Abstract
Purpose
To determine the safety, efficacy, and tolerability of combinations of pilocarpine (Pilo) and oxymetazoline (Oxy) ocular drops dosed once daily and identify the optimal concentration of each for the pharmacologic treatment of presbyopia.
Design
Two concurrent Phase 2, multicenter, double-masked, randomized, vehicle-controlled studies, 1 short-term and 1 extended study.
Participants
Emmetropic individuals affected by presbyopia and in good general health.
Methods
Uncorrected near visual acuity (UNVA) was measured throughout both studies with various concentrations and combinations of Pilo (0%, 0.5% 1.0%, and 1.5%) and Oxy (0%, 0.0125%, 0.05%, and 0.125%). For safety, uncorrected distance visual acuity (UDVA) was measured, treatment-emergent adverse events (TEAEs) were recorded, and a temporal/supraorbital headache assessment was completed.
Main Outcome Measures
The primary efficacy end point was mean change from baseline in UNVA.
Results
In the short-term study, Pilo was shown to produce a significant dose response in the average increase of letters (P < 0.001), whereas Oxy did not have a significant impact (P = 0.4797). The addition or increase in concentration of Oxy did not reduce incidence or severity of headaches when compared with Pilo alone. Efficacy results from the extended study supported the results from the short-term study. As early as 15 minutes postadministration, a dose response could be seen, with peak effect at 1 hour. Peak improvement increased from day 1 to day 14 and was maintained up to day 28. The most common TEAE was headache. There was no clinically significant reduction in UDVA. A polynomial regression model was developed and determined that the optimal concentration range of Pilo is between 1.16% and 1.32%.
Conclusions
On the basis of the results of the 2 Phase 2 studies, AGN-190584, a reading drop containing an optimized concentration of pilocarpine HCl (1.25%) delivered using a proprietary formulation, was developed and is currently under investigation in Phase 3 studies.
Keywords: Accommodation, Oxymetazoline, Pharmacological Treatment, Pilocarpine, Pinhole, Presbyopia, Uncorrected near visual acuity
Abbreviations and Acronyms: AE, adverse event; ANCOVA, analysis of covariance; D, diopters; DE, dominant eye; IxRS, interactive response system; mITT, modified intent-to-treat; NDE, nondominant eye; Oxy, oxymetazoline; Pilo, pilocarpine; SD, standard deviation; TEAE, treatment-emergent adverse event; UDVA, uncorrected distance visual acuity; UNVA, uncorrected near visual acuity; VAS, visual analog scale
Presbyopia is a highly prevalent, progressive condition in which the eye’s ability to focus on near objects reduces with increasing age. This reduction is due to the decreased ability of the lens to change shape to focus on near objects, known as “accommodation.” Loss of accommodation is likely due to lens hardening caused by the loss of viscoelasticity of the crystalline lens and contraction power in the ciliary body.1,2 Uncorrected presbyopia is a worldwide vision impairment condition that affects approximately 1.8 billion individuals globally.3 It impacts an individual’s daily activities and emotional well-being, and decreases the quality of life.4, 5, 6, 7
Despite its prevalence, there are limited treatment options for presbyopia. Current nonsurgical methods include the use of reading, bifocal, or multifocal glasses and monovision or multifocal contact lenses. However, none can restore dynamic range of accommodation,8 and multifocal glasses can impair depth perception, which increases the risk of falls.9,10 There are also surgical treatment options, but similar to the nonsurgical options, vision quality is reduced by at least at 1 viewing distance.8,11 Surgery also has its own set of challenges and risks, such as infection, loss of contrast sensitivity, optical aberrations, and potential risk of retinal detachment.12, 13, 14, 15
Because many individuals have expressed a preference for eye drops,16 there is a need for a noninvasive, reversible, pharmacological treatment for presbyopia. Pilocarpine (Pilo) is a cholinergic muscarinic receptor agonist that has been used as a glaucoma treatment for decades but has largely been replaced by topical prostaglandins and beta-blockers as first-line therapies for glaucoma.17 The mechanism of action of Pilo, for the treatment of presbyopia, is through enhancing both depth of focus and accommodation.11,18,19 Pilo contracts iris sphincter muscles and ciliary muscles by binding to and activating muscarinic M3 receptors.20,21 Contraction of the iris sphincter causes pupil constriction, creating a pinhole effect that increases depth of focus and improves the ability to focus on near objects.11,22 In addition, contraction of the ciliary muscle enhances accommodation and can also contribute to improving near vision. In this study, the Pilo formulation has been optimized with a proprietary vehicle that reduces ocular irritation and blurry vision when compared with a commercially available pilocarpine hydrochloride ophthalmic solution for the treatment of glaucoma23 and is being investigated for the treatment of symptoms associated with presbyopia.
Oxymetazoline (Oxy) is a selective α1-adrenergic receptor agonist.24,25 As an ocular therapy, Oxy has been used to treat minor eye redness26 and ptosis.27 When applied topically to the eye, it can cause the contraction of the dilator muscles, subsequently dilating the pupils.28 Oxy also acts as a vasoconstrictor29 and has been added to tetracaine, an anesthetic, to slow its absorption and improve duration of action for intranasal applications.30,31 Although the addition of Oxy to Pilo is hypothesized to dampen the initial peak effect of Pilo, it may also extend the duration of action and potentially reduce the rate and severity of Pilo’s adverse events (AEs) (JC Abad, personal communications, December 24, 2013). Based on the expectation that Oxy would reduce Pilo’s AEs and potentially prolong its duration of action, combinations of Pilo and Oxy were investigated for the treatment of presbyopia.
The purpose of these dose-ranging, Phase 2 studies was to determine the optimal concentrations of Pilo and Oxy for the improvement of near vision in individuals with presbyopia. The safety, efficacy, and tolerability of fixed and unfixed Pilo/Oxy combinations were evaluated for this purpose in 2 Phase 2 studies (NCT02595528, NCT02780115).
Methods
The studies were registered on clinicaltrials.gov with the identifiers NCT02595528 and NCT02780115. They were performed in compliance with Good Clinical Practice, the principles of the Declaration of Helsinki, or the laws and regulations of the country in which the studies were conducted. Institutional Review Board or independent Ethics Committee approval was obtained at each site before the study began, and all patients provided written informed consent before undergoing any study-related procedure.
Study Design
The 2 concurrent Phase 2 studies had different study designs. Both studies were randomized, dose-ranging studies with either unfixed (i.e., drugs applied from individual bottles and dosed 5 minutes apart) or fixed (i.e., both drugs applied from a single bottle) combinations of Pilo (0%, 0.5% 1.0%, and 1.5%) and Oxy (0%, 0.0125%, 0.05%, and 0.125%). Patients were randomized by the interactive response system (IxRS). In the NCT02595528 study (i.e., short-term study), patients were randomized in a 1:1:1:1 ratio and were stratified by baseline uncorrected near visual acuity (UNVA) (≤20/80 and >20/80). At the time of randomization on visit 1, eligible patients were placed into 1 of 2 strata and were assigned to 1 of the 4 treatment groups. In the NCT02780115 study (i.e., extended study), patients were randomized by IxRS in a 1:1:1:1:1 ratio stratified by age (≤50 and >50 years) and iris color (brown and not brown). At the time of randomization, eligible patients were placed into 1 of 4 strata and assigned to 1 of the 5 treatment groups. For both studies, treatment group assignment was based on the randomization scheme within the patient's stratum according to the order of enrollment. The IxRS will assign the next available randomization number for the appropriate stratum to the patient at the time the investigator requests randomization. Study medication was labeled with a medication kit number, study number, and dominant eye (DE) or nondominant eye (NDE). The IxRS provided the site with the specific medication kit numbers for each patient at the time of randomization. Randomization schemes were prepared by Allergan Biostatistics. Sites were assigned study medication kits according to the IxRS instructions and received the IxRS confirmation notifications for each transaction. All notifications were maintained with the study source documents.
In the short-term study, there were 4 treatment groups that went through five 2-day dosing periods with a 7- to 21-day washout in between each dosing period. This study was conducted at 15 study centers. This study was designed to quickly determine the efficacy of 16 different combinations of Pilo and Oxy over a short period. The 4 treatment groups were defined by Pilo concentration (0%, 0.5% 1.0%, and 1.5%). In 4 of the dosing periods, participants within each group received an unfixed combination of a single concentration of Pilo and 4 different concentrations of Oxy (0%, 0.0125%, 0.05%, and 0.125%). All participants also received a fixed combination of Pilo 1.0% and Oxy 0.125% in a fifth dosing period. The orders in which the participants received the 5 combinations were random, and all study treatments were masked. Treatment was dosed once daily in the NDE, and vehicle (placebo) was dosed in the DE (Fig 1).
Figure 1.
The treatment groups for the short-term and extended studies are shown. In the short-term study, participants were placed into 1 of 4 treatment groups based on pilocarpine (Pilo) concentration (0%, 0.5%, 1.0%, and 1.5%) and went through 5 different dosing periods. In 4 of the dosing periods, participants received the same concentration of Pilo and randomly cycled through different concentrations of oxymetazoline (Oxy) (0%, 0.0125%, 0.05%, and 0.125%). In 1 of the 5 dosing periods, participants received a fixed combination of Pilo 1.0%/Oxy 0.125%. The stars represent the combinations that were chosen as treatment groups for the extended study. NDE = nondominant eye; OU = both eyes.
To accelerate the development and understanding of dosing Pilo and Oxy as fixed combinations over an extended period of time, the extended study was performed concurrently at 14 study centers. This study consisted of 5 fixed combination treatment groups that went through a single dosing period of 28 consecutive days with a 14-day follow-up. To test low, medium, and high fixed combinations of Pilo and Oxy concentrations, the following treatment groups were chosen from the short-term study for the extended study: Pilo 0%/Oxy 0% dosed in both eyes (OU) (Vehicle OU), Pilo 0.5%/Oxy 0.0125% dosed OU (Low OU), Pilo 1.0%/Oxy 0.05% dosed OU (Medium OU), Pilo 1.5%/Oxy 0.125% dosed OU (High OU), and Pilo 1.5%/Oxy 0.125% dosed in the NDE with the DE receiving vehicle (High NDE) (Fig 1). Treatment was dosed once daily. In contrast to the short-term study, all treatments were fixed combinations, and all but 1 treatment group was dosed in OU. The age range for the extended study was larger than in the short-term study, and efficacy measurements were taken at earlier time points to pinpoint onset of action. In both studies, the drops were delivered with a proprietary formulation that quickly equilibrates with tear pH upon instillation, and the NDE was used as the study eye. For both studies, the investigators and participants were fully masked to study drug and vehicle treatments.
Inclusion/Exclusion
The inclusion criteria for the short-term study were individuals ≥40 and <51 years of age; in the extended study, the age was raised to include individuals ≥40 and ≤55 years of age. Individuals had to be in general good health, emmetropes (defined as sphere −0.50 diopters [D] to +0.75 D and/or a cylinder <0.75 D) with presbyopia in each eye, photopic, high-contrast uncorrected distance visual acuity (UDVA) of 20/25 or better in each eye at the screening and baseline visits, mesopic, high-contrast UNVA of 20/40 (J3) to 20/200 (J17) in each eye at the screening and baseline visits, magnitude +1.00 to +2.50 reading resulting in mesopic, high-contrast UNVA of 20/20 or better in each eye, maximal accommodative amplitude >1.25 D and <5 D in OU for the NCT02595528 study and maximal accommodative amplitude ≥1.25 D in OU in the NCT02780115 study, mesopic pupil diameter <8.0 mm and photopic pupil diameter >3.0 mm in OU, phakic in each eye, intraocular pressure >10 mmHg and <21 mmHg. Individuals with severe dry eye disease, corneal abnormalities that would likely interfere with visual acuity, history of cataract surgery, phakic intraocular lens surgery, corneal inlay surgery, or any intraocular surgery (exception: LASIK or photorefractive keratectomy with UDVA and UNVA meeting inclusion criteria), narrow iridocorneal angles, history of angle-closure glaucoma, or previous iridotomy, iris heterochromia, abnormal pupil shape, anisocoria >1 mm between pupils under mesopic conditions, history of migraine headaches requiring treatment, or on any concurrent use of topical ophthalmic medications during the study were excluded.
Efficacy
For the short-term study, mesopic, high-contrast UNVA was measured at hours 0, 1, 3, 6, 8, and 10 on day 1 and 2 for efficacy. The primary efficacy end point for the short-term study was the average change from baseline in UNVA letters in the NDE over the 2-day dosing period. In the extended study, measurements were taken on day 1 at 0.25, 0.5, 1, 3, 6, 8, and 10 hours, on day 28 at 0.5, 1, 3, 6, 8, and 10 hours postadministration, and on days 14 and 21 at 0, 0.5, and 1-hour postadministration. The primary end point for the extended study was the mean change from baseline in mesopic, high-contrast UNVA letters in the NDE at day 28. Pupil diameter was also measured at each time point. All measurements were for monocular vision from the NDE.
Mesopic conditions were defined by lighting, 10 to 11 lux, measured at the target. Ten different near logarithm of the minimum angle of resolution charts (Cat No. 23212-1A, -1B, -2A, -2B, -3A, -3B, -4A, -4B, -5A, and -5B) and 3 different distance logarithm of the minimum angle of resolution charts (Cat No. 2144, 2144B, 2144C) were used for UDVA and UNVA measurements. The charts had the same settings but different SLOAN letters. The sites selected which charts to use but were required to rotate charts throughout each day to avoid patient memorization of the letters. The chart used at each measurement was tracked by the source worksheet. For UNVA, charts were mounted 40 cm from the participant’s eye by a metal bar attached to the top of the Grand Seiko that was designed to suspend the charts at the desired distance. For UDVA, charts were mounted 4 m away from the participant’s eye, approximately in the middle of the visual field. For visual acuity, participants were asked to read the lowest line they could read on the chart and continued reading until 3 or more errors were made on 1 line. The number of letters correctly read, including the letters in the preceding lines, were counted and recorded. Distance and near pupil size were measured by a pupilometer (NeurOptics, VIP-200) or a Grand-Seiko autorefractor while the participants were looking at the distance or near chart.
Safety
For safety, high-contrast UDVA was measured, and treatment-emergent adverse events (TEAEs) were recorded in both studies. A temporal and supraorbital headache assessment was also completed using a 100-mm long visual analog scale (VAS) to rate the headaches that were experienced in each eye at hours 0.5 and 1 at every study visit in the extended study. Tolerability was determined through a participant-reported ocular tolerability and drop comfort assessment after drop administration at each study visit. Participants were asked to rate symptom severity as “none,” “trace,” “mild,” “moderate,” or “severe” upon drop instillation, as well as the duration of symptoms as “<1 minute,” “1 to 5 minutes,” and “>5 minutes.” The symptoms included blurred vision, foreign body sensation, pain, burning/stinging, and itching. Participants also rated the comfort of the drops as “soothing,” “very comfortable,” “comfortable,” “uncomfortable,” “very uncomfortable,” or “intolerable.”
Statistics
Statistical analysis was performed using analysis of covariance (ANCOVA) techniques and 2-sample t tests for between-group comparisons. Categorical variables were analyzed using Pearson’s chi-square test. In the extended study, 26 patients per treatment group was determined as the sample size that would provide 99% power to observe a difference between an active group and the vehicle group for ≥1 line of change from baseline in mesopic, high-contrast UNVA and 68% power for ≥1 line of change from baseline in photopic, high-contrast UNVA, with a type 1 error of 0.05 and accounting for a 12.5% dropout rate. Statistical analysis was carried out using SAS version 9.3 (SAS Institute Inc.).
Computational Model
A polynomial regression model was developed using SAS, based on the short-term study results, to determine the optimal concentration range for Pilo. The Pilo concentration profile, with no Oxy, was based on a cubic regression model to find the average letter change from baseline.
Results
In the short-term study, a total of 163 participants were enrolled, of whom 157 were included in the modified intent-to-treat (mITT) population and 161 were included in the safety population. For the mITT population, the mean age was 46.8 years, ranging from 40 to 50 years. Most participants were female (69.4%) and White (79.0%) (Table 1). A total of 151 participants were enrolled in the extended study, and all were included in the mITT and safety populations. The mean age was 48.6 years, ranging from 40 to 55 years. The majority of study participants were female (69.5%) and White (79.5%) (Table 2).
Table 1.
Study Participants’ Demographics and Baseline Characteristics for Short-term Study
| Treatment Groups |
Total (N = 157) | ||||
|---|---|---|---|---|---|
| Pilo 0% (N = 40) | Pilo 0.5% (N = 37) | Pilo 1.0% (N = 42) | Pilo 1.5% (N = 38) | ||
| Mean age (SD), yrs | 46.6 (2.9) | 47.1 (2.6) | 46.8 (2.8) | 46.6 (2.2) | 46.8 (2.6) |
| Min, Max | 40, 50 | 40, 50 | 40, 50 | 41, 50 | 40, 50 |
| Sex - male, % | 20.0 | 32.4 | 31.0 | 39.5 | 30.6 |
| Race, % | |||||
| White | 77.5 | 81.1 | 66.7 | 92.1 | 79.0 |
| Black | 15.0 | 13.5 | 31.0 | 7.9 | 17.2 |
| Asian | 7.5 | 0 | 0 | 0 | 1.9 |
| Other | 0 | 5.4 | 2.4 | 0 | 1.9 |
| Baseline UNVA, % | |||||
| 20/40–20/80 | 65.0 | 70.3 | 66.7 | 68.4 | 67.5 |
| 20/100 or worse | 35.0 | 29.7 | 33.3 | 31.6 | 32.5 |
Pilo = pilocarpine; SD = standard deviation; UNVA = uncorrected near visual acuity.
Table 2.
Study Participants’ Demographics and Baseline Characteristics for the Extended Study
| Vehicle OU Pilo 0%/Oxy 0% (N = 28) |
Low OU Pilo 0.5%/Oxy 0.0125% (N = 30) |
Medium OU Pilo 1.0%/Oxy 0.05% (N = 30) |
High OU Pilo 1.5%/Oxy 0.125% (N = 32) |
High NDE Pilo 1.5%/Oxy 0.125% (N = 31) |
Total (N = 151) | |
|---|---|---|---|---|---|---|
| Mean age (SD), yrs | 48.3 (3.9) | 49.4 (2.7) | 47.9 (3.9) | 49.2 (3.8) | 48.6 (3.6) | 48.6 (3.6) |
| Min, Max | 40, 55 | 43, 54 | 41, 54 | 41, 55 | 40, 55 | 40, 55 |
| Iris color brown, n (%) | 18 (64.3) | 19 (63.3) | 18 (60.0) | 20 (62.5) | 24 (77.4) | 99 (65.6) |
| Sex - male, % | 32.1 | 33.3 | 40 | 21.9 | 25.8 | 30.5 |
| Race, n (%) | ||||||
| White | 23 (82.1) | 23 (76.7) | 25 (83.3) | 23 (71.9) | 26 (83.9) | 120 (79.5) |
| Black | 3 (10.7) | 5 (16.7) | 5 (16.7) | 8 (25) | 4 (12.9) | 25 (16.6) |
| Asian | 0 | 1 (3.3) | 0 | 0 | 1 (3.2) | 2 (1.3) |
| American Indian or Alaska native | 1 (3.6) | 1 (3.3) | 0 | 0 | 0 | 2 (1.3) |
| Native Hawaiian/Pacific Islander | 1 (3.6) | 0 | 0 | 0 | 0 | 1 (0.7) |
| Multiple | 0 | 0 | 0 | 1 (3.1) | 0 | 1 (0.7) |
| Baseline UNVA, % | ||||||
| 20/40–20/80 | 71.4 | 66.7 | 60 | 59.4 | 71 | 65.6 |
| 20/100 or worse | 28.6 | 33.3 | 40 | 40.6 | 29 | 34.4 |
NDE = nondominant eye; OU = both eyes; Oxy = oxymetazoline; Pilo = pilocarpine; UNVA = uncorrected near visual acuity.
Efficacy
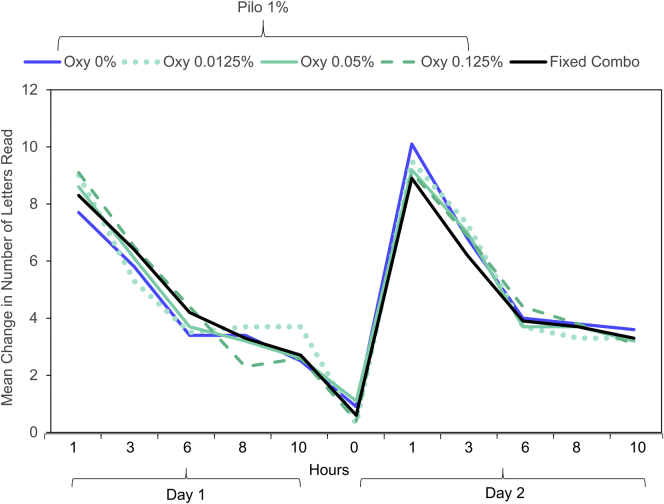
The mean change from baseline in mesopic, high-contrast UNVA letters for all treatment combinations in the short-term study is shown in Table 3. The number of letters gained increased as the Pilo concentration increased, up to a concentration of 1.0%. Both the Pilo 1.0% and 1.5% groups had a similar mean change in mesopic, high-contrast UNVA letter gain of approximately 5 letters across all Oxy concentrations. The addition of Oxy did not appear to improve the efficacy of Pilo alone, because letter gain did not significantly increase as Oxy concentration increased at any of the 4 Pilo concentrations. Analysis of covariance showed that Pilo produced a significant dose response in the average increase of letters across multiple time points compared with baseline (P < 0.001), but Oxy concentration did not have a significant impact (P = 0.4797). Although a clear dose response was seen for Pilo, there did not appear to be a dose response for Oxy. Figure 2 demonstrates minimal differences in the change from baseline in UNVA letters between any concentration of Oxy (0%, 0.0125%, 0.05%, 0.125%) when dosed in combination with 1.0% Pilo at any time point over the 2-day dosing period. There were no differences in efficacy between the fixed and unfixed combinations.
Table 3.
Mean Change in Mesopic, High-Contrast UNVA Letters from Baseline over the 2-Day Dosing Period in the Short-term Study
| Treatment Group Pilo % |
Oxy 0% | Oxy 0.0125% | Oxy 0.05% | Oxy 0.125% | Fixed Combination |
|---|---|---|---|---|---|
| Pilo 0% | |||||
| N | 39 | 38 | 39 | 39 | 40 |
| LS Mean (SE) | 1.12 (0.61) | 1.27 (0.62) | 1.62 (0.61) | 1.02 (0.61) | |
| Mean (SD) | 1.00 (2.70) | 1.13 (3.15) | 1.48 (3.77) | 0.85 (4.91) | 3.01 (3.60) |
| Median | 1.00 | 0.70 | 1.20 | 0.90 | 2.40 |
| Min, Max | −4.6, 7.5 | −6.5, 8.5 | −6.8, 11.3 | −18.2, 10.5 | −2.8, 13.3 |
| Pilo 0.5% | |||||
| N | 37 | 35 | 35 | 35 | 35 |
| LS Mean (SE) | 3.40 (0.63) | 3.96 (0.65) | 4.89 (0.65) | 4.27 (0.65) | |
| Mean (SD) | 3.20 (2.93) | 3.75 (4.12) | 4.68 (3.92) | 4.06 (3.64) | 4.61 (4.57) |
| Median | 3.40 | 3.20 | 6.10 | 4.10 | 4.20 |
| Min, Max | −3.0, 9.1 | −3.8, 11.5 | −3.6, 13.7 | −2.3, 13.6 | −1.9, 17.8 |
| Pilo 1.0% | |||||
| N | 42 | 41 | 41 | 41 | 42 |
| LS Mean (SE) | 5.25 (0.59) | 5.36 (0.60) | 5.17 (0.60) | 5.34 (0.60) | |
| Mean (SD) | 5.09 (4.39) | 5.22 (3.54) | 5.05 (3.86) | 5.22 (3.47) | 5.07 (4.93) |
| Median | 4.75 | 4.60 | 4.80 | 4.70 | 3.80 |
| Min, Max | −2.5, 16.2 | −0.5, 13.3 | −3.1, 13.7 | −2.2, 11.4 | −4.1, 19.6 |
| Pilo 1.5% | |||||
| N | 36 | 36 | 35 | 37 | 37 |
| LS Mean (SE) | 5.11 (0.64) | 6.65 (0.64) | 4.83 (0.65) | 5.64 (0.63) | |
| Mean (SD) | 5.01 (4.10) | 6.56 (4.79) | 4.73 (3.30) | 5.49 (4.36) | 5.18 (4.23) |
| Median | 4.40 | 6.50 | 4.90 | 4.6 | 5.00 |
| Min, Max | −5.9, 14.4 | −1.1, 22.0 | −1.5, 10.6 | −4.8, 18.3 | −1.4, 16.2 |
LS = least squares; Oxy = oxymetazoline; Pilo = pilocarpine; SD = standard deviation; SE = standard error; UNVA= uncorrected near visual acuity.
Figure 2.
The mean change from baseline in mesopic, high-contrast uncorrected near visual acuity (UNVA) letters was consistent in the Pilo 1.0% treatment group through all dosing periods with various oxymetazoline (Oxy) concentrations in the short-term study. There were no statistically significant differences between any of the combinations at any time point measured.
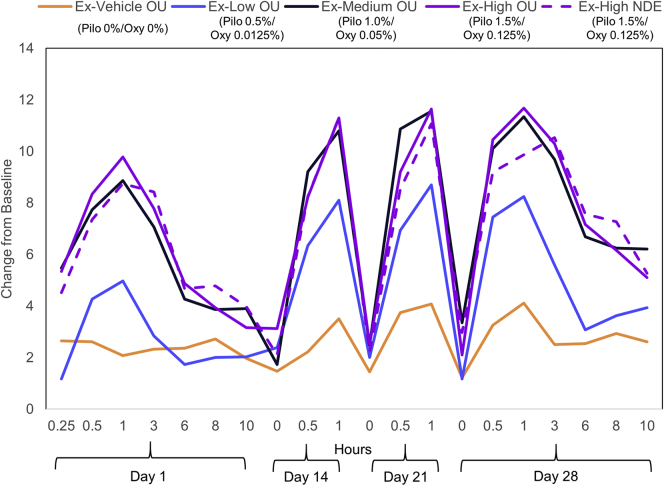
The mean change from baseline in mesopic, high-contrast UNVA letters at day 28 in the extended study for the Vehicle OU (Pilo 0%/Oxy 0% OU), Low OU (Pilo 0.5%/Oxy 0.0125% OU), Medium OU (Pilo 1.0%/Oxy 0.05% OU), High OU (Pilo 1.5%/Oxy 0.125% OU), and High NDE (Pilo 1.5%/Oxy 0.125% NDE) groups is shown in Table 4. The Medium OU group had the highest mean change from baseline of the groups dosed OU. Based on an ANCOVA comparison between the vehicle group and treatment groups, the difference was significant for the Medium OU (P = 0.0009), High OU (P = 0.0014), and High NDE (P = 0.0008) groups, whereas the Low OU group was not significant (P = 0.1663). Mean change from baseline in mesopic, high-contrast UNVA letters at all time points from all treatment groups is shown in Figure 3. Uncorrected near visual acuity was measured up to 10 hours after dosing on day 1 and day 30, but only measured up to hour 1 on day 14 and day 21. The 3 treatment groups with at least Pilo 1.0% had similar changes in UNVA letters at all time points. In these groups, efficacy is seen as early as 15 minutes after drop administration on day 1, with a mean (standard deviation [SD]) letter gain ranging from 4.5 (5.2) to 5.5 (6.2) letters. Peak effect on each day was seen at 1 hour postdose. On day 1, it ranged from 8.7 (6.6) to 9.8 (7.1) letters. Efficacy gradually reduced after hour 1 and reached a plateau at hour 6 on day 1. There was a slight increase in daily peak effect from day 1 to day 14. On day 14, the mean (SD) daily peak effect ranged between 10.8 (7.7) and 11.3 (6.2) letters and remained high at day 28, ranging between 9.9 (6.6) and 11.7 (6.6) letters with no tachyphylaxis effect observed. In the Medium OU group on days 1 and 28, the mean (SD) change from baseline in letters decreased between hour 1 and 6, from 8.9 (7.2) to 4.3 (6.6) letters and 11.3 (9.5) to 6.7 (8.5) letters, respectively. However, this decrease was less variable between hours 6 and 10, only changing from 4.3 (6.6) to 3.9 (5.2) letters and from 6.7 (8.5) to 6.2 (7.1) letters, on days 1 and 28, respectively.
Table 4.
Mean Change from Baseline in Mesopic, High-Contrast UNVA Letters at Day 28 in the Extended Study
| Statistics | Vehicle OU Pilo 0%/Oxy 0% |
Low OU Pilo 0.5%/Oxy 0.0125% |
Medium OU Pilo 1.0%/Oxy 0.05% |
High OU Pilo 1.5%/Oxy 0.125% |
High NDE Pilo 1.5%/Oxy 0.125% |
|---|---|---|---|---|---|
| LS Mean (SE) | 3.00 (1.03) | 4.96 (1.00) | 7.77 (1.00) | 7.54 (0.97) | 7.81 (1.02) |
| Mean (SD) | 2.70 (4.62) | 4.77 (5.03) | 7.66 (7.16) | 7.56 (4.84) | 7.52 (4.88) |
| Median | 2.86 | 3.29 | 5.71 | 7.29 | 7.57 |
| Min, Max | −6.0, 16.4 | −7.0, 13.4 | −4.1, 20.9 | −1.1, 19.6 | −0.3, 23.3 |
| n | 28 | 29 | 29 | 31 | 30 |
| P value∗ | -- | 0.1663 | 0.0009 | 0.0014 | 0.0008 |
LS = least squares; OU = both eyes; Oxy = oxymetazoline; Pilo = pilocarpine; SD = standard deviation; SE = standard error; UNVA = uncorrected near visual acuity.
Analyses were based on ANCOVA model with treatment group, age group (<50 or >50 yrs), iris color (brown or not brown), and baseline UNVA. The pairwise comparison was between active treatment group and vehicle group.
Figure 3.
The graph shows the mean change from baseline in mesopic, high-contrast uncorrected near visual acuity (UNVA) letters over time in the extended study. NDE = nondominant eye; OU = both eyes.
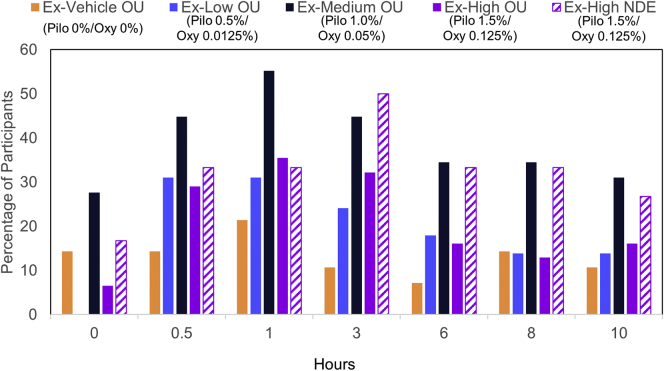
For the extended study, the percentage of participants gaining 2 lines (≥10 letters) and 3 lines (≥15 letters) or more from baseline in mesopic, high-contrast UNVA was also calculated at each time point on day 28. The Medium OU group was the only group to have a statistically significant percentage of participants maintain 2-line improvement at every time point on day 28 from 0.5 to 8 hours. Likewise, it was the only group to have a statistically significant percentage of participants maintain 3-line improvement at day 28 from 0.5 to 8 hours, whereas the other groups maintained a significant percentage only up to 3 hours (Fig 4A and B). The Medium OU group also had the highest percentage of participants who achieve UNVA of 20/40 or better at every time point on day 28, except at hour 3 (Fig 5).
Figure 4.
The graphs show the percentage of study participants with 2-line improvement (A) and 3-line improvement (B) in mesopic, high-contrast uncorrected near visual acuity (UNVA) compared with baseline on day 28 in the extended study. At each time point, treatment groups with a statistically significant difference compared with the vehicle group are indicated. NDE = nondominant eye; OU = both eyes.
Figure 5.
The graph shows the percentage of participants who achieved a visual acuity score of 20/40 or better in mesopic, high-contrast uncorrected near visual acuity (UNVA) at each time point on day 28 in the extended study. NDE = nondominant eye; OU = both eyes.
Pupil Diameter
Near vision pupil diameter, measured in the extended study, showed a decrease in all 4 treatment groups compared with vehicle. The pupil diameters at each time point on day 28 are shown in Figure 6.
Figure 6.
The graph shows the mean mesopic near vision pupil diameter on day 28 in the extended study. All treatment groups had a reduction in pupil diameter. NDE = nondominant eye; OU = both eyes.
Treatment-Related TEAEs
There were no unexpected safety findings in either the short-term or extended study. There was no clinically meaningful change in UDVA from baseline observed at any combination or time point in either study. Figure 7 shows the mean change from baseline in letters for mesopic, high-contrast UDVA in the extended study. The worst loss in UDVA occurred in the medium OU group and was 2.6 letters on day 1 at 0.5 hour.
Figure 7.
The graph shows the mean change from baseline in letters for mesopic, high-contrast uncorrected distance visual acuity (UDVA) throughout the extended study. NDE = nondominant eye; OU = both eyes.
Treatment-related TEAEs of >5% in any group are shown in Tables 5 and 6 for the short-term or extended study, respectively. Table 5 shows that increased Oxy concentrations did not reduce the rate of treatment-related TEAEs within each group. Additionally, increased concentrations of Oxy did not decrease the incidence of headaches in each treatment group. There were no differences in safety and tolerability between the fixed and unfixed combinations. Table 6 shows that the most common treatment-related TEAE was headache, and all groups reported it as a TEAE, including the vehicle group. Most headaches started at approximately 1 hour postdose and resolved by hour 3. Only 2 headaches were moderate in severity, with the majority being considered mild. All headaches in the Medium OU group were considered mild in severity, and the Medium OU group had a lower incidence of headache when compared with the High OU group. The supraorbital and temporal VAS headache assessment showed that all headaches from the vehicle, Medium OU, and High OU groups scored between 0 and ≤20. In the supraorbital VAS assessment, 7.14% of headaches in the Low OU group scored between 20 and ≤40 and 3.70% in the High NDE group scored between 40 and ≤60. In the temporal VAS assessment, 3.45% and 3.23% scored between 20 and <40 in the Low OU and High NDE groups, respectively. Overall, there were no clinically meaningful signals detected from intraocular pressure evaluations at multiple time points in any treatment group.
Table 5.
Treatment-Related Treatment-Emergent Adverse Events of >5% in the Short-term Study
| Treatment Group (Pilo %) | Preferred Term | Oxy 0% N (%) |
Oxy 0.0125% N (%) |
Oxy 0.05% N (%) |
Oxy 0.125% N (%) |
Fixed Combination |
|---|---|---|---|---|---|---|
| 0% | N | 40 | 39 | 40 | 40 | 40 |
| Headache | 0 | 0 | 1 (2.5) | 1 (2.5) | 3 (7.3) | |
| Instillation site pain | 1 (2.5) | 1 (2.6) | 1 (2.5) | 1 (2.5) | 0 | |
| Vision blurred | 1 (2.5) | 1 (2.6) | 1 (2.5) | 2 (5.0) | 1 (2.4) | |
| 0.5% | N | 38 | 37 | 37 | 36 | 37 |
| Headache | 0 | 1 (2.7) | 1 (2.7) | 1 (2.8) | 4 (10.3) | |
| Lacrimation increased | 2 (5.3) | 0 | 2 (5.4) | 2 (5.6) | 3 (7.7) | |
| Foreign body sensation in eyes | 0 | 0 | 1 (2.7) | 0 | 1 (2.7) | |
| 1.0% | N | 42 | 41 | 41 | 41 | 42 |
| Headache | 0 | 1 (2.4) | 1 (2.4) | 1 (2.4) | 1 (2.4) | |
| Punctate keratitis | 2(4.8) | 1 (2.4) | 2 (4.9) | 1 (2.4) | 2 (4.8) | |
| 1.5% | N | 37 | 37 | 36 | 38 | 38 |
| Headache | 1 (2.7) | 1 (2.7) | 2 (5.6) | 2 (5.3) | 2 (5.3) | |
| Vision impairment | 3 (8.1) | 3 (8.1) | 3 (8.3) | 3 (7.9) | 3 (7.9) | |
| Instillation site pain | 0 | 0 | 0 | 2 (5.3) | 2 (5.3) | |
| Punctate keratitis | 1 (2.7) | 0 | 0 | 1 (2.6) | 2 (5.3) | |
| Visual acuity reduced | 1 (2.7) | 2 (5.4) | 2 (5.6) | 2 (5.3) | 0 | |
| Vitreous floaters | 1 (2.7) | 0 | 1 (2.8) | 1 (2.6) | 1 (2.6) |
Oxy = oxymetazoline; Pilo = pilocarpine.
Table 6.
Treatment-Related Treatment-Emergent Adverse Events of >5% in the Extended Study
| Preferred Term | Vehicle OU Pilo 0%/Oxy 0% |
Low OU Pilo 0.5%/Oxy 0.0125% |
Medium OU Pilo 1.0%/Oxy 0.05% |
High OU Pilo 1.5%/Oxy 0.125% |
High NDE Pilo 1.5%/Oxy 0.125% |
|---|---|---|---|---|---|
| Headache | 3 (10.7) | 5 (16.7) | 6 (20.0) | 9 (28.1) | 6 (19.4) |
| Vision blurred | 0 | 2 (6.7) | 3 (10.0) | 3 (9.4) | 2 (6.5) |
| Instillation site foreign body sensation | 3 (10.7) | 3 (10.0) | 1 (3.3) | 0 | 1 (3.2) |
| Instillation site pain | 3 (10.7) | 4 (13.3) | 1 (3.3) | 3 (9.4) | 1 (3.2) |
| Instillation site pruritus | 0 | 4 (13.3) | 0 | 1 (1.31) | 2 (6.5) |
| Instillation site lacrimation | 3 (10.7) | 3 (10.0) | 2 (6.7) | 1 (1.31) | 0 |
NDE = nondominant eye; OU = both eyes; Oxy = oxymetazoline; Pilo = pilocarpine.
The results for the participant-reported ocular tolerability and drop comfort assessment were similar for both studies. A majority reported symptom severity levels of “none” and that symptom duration was “<1 minute” or “1 to 5 minutes.” No participants reported drop comfort as “intolerable,” with a majority reporting them as “soothing,” “very comfortable,” and “comfortable.”
Model
The computational polynomial regression model indicated that the optimal Pilo concentration range is between 1.16% and 1.32% (Fig 8).
Figure 8.
Scatterplot used to generate the polynomial regression computational model presented with 95% confidence intervals of the fitted values.
Discussion
The 2 Phase 2 studies intended to identify the optimum concentration of Pilo and Oxy for the treatment of presbyopia. However, based on the results from the short-term study, Oxy did not contribute to efficacy or safety of the Pilo and Oxy combinations. Increased concentrations of Oxy did not improve UNVA when compared with Pilo alone, and ANCOVA analysis did not show that Oxy had a significant impact on the increase in UNVA letter gain. In contrast, Pilo drove a significant efficacy response and the polynomial regression model determined that the optimal concentration range for Pilo would be between 1.16% and 1.32%.
Efficacy results from the extended study supported this; results showed that the Medium OU, High OU, and High NDE groups outperformed the Low OU group. In addition, the Medium OU group was the only group to have a significant percentage of participants maintain both 2-line and 3-line improvement out to 8 hours. Both 2- and 3-line improvement were included, as 2-line improvement is considered clinically meaningful and 3-line improvement is the Food and Drug Administration recommended outcome measurement.32 The Medium OU group also had the highest mean change from baseline in UNVA letters out of the 4 groups dosed OU, and mean change from baseline in UNVA letters was stable from hours 6 to 10, indicating that efficacy could be maintained to 10 hours in some patients. The extended study showed that there were no signs of tachyphylaxis, as dose-response and peak effect were maintained at day 28. Although the short-term study indicated that Oxy did not contribute to efficacy or safety of the Oxy and Pilo combinations, the 2 study protocols were designed at the same time, so the findings from the short-term study could not be taken into consideration for the study design of the extended study. Because of the addition of Oxy to all treatment combinations, it is unclear if efficacy results from the extended study can be attributed to Pilo alone.
Two potential mechanisms of action are involved for Pilo to treat presbyopia: pupil constriction to increase depth of focus and ciliary muscle contraction to increase accommodation. The pinhole effect, created by the pupil constriction, reduces blurry circle and blocks aberrated or scattered light.11,18,19 This results in an increased depth of focus and likely contributes to the improved near vision after Pilo treatment. Other presbyopia treatment options, such as pinhole spectacles, pinhole contact lenses, or corneal inlay surgery, create an artificial pinhole(s) with fixed aperture(s).33 However, these options all suffer from limitations. For example, corneal inlay surgery can be associated with complications, including difficulty with contrast sensitivity, problems with night vision, double vision, ghost images, glare, halos, and corneal scarring.12, 13, 14 Furthermore, the pinhole is usually located on the cornea, which is away from the iris plane, restricting the field of vision.18,33 In contrast to other treatment options, our pharmacological approach uses the eye’s own iris to create a natural pinhole, increasing the depth of focus and allowing dynamic pupil modulation in response to varying lighting and distance conditions. The pinhole is located on the pupil plane, which helps avoid excessive visual field restriction.33 A reduction in pupil size was seen in all treatment groups. Overall, the largest reduction was observed in the High NDE group, and the smallest reduction was in the Low OU group. Pilo also causes a mild contraction of the ciliary muscles, leading to increased accommodation, another factor contributing to the improvement of near vision.34 With contraction of the ciliary muscles, the strain on the suspensory ligaments is reduced and the lens is relaxed, causing the lens to become more rounded, improving the ability to focus on near objects.35 Given the age range of the participants in the 2 studies, some would have early presbyopia with a certain amount of accommodation amplitude. Therefore, both mechanisms of action could contribute to efficacy. Based on the change from baseline on UDVA (Fig 7), the increased accommodation did not result in clinical meaningful loss in distance vision, which may be partially counteracted by the increased depth of focus. Together, dynamic pupil modulation and mild contraction of the ciliary muscles caused by Pilo improved near vision without substantially impacting distance vision.
Although the studies used the Grand-Seiko auto-refractor with the intention of measuring accommodation, the instrument did not generate reliable refraction data for calculating accommodation. The Grand-Seiko has a limited pupil size range; when the pupil diameter was constricted to near and below 2.3 mm, an erroneous reading was generated in refraction.36 The lack of accommodation data is a limitation and should be investigated using a different approach in future studies.
Overall, there were no unexpected safety findings. The safety results, from both studies, showed that Pilo concentrations up to 1.5% and Oxy concentrations up to 0.125% have acceptable safety and tolerability profiles. Although loss in UDVA when accommodation increases (myopic shift) is a concern, there were no clinically significant losses in UDVA in any treatment group. This is in agreement with another study that showed that smaller pupils, between 2 and 3 mm, can improve focus on near objects without significantly impacting distance acuity or quality37 and may be due to the increased depth of focus from the pinhole effect offsetting the myopic shift. As previously mentioned, the addition of Oxy did not improve safety when compared with Pilo alone. The incidence of TEAEs did not decrease with the addition or increased concentration of Oxy in the short-term study, and participants who were treated with Oxy alone still had incidences of TEAEs.
Previous studies have shown that headaches/brow aches are common AEs of Pilo38, 39, 40, 41, 42, 43 and have even been the cause of discontinuation from a study.39 The incidence of headaches was variable between studies, with some showing that >20% of patients reported headaches with the use of Pilo alone.41, 42, 43 Although headaches were also an AE in our studies, based on the VAS headache assessment, almost all headaches were considered mild, and incidence was <5% when Pilo was dosed alone in the short-term study. Incidence of headaches was higher in the extended study and similar to what has been reported in the literature: 20.0% in the Medium OU group and 28.1% in the High OU group. It is important to note that the rate of headache in the vehicle group was 10.7%. Additionally, the majority of headaches were mild, and none required treatment. However, it is difficult to make direct comparisons between our studies and what has been reported in the literature as different methods of assessing for headaches were used. In addition, patients were specifically queried about headaches for the VAS assessment, which may have brought out a higher incidence of headaches.
Burning, stinging, ocular discomfort, and blurred vision are also AEs associated with Pilo.38,39,42,43 In one study, 14% of patients reported blurred vision and 11% reported burning/stinging,43 whereas in another study, up to 67% of patients have reported blurred vision lasting less than 20 minutes.39 In contrast, our participants reported ocular tolerability and drop comfort assessment showed that the majority reported symptom severity levels of “none” and the duration of any symptom lasted less than 5 minutes. Our study results also showed that when Pilo was dosed alone, the rate of similar AEs decreased when compared with these previous studies. This decrease may be due to the optimized formulation in the proprietary vehicle, which was designed for fast equilibration to the physiologic pH on the ocular surface to reduce stinging and burning when instilling the eye drop. The proprietary vehicle also helped mitigate blurring.23 In addition, previous studies used higher doses (2% or 4%) and dosing frequencies (twice daily) of Pilo compared with our study, which may contribute to the increase in AEs observed in the previous studies. The overall safety and tolerability profile of Pilo in our studies may be attributed to the proprietary vehicle, as well as the relatively low concentrations and dosing frequency when compared with other studies using Pilo formulations for treating glaucoma.
Although these 2 studies were designed to work together to determine the best treatment option, the differences between the study designs are a limitation. These differences prevented pooled analyses and direct comparison. Another limitation was that treatment groups of Pilo alone and Oxy alone were not tested in the extended study. Nevertheless, the Phase 2 studies informed the future development using the optimal drug concentration range to achieve clinically significant improvements in near vision. Population bias is another limitation, because the majority of participants were female and White, and had brown eyes, in both studies. It is possible the results could be different for a cohort of patients with different gender, race, and iris color demographics.
In conclusion, based on the results from both studies and the polynomial regression model, the ocular reading drop AGN-190584, containing an optimal concentration of pilocarpine HCl (1.25%) delivered using a proprietary formulation, was developed. Oxy was removed from the formulation because of the lack of contribution in efficacy and safety shown in the short-term study. AGN-190584 is under investigation in Phase 3 studies, GEMINI I and II, to determine its efficacy and safety for the treatment of presbyopia.
Manuscript no. D-21-00046.
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): F.P.: Consultant – Alcon, Eyepoint, Gebauer, Haag-Streit; Equity/stock owner –RxSight, Staar Surgical, Strathspey Crown; Grant support – Aerie Pharmaceuticals, Allergan.
M.H.: Conducted clinical trials – Allergan, Bausch Health, Novartis, Kala, Tarsus, Hovione, Silk-tech, Eyenovia, Surface, Nevakar; Consultant – Sun Pharma, Topcon, Laboratoires Thea, Aurinia, Eyevance, Visus, Azura, Aldeyra, Aperta Biosciences.
M.M.: Grant support – Allergan.
D.E.: Conducted clinical trials – Allergan, Alcon Vision, LLC, Bausch & Lomb, Vistakon, Kala, Hovione, AxeroVision, Novaliq, Novartis, Ocular Therapeutix.
M.R., J.P., H.L., and S.L.: Full-time employees – AbbVie Inc.
D.W.: Consultant – Allergan, Eyenovia; Grant support – Allergan, Novartis, Dompe, Mallinckrodt, SilkTech, Novaliq, Santen, Nicox, Aerpio, Annexon, Eyenovia.
This study was sponsored by Allergan plc, Dublin, Ireland (prior to its acquisition by AbbVie Inc.). Writing and editorial assistance was provided to the authors by Stephanie Kuwahara, PhD, of AbbVie. Neither honoraria nor payments were made for authorship.
Presented at: The American Academy of Ophthalmology Annual Meeting, November 13–15, 2020. Virtual.
Author Contributions:
Conception and design: Liu, Penzner, Robinson
Data collection: Price, Hom, Moshirfar, Evans, Liu, Penzner, Robinson, Wirta
Analysis and interpretation: Price, Hom, Moshirfar, Evans, Liu, Penzner, Robinson, Lee, Wirta
Obtained funding: N/A
Overall responsibility: Price, Hom, Moshirfar, Evans, Liu, Penzner, Robinson, Lee, Wirta
HUMAN SUBJECTS: Human subjects were included in this study. Institutional Review Board or Independent Ethics Committee approval was obtained at each site before the study began, and all patients provided written informed consent before undergoing any study-related procedure. All research adhered to the tenets of the Declaration of Helsinki.
No animal subjects were used in this study.
References
- 1.Goertz A.D., Stewart W.C., Burns W.R., et al. Review of the impact of presbyopia on quality of life in the developing and developed world. Acta Ophthalmol (Copenh) 2014;92:497–500. doi: 10.1111/aos.12308. [DOI] [PubMed] [Google Scholar]
- 2.Radhakrishnan H., Charman W.N. Age-related changes in static accommodation and accommodative miosis. Ophthalmic Physiol Opt. 2007;27:342–352. doi: 10.1111/j.1475-1313.2007.00484.x. [DOI] [PubMed] [Google Scholar]
- 3.Fricke T.R., Tahhan N., Resnikoff S., et al. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology. 2018;125:1492–1499. doi: 10.1016/j.ophtha.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 4.McDonnell P.J., Lee P., Spritzer K., et al. Associations of presbyopia with vision-targeted health-related quality of life. Arch Ophthalmol. 2003;121:1577–1581. doi: 10.1001/archopht.121.11.1577. [DOI] [PubMed] [Google Scholar]
- 5.Frick K.D., Joy S.M., Wilson D.A., et al. The global burden of potential productivity loss from uncorrected presbyopia. Ophthalmology. 2015;122:1706–1710. doi: 10.1016/j.ophtha.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Lu Q., Congdon N., He X., et al. Quality of life and near vision impairment due to functional presbyopia among rural Chinese adults. Invest Ophthalmol Vis Sci. 2011;52:4118–4123. doi: 10.1167/iovs.10-6353. [DOI] [PubMed] [Google Scholar]
- 7.Wolffsohn J.S., Leteneux-Pantais C., Chiva-Razavi S., et al. Social media listening to understand the lived experience of presbyopia: systematic search and content analysis study. J Med Internet Res. 2020;22 doi: 10.2196/18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolffsohn J.S., Davies L.N. Presbyopia: effectiveness of correction strategies. Prog Retin Eye Res. 2019;68:124–143. doi: 10.1016/j.preteyeres.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Lord S.R., Dayhew J., Sc B.A., Howland A. Multifocal glasses impair edge-contrast sensitivity and depth perception and increase the risk of falls in older people. J Am Geriatr Soc. 2002;50:1760–1766. doi: 10.1046/j.1532-5415.2002.50502.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson L., Buckley J.G., Scally A.J., Elliott D.B. Multifocal spectacles increase variability in toe clearance and risk of tripping in the elderly. Invest Ophthalmol Vis Sci. 2007;48:1466–1471. doi: 10.1167/iovs.06-0586. [DOI] [PubMed] [Google Scholar]
- 11.Tucker J., Charman W.N. The depth-of-focus of the human eye for Snellen letters. Am J Optom Physiol Opt. 1975;52:3–21. doi: 10.1097/00006324-197501000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Abdelkader A. Improved presbyopic vision with miotics. Eye Contact Lens. 2015;41:323–327. doi: 10.1097/ICL.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 13.Seyeddain O., Bachernegg A., Riha W., et al. Femtosecond laser-assisted small-aperture corneal inlay implantation for corneal compensation of presbyopia: two-year follow-up. J Cataract Refract Surg. 2013;39:234–241. doi: 10.1016/j.jcrs.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Seyeddain O., Hohensinn M., Riha W., et al. Small-aperture corneal inlay for the correction of presbyopia: 3-year follow-up. J Cataract Refract Surg. 2012;38:35–45. doi: 10.1016/j.jcrs.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Horgan N., Condon P.I., Beatty S. Refractive lens exchange in high myopia: long term follow up. Br J Ophthalmol. 2005;89:670–672. doi: 10.1136/bjo.2004.052720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stokes J, Shirneshan E, Graham CA, et al. American Academy of Optometry. 2020; October. Exploring the experience of living with and treating presbyopia. Paper presented at. 7, 2020; October, Virtual Poster, 2020. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S.K., Niranjan D.G., Agrawal S.S., et al. Recent advances in pharmacotherapy of glaucoma. Indian J Pharmacol. 2008;40:197–208. doi: 10.4103/0253-7613.44151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H.H., Park I.K., Moon N.J., Chun Y.S. Clinical feasibility of pinhole glasses in presbyopia. Eur J Ophthalmol. 2018;29:133–140. doi: 10.1177/1120672118810999. [DOI] [PubMed] [Google Scholar]
- 19.Kim W.S., Park I.K., Chun Y.S. Quantitative analysis of functional changes caused by pinhole glasses. Invest Ophthalmol Vis Sci. 2014;55:6679–6685. doi: 10.1167/iovs.14-14801. [DOI] [PubMed] [Google Scholar]
- 20.Skaat A., Rosman M.S., Chien J.L., et al. Effect of pilocarpine hydrochloride on the Schlemm canal in healthy eyes and eyes with open-angle glaucoma. JAMA Ophthalmol. 2016;134:976–981. doi: 10.1001/jamaophthalmol.2016.1881. [DOI] [PubMed] [Google Scholar]
- 21.Drummond P.D. The effect of light intensity and dose of dilute pilocarpine eyedrops on pupillary constriction in healthy subjects. Am J Ophthalmol. 1991;112:195–199. doi: 10.1016/s0002-9394(14)76701-7. [DOI] [PubMed] [Google Scholar]
- 22.García-Lázaro S., Ferrer-Blasco T., Radhakrishnan H., et al. Visual function through 4 contact lens-based pinhole systems for presbyopia. J Cataract Refract Surg. 2012;38:858–865. doi: 10.1016/j.jcrs.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 23.Giyanani J, Shabaik Y, Penzner J, Gore A. Novel, fast-equilibrating, ophthalmic vehicle for enhanced patient comfort and tolerability for pilocarpine delivery. Paper presented at: American Association of Pharmaceutical Scientists 2020 Annual Meeting and Exposition; October 26, 2020. Virtual Poster.
- 24.Schoeffter P., Hoyer D. Interaction of the alpha-adrenoceptor agonist oxymetazoline with serotonin 5-HT1A, 5-HT1B, 5-HT1C and 5-HT1D receptors. Eur J Pharmacol. 1991;196:213–216. doi: 10.1016/0014-2999(91)90432-p. [DOI] [PubMed] [Google Scholar]
- 25.Chu T.C., Ogidigben M.J., Potter D.E. Oxymetazoline: potential mechanisms of inhibitory effects on aqueous humor dynamics. Pharmacology. 1996;53:259–270. doi: 10.1159/000139438. [DOI] [PubMed] [Google Scholar]
- 26.Xuan B., Chiou G.C. Efficacy of oxymetazoline eye drops in non-infectious conjunctivitis, the most common cause of acute red eyes. J Ocul Pharmacol Ther. 1997;13:363–367. doi: 10.1089/jop.1997.13.363. [DOI] [PubMed] [Google Scholar]
- 27.Slonim C.B., Foster S., Jaros M., et al. Association of oxymetazoline hydrochloride, 0.1%, solution administration with visual field in acquired ptosis: a pooled analysis of 2 randomized clinical trials. JAMA Ophthalmol. 2020;138:1168–1175. doi: 10.1001/jamaophthalmol.2020.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa H., Miller D.D., Patil P.N. Comparison of post-junctional alpha-adrenoceptors in iris dilator muscle of humans, and albino and pigmented rabbits. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:765–772. doi: 10.1007/BF00166903. [DOI] [PubMed] [Google Scholar]
- 29.Haenisch B., Walstab J., Herberhold S., et al. Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam Clin Pharmacol. 2010;24:729–739. doi: 10.1111/j.1472-8206.2009.00805.x. [DOI] [PubMed] [Google Scholar]
- 30.Hersh E.V., Pinto A., Saraghi M., et al. Double-masked, randomized, placebo-controlled study to evaluate the efficacy and tolerability of intranasal K305 (3% tetracaine plus 0.05% oxymetazoline) in anesthetizing maxillary teeth. J Am Dent Assoc. 2016;147:278–287. doi: 10.1016/j.adaj.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Ciancio S.G., Hutcheson M.C., Ayoub F., et al. Safety and efficacy of a novel nasal spray for maxillary dental anesthesia. J Dent Res. 2013;92(7 Suppl):43s–48s. doi: 10.1177/0022034513484334. [DOI] [PubMed] [Google Scholar]
- 32.Center for Drug Evaluation and Research, U.S. Food and Drug Administration. Ozurdex (dexamethasone implant). In: Company: Allergan, Inc. Application No 22-315. Approval Date 6/17/2009. 2011.
- 33.Charman W.N. Pinholes and presbyopia: solution or sideshow? Ophthalmic Physiol Opt. 2019;39:1–10. doi: 10.1111/opo.12594. [DOI] [PubMed] [Google Scholar]
- 34.Gil D.W., Krauss H.A., Bogardus A.M., WoldeMussie E. Muscarinic receptor subtypes in human iris-ciliary body measured by immunoprecipitation. Invest Ophthalmol Vis Sci. 1997;38:1434–1442. [PubMed] [Google Scholar]
- 35.Waller D.G., Sampson A.P. In: Medical Pharmacology and Therapeutics. 5th Ed. Waller D.G., Sampson A.P., editors. Elsevier; New York, NY: 2018. pp. 569–578. [Google Scholar]
- 36.Davies L.N., Mallen E.A., Wolffsohn J.S., Gilmartin B. Clinical evaluation of the Shin-Nippon NVision-K 5001/Grand Seiko WR-5100K autorefractor. Optom Vis Sci. 2003;80:320–324. doi: 10.1097/00006324-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Xu R., Thibos L., Bradley A. Effect of target luminance on optimum pupil diameter for presbyopic eyes. Optom Vis Sci. 2016;93:1409–1419. doi: 10.1097/OPX.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 38.Ozdemir M., Ozdemir G. Comparison of the intraocular pressure lowering effect of latanoprost and carteolol-pilocarpine combination in newly diagnosed glaucoma. Jpn J Ophthalmol. 2003;47:72–76. doi: 10.1016/s0021-5155(02)00628-7. [DOI] [PubMed] [Google Scholar]
- 39.Kaluzny J., Sobecki R., Czechowicz-Janicka K., et al. Efficacy and safety of latanoprost versus pilocarpine/timolol maleate fixed combination in patients with primary open-angle glaucoma or ocular hypertension. Acta Ophthalmol. 2008;86:860–865. doi: 10.1111/j.1755-3768.2008.01324.x. [DOI] [PubMed] [Google Scholar]
- 40.Boger W.P., 3rd, Steinert R.F., Puliafito C.A., Pavan-Langston D. Clinical trial comparing timolol ophthalmic solution to pilocarpine in open-angle glaucoma. Am J Ophthalmol. 1978;86:8–18. doi: 10.1016/0002-9394(78)90006-5. [DOI] [PubMed] [Google Scholar]
- 41.Nagasubramanian S. Springer; Berlin, Heidelberg: 2000. A Comparison of the Ocular Hypotensive Efficacy, Safety and Acceptability of Brimonidine 0.2% Twice Daily Versus Pilocarpine 2.0% Thrice Daily as Adjunct Therapy with Beta-Blockers. [Google Scholar]
- 42.Laibovitz R., Boyle J., Snyder E., et al. Dorzolamide versus pilocarpine as adjunctive therapies to timolol: a comparison of patient preference and impact on daily life. Clin Ther. 1996;18:821–832. doi: 10.1016/s0149-2918(96)80042-7. [DOI] [PubMed] [Google Scholar]
- 43.Hartenbaum D., Maloney S., Vaccarelli L., et al. Comparison of dorzolamide and pilocarpine as adjunctive therapy in patients with open-angle glaucoma and ocular hypertension. Clin Ther. 1999;21:1533–1538. doi: 10.1016/s0149-2918(00)80008-9. [DOI] [PubMed] [Google Scholar]