warranted.19
Aim and objectives
The overall aim of the randomised controlled trial (RCT) SWEET (S&SEs: Prolonged effects on health, obesity and safety) is to investigate the efficacy and safety of combined (foods and drinks) and prolonged use of S&SEs—as part of a whole healthy ad libitum diet approach—in a population of overweight adults and children. The two primary outcomes on efficacy and safety will be assessed in adults by 1-year changes in body weight and 1-year changes in gut microbiota related to metabolic health outcomes, respectively. Secondary objectives concern effects on obesity-related risk factors such as fat mass, glucose metabolism and lipidemia as well as safety aspects such as allergenicity. Other outcomes include appetite sensations, food cravings, food preferences and preference for sweet taste.
Hypothesis
We hypothesise that prolonged use of S&SEs in foods and drinks will increase palatability of the diet and thereby increase compliance to the recommendations for a healthy sugar-reduced diet, resulting in improved body weight control. Further, we hypothesise that there will be no safety concerns using S&SEs in the long-term.
Methods and analysis
Study design
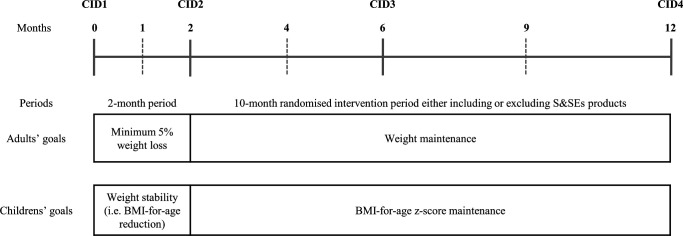
SWEET is conducted in four intervention sites; Athens (Harokopio University of Athens, Greece), Copenhagen (University of Copenhagen, Denmark), Maastricht (Maastricht University, the Netherlands) and Navarra (University of Navarra, Spain) covering North, Central, South and East Europe, thereby reflecting different geographic distributions of obesity in Europe. In the 1-year RCT, both adults and families (at least one adult and one child) are included. The RCT consists of an initial 2-month period followed by a 10-month randomised two-armed parallel intervention period. For adults, the goals in these periods are first to achieve a weight loss (WL) and second to maintain the WL. For children, the goals are first to achieve weight stability and second to maintain body mass index (BMI)-for-age z-score. The 10-month randomised intervention period will be carried out by using a ‘fading visit’ approach (figure 1). During the RCT, all participants will undergo four clinical investigation days (CIDs) and will be supervised by dieticians individually/familywise and/or in groups at least every third month.
Figure 1.
Overall study design. Solid lines are CIDs and dashed lines are dietary counselling sessions where non-fasting body weight of adults is measured. Additionally, LED products for adults will be collected from the intervention site every second or third week during the initial 2-month period. BMI, body mass index; CID, clinical investigation day; LED, low-energy diet; S&SEs, sweeteners and sweetness enhancers.
Originally, a 1-year follow-up period was planned after the 10-month intervention period; however, it was omitted due to recruitment delay caused by the COVID-19 pandemic. Furthermore, the initial plan was to include at least one child per adult (ie, only families). However, recruitment turned out to be very difficult and the strategy was changed to also include adults without children because the primary outcomes and sample size determinations were based solely on adults. Screening visits were conducted between 29 June 2020 and 27 September 2021, and the last patient last visit (CID at month 12) is scheduled for 30 September 2022.
Patient and public involvement
Neither patients nor the public were involved in the design and conduct of the study and they will not be involved in interpretation, reporting or dissemination of the trial.
Participants
Recruitment and screening
Participants were recruited continuously by multiple routes, for example, web pages, social media, newspapers and registries (local databases or civil registration numbers). Potential adult participants were prescreened by phone and answered questions on behalf of their child(ren). If still eligible and interested after prescreening, they received written information and were invited to an information meeting. After the information meeting, an informed consent form and a general data protection regulation form were signed by the adult participant, and for children by the parents or person(s) having custody and the site-PI or delegated staff. Thereafter, the screening visit was scheduled. Participants were screened in the fasting state, where all inclusion and exclusion criteria were assessed. The recruitment has ended with inclusion of 341 adults and 38 children.
Eligibility criteria
Adults (men and women), 18–65 years, BMI ≥25 kg/m2 and children (boys and girls), 6–12 years and BMI-for-age >85th percentile were included. Children were only included if they had an eligible adult family member (ie, as a family)—a biological relationship was not required. However, it was required that the family lived in the same household at least 4 days/week. Participants were required to have a regular consumption of sugar-containing/sugar-sweetened products and be motivated and willing to be randomised to any of the two intervention groups. All exclusion criteria are listed in table 1. Inclusion and exclusion criteria were assessed at screening; however, the site-PI or delegated personnel has the right to terminate participation at any time if deemed in the participant’s best interest, and children are excluded if their adult family member’s participation is discontinued.
Table 1.
List of exclusion criteria
| Adults | Children |
| General | |
| Weight change >5% 2 months prior to screening Surgical treatment of obesity Blood donation <3 months prior to study initiation Change in smoking habits during the last month. (Smoking was allowed and monitored throughout the study) Regularly drinking >21 (men) or >14 (women) units of alcohol per week Intensive physical training (>10 hours per week) Self-reported eating disorders Intolerance and allergies expected to interfere with the study Self-reported drug abuse within the previous 12 months Night- or shift work that ends later than 23:00 For women: pregnancy, lactation Persons who do not have access to either (mobile) phone or Internet Insufficient communication with national language Inability, physically or mental, to comply with the procedures required by the study protocol Participant’s general condition contraindicates continuing the study Simultaneous participation in other clinical intervention studies |
Intensive physical training (>10 hours of per week) Self-reported eating disorders Intolerance and allergies expected to interfere with the study Insufficient communication with national language Inability, physically or mental, to comply with the procedures required by the study protocol Participant’s general condition contraindicates continuing the study Simultaneous participation in other clinical intervention studies |
| Medical conditions | |
| Diagnosed diabetes mellitus Medical history of CVD (eg, current angina; myocardial infarction or stroke within the past 6 months; heart failure; symptomatic peripheral vascular disease) Systolic blood pressure above 160 mmHg and/or diastolic blood pressure above 100 mmHg (measured at screening) whether on or off treatment for hypertension Significant liver diseases for example, cirrhosis (fatty liver disease allowed) Malignancy which was active or in remission for less than 5 years after last treatment (local basal and squamous cell skin cancer allowed) Active inflammatory bowel disease, coeliac disease, chronic pancreatitis or other disorder potentially causing malabsorption Thyroid diseases, except Levothyroxine treatment of hypothyroidism if not on a stable dose for at least 3 months Psychiatric illness (eg, major depression, bipolar disorders) |
Diagnosed diabetes mellitus Other diseases that may influence the study outcomes |
| Medication | |
| Use currently or within the previous 3 months of prescription or over the counter medication that had the potential of affecting body weight including food supplements Exceptions related to medical conditions: I) Cholesterol or blood pressure lowering medication was allowed if the participant’s dose had not changed during the last 3 months II) Low dose antidepressants if they, in the judgement of the investigator, did not affect weight or study participation. III) Levothyroxine for treatment of hypothyroidism if on a stable dose for at least 3 months |
Use currently or within the previous 3 months of prescription or over the counter medication that had the potential of affecting body weight including food supplements |
| Laboratory screening* | |
| Glucose >7.0 mmol/L Haemoglobin: women; <7.5 mmol/L (Copenhagen, Maastricht, Navarra, Athens) men; <8.5 mmol/L (Copenhagen, Maastricht) and<8.1 mmol/L (Navarra, Athens) For Maastricht participants only: Creatinine <50 µmol/L and >100 µmol/L ALT >34 IU |
– |
*Fasting blood sample was collected from adults and locally analysed to assess glucose and haemoglobin levels, and some additional values at Maastricht.
ALT, Alanine transaminase; CVD, cardiovascular diseases; IU, international unit.
Randomisation
After screening, eligible participants were randomly assigned to one of the two intervention groups in a 1:1 ratio by a site-specific randomisation list created by a person in Copenhagen not involved in the RCT. The randomisation was stratified by gender, age (<40 years or ≥40 years) and BMI (<30 or ≥30 kg/m2), and stratification was implemented by sequentially assigning families and adults from each stratum to the two interventions in blocks of 4, using the software R. Each household was randomised to the same intervention determined by the oldest member of the household. Although randomisation was done after screening, it was not revealed to the household/participant before completion of the initial 2-month period.
Intervention
The two trial periods are illustrated in figure 1.
Two-month period
In the initial 2-month period, adults—regardless of randomisation—received a low-energy diet (LED) (Cambridge Weight Plan, Northants, United Kingdom). If the clinically relevant criteria for WL of ≥5%21 was not achieved, the participant was excluded. During the 2-month period, adults visited the intervention site 2–3 times for collection of LED products, weighing and dietetic counselling. The LED consisted of 3347–4184 kJ/day, 15–20 E% fat, 35–40 E% protein and 45–50 E% carbohydrate. Four products per day were provided as shakes, soups, ready-to-drink products and bars. Additionally, 200 g tomatoes, 125 g cucumber, 50 g lettuce and chewing of maximum six pieces of sugar-free chewing gum or pastilles per day were allowed. For some adults (eg, BMI >40 kg/m2 or achieving a BMI ≤23 kg/m2 during the LED without a wish to lose more weight), the LED was supplemented with milk/yoghurt, but only if it was expected that the required 5% WL could be achieved.
In the initial 2-month period, children were encouraged to follow the dietary recommendations of the American Academy of Paediatrics on the prevention, assessment and treatment of overweight and obesity.22 The goal was to obtain weight stability, which would reduce BMI-for-age z-score. For children, no weight criterion existed. Therefore, all children could continue into the WM period as long as their adult family member was included. Children were welcome to visit the intervention site for weighing and dietician counselling; however, it was not mandatory.
Ten-month period with S&SEs and sugar diets
During the 10-month randomised intervention period, dietary counselling sessions will be practiced as individual (ie, household) counselling sessions at months 2 and 6 and when COVID-19 restrictions allow in intervention groups (months 4 and 9). Otherwise, individual counselling sessions will be scheduled. The goals are to maintain WL for adults and BMI-for-age z-score for children. Further reduction in weight or BMI-for-age z-score is allowed, if the participant is compliant with the intervention, but counselling sessions will only cover maintenance aspects.
The two intervention diets are (1) a healthy diet with <10 E% added sugar allowing foods and drinks with S&SEs (S&SEs group) and (2) a healthy diet with <10 E% added sugar not allowing foods and drinks with S&SEs (sugar group). Both diets are ad libitum and S&SEs cover all types (artificial, natural, low-calorie, sugar alcohols, non-caloric) available on the market. To secure dietary adherence calculation of maximum sugar intake (g) will be based on a diet with 9.5 E% sugar. The maximum allowed sugar intake will be calculated individually based on body weight at month 2 (recalculated at month 6), using the formula by Henry23 multiplied by the physical activity level. A unit system for the sugar and S&SEs intake has been developed, where individual maximum sugar intake is converted to a certain number of units per day (and week) (one unit corresponding to 10 g sugar). One unit of S&SE product in the S&SE group is equal to the amount—in weight or volume—of 1 unit sugar-rich product in the sugar group. For the S&SE group, as many sugar-containing products as possible should be replaced by S&SE products. Food exchange lists, covering categories listed in table 2, including pictures of products, amounts and units per product, guide the participants in the two groups. Additional details and examples of the two interventions are provided in table 3. Due to the characteristics of the study, blinding is not possible; however, all efforts to blind study staff taking measurements and persons doing statistical analysis will be done.
Table 2.
Foods and drinks relevant for the 10-month randomised intervention period
| Category | Examples |
| Drinks | Carbonated soft drinks, fruit juice, non-carbonated soft drinks, cocoa powder, mixture of fruit syrup and water, energy drinks, pre-packed juices and nectars, protein shakes, energy drinks |
| Milk products | Flavoured yoghurts, yoghurt drinks, milk shakes, chocolate milk, fermented milk, cold butter milk |
| Breakfast cereals | Breakfast cereals, muesli, cereals bars, rolled oats |
| Sugar, honey and marmalade | Sugar, syrup, honey, marmalade, jam, compote |
| Chocolate and bars | Chocolate with and without filling, chocolate bars, chocolate/hazelnut paste/spread, thin sliced chocolate |
| Desserts | Pudding, mousse, cold soufflé, custard, strained stewed fruit, Greek jelly, pancakes |
| Ice cream | Ice cream, sorbet, ice lolly |
| Candy | Wine gum, liquorice, bon-bon mix, marshmallow, marzipan |
| Cake and biscuits | Cake, cookies, biscuits, Danish pastry, sponge cake |
Table 3.
Description of diets in the 10-month randomised intervention period
| Sugar group | S&SE group | |
| Sugar-containing products | <10 E% added sugar. | <10 E% added sugar and as little as possible. |
| S&SE products | Not allowed. Except for up to two pieces of sugar-free chewing gum per day. |
Allowed. No restrictions on specific types of S&SEs. |
| Units | Consumption of a maximum number of units (corresponding to 9.5 E% added sugar) of sugar-containing products each day/week. | Unit calculation (corresponding to 9.5 E% added sugar in weight/volume) will guide the participant to ensure intake of <10 E% added sugar. As many sugar-containing products in the diet as possible should be replaced with S&SE-containing products. Ideally, the amount of S&SEs products corresponding to the maximal units from sugar-containing products should be consumed. However, if a participant experiences an adverse event, they are recommended to consume less than the calculated units and change to other S&SE products (eg, avoid sugar-alcohols). |
| Example | For a participant with an energy requirement of 9000 kJ/d, 9.5 E% from sugar corresponds to 50 gram added sugar=5 units. 5 units per day or 35 units per week is then the maximum allowed intake of added sugar for this participant. |
For a participant with an energy requirement of 9000 kJ/d, 9.5 E% from sugar corresponds to 50 gram added sugar=5 units. Ideally, this participant should consume 5 units per day or 35 units per week of S&SE containing products. One unit is equivalent to 1 unit in the sugar group (in weight or volume). |
E%, energy percentage; S&SEs, sweeteners and sweetness enhancers.
Compliance
Participants are required to record intake of all foods and drinks (pen and paper) for 4 days (3 weekdays and one weekend day) at months 0 and 12 with information on time, type/brand names, cooking and processing methods, weight or household measures. Daily average intake of energy, macronutrients and micronutrients will be calculated by national dietary software in the four intervention sites. From the food records, amount and intake of sugar and S&SEs in units from the products listed in table 2 is also assessed at months 0 and 12. Furthermore, adults complete a food frequency questionnaire about intake of sweet products during the past month and they collect 24-hour urine samples at months 0, 6 and 12. Urinary biomarkers of S&SEs (acesulfame-K, saccharin, sucralose, cyclamate and steviol glycoronide) as well as fructose and sucrose will be analysed by Wageningen University, the Netherlands and urinary excretion of urea/nitrogen will be analysed locally. Based on the above listed data, the participants’ compliance with the intervention diets will be assessed including associations between changes in outcomes, for example, body weight, energy consumption and sugar consumption.
Data collection and outcomes
Including information meeting, screening, counselling sessions and CIDs, the trial consists of a minimum of 10 or 6 visits for adults and children, respectively. Data are collected according to standard operation procedures (SOPs) and table 4 shows activities/data collection at each visit. Most data will be collected at months 0, 2, 6 and 12, where participants have fasted for a minimum of 10 hours and avoided intensive physical exercise, coffee and smoking for 12 hours prior to the CIDs.
Table 4.
Flowchart for adults (A) and children (C) (full sampling at months 0 and 12)
| Pre-screening | Information meeting | Screening | Baseline | 2-month period (CID1 to CID2) |
10-month randomised intervention period (CID2 to CID4) |
1-year assessment | |||||
| CID | – | – | – | CID1 | – | – | CID2 | – | CID3 | – | CID4 |
| Visit | – | V0 | V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | V9 |
| Month | – | – | 0 | 0.5 | 1 | 2 | 4 | 6 | 9 | 12 | |
| Inclusion/exclusion criteria | A+C | A+C | |||||||||
| Signing Informed Consent | A+C* | ||||||||||
| Med. hist., medication etc. | A+C | ||||||||||
| Randomisation of the oldest family/household member | A | ||||||||||
| Supervision/counselling | A+C† | A(+C)‡ | A+C† | A(+C)‡ | A+C† | A(+C)‡ | A+C† | ||||
| Collection of LED products | A§ | A§ | A§ | A§ | |||||||
| Body weight and height¶ | A+C | A+C | A** | A** | A+C | A** | A+C | A** | A+C | ||
| Waist and hip circumference | A+C | A+C | A+C | A+C | |||||||
| Body composition | A+C†† | A | A+C†† | ||||||||
| Blood pressure and heart rate | A+C | A+C | A+C | A+C | A+C | ||||||
| Fasting blood samples | A‡‡ | A+C | A+C§§ | A+C | A+C | ||||||
| Adverse events and concomitant medication | A+C | A+C | A+C | A+C | |||||||
| Allergenicity (skin prick test) | A | A | |||||||||
| 24 hours urine collection (content of S&SEs) | A | A | A | ||||||||
| Faecal spot sample | A | A | A | A | |||||||
| 4-day dietary record | A+C | A+C | |||||||||
| Questionnaires (electronic platforms): | |||||||||||
| General background questionnaire | A+C | ||||||||||
| Physical activity | A+C | A+C | |||||||||
| Three factor eating questionnaire | A+C | A+C | |||||||||
| Leeds food preference questionnaire | A+C | A+C | A+C | A+C | |||||||
| Allergenicity | A+C | A+C | |||||||||
| Craving for sweet taste | A | A | |||||||||
| Perception of S&SEs | A | A¶¶ | A¶¶ | ||||||||
| Control of eating | A | A | A | A | |||||||
| Subjective appetite sensations | A | A | A | A | |||||||
| Sweet food frequency questionnaire (FFQ) | A | A | |||||||||
| Diet satisfaction | A | A | A | ||||||||
| Perception and evaluation of the intervention | A | A | |||||||||
| Quality of life | A | A | |||||||||
| Puberty | C | C | C | ||||||||
*For children, the informed consent is signed by the parents/guardians.
†Individual/family counselling is preferably scheduled at the same day as the CID.
‡Group counselling, children participation is preferred, but not mandatory.
§Adults will collect LED products from the intervention site every second or third week during the 2-month period. At months 0.5 and 1.5 (optional), the adults will be weighed and have the opportunity to consult a dietician.
¶Height is only measured at screening for adults.
**Fasting is not required for this body weight measurement.
††At University of Maastricht, body composition is not measured in children.
‡‡At screening, fasting blood samples will be analysed at each intervention site. All other blood samples are analysed at the Central Laboratory (Bioiatriki).
§§At University of Maastricht, a fasting blood sample is not drawn from children at CID2.
¶¶A shorter version of the questionnaire is used at CID3-4.
A, adult; C, child; CID, clinical investigation day; LED, low-energy diet; S&SEs, sweeteners and sweetness enhancers.
Primary outcomes
This trial has two independent primary outcomes. The primary outcome for efficacy is 1-year change in body weight. The primary outcome for safety is 1-year change in gut microbiota composition associated with impaired health (eg, change in microbial beta-diversity and composition). Both outcomes relate only to adults, and, hence, the required sample size was calculated for adults only.
Body weight
Body weight is measured to the nearest 0.1 kg using a digital scale with the participant wearing underwear/light clothes. Fasting body weight will be measured at screening and CIDs; however, fasting is not required, when body weight is measured at other visits.
Gut microbiota
Gut microbiota composition will be assessed from faecal spot samples collected at home prior to all CIDs. Samples are immediately frozen (−20°C) and later transported to the intervention site in cooling bags, whereafter they are stored at −80°C. Samples will be analysed targeting the V3–V4 regions of 16 s rRNA genes by Illumina sequencing at Maastricht University, the Netherlands.
Secondary outcomes
Secondary outcomes include changes in anthropometry and body composition (for children, BMI-for-age z-score), risk factors for T2D and CVD, allergenicity, adverse events (AEs) and concomitant medication. Additionally, some secondary outcomes will be assessed in adult subgroups, for example, gut-brain signalling markers, postprandial energy expenditure and substrate oxidation, physical activity, liver fat, adipose tissue and lipid metabolism, brain reward, insulin sensitivity markers and composition and functionality of the human gut microbiota in vitro. Furthermore, children’s gut microbiota composition may be analysed depending on the final sample size (table 5).
Table 5.
Secondary and other outcomes investigated in substudies and in subgroups
| Outcome | Measurements and method | Participants | Time points of data collection | |||
| Baseline | After WL | After WM | ||||
| Month 0 CID1 |
Month 2 CID2 |
Month 6 CID3 |
Month 12 CID4 |
|||
| Substudies (include an intervention) | ||||||
| Brain reward activity | Brain activity is measured by fMRI after consumption of a drink with sugar, S&SEs, water | Substudy including a subgroup of adults in Maastricht | A | A | A | |
| Postprandial responses (energy expenditure, substrate oxidation, blood biochemistry and appetite) | Indirect calorimetry, blood sampling, appetite sensation based on VAS and ad libitum energy intake after consumption of a drink with S&SE or water | Substudy including a subgroup of adults in Copenhagen | A | A | A | |
| Subgroups | ||||||
| Physical activity | 7-day measurements by accelerometer | Adults in Maastricht | A | A | A | |
| Gut-brain signalling markers | Analyses of GLP-1, CCK and ghrelin from fasting blood samples | Adults in Copenhagen and Maastricht | A | A | A | A |
| Liver fat | 1H-MRS | Subgroup of adults in Maastricht | A | A | A | |
| Adipose tissue function and lipid metabolism | Adipocyte morphology, ex vivo lipolysis, gene and protein expression analyses of adipose tissue samples (biopsy) | Adults in Maastricht | A | A | A | |
| Insulin sensitivity markers | Indices for example, HOMA-IR, Matsuda index, Disposition index etc. calculated from a 7-point OGTT | Adults in Maastricht | A | A | A | |
| Gut microbiota | 16S rRNA illumine sequencing of faecal samples | Children in Maastricht | C | C | C | |
| Composition and functionality of the human gut microbiota in vitro | Microbial metabolites, for example, SCFA and 16S rRNA illumine sequencing of faecal samples | Subgroup of adults in Maastricht | A | |||
1H-MRS, Proton Magnetic Resonance Spectroscopy; A, adults; C, children; CCK, cholecystokinin; CID, clinical investigation day; fMRI, functional MRI; GLP-1, glucagon-like peptide 1; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; OGTT, oral glucose tolerance test; SCFA, short chain fatty acids; S&SEs, sweeteners and sweetness enhancers; VAS, visual analogue scales; WL, weight loss; WM, weight maintenance.
Anthropometry
Height is measured to the nearest 0.5 cm using a stadiometer at screening and for children at all CIDs. For adults, BMI is calculated as body weight (kg)/height2 (m2). For children, the WHO AnthroPlus software (www.who.int/tools/growth-reference-data-for-5to19-years/application-tools) is applied to calculate BMI-for-age percentile and z-score. Waist and hip circumferences are measured two times with a non-elastic tape measure on the skin to the nearest 0.5 cm, and the average is calculated. Waist circumference is measured halfway between the lowest rib and iliac crest during exhalation. Hip circumference is measured as the largest circumference in the area around the buttock. Dual-energy X-ray absorptiometry (DXA) scans are performed in underwear to assess body composition, including fat percentage, fat mass and fat-free mass.
Blood pressure and heart rate
After minimum 5 min rest in a sitting position, blood pressure (mm Hg) and heart rate (beats per minute) are measured three times on the right arm with an automatically inflated cuff. An average is calculated from the last two measurements if the two measurements differ with ≤5 mm Hg. If either the systolic or diastolic blood pressure differs by >5 mm Hg, a fourth measurement is performed and the average was calculated from the third and fourth measurements.
Blood samples
Fasting venous blood samples are drawn at all CIDs, except at month 2 for children at Maastricht due to Ethical concerns. Serum samples are collected for analyses of lipids (triglycerides, total, low-density lipoprotein and high-density lipoprotein cholesterol), alanine aminotransferase, aspartate aminotransferase, insulin, C reactive protein and immunoglobulin E. Plasma is collected for glucose analysis and full blood for haemoglobin A1c analysis. All samples are stored locally at −80°C until shipment to the central lab at Bioiatriki S.A., Greece.
Skin prick test
For adults, a skin prick test is performed on the forearm. One drop of the allergens hazel, alder, birch, grass mix, artemisia absinthium, ragweed, alternaria, moulds mix, cat, dog, dermatophagoides pteronyssinus and dermatophagoides mix as well as positive and negative control solutions are applied. The response is recorded after 15 min.
AEs and concomitant medication
All AEs experienced after inclusion and during the trial are registered. At CIDs, the participant is asked whether he/she has noticed any unfavourable events since the last CID. During the 10-month randomised intervention period, participants—regardless of intervention—are asked directly about certain AEs that may be related to consumption of S&SE, that is, gastrointestinal symptoms and headache. All medications necessary for the participants’ health, not listed in the protocol exclusion criteria, may be continued during the trial. At CIDs, the participant is asked whether he/she has taken any new medicine or has changed dosage of already registered medicine.
Other outcomes
Questionnaires are used to obtain information about sociodemographic characteristics such as education, occupation, household income, etc, physical activity, quality of life, and to investigate subjective neurobehavioural indices, for example, food preferences and preference for sweet taste, perception of S&SEs, cravings, subjective appetite sensations and perception and evaluation of the 10-month randomised intervention period. Furthermore, puberty is assessed for children. All questionnaires are prepared in English and translated into local language. The majority of questionnaires will be delivered by a Questionnaire Delivery Platform (QDP) implemented by NetUnion, Switzerland. At baseline, all questionnaires are completed at the intervention sites, but before other CIDs, adults can complete those delivered by the QDP at home prior to the CID. Children always complete all questionnaires at the intervention site. Two questionnaires are always completed at the intervention site; one about perception of S&SEs (delivered by the Qualtrics platform via a weblink) and one about food rewards assessed by the Leeds Food Preference Questionnaire (E-prime software).
Subgroups and substudies
Some of the secondary and other outcomes are collected in substudies and in subgroups of different participants (table 5).
Statistical methods
Sample size determination
The sample size calculation is based on adults for the two primary outcomes. For body weight, a clinically meaningful effect of 1.5 kg placebo subtracted body weight has previously been approved by the European Food Safety Authority (EFSA).24 Based on a similar trial,25 we estimated that a difference of 1.5 kg with an SD of ±3.5 kg, a 90% power, a two-sided α of 0.05, would require 231 completers. With an estimated drop out of 30%, a minimum of 330 adult participants should be included. For change in gut microbiota, a ±10% change in 20 of the 50 most abundant operational taxonomic units with an alpha of <0.05% would require 100 complete samples. The inclusion of at least 330 adults (approximately 25% of the participants per intervention site) is, therefore, also sufficient to detect possible changes in gut microbiota.
Statistical analysis plan
As part of the SWEET project, a statistical analysis plan has been developed. For body weight, 1-year change between the two interventions will be analysed by analysis of covariance (ANCOVA) linear mixed model, with change in body weight as response; treatment group and relevant covariates, for example, age, gender and BMI are fixed effects. Participant ID and intervention site are random (intercept) effects. Intention-to-treat principle will be applied on those completing the initial 2-month period. Additionally, complete-case analyses (all dropouts omitted) and per-protocol analyses (only compliant participants) as well as analyses including additional covariate adjustments (eg, energy density) and intermediate time points will be applied.
For gut microbiota, 1-year change in microbial diversity and microbial composition (relative abundance at phyla and genera level) will be analysed. Paired Wilcoxon test is used to study within intervention changes in relative abundance. Linear mixed models with Benjamin-Hochberg correction for multiple testing will be used for between-intervention comparisons.
Data will be presented with the use of standard descriptive statistics shown as mean (SD) or median (Q1:Q3) for normally and non-normally distributed data, respectively, and categorical data by percentages. Results will be presented as mean difference in changes ±SEM or 95% CIs and p values when relevant. A statistical level of 0.05 will be applied and graphical models will be carried out to assess model assumptions. When relevant, transformation, for example, logarithm will be applied or non-parametric statistical tests will be performed.
For secondary outcomes on continuous data, the main analysis will compare the 1-year mean change between the two treatment groups by use of the ANCOVA-type linear mixed model defined above without any multiplicity adjustment or imputation of missing values (ie, available-case analyses). Additional sensitivity analyses may be carried out as appropriate in the same way as for the primary outcome. Furthermore, analysis of repeated measures will be performed using linear mixed models, including time×treatment interaction, time and treatment effects, covariates (eg, age, gender, BMI) as fixed effects, and participant ID and intervention sites as random effects. In case of significant time×treatment interaction, differences between treatments will be identified per time point. Mean changes will be compared between the groups using the estimated mean difference and approximate t tests derived from the fitted linear mixed models (assuming a two-sided alternative). For secondary categorical outcome (eg, yes/no, 0/1/2, etc), logistic or ordinal mixed effects model will be used, including the same fixed and random effects as the linear mixed models.
Ethics and dissemination
The RCT will be conducted in accordance with the Declaration of Helsinki26 and this master protocol (V.3.0, 28 October 2020) is approved by the responsible national/regional committee in the four countries from where consent to all previous and future amendments to the protocol was and will be obtained. All adults receive the LED products free of charge. At Copenhagen, Navarra and Athens participants will not receive reimbursement for their participation. At Maastricht, travel expenses and financial compensation are provided to all eligible participants (125 Euros for adults without child(ren) and 250 Euros for 1 adult and one child +80 Euros per extra family member).
There are no risks related to the dietary interventions; however, discomfort may occur. The LED (not provided for children) contains all needed nutrients, but only little energy and, therefore, adults may experience headaches, dizziness, tiredness and nausea, particularly in the first few days. Constipation, stomach cramps or more profound nausea can occur and information on this is given before inclusion. However, allergic reactions to the LED are rare. The sugar and S&SE intervention products are commercially available foods and drinks purchased in the supermarket and no adverse side effects are expected. However, changes in gastrointestinal symptoms (eg, bloating and excess gas production) may occur depending on the participant’s habitual intakes of fibre and types of S&SEs, for example, sugar alcohols. At each intervention site, a physician can be consulted in case of medical uncertainties.
Some study procedures involve risks, but the procedures implemented are designed to minimise these. Drawing blood samples will seldom cause harm besides that associated with the insertion; however, children will be offered local anaesthetic Emla patches to reduce pain. A maximum of 80 mL and 125 mL of blood is drawn during the 1-year trial for children and adults, respectively. For children, this is less than 1 mL blood/kg body weight per donation, which is considered safe. Fertile women will be tested for pregnancy before DXA scanning and excluded from the trial if pregnant. The DXA scans will induce minor radiation (<0.010 mSv per scan). Scanning will be done 2 and 3 times during the 1-year trial for children and adults, respectively, and only one rescan will be allowed per CID. The skin prick test, only performed in adults, is anticipated to cause very little discomfort. A positive reaction may cause itching, which will be treated with a salve. In very rare cases, a systemic anaphylactic reaction can occur and emergency equipment is in place.
For children, special attention is given to ensure that the child is not forced to participate by the adult family member. Furthermore, a child cannot remain included if the adult family member drops out or is excluded from the trial.
All participants will be insured against injury caused by their participation according to local legal requirements. The trial is monitored by the European Clinical Research Infrastructure Network to ensure compliance with the protocol and SOPs. All trial-related information will be recorded, handled and stored safely allowing accurate reporting, interpretation and verification. All data will be collected in a central DataHub at Copenhagen from where pseudo-anonymised data can be requested until 2032 via a data sharing contract. From 2032, fully anonymised data can be transferred. Source data are collected on paper first or is entered directly into the electronic systems, for example, the QDP, the Qualtrics platform and/or the Research Electronic Data Capture (REDCap) tool hosted at the University of Copenhagen.27 28 REDCap is a secure, web-based software platform designed to support data capture. Source data from DXA scans and analysis of biological material are registered on the device or related hardware, whereas the source data from dietary records (handwritten on paper) will be entered into a national dietary software programme for analysis. The sponsor/investigator will provide direct access to source data/documents for inspection.
Results will be published in international peer-reviewed scientific journals regardless of whether the findings are positive, negative or inconclusive.
Discussion
The strengths of this RCT are the investigation of the long-term effects of S&SEs in the contexts of an ad libitum healthy diet not only including drinks but also foods, compared with added sugar. The 10-month weight loss maintenance period—where the effects of S&SEs are investigated—is a very critical period. Thus, based on previous research, individuals are expected to regain at least some of their lost body weight.29 30 The long duration of the weight loss maintenance period is important when studying changes in body weight (one of the two primary outcomes) as highlighted by the EFSA.31 In 2020, the effect of S&SEs on body weight was extensively reviewed by Rogers & Appleton.11 They identified nine studies where effects of S&SEs, compared with sugar, were investigated in participants with overweight or obesity. Of these nine studies, only two studies investigated effects of both foods and drinks32 33 and five of the nine studies investigated effects of a single S&SE. Furthermore, only two studies34 35 had a duration of a minimum of 6 months, but none of these had the same weight loss and weight maintenance design as the current RCT. Thus, studies investigating the effect of S&SEs, compared with sugar, for weight loss maintenance are lacking. Other strengths are that the trial is conducted as a multicentre trial covering four European countries with both adults and children included. Furthermore, data will cover a broad range of measurements related to health (body weight management, risk factors for CVD, T2D, etc) and safety (microbiology, allergy, AEs), appetite and food preferences. These will be measured at baseline, after a 2-month WL (adults) or 2-month weight stability (children) period, and after a 10-month intervention period. A trial limitation is that the number of included children was lower than initially planned, but this will not affect the two primary outcomes. Another limitation is that data related to energy and nutrient intakes (food records) are only collected at baseline and at months 12. However, urinary S&SE biomarkers collected at baseline, month 6 and month 12 will indicate if compliance decreases before month 12.
Supplementary Material
Footnotes
Contributors: The SWEET EU project was initiated by JCGH, AR and JAH. The protocol for the SWEET intervention trial was written by LK, AR and YM, with contribution from EEB and JAM. AR, YM, EEB and JAM are principal investigators (PI) at the four intervention sites, where LK, SSHA, SN-C, KR and TA are investigators. EF, GF, CEH, TL and HM are responsible for specific methods, platforms, or analyses, and MdA is responsible for monitoring of the trial. LK and AR drafted the manuscript and YM, EEB and JAM critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding: The PI, AR, is also the sponsor (e-mail: ara@nexs.ku.dk, Phone: +45 21 30 69 12, Department of Nutrition, Exercise and Sports, University of Copenhagen, Rolighedsvej 26, 1958 Frederiksberg, Denmark). The trial is funded by the Horizon2020 program: Sweeteners and sweetness enhancers: Impact on health, obesity, safety and sustainability (acronym: SWEET, grant #774293) and funding covers salary for project personal, supplies, remuneration, and dissemination of results. The amount is deposited in a project account subject to audits/public revision.
Competing interests: AR has received honoraria from Unilever and the International Sweeteners Association. CEH’s research centre provides consultancy to, and has received travel funds to present research results from organisations supported by food and drink companies. JCGH and JH have received project funds from the American Beverage Association. TL works for a company, NetUnion sarl, which has no conflict of interest in the study outcome.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.World Health Organization (WHO) . Noncommunicable diseases country profiles 2018. Geneva, 2018. [Google Scholar]
- 2.World Health Organization (WHO) . Guideline: sugar intake for adults and children. Geneva, 2015. [PubMed] [Google Scholar]
- 3.Nordic Council of Ministers . Nordic nutrition recommendations 2012. integrating nutrition and physical activity. Norden, 2014. [Google Scholar]
- 4.Technical University of Denmark (DTU) The National Food Institute. . Dietary habits in Denmark 2011-2013 [Report in Danish: Danskernes kostvaner 2011-2013]. Denmark 2015. [Google Scholar]
- 5.Institute of Preventive Medicine Environmental and Occupational Health . National dietary guidelines for Greek adults and children. Greece, 2014. [Google Scholar]
- 6.Ruiz E, Rodriguez P, Valero T, et al. Dietary intake of individual (free and intrinsic) sugars and food sources in the Spanish population: findings from the ANIBES study. Nutrients 2017;9. 10.3390/nu9030275. [Epub ahead of print: 14 Mar 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sluik D, van Lee L, Engelen AI, et al. Total, free, and added sugar consumption and adherence to guidelines: the Dutch national food consumption survey 2007-2010. Nutrients 2016;8:70. 10.3390/nu8020070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance? Physiol Behav 2016;164:488–93. 10.1016/j.physbeh.2016.04.029 [DOI] [PubMed] [Google Scholar]
- 9.Sylvetsky AC, Rother KI, Sciences N. Hhs public access. 2017;164:446–50. [Google Scholar]
- 10.O'Connor D, Pang M, Castelnuovo G, et al. A rational review on the effects of sweeteners and sweetness enhancers on appetite, food reward and metabolic/adiposity outcomes in adults. Food Funct 2021;12:442–65. 10.1039/D0FO02424D [DOI] [PubMed] [Google Scholar]
- 11.Rogers PJ, Appleton KM. The effects of low-calorie sweeteners on energy intake and body weight: a systematic review and meta-analyses of sustained intervention studies. Int J Obes 2021;45:464–78. 10.1038/s41366-020-00704-2 [DOI] [PubMed] [Google Scholar]
- 12.Toews I, Lohner S, Küllenberg de Gaudry D, et al. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ 2019;364:k4718. 10.1136/bmj.k4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anker CCB, Rafiq S, Jeppesen PB. Effect of steviol glycosides on human health with emphasis on type 2 diabetic biomarkers: a systematic review and meta-analysis of randomized controlled trials. Nutrients 1965;2019:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters JC, Beck J, Cardel M, et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: a randomized clinical trial. Obesity 2016;24:297–304. 10.1002/oby.21327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Normand M, Ritz C, Mela D, et al. Low-Energy sweeteners and body weight: a citation network analysis. BMJ Nutr Prev Health 2021;4:319–32. 10.1136/bmjnph-2020-000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greyling A, Appleton KM, Raben A, et al. Acute glycemic and insulinemic effects of low-energy sweeteners: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2020;112:1002–14. 10.1093/ajcn/nqaa167 [DOI] [PubMed] [Google Scholar]
- 17.Nichol AD, Holle MJ, An R. Glycemic impact of non-nutritive sweeteners: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr 2018;72:796–804. 10.1038/s41430-018-0170-6 [DOI] [PubMed] [Google Scholar]
- 18.Del Pozo S, Gómez-Martínez S, Díaz LE, et al. Potential effects of Sucralose and saccharin on gut microbiota: a review. Nutrients 2022;14:1682. 10.3390/nu14081682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Y, Liu H, Qin N, et al. Impact of food additives on the composition and function of gut microbiota: a review. Trends in Food Science & Technology 2020;99:295–310. 10.1016/j.tifs.2020.03.006 [DOI] [Google Scholar]
- 20.Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181–6. 10.1038/nature13793 [DOI] [PubMed] [Google Scholar]
- 21.Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity 2015;23:2319–20. 10.1002/oby.21358 [DOI] [PubMed] [Google Scholar]
- 22.Barlow SE, Expert Committee . Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–92. 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 23.Henry CJK. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 2005;8:1133–52. 10.1079/PHN2005801 [DOI] [PubMed] [Google Scholar]
- 24.European Food Safety Authority (EFSA). Nutrition and Allergies (NDA). . Scientific opinion on the substantiation of health claims related to konjac mannan (glucomannan) and reduction of body weight (ID 854, 1556, 3725), reduction of post-prandial glycaemic responses (ID 1559), maintenance of normal blood glucose concentration.. EFSA Journal 2010;8. [Google Scholar]
- 25.Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non‐nutritive sweetened beverages on weight loss during a 12‐week weight loss treatment program. Obesity 2014;22:1415–21. 10.1002/oby.20737 [DOI] [PubMed] [Google Scholar]
- 26.General Assembly of the World Medical Association . World Medical association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 2014;81:14–18. [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouvelioti R, Vagenas G, Langley-Evans S. Effects of exercise and diet on weight loss maintenance in overweight and obese adults: a systematic review. J Sports Med Phys Fitness 2014;54:456–74. [PubMed] [Google Scholar]
- 30.Kraschnewski JL, Boan J, Esposito J, et al. Long-Term weight loss maintenance in the United States. Int J Obes 2010;34:1644–54. 10.1038/ijo.2010.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EFSA panel on dietetic products nutrition and allergies (NDA). guidance on the scientific requirements for health claims related to appetite ratings, weight management, and blood glucose concentrations. EFSA Journal 2012;10. [Google Scholar]
- 32.Blackburn GL, Kanders BS, Lavin PT, et al. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr 1997;65:409–18. 10.1093/ajcn/65.2.409 [DOI] [PubMed] [Google Scholar]
- 33.Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on AD libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9. 10.1093/ajcn/76.4.721 [DOI] [PubMed] [Google Scholar]
- 34.Engel S, Tholstrup T, Bruun JM, et al. Effect of high milk and sugar-sweetened and non-caloric soft drink intake on insulin sensitivity after 6 months in overweight and obese adults: a randomized controlled trial. Eur J Clin Nutr 2018;72:358–66. 10.1038/s41430-017-0006-9 [DOI] [PubMed] [Google Scholar]
- 35.Tate DF, Turner-McGrievy G, Lyons E, et al. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the C hoose H ealthy O pt i ons C onsciously E veryday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012;95:555–63. 10.3945/ajcn.111.026278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.