Abstract
Purpose
To understand consequences of reconstituting cone photoreceptor function in congenital binocular blindness resulting from mutations in the centrosomal protein 290 (CEP290) gene.
Design
Phase 1b/2 open-label, multicenter, multiple-dose, dose-escalation trial.
Participants
A homogeneous subgroup of 5 participants with light perception (LP) vision at the time of enrollment (age range, 15–41 years) selected for detailed analyses. Medical histories of 4 participants were consistent with congenital binocular blindness, whereas 1 participant showed evidence of spatial vision in early life that was later lost.
Intervention
Participants received a single intravitreal injection of sepofarsen (160 or 320 μg) into the study eye.
Main Outcome Measures
Full-field stimulus testing (FST), visual acuity (VA), and transient pupillary light reflex (TPLR) were measured at baseline and for 3 months after the injection.
Results
All 5 participants with LP vision demonstrated severely abnormal FST and TPLR findings. At baseline, FST threshold estimates were 0.81 and 1.0 log cd/m2 for control and study eyes, respectively. At 3 months, study eyes showed a large mean improvement of –1.75 log versus baseline (P < 0.001), whereas untreated control eyes were comparable with baseline. Blue minus red FST values were not different than 0 (P = 0.59), compatible with cone mediation of remnant vision. At baseline, TPLR response amplitude and latency estimates were 0.39 mm and 0.72 seconds, respectively, for control eyes, and 0.28 mm and 0.78 seconds, respectively, for study eyes. At 3 months, study eyes showed a mean improvement of 0.44 mm in amplitude and a mean acceleration of 0.29 seconds in latency versus baseline (P < 0.001), whereas control eyes showed no significant change versus baseline. Specialized tests performed in 1 participant confirmed and extended the standardized results from all 5 participants.
Conclusions
By subjective and objective evidence, intravitreal sepofarsen provides improvement of light sensitivity for individuals with LP vision. However, translation of increased light sensitivity to improved spatial vision may occur preferentially in those with a history of visual experience during early neurodevelopment. Interventions for congenital lack of spatial vision in CEP290-associated Leber congenital amaurosis may lead to better results if performed before visual cortex maturity.
Keywords: Antisense oligonucleotides, Cone photoreceptors, Fovea, Inherited retinal degenerations, Low vision
Abbreviations and Acronyms: CEP290, centrosomal protein 290; FST, full-field stimulus testing; LCA, Leber congenital amaurosis; LP, light perception; NLP, no light perception; TPLR, transient pupillary light reflex; VA, visual acuity
Retinal photoreceptors transduce light energy into chemical energy and drive a complex synaptic signaling cascade within the retina, through the optic nerve, and to the brain, culminating in visual perception. Low-level features such as spatial contrast, colors, and motion as well as high-level features such as object recognition combine to form typical vision. Importantly, attainment of visual perception is a gradual process driven by visual experience over many months to years of life during early postnatal neurodevelopment. In nonhuman animals, lack of visual experience in the early postnatal period is known to result in major deficits in neuroanatomic features and neurochemistry that are only partially reversible when vision is regained beyond a critical period.1, 2, 3, 4 Translation of these experimental findings to human vision loss and later recovery has been studied extensively in amblyopia with early monocular visual impairment and in congenital bilateral dense cataracts with early binocular blindness.5, 6, 7 Treatment of severe visual deprivation must be corrected during the first 4 months of life to restore near-normal vision. For less severe visual deprivation, treatment before 9 years of age during a window of cortical plasticity is usually necessary for good outcomes. The relevance of a similarly limited window of successful treatment for Leber congenital amaurosis (LCA) is less studied. Leber congenital amaurosis, caused by > 20 distinct monogenic diseases,8 tends to result in early binocular blindness originating from molecular defects within rod and cone photoreceptors, which contrasts with normal photoreceptors in individuals with congenital cataracts or typical amblyopia.
Until recently, LCA was not treatable and questions regarding structural and functional changes at the visual cortex resulting from congenital blindness and the potential to regain vision were academic.9, 10, 11, 12, 13 However, one genetic form of LCA has already become treatable,14,15 and promising interventions are being evaluated for other genetic forms of LCA.16, 17, 18, 19 Improvement of partially retained vision seems to be possible, at least for some of the treatment methods and some of the individuals. What is least known is the recovery potential of visual perception for the most severe forms of LCA undergoing successful reversal of the molecular defect within photoreceptors. Do congenitally blind individuals start seeing when photoreceptor function improves as an adult?
Blindness resulting from LCA covers a very wide spectrum from impaired visual acuity (VA) with reduced light sensitivity and limited visual field to complete blindness, or no light perception (NLP).9,20, 21, 22, 23, 24 Toward the most severe end of this spectrum are eyes that can detect light, but lack any ability to distinguish spatial pattern or directionality of light; the clinical label is light perception (LP) without projection. Individuals with centrosomal protein 290 (CEP290) mutations associated with LCA (CEP290-LCA) tend to include the largest proportion of those with severe blindness in the LP or NLP category as compared with LCA with different molecular causation.25 The current study focused on 5 participants with CEP290-LCA and binocular severe photoreceptor blindness who were treated monocularly with sepofarsen, which is an intravitreal antisense oligonucleotide that has shown preliminary evidence of vision improvement.16,17,19
Methods
Participants and Intervention
Eleven individuals with CEP290-LCA participated in the phase 1b/2 trial of sepofarsen (ClinicalTrials.gov identifier, NCT03140969; European Union Drug Regulating Authorities Clinical Trials Database identifier, 2017-000813-22). All participants harbored biallelic mutations in CEP290, at least 1 of which was the common c.2991+1655A→G allele. Some of the results from the trial have been previously published.16,17,19 The current work provides additional details in a subset of 5 participants (age range, 15–41 years) who formed a homogeneous cohort of special interest having LP vision binocularly at the time of treatment (Supplemental Table 1). The participants selected for the current work have been previously published16,17,19 identified as patients P1, P2, P6, P9, and P10, and the same nomenclature is retained here for ease of comparability. Importantly, participants were enrolled at different centers and tested by different investigative groups: patients P1 and P6 in Iowa (United States), patients P2 and P10 in Philadelphia (United States), and patient P9 in Ghent (Belgium).
Sepofarsen is an antisense oligonucleotide designed for potential treatment of the vision loss experienced by those with LCA resulting from the CEP290 c.2991+1655A→G/p.(Cys998∗) mutation.26 Sepofarsen is thought to bind to the exonic splicing enhancer sequence at intron 26 of the CEP290 pre-mRNA and to modulate the RNA splicing process, blocking access to the active cryptic splicing site and restoring preference for the wild-type splicing sites. A resulting increase of wild-type mRNA transcript is predicted to lead to an increase of functional CEP290 protein. An open-label study was designed to evaluate the safety and tolerability of sepofarsen administered via unilateral intravitreal injection.
This study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review boards of the University of Pennsylvania, University of Iowa, and Ghent University. Adult participants provided informed written consent, whereas children provided informed written assent, with the parents providing informed written consent. Contemporaneous with the clinical trial, patient P10 was enrolled in additional research studies that were approved by the University of Pennsylvania institutional review board. These specialized, noninvasive assessments provided further details of visual function and retinal structure that expanded the findings from the clinical trial protocol.
Visual Acuity and Rudimentary Spatial Vision
The cohort of 5 participants had clinical LP vision (with no light localization, also known as LP with no projection) at the time of enrollment. It is important to note that LP, bare LP, and NLP designations in the clinic tend to overlap. Depending on the amount of light, attention, and effort used, it is not uncommon for some NLP eyes to be renamed as LP or vice versa. Best-corrected visual acuity measurements with ETDRS and tumbling E charts were attempted as part of standard protocol at all visits throughout the trial. In addition, the Berkeley rudimentary vision test27 was used to allow more precise measurement and categorization of remnant spatial vision.
Light Sensitivity
Sensitivity to chromatic light flashes with full-field stimulus testing (FST) was specifically developed for participants with severe vision loss and lack of oculomotor control.28, 29, 30, 31 Full-field stimulus testing provides a measure of best light sensitivity retained across the retina, and use of chromatic stimuli allows estimation of the photoreceptor source. In all 5 participants, per clinical trial protocol, chromatic FST was performed with dark adaptation with commercial software using a binary thresholding algorithm and an unconstrained response-acceptance window.32,33 In addition, patient P10 underwent chromatic FST as a specialized assessment under dark-adapted and light-adapted conditions using a 4/2-dB staircase with 2 response reversals and a limited response-acceptance window to minimize the effect of extraneous responses not synchronized with the stimulus presentation.16, 17, 18,30 The homogeneous white 10 cd/m2 background for light-adapted FST was provided by a light-emitting diode (EWL5FW12-P01 [Electrospell]; International Commision on Illumination chromaticity coordinates, x = 0.32, y = 0.33) centered on a plastic bracket fitting the Colordome (Diagnosys) filter holder. Light output was controlled by a constant-current pulse-width modulator circuit (TLC59711; Texas Instruments) powered by a rechargeable battery (YB1206000-USB; TalentCell).
Pupillometry
Pupillometric recordings (RETIport; Roland Consult Stasche & Finger GmbH) were performed for all participants per protocol after at least 40 minutes of binocular dark adaptation. An infrared-sensitive video camera (uEye; IDS) acquired the direct pupil constriction videos (in the stimulated eye with the contralateral eye patched) at 30 frames per second, starting from 1 second before the stimulus onset (baseline before stimulus) for a total duration of 15 seconds. Pupil magnification was fixed (cornea to camera distance, 0.3 m, 0.05 mm/pixel). The pupil boundaries were detected and tracked automatically by the commercial software from the video record (Roland Consult version 1017.2.0.6). Full-field brief (1-second) white stimuli over a range of increasing luminances from 0.6 to 3.6 log cd/m2 were used to evoke transient pupillary light responses (TPLRs) similar to previous publications.17,22,24
Transient pupillary light response amplitude was defined as the difference between the pupil diameter at a fixed time (0.9 second) after the onset of the stimulus and the baseline before stimulus. Transient pupillary light response latency was defined as the time to reach 0.2-mm criterion constriction. Typically, 2 to 4 repetitions were attempted at each intensity. All evaluable responses were included in analyses. Luminance response functions as defined below were fit to all measurable responses by minimizing the residuals squared and a GRG nonlinear engine (Excel; Microsoft). For TPLR amplitude, the following formula was used:
where R is the response amplitude (in millimeters) at 0.9 second measured at each luminance (L, in candelas per square meter), Rmax is the maximum amplitude (in millimeters), KR is the luminance (in candelas per square meter) to reach half Rmax, and n is the exponent defining the steepness of the function. Rmax was fixed to the average of the amplitudes recorded for the highest luminance (to avoid extrapolation), and KR and n were determined by minimizing the error. Note that the term in parenthesis is a nonlinear function between 0 and 1 corresponding to the extremes when L << KR and L >> KR, respectively. Constraints used for curve fitting were 0.5 ≤ n ≤ 2, 10–5 ≤ KR ≤ 105 cd/m2. For TPLR latency, the following formula was used:
where D is the response latency (in seconds) to reach 0.2-mm amplitude measured at each luminance (L, in candelas per square meter), Dmin is the minimum latency (in seconds), AD is a delay factor (in seconds), KD is the luminance (in candelas per square meter) that delays the latency by half of AD, and m is the exponent defining the steepness of the function. Dmin was fixed to the average of the latencies recorded for the highest luminance (to avoid extrapolation), and AD, KD, and m were determined by minimizing the error. Note that the term in parenthesis is a nonlinear function between 1 and 0 corresponding to the extremes when L << KD and L >> KD, respectively. Constraints used for curve fitting were 0.1 ≤ m ≤ 2, 0.2 ≤ AD ≤ 3 seconds, 10–5 ≤ KD ≤ 105 cd/m2. In addition, red TPLRs were recorded in patient P10 as previously published in others with CEP290 LCA.17,24
Imaging
Spectral-domain OCT was used to obtain cross-sectional imaging of the retina (RTVue-100; Optovue). En face imaging with near-infrared illumination was performed with autofluorescence mode using a confocal scanning laser ophthalmoscope (Spectralis; Heidelberg Engineering). For 3 of the participants (patients P1, P2, and P6) baseline images before treatment and images obtained 1 and 3 months after treatment were published previously, and for 2 of the participants (patients P9 and P10), baseline images before treatment images and images obtained 1 month after treatment were published previously.16 Additional imaging data for patient P10 from baseline to 3 months after treatment are included here to estimate the treatment potential from retinal structure using an artificial intelligence approach.17,23 In short, a cohort of patients with retinitis pigmentosa with cone-only function in the macula were selected, and a machine learning algorithm was trained to predict local cone sensitivity from the local retinal structure.17,23 We applied this method to patient P10 and estimated the treatment potential by assuming that the FST sensitivity in patient P10 originates from the fovea. We compared measured change in FST sensitivity with the expected treatment potential.
Statistical Analysis
A mixed-effects model was used to analyze the treatment effect from FST data, with color (blue or red levels), visit (before and after levels), treatment (control and treated levels), and the interaction of the last 2 items as fixed effects and with intercept plus slope with respect to visit, grouping by participant identification as random effects. Significance of the treatment effect was assessed from the interaction term. The effect of dose was analyzed with a similar model (with color, visit, dose with levels of 160 μg or 320 μg, and the interaction of the last 2 items as fixed effects). All available observations were used (median number of threshold measurements per eye, visit, and color was 17 [range, 6–27]). The within-subject variability at baseline was assessed as ± 1.96 × standard deviation (SD), where SD is the residual SD from the model using data from both colors and eyes, with color as a fixed effect and participant identification or eye as nested random effects. The TPLR data obtained with a single stimulus luminance were analyzed similarly with a mixed-effects model. Fixed effects were visit and treatment and their interaction for treatment effect and visit and dose for dose effect. Only the intercept was modeled to assess variability. Random effects were the same as for FST. The median number of TPLR observations per eye and visit was 3.5 (range, 2–5). Analyses were conducted separately for pupillary constriction amplitude and latency.
Results
The current study is a subanalysis in a subset of patients who took part in the first sepofarsen trial (ClinicalTrials.gov identifier, NCT03140969; European Union Drug Regulating Authorities Clinical Trials Database identifier, 2017-000813-22). Results published to date16,17,19 from 11 treated patients with CEP290-LCA demonstrated improvements in light sensitivity that averaged 0.85 log units at 12 months after ≥ 1 injections. However, distinct differences were found among the participants enrolled regarding baseline VA before treatment (range, 0.6 logarithm of the minimum angle of resolution to LP), light sensitivity (range, near normal to near 8 log10-unit deficit), number of injections (range, 1–4), and recorded adverse events (range, none to lenticular and retinal changes). For the current work, we focused on a subset of 5 participants with binocular LP vision at enrollment and considered the first 3 months after the first injection to avoid potential confounders such as differences in the number of subsequent (maintenance) doses injected after the 3-month time point.
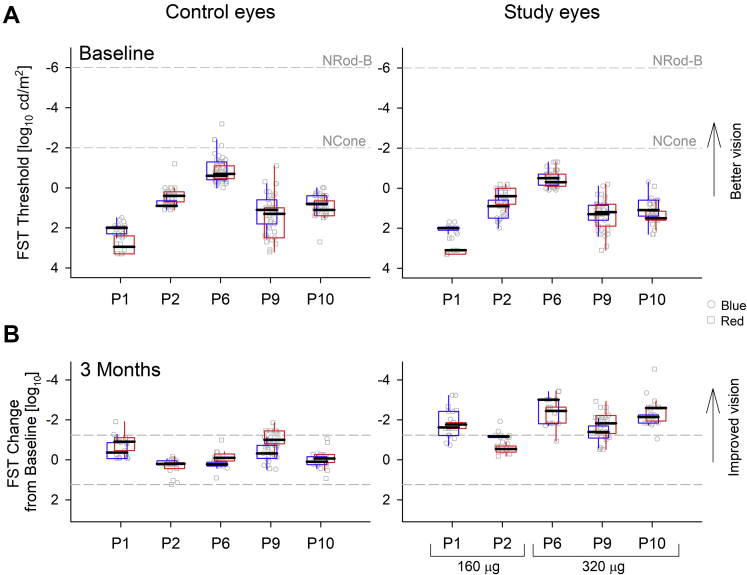
We first evaluated the loss of light sensitivity in the 5 severely affected LP eyes measured at 2 baseline visits before treatment. Dark-adapted red FST thresholds ranged from –0.2 to +3.2 log phot-cd/m2, and blue FST thresholds from –1.0 to +2.3 log phot-cd/m2. Patient P6 showed the lowest thresholds (best sensitivity; Fig 1A). Before treatment, median sensitivities from all 5 participants were worse than the normal dark-adapted cone plateau threshold of –2.0 log phot-cd/m2 and were substantially worse than normal dark-adapted rod thresholds of –4.0 and –6.5 log phot-cd/m2 for red and blue, respectively.
Figure 1.
Graphs demonstrating full-field stimulus testing (FST) results show subjective light sensitivity improvements recorded with sepofarsen treatment in a cohort of 5 participants with light perception vision. A, Dark-adapted chromatic FST thresholds at 2 baseline visits for the control and study eyes. Normal dark-adapted cone threshold (NCone) and normal dark-adapted rod thresholds for blue stimuli (NRod-B) are shown for reference. B, Change in FST thresholds from mean baseline value at 3 months. Test–retest variability limits estimated from all baseline data are shown (dashed lines). Study eyes of patients P1 and P2 received the 160-μg dose, whereas patients P6, P9, and P10 received the 320-μg dose. In all panels, each cluster of points shows individual FST thresholds with blue (squares) and red (circles) stimuli. Boxplots (colored blue and red for respective stimuli) show the extreme of the lower and upper whisker, lower and upper hinge, and median.
Photopically matched blue and red FST threshold pairs for each eye supported the likelihood that mediation was by cone photoreceptors. Specifically, there was no apparent reason to suspect involvement of desensitized rods or intrinsically photosensitive retinal ganglion cells, both of which would have caused blue minus red differences to be more than +2 log10 units. Thus, despite the extremely severe vision loss, 5 selected participants retained abnormally reduced, but detectable, cone sensitivity that could potentially be improved.
To evaluate treatment effects, FST change from average baseline was calculated at 3 months after injection in control and study eyes (Fig 1B). The 3-month time point was chosen because previous data implied potential peak effect of sepofarsen by this time and it preceded variability in the number of maintenance injections. Previously, we reported that the average values of FST sensitivity increase16,19; however, individual estimates and their variability were not considered. Individual FST thresholds in uninjected control eyes tended to be similar to baseline thresholds, whereas in injected study eyes, FST improvement was found across all participants (Fig 1B).
Statistical analysis of all the data across the 5 participants was performed using mixed-effects models to account for internal correlation of the data. At baseline, variability estimates were ±1.24 log units (95% confidence interval of residuals), which was higher than previous estimates of ±0.40 log units in a larger population of patients that included mostly those retaining better vision than LP.30 Increased variability of vision-based perceptual measurements was expected in severely affected participants who tended to not be visual. The model estimates for FST thresholds at baseline were 0.81 and 1.0 log cd/m2 for control and study eyes, respectively. At 3 months, the difference from baseline was not significant for uninjected control eyes (–0.22 log; P = 0.3). In contrast, study eyes showed a large mean improvement of –1.75 log versus baseline (P < 0.001). The blue minus red differences at both baseline and 3 months were not different than 0 (P = 0.59; range, –1.1 to 0.72 log units), compatible with cone mediation after taking into account the higher variability in these participants. In terms of dose, low- and high-dose groups showed similar thresholds at baseline (P = 0.44). Improvements after treatment from baseline were –1.27 and –2.11 log units for the 160-μg and 320-μg dose groups, respectively, but this difference did not reach significance (P = 0.44).
Considering the difficulty and variability of making perceptual FST measurements in patients with LP vision, we next evaluated whether objective evidence of improvements in the visual system existed. One important objective outcome is TPLR,31 and we previously predicted that improvements in cone photoreceptor function of individuals with CEP290-LCA should accelerate the TPLR.24 Consistent with this prediction, in one participant (patient P11), sepofarsen injection did indeed accelerate TPLR latency, as previously shown.17 What remained unknown was whether such objective improvements could also be recorded with a standardized protocol across multiple international centers, especially in severely affected patients with LP vision and wandering eyes, providing a severe challenge to stable imaging of the pupil.
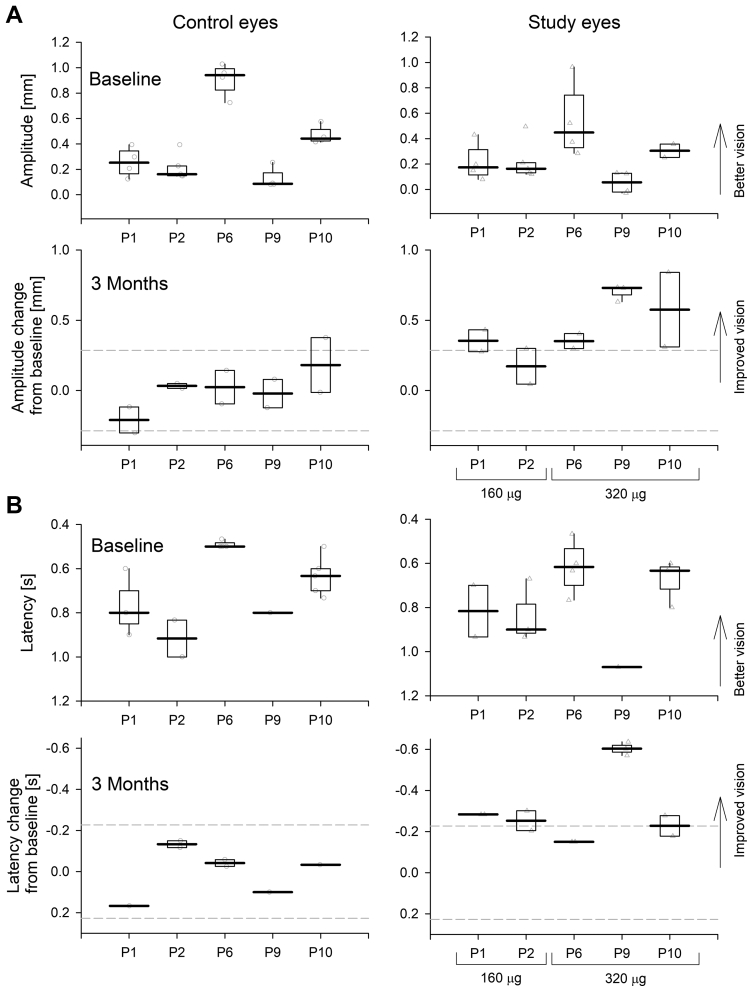
Families of TPLR responses evoked with 4 increasing luminances of white stimuli in dark-adapted eyes were quantified for response amplitude and latency and were modeled with luminance-response functions. The raw data, models (Supplemental Fig 1), and model parameters (Supplemental Table 2) are provided. At baseline before treatment, dark-adapted pupil diameters ranged from 3.7 to 7.5 mm, maximum constriction amplitude ranged from 0.6 to 1.8 mm, luminance at half maximum response ranged from 0.7 to 2.9 log cd/m2, and minimum latencies ranged from 0.4 to 0.7 seconds. Results from contralateral eyes tended to be symmetric. It was notable that the TPLR parameters of patient P6 were distinctly better than those of the other 4 participants, consistent with a similar trend seen in that patient’s FST results when compared with those of the other participants. At 3 months after the treatment, a tendency was found for larger maximum constriction amplitudes and faster latencies in the treated eyes (Supplemental Fig 1).
Statistical analysis of TPLR data across the 5 participants was performed using mixed-effects models to account for internal correlation of the data (Fig 2). For these analyses, a single luminance was chosen based on previous literature,17,24 as well as to balance considerations showing saturation at higher luminances and lack of response at lower luminances (Supplemental Fig 1). At baseline, variability estimates for response amplitude and latency were ±0.286 mm and ±0.227 second (95% confidence interval of residuals), respectively. The model estimates for TPLR response amplitude and latency at baseline were 0.39 mm and 0.72 second, respectively, for uninjected control eyes, and 0.28 mm and 0.78 second, respectively, for study eyes. At 3 months, control eyes showed no significant mean change in amplitude or latency (P = 0.96 and P = 0.88, respectively). In contrast, study eyes showed a mean improvement in visual function: 0.44-mm increase in amplitude and 0.29-second acceleration in latency (P < 0.001 in both metrics, interaction term). No evidence was found of a difference in treatment effect related to dose on the pupillary response parameters (P = 0.7 and P = 0.78 for amplitude and latency, respectively). No significant correlation was found between the magnitude of the FST changes and TPLR changes (P = 0.6 and P = 0.53 for FST vs. TPLR amplitude and vs. TPLR latency changes, respectively, Pearson’s correlation) likely because of the FST originating from changes at the fovea, whereas TPLR is driven by the parafovea.17,24
Figure 2.
Graphs demonstrating transient pupillary light reflex (TPLR) results show objective light sensitivity improvements recorded with sepofarsen treatment in a cohort of 5 participants with light perception vision. Both (A) amplitude and (B) latency of the TPLR evoked with a white 40 cd/m2 stimulus in dark-adapted eyes are evaluated. In patient P6, a white 4-cd/m2 stimulus was used (Supplemental Fig 1). Upper panels show results at baseline and lower panels show the change from mean baseline at 3 months in control and study eyes. Boxplots show the extreme of the lower and upper whisker, lower and upper hinge, and median. Test–retest variability limits estimated from all baseline data are shown (dashed lines) in lower panels.
Subjective (FST) and objective (TPLR) evaluation of the visual system in participants with LP vision treated with sepofarsen provided incontrovertible evidence of significant improvement in the cone photoreceptors’ ability to catch light quanta. What about improvements in spatial vision? As previously reported, only 1 of the participants, patient P2, showed a large increase in VA, from LP to 20/400,16 whereas patients P1, P6, P9, and P10 showed no evidence of VA improvements and remained at LP vision bilaterally.16,17,19 Patients P1, P6, P9, and P10 also failed results for the coarsest measures of spatial vision with Berkeley rudimentary vision testing27 in both eyes at all visits.
To better understand the potential causes of the apparent incongruity between light sensitivity improvements recorded with subjective and objective methods in all 5 participants, but VA improvement recorded in only 1 participant, we further evaluated potential individual differences in neurodevelopmental history (Supplemental Material). Surprisingly, patient P2, who showed VA improvement, had a medical history consistent with having spatial vision and measurable chart acuity at least in 1 eye in early childhood that was progressively lost after the second decade of life. This was in distinct contrast to medical histories of patients P1, P6, P9, and P10, who were consistent with showing congenital binocular complete blindness with no evidence of spatial vision from early childhood.
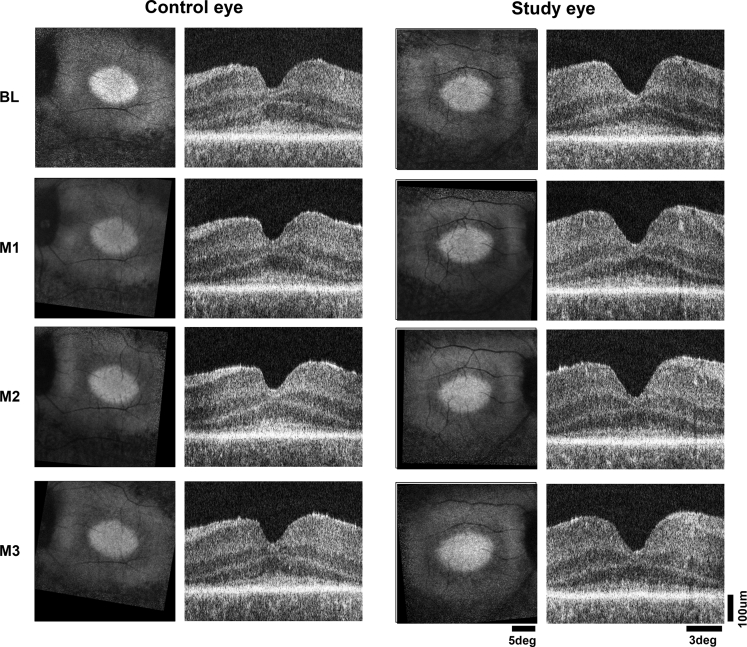
To obtain potential clues for understanding the relationship in retinal structure and visual function, additional research investigations were performed in patient P10 as a representative of the 4 participants who showed light sensitivity improvements without spatial vision improvement. Patient P10 harbored a retinal structure stereotypical for CEP290-LCA consisting of an elliptical central island of retained retinal pigment epithelium melanization and photoreceptors as apparent on near-infrared autofluorescence and OCT imaging in both eyes (Fig 3). Three months after a single uniocular injection of intravitreal sepofarsen, no changes were visible to the en face or cross-sectional retinal structure, thus ruling out a major loss of photoreceptors as a potential reason for the lack of spatial vision response (Fig 3).
Figure 3.
Images showing the retinal structure of subject P10 at baseline (BL) and months 1 through 3 (M1, M2, and M3, respectively) in the untreated control eye and study eye, which received an intravitreal injection of 320 μg sepofarsen. En face images (square panels on left) display near-infrared autofluorescence representing retinal pigment epithelium melanization. The brighter central ellipse is the stereotypically retained region in CEP290-Leber congenital amaurosis, surrounded by loss of signal resulting from retinal degeneration and demelanization of the retinal pigment epithelium. The near-infrared autofluorescence images from months 1 through 3 have been registered to the BL image of each eye to allow comparison. Cross-sectional images (rectangular panels on right) are OCT scans along the horizontal meridian crossing the fovea. Calibration shown on lower right.
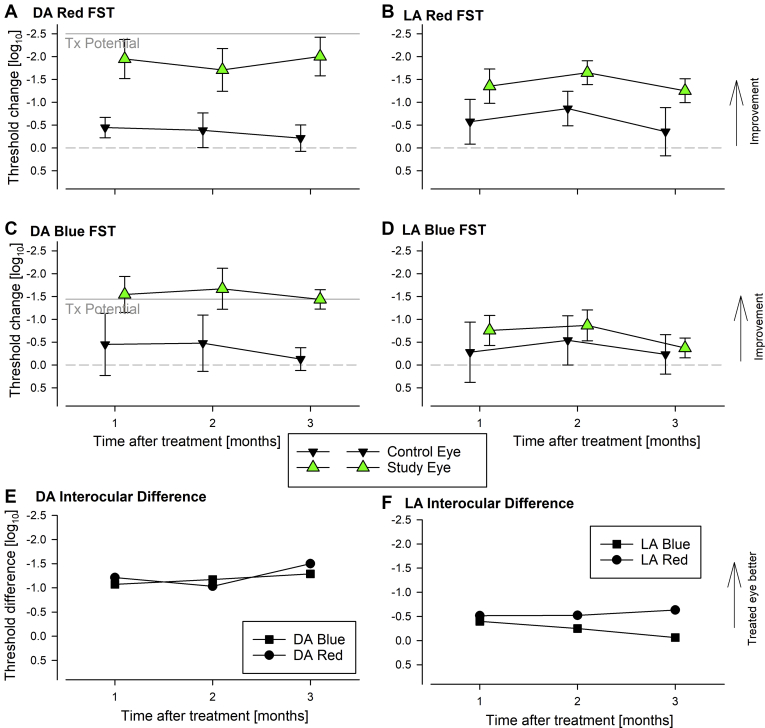
We used specialized chromatic FSTs16,17 to better understand the magnitude and kinetics of changes in light sensitivity after treatment. Under dark-adapted conditions, both red and blue FSTs substantially increased by 2 and 1.5 log, respectively, in the treated eye at 1 month and remained improved through 3 months (Fig 4A, C). A smaller increase in the untreated eye was found such that interocular differences increased from near 0 to > 1 log unit for both colors (Fig 4E). Photopically matched thresholds at baseline followed by synchronized chromatic changes suggested that remnant cone function was the source of this rudimentary vision and that improvement was originating from cone photoreceptors.
Figure 4.
Graphs showing specialized chromatic full-field stimulus testing (FST) results of participant P10 at baseline and months 1 through 3 in the untreated control eye and study eye, which received an intravitreal injection of 320 μg sepofarsen. A–D, Full-field stimulus testing threshold changes from the baseline mean are shown for red (A, B) and blue (C, D) stimuli under dark-adapted (DA) and light-adapted (LA) conditions. Maximum treatment (Tx) potential predicted from artificial intelligence evaluation of patient P10’s foveal OCT data are shown for DA FST (A, C). Gray dashed line represents no change from baseline. Symbols and error bars represent mean and ±1 standard deviation. E, F, Interocular difference of mean DA and LA FST thresholds.
The FST sensitivity improvements of patient P10 after treatment were reasonably predictable from the retinal structure,23 assuming the light sensitivity was originating from the fovea (Fig 4A, C). Red FST sensitivities after treatment were within approximately 0.5 log of prediction, and blue FSTs were within approximately 0.2 log of prediction. Under light-adapted conditions, FST improvements in the treated eye and the interocular difference showed smaller magnitude (Fig 4B, D, F), as previously shown in 2 other patients treated with sepofarsen.16,17
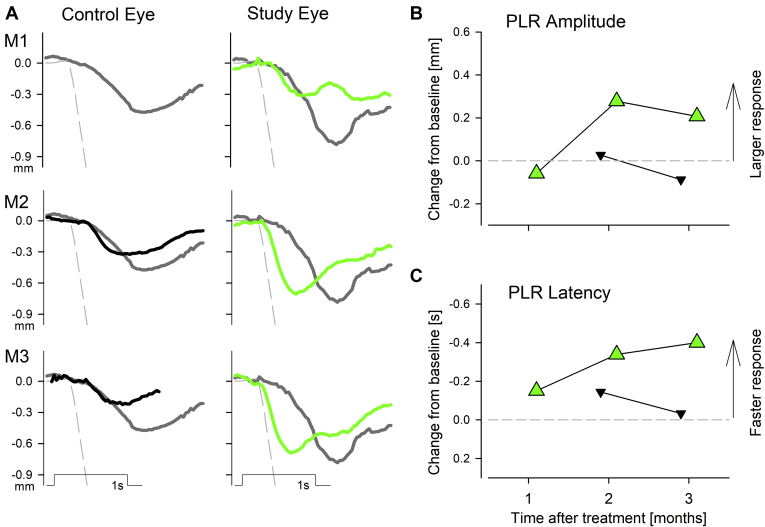
Objective evidence of changes to the visual system in patient P10 were then examined with TPLR using red stimuli, which would be expected to stimulate cone photoreceptors preferentially (Fig 5).17,18,24 At baseline, red TPLRs were small in amplitude and slow in latency. At 2 and 3 months after the sepofarsen injection, a substantial acceleration of the TPLR was found in the study eye qualitatively (Fig 5A) as well as quantitatively (Fig 5B, C).
Figure 5.
Graphs showing specialized transient pupillary light reflex (TPLR) results of participant P10 at baseline and months 1 through 3 (M1, M2, and M3, respectively) in the untreated control eye and study eye, which received an intravitreal injection of 320 μg sepofarsen. A, Traces of mean pupillary diameter as a function of time for brief (1-second) red full-field flashes of 50 cd/m2 luminance at baseline (thick gray lines, duplicated at each panel) and at M1, M2, and M3 after injection. Thick black traces are uninjected control eyes and green traces are injected study eyes. Evaluable TPLRs were missing at M1 for the control eye. A representative normal response (dashed line) shown for comparison. Stimulus marker and scale bar are shown. B, C, Constriction amplitude and latency changes from mean baseline for control (black symbols) and study (green symbols) eyes. Gray dashed line represents no change from baseline.
Discussion
The pathophysiology of CEP290-LCA is defined by lack of light sensitivity in central cone photoreceptors that can survive structurally for decades.9,20 For interventions aiming to treat CEP290-LCA, a key efficacy outcome involves measurement of light sensitivity. Previous results with sepofarsen demonstrated improvements in light sensitivity that could also be associated with clinically meaningful and statistically significant gains in VA in some participants, but not in others.16,17,19 Among the special cohort of 5 participants with LP vision treated with sepofarsen, 1 participant (patient P2) was notable for improving acuity from LP to 20/400, whereas 4 other participants (patients P1, P6, P9, and P10) showed apparent light sensitivity improvements by FST without any changes in VA.16,17,19 Why these 4 participants differed from participant P2 was not understood. The current work was an in-depth analysis into the data, with standard as well as novel methods, together with examination of the neurodevelopmental history of each participant, to better understand visual consequences of sepofarsen injected into eyes with LP vision. Duration of analysis was limited to the 3 months after the first injection to retain as much experimental homogeneity as possible. After 3 months, the trial continued for 9 more months,17,19 but different participants received variable numbers of additional injections, thus making comparisons more difficult. Also, the 3-month time point seemed to correspond to the approximate peak of sepofarsen pharmacodynamics based on 1 eye that received a single injection and was followed up for 15 months.17
Light perception vision is agreed to represent one of the most severe forms of blindness because it corresponds to the ability to distinguish between light and dark without being able to distinguish spatial pattern or, in many cases, directionality of light. Despite a long history of being a clinical descriptor, the LP designation is rarely defined in detail. For example, what is the amount of light required? Does the color of light make a difference? What are the sources of photoreceptors providing LP vision? Full-field stimulus testing is a useful quantitative technique we have developed that can provide answers to such questions.28, 29, 30, 31 In the case of the current cohort of participants with LP vision, chromatic FST results suggested remnant cone photoreceptors mediating the light sensitivity, which would be consistent with the primary pathophysiologic features of CEP290-LCA, in which rod photoreceptors tend to degenerate completely within the first decade of life and dysfunctional central cones survive.9,20 In general, FST sensitivity for 5 participants with LP vision was lower (worse) than 6 patients with retained spatial vision. However, the patients with LP vision showed a wide spectrum of light sensitivity ranging over 3 log units, and some overlap occurred between groups. For example, patient P6 with LP vision showed equal or better light sensitivity than patients P3 with spatial vision of 2.5 logarithm of the minimum angle of resolution units detectable with the Berkeley rudimentary vision test method. It could be hypothesized that patient P2’s acuity gain was driven by relatively better light sensitivity, but this hypothesis was not supported because the baseline FST results for patient P2 were not the best (or the worst) among participants with LP vision.
The incongruence between FST and acuity improvements among LP participants could have been the result of the variability of performing a perceptual test in participants with very little vision or driven by motivational differences that are especially prevalent in open-label trials where the participant knows which eye has been injected. Therefore, we examined objective TPLR results in participants with LP vision, as we had done previously in a different participant receiving sepofarsen treatment.17 Participants with LP vision showed improvements in pupillary constriction parameters in treated eyes (Figs 2 and 5; Supplemental Fig 1), thus providing objective support to the subjective FST results. Importantly, TPLR provides evidence of the function of brainstem visual pathways, but does not provide evidence of cortical activation. Future studies could be planned to measure cortical activation with specific functional magnetic resonance imaging stimuli designed for patients with severe vision loss.10, 11, 12, 13,34
Among other choices to explain differences in outcome among participants with LP vision was consideration of potential differences in available cortical plasticity. All 5 participants retained the capacity to perceive improved level of light sensitivity, but only patient P2 retained the plasticity to gain spatial vision. Normally, participants treated within the first decade of life are thought to have the largest plasticity, but counterintuitively, patient P2 was substantially older at the time of the treatment than the 4 remaining participants with LP vision, although none were treated within the first decade of life. On careful evaluation of early visual neurodevelopmental history, it became clear that patient P2 had a history of spatial vision in early life that was progressively lost over several decades, whereas the remaining participants with LP vision never showed evidence of spatial vision. Assuming the results from the single patient P2 can be extrapolated to others, our data lead to the parsimonious hypothesis that a subset of (older) adult participants with LP vision with CEP290-LCA and a developmental history of having spatial vision may gain the most spatial vision with sepofarsen. Additionally, adult participants with LP vision with CEP290-LCA and no developmental history of having spatial vision could potentially benefit from sepofarsen via retinal sensitivity improvement, which could translate into improvement in quality of life and potentially long-term improvement in spatial vision. This speculation will require evaluation of data well beyond 1 year.
If sepofarsen were to be approved for use in individuals with CEP290-LCA in the future, physicians may have to provide guidance for the expectations of a large subset of adult patients with CEP290-LCA and LP vision regarding the potential for dramatic gain of spatial vision versus gain of light sensitivity without spatial vision. Early developmental history of vision could help in this regard. In addition, evaluation of oculomotor instability may also assist in prognosis. Congenital complete binocular vision loss tends to lead to wandering or roving eye movements, which show large amplitude involuntary deviations from primary gaze.21 Individuals who completely lose their vision in later life tend to be able to control eyes closer to primary gaze.
Hypotheses reported in this article are based on efficacy data from a limited number of participants older than 15 years with LP vision treated with sepofarsen in a phase 1b/2 trial. Data from an ongoing study designed specifically for participants younger than 8 years (ClinicalTrials.gov identifier, NCT04855045; European Union Drug Regulating Authorities Clinical Trials Database identifier, 2020-000535-45) will potentially answer questions regarding differences in spatial vision gains as a function of age at treatment.
Manuscript no. XOPS-D-22-00003.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): S.R.R.: Financial support – Spark Therapeutics, Inc.; Lecturer – Novartis; Equity owner – Digital Diagnostics, Inc.
A.V.D.: Scientific Advisory Board – ProQR Therapeutics; Patent – Novartis Visual Functioning Questionnaire
B.P.L.: Consultant, Financial support, Data Safety Monitoring Board or Advisory Board – ProQR Therapeutics
M.R.S.: Employee and Equity owner – ProQR Therapeutics
A.G.: Employee and Equity owner – ProQR Therapeutics
Supported by ProQR Therapeutics, Leiden, The Netherlands.
HUMAN SUBJECTS: Human subjects were included in this study. This study adhered to the Declaration of Helsinki and was approved by the institutional review boards of the University of Pennsylvania, University of Iowa and Ghent University. Adult participants provided informed written consent, whereas children provided informed written assent, with the parents providing informed written consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Cideciyan, Jacobson, Ho, Roman, Russell, Drack, Leroy, Schwartz, Girach
Analysis and interpretation: Cideciyan, Jacobson, Ho, Krishnan, Roman, Garafalo, Wu, Swider, Sumaroka, Van Cauwenbergh, Russell, Drack, Leroy
Data collection: Cideciyan, Jacobson, Ho, Krishnan, Roman, Garafalo, Wu, Swider, Sumaroka, Van Cauwenbergh, Russell, Drack, Leroy
Obtained funding: Cideciyan, Jacobson, Ho, Russell, Drack, Leroy
Overall responsibility: Cideciyan, Jacobson, Roman
Supplementary Data
Supplemental Fig 1.
References
- 1.Hubel D., Wiesel T. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160(1):106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiesel T., Hubel D. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- 3.Hubel D., Wiesel T., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 4.Park W., Fine I. New insights into cortical development and plasticity: from molecules to behavior. Curr Opin Physiol. 2020;16:50–60. doi: 10.1016/j.cophys.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fine I., Smallman H., Doyle P., MacLeod D. Visual function before and after the removal of bilateral congenital cataracts in adulthood. Vision Res. 2002;42(2):191–210. doi: 10.1016/s0042-6989(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 6.Birch E. Amblyopia and binocular vision. Prog Retin Eye Res. 2013;33(1):67–84. doi: 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalia A., Lesmes L., Dorr M., et al. Development of pattern vision following early and extended blindness. Proc Natl Acad Sci U S A. 2014;111(5):2035–2039. doi: 10.1073/pnas.1311041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.den Hollander A.I., Roepman R., Koenekoop R.K., Cremers F.P.M. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27(4):391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Cideciyan A.V., Aleman T.S., Jacobson S.G., et al. Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis. Hum Mutat. 2007;28(11):1074–1083. doi: 10.1002/humu.20565. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre G.K., Komáromy A.M., Cideciyan A.V., et al. Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation. PLoS Med. 2007;4(6):1117–1128. doi: 10.1371/journal.pmed.0040230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cideciyan A.V., Aguirre G.K., Jacobson S.G., et al. Pseudo-fovea formation after gene therapy for RPE65-LCA. Invest Ophthalmol Vis Sci. 2015;56(1):526–537. doi: 10.1167/iovs.14-15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguirre G.K., Datta R., Benson N.C., et al. Patterns of individual variation in visual pathway structure and function in the sighted and blind. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0164677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguirre G.K., Butt O., Datta R., et al. Postretinal structure and function in severe congenital photoreceptor blindness caused by mutations in the GUCY2D gene. Invest Ophthalmol Vis Sci. 2017;58(2):959–973. doi: 10.1167/iovs.16-20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cideciyan A.V. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29(5):398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maguire A.M., Russell S.R., Wellman J., et al. Efficacy, safety, and durability of voretigene neparvovec-rzyl in RPE65 mutation-associated inherited retinal dystrophy: results of phase 1 and 3 trials. Ophthalmology. 2019;126(9):1273–1285. doi: 10.1016/j.ophtha.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Cideciyan A.V., Jacobson S.G., Drack A.V., et al. Effect of an intravitreal antisense oligonucleotide on vision in Leber congenital amaurosis due to a photoreceptor cilium defect. Nat Med. 2019;25(2):225–228. doi: 10.1038/s41591-018-0295-0. [DOI] [PubMed] [Google Scholar]
- 17.Cideciyan A.V., Jacobson S.G., Ho A.C., et al. Durable vision improvement after a single treatment with antisense oligonucleotide sepofarsen: a case report. Nat Med. 2021;27(5):785–789. doi: 10.1038/s41591-021-01297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson S.G., Cideciyan A.V., Ho A.C., et al. Safety and improved efficacy signals following gene therapy in childhood blindness caused by GUCY2D mutations. iScience. 2021;24(5):102409. doi: 10.1016/j.isci.2021.102409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell SR, Drack AV, Cideciyan AV, et al. Intravitreal sepofarsen for Leber congenital amaurosis 10. Nat Med. 2022;28(5):1014-1021. [DOI] [PMC free article] [PubMed]
- 20.Cideciyan A.V., Rachel R.A., Aleman T.S., et al. Cone photoreceptors are the main targets for gene therapy of NPHP5 (IQCB1) or NPHP6 (CEP290) blindness: generation of an all-cone Nphp6 hypomorph mouse that mimics the human retinal ciliopathy. Hum Mol Genet. 2011;20(7):1411–1423. doi: 10.1093/hmg/ddr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson S.G., Cideciyan A.V., Sumaroka A., et al. Outcome measures for clinical trials of Leber congenital amaurosis caused by the intronic mutation in the CEP290 gene. Invest Ophthalmol Vis Sci. 2017;58(5):2609–2622. doi: 10.1167/iovs.17-21560. [DOI] [PubMed] [Google Scholar]
- 22.Charng J., Jacobson S.G., Heon E., et al. Pupillary light reflexes in severe photoreceptor blindness isolate the melanopic component of intrinsically photosensitive retinal ganglion cells. Invest Ophthalmol Vis Sci. 2017;58(7):3215–3224. doi: 10.1167/iovs.17-21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumaroka A., Garafalo A.V., Semenov E.P., et al. Treatment potential for macular cone vision in Leber congenital amaurosis due to CEP290 or NPHP5 mutations: predictions from artificial intelligence. Invest Ophthalmol Vis Sci. 2019;60(7):2551–2562. doi: 10.1167/iovs.19-27156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan A.K., Jacobson S.G., Roman A.J., et al. Transient pupillary light reflex in CEP290- or NPHP5-associated Leber congenital amaurosis: latency as a potential outcome measure of cone function. Vision Res. 2020;168:53–63. doi: 10.1016/j.visres.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walia S., Fishman G.A., Jacobson S.G., et al. Visual acuity in patients with Leber’s congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology. 2010;117(6):1190–1198. doi: 10.1016/j.ophtha.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 26.Dulla K., Aguila M., Lane A., et al. Splice-modulating oligonucleotide QR-110 restores CEP290 mRNA and function in human c.2991+1655A>G LCA10 models. Mol Ther Nucleic Acids. 2018;12:730–740. doi: 10.1016/j.omtn.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey I.L., Jackson A.J., Minto H., et al. The Berkeley rudimentary vision test. Optom Vis Sci. 2012;89(9):1257–1264. doi: 10.1097/OPX.0b013e318264e85a. [DOI] [PubMed] [Google Scholar]
- 28.Roman A.J., Schwartz S.B., Aleman T.S., et al. Quantifying rod photoreceptor-mediated vision in retinal degenerations: dark-adapted thresholds as outcome measures. Exp Eye Res. 2005;80(2):259–272. doi: 10.1016/j.exer.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Roman A.J., Cideciyan A.V., Aleman T.S., Jacobson S.G. Full-field stimulus testing (FST) to quantify visual perception in severely blind candidates for treatment trials. Physiol Meas. 2007;28(8):N51–N56. doi: 10.1088/0967-3334/28/8/N02. [DOI] [PubMed] [Google Scholar]
- 30.Roman A.J., Cideciyan A.V., Wu V., et al. Full-field stimulus testing: role in the clinic and as an outcome measure in clinical trials of severe childhood retinal disease. Prog Retin Eye Res. 2022;87:101000. doi: 10.1016/j.preteyeres.2021.101000. [DOI] [PubMed] [Google Scholar]
- 31.Cideciyan A.V., Krishnan A.K., Roman A.J., et al. Measures of function and structure to determine phenotypic features, natural history, and treatment outcomes in inherited retinal diseases. Annu Rev Vis Sci. 2021;7:747–772. doi: 10.1146/annurev-vision-032321-091738. [DOI] [PubMed] [Google Scholar]
- 32.Klein M., Birch D.G. Psychophysical assessment of low visual function in patients with retinal degenerative diseases (RDDs) with the Diagnosys full-field stimulus threshold (D-FST) Doc Ophthalmol. 2009;119(3):217–224. doi: 10.1007/s10633-009-9204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collison F.T., Fishman G.A., McAnany J.J., et al. Psychophysical measurement of rod and cone thresholds in Stargardt disease with full-field stimuli. Retina. 2014;34(9):1888–1895. doi: 10.1097/IAE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castaldi E., Cicchini G.M., Falsini B., et al. Residual visual responses in patients with retinitis pigmentosa revealed by functional magnetic resonance imaging. Transl Vis Sci Technol. 2019;8(6):44. doi: 10.1167/tvst.8.6.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.