Abstract
Thyroid storm is a rare and life-threatening condition associated with excess thyroid hormones. Early detection of thyroid storm is the key to decreasing the morbidity and mortality associated with this condition. We present a rare case of thyroid storm induced by combination therapy with nivolumab and ipilimumab in a patient with advanced non-small cell lung cancer (NSCLC). Because of prominent hyperthyroidism with gastrointestinal symptoms and signs of heart failure, the patient was diagnosed with thyroid storm 3 weeks after initiating this combination immunotherapy. The patient had no history of thyroid disease but was positive for antithyroid antibodies. This case report suggests that thyroid function and symptoms of suspected thyroid storm should be evaluated routinely within 3 weeks from the initiation of therapy when combination therapy is administered in patients with NSCLC positive for antithyroid antibodies.
Keywords: Endocrine system, Thyroid disease, Lung cancer (oncology)
Background
Immune checkpoint inhibitors (ICIs) are widely used to treat various malignant tumours, including malignant melanoma and non-small cell lung cancer (NSCLC). The ipilimumab is a monoclonal antibody acting against cytotoxic T-lymphocyte 4 (CTLA-4), and nivolumab is a human programmed death receptor-1 (PD-1)-blocking antibody. Combination therapy with nivolumab and ipilimumab has demonstrated consistent efficacy in patients with advanced NSCLC. However, the incidence of immune-related adverse events (irAEs), including endocrine disorders such as pituitary and thyroid dysfunctions, has been an important issue with ICI treatment. The nivolumab and ipilimumab have notably similar irAE incidence rates. An increase in irAE incidence rate is observed when these drugs are administered in combination. According to a systematic review, the incidence rate of hyperthyroidism was observed to be 8% (34/407) in patients with advanced solid tumours.1 According to the Common Terminology Criteria for Adverse Events V.5.0, the severity of thyroid dysfunction is low,2 and there has been only one reported case of thyroid storm in a patient with advanced melanoma induced by combination therapy with nivolumab and ipilimumab.3
A previous study suggested that the risk of thyroid dysfunction induced by anti-PD-1 therapy can be increased in patients positive for antithyroglobulin antibodies (TgAbs) and/or antithyroid peroxidase antibodies (TPOAbs) before treatment.4
We have reported a rare case of thyroid storm in a patient with advanced NSCLC receiving nivolumab and ipilimumab combination therapy.
Case presentation
The patient was a woman in her 70s with advanced NSCLC. The chief complaints were dyspnoea on exertion and left-sided chest pain. Chest CT revealed a mass lesion in the left upper lung, and a bronchoscopy was performed. Based on the results of positron emission tomography-CT and contrast-enhanced MRI of the head, the patient was diagnosed with lung adenocarcinoma, cT4N3M0, stage IIIC; genetic testing revealed no driver gene mutations, and the programmed death ligand-1 expression rate (22c3) was less than 1%. The patient’s Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 1. She was admitted to our hospital and combination therapy with nivolumab and ipilimumab was initiated. Although the patient was positive for TgAb before chemotherapy, all other thyroid function test results were normal. She was asymptomatic and had never been diagnosed with thyroid goitre. After initial treatment, nausea (grade 1) and sinus tachycardia (grade 1) were observed on days 5 and 10, respectively. The treatment course was otherwise uneventful, and the second course of nivolumab was administered on day 21 after the initiation of treatment. The patient’s general condition was stable, and she was discharged 24 days after treatment initiation. However, on the day after discharge, she was rushed to the emergency room because of dyspnoea, nausea and cold sweats. The patient was tachycardic at 131 beats per minute and agitated, with symptoms of nausea and vomiting. Her blood pressure was 103/74 mm Hg, and her Glasgow Coma Scale score was 15. Most notably, no thyromegaly, exophthalmos, or thyroid tenderness were appreciable on physical examination. The Burch-Wartofsky Point Scale (BWPS) score was 50. The patient was admitted to the intensive care unit (ICU) for thyroid storm management after endocrinology and cardiology consultation.
Investigations
The initial complete blood count was normal, but the C reactive protein level was slightly high. Blood cultures were found to be negative after 48 hours. An echocardiogram showed sinus tachycardia, and the N-terminal prohormone B-type natriuretic peptide levels were also high. The patient’s thyroid function test results on the day of presentation were as follows: thyroid-stimulating hormone (TSH), 0.01 μIU/mL; free thyroxine (FT4), 7.2 ng/dL; and free triiodothyronine (FT3), 16.0 pg/mL (table 1).
Table 1.
Summary of laboratory test results taken before chemotherapy and on admission
| On presentation | Before chemotherapy | |||
| Complete blood count | Units | Reference range | ||
| White cell count | 109/L | 3.6–8.9 | 6.7 | 3.2 |
| Haemoglobin | g /L |
115–150 | 114 | 129 |
| Platelet count | 109/L | 150–350 | 256 | 185 |
| C reactive protein | mg/dL | <0.3 | 2.5 | 0.68 |
| Coagulation screen | ||||
| Prothrombin time | INR | 1.27 | 1.06 | |
| D-dimer | μg/mL | 1< | 2 | 0.9 |
| Blood chemistry | ||||
| Sodium | mEq/L | 135–145 | 137.7 | 140.2 |
| Potassium | mEq/L | 3.5–5 | 4.1 | 4.5 |
| Blood urea nitrogen | mg/dL | 9–21 | 17 | 16 |
| Creatinine | mg/dL | 0.5–0.8 | 0.41 | 0.65 |
| Lactate dehydrogenase | U/L | 119–221 | 174 | 193 |
| Creatine kinase | U/L | 47–200 | 109 | 119 |
| NT-proBNP | pg/mL | <125 | 458.3 | 54.5 |
| Thyroid hormones | ||||
| Free triiodothyronine | pg/mL | 2.4–4.5 | 16 | 3.3 |
| Free thyroxine | ng/dL | 1–1.7 | 7.2 | 1.3 |
| Thyroid-stimulating hormone | μIU/mL | 0.56–4.5 | 0.01 | 1.51 |
| Antithyroglobulin antibody | IU/mL | <28 | 718 | 106 |
| Antithyroid peroxidase antibody | IU/mL | <16 | 4.2 | 9 |
Her thyroid function test results prior to initial combination treatment were as follows: TSH, 1.51 μIU/mL; FT4, 1.3 ng/dL; and FT3, 3.3 pg/mL. However, her thyroid peroxidase immunoglobulin levels were normal, and TgAbs were positive at 106 IU/mL (table 1). Chest radiography revealed a new left pleural effusion.
Differential diagnosis
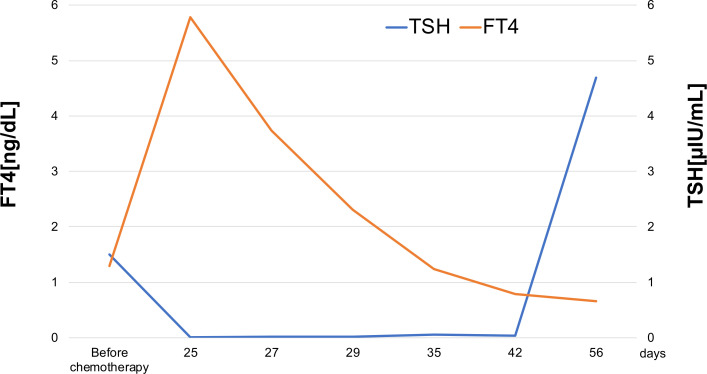
The patient exhibited several signs and symptoms of thyroid storm based on the BWPS diagnostic criteria for thyroid storm, including agitation, nausea with vomiting, tachycardia, fever and history of nivolumab and ipilimumab treatment. Differential diagnoses included medication-induced thyroid storm, Graves’ disease, thyroiditis, thyroid adenoma, toxic multinodular goitre and Hashimoto’s thyroiditis. We considered Graves’ disease and Hashimoto’s disease to be less likely based on the negative results for thyrotropin receptor autoantibodies and TPOAb. The chest CT scan showed neither adenoma nor goitre before chemotherapy. Based on these findings, the most likely diagnosis was an immune-mediated thyroid storm. The clinical course of the thyroid function tests before and after the initially combined immunotherapy supported this diagnosis (figure 1).
Figure 1.
Changes in thyroid function tests over the course of treatment. Day 25 represents the day from initiation of chemotherapy. FT4, free thyroxine; TSH, thyroid-stimulating hormone.
Treatment
The patient was admitted to the ICU for a thyroid storm and was initiated on propranolol (20 mg per day) and landiolol (750 mg per day) to achieve appropriate control of her heart rate. Hydrocortisone (100 mg every 8 hour) and potassium iodide (200 mg per day) were also administered. Antithyroid drugs were not administered because anti-TSH receptor antibodies were negative and the cause of the thyroid storm was considered to be drug-induced destructive thyroiditis.5 On day 2, she was febrile up to 40°C and exhibited signs of heart failure, therefore, she was initiated on non-invasive positive pressure ventilation. On day 3, her general condition worsened, resulting in a further decline in cardiac function and circulatory failure despite treatment; therefore, norepinephrine therapy was started, and respiratory management was switched to invasive positive pressure ventilation. As acute renal failure was also observed, continuous haemodiafiltration was initiated to prevent her clinical condition from worsening. As a result of continued intensive management, thyroid function gradually recovered, and cardiac function improved accordingly. As her respiratory condition stabilised, she was extubated 9 days after intubation. The patient’s thyroid function improved over time and remained within the normal range; thus, thyroid medication was not administered.
Outcome and follow-up
After discharge from the ICU, rehabilitation was initiated to improve activities of daily living. However, her ECOG PS only improved by 3, and chemotherapy for NSCLC was terminated. She was discharged on day 40 after admission, and palliative care was continued at home.
Discussion
This case report is an example of a nivolumab and ipilimumab combination therapy-induced immune-mediated thyroid storm, which occurred within 3 weeks of treatment initiation. The clinical diagnosis of thyroid storm was confirmed by the BWPS system. This scale system assigns points in seven domains among five categories of thermoregulatory dysfunction, central nervous system effects, gastrointestinal-hepatic dysfunction, cardiovascular dysfunction and precipitant history. Scores were added, and scores of 45 or greater were considered highly suggestive of a thyroid storm.6 Our patient’s score was 50 on presentation.
A notable point, in this case, was that the patient was positive for antithyroid antibodies. The incidence of thyroid dysfunction in patients positive for thyroid antibodies is high. Okada et al reported that the incidence of thyroid dysfunction, including destructive thyroiditis and hypothyroidism, was higher in the thyroid autoantibody-positive group than in the autoantibody-negative group. It was also reported that the presence of TgAb or TPOAb or both at baseline was a general risk factor for immunotherapy-induced thyroid dysfunction.4
On searching several databases, we found only one case report in which ipilimumab and nivolumab combination induced thyroid storm in a patient receiving immunotherapy for advanced melanoma. In that case, the patient was negative for antithyroid antibodies but developed a thyroid storm 21 days after initiation of the combination therapy.
The combination of anti-PD-1 and anti-CTLA-4 antibodies has been reported to increase the incidence of thyroid dysfunction and shorten the time for the onset of thyroid dysfunction. Iyer et al reported that the median time to thyrotoxicosis after treatment with ipilimumab and nivolumab combination was 2 weeks, whereas that with nivolumab monotherapy was 6 weeks.7
To summarise, we have reported a rare case of nivolumab and ipilimumab combination-induced thyroid storm. When combined anti-PD-1 and anti-CTLA-4 antibody therapy is administered in patients who are positive for antithyroid antibodies, it is desirable to perform thyroid function tests every 2 weeks, if possible. It may also be beneficial to monitor the thyroid function every 3 weeks after anti-PD-1 antibody administration because of the risk of development of thyroid-related adverse events in the early phase.
Learning points.
Combined nivolumab and ipilimumab immunotherapy for non-small cell lung cancer (NSCLC) is an effective treatment, but it should be recognised that severe immune-related adverse events, such as thyroid storm, may be present.
In patients with positive antithyroglobulin antibodies (TgAbs) or antithyroid peroxidase antibodies (TPOAbs) or both, consultation with an endocrinologist is recommended before initiating nivolumab plus ipilimumab, even if there is no history of thyroid disease.
In patients with NSCLC positive for TgAbs or TPOAbs or both, treated with nivolumab and ipilimumab combination therapy, thyroid function and symptoms of suspected thyroid storm should be monitored every 2 or 3 weeks.
When nivolumab and ipilimumab combination therapy is administered, confirmation of thyroid function before the second course of nivolumab treatment is recommended.
Footnotes
Contributors: SK and KM were the physicians directly responsible for this case. SK and KM wrote the manuscript in consultation with KS and KT. KM took the lead in writing the manuscript. KS is the head of the respiratory medicine department of the hospital where this case was treated. KT is responsible for the entire Department of Respiratory Medicine at Juntendo University.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018;4:173–82. 10.1001/jamaoncol.2017.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services . Common terminology criteria for adverse events (CTCAE) version 5.0. National Institutes of health, National cancer Institute 2017;4. [Google Scholar]
- 3.McMillen B, Dhillon MS, Yong-Yow S. A rare case of thyroid storm. BMJ Case Rep 2016;2016:bcr2016214603. 10.1136/bcr-2016-214603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada N, Iwama S, Okuji T, et al. Anti-Thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer 2020;122:771–7. 10.1038/s41416-020-0736-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satoh T, Isozaki O, Suzuki A, et al. 2016 guidelines for the management of thyroid storm from the Japan thyroid association and Japan endocrine Society (first edition). Endocr J 2016;63:1025–64. 10.1507/endocrj.EJ16-0336 [DOI] [PubMed] [Google Scholar]
- 6.Bahn Chair RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American thyroid association and American association of clinical endocrinologists. Thyroid 2011;21:593–646. 10.1089/thy.2010.0417 [DOI] [PubMed] [Google Scholar]
- 7.Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-Related thyroiditis with immune checkpoint inhibitors. Thyroid 2018;28:1243–51. 10.1089/thy.2018.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]