Abstract
Purpose
To evaluate whether cataract surgery is associated with decreased risks of central retinal vein occlusion (CRVO) or branch retinal vein occlusion (BRVO) development using the American Academy of Ophthalmology Intelligent Research in Sight (IRIS®) Registry.
Design
Retrospective database study of the IRIS Registry data.
Participants
Patients in the IRIS Registry who underwent cataract surgery and 1:1 matched control participants from the IRIS Registry using a decision tree classifier as a propensity model.
Methods
Control and treatment groups initially were selected using Current Procedural Terminology codes for uncomplicated cataract surgery and other straightforward criteria. To accomplish treatment–control matching, a decision tree classifier was trained to classify patients as treatment versus control based on a set of chosen predictors for treatment, where best-corrected visual acuity and age were the most important predictors. Treatment and control participants subsequently were matched using the classifier, the visit dates, and the identifications of the practice. Cox regression was performed on the matched groups to measure the hazard ratio (HR) of retinal vein occlusion development adjusted for age, sex, race, primary insurance type, and previous diagnosis of diabetic retinopathy (DR), glaucoma, and narrow angles.
Main Outcome Measure
The HR of retinal vein occlusion developing in patients who underwent cataract surgery compared with matched control participants.
Results
The HRs for CRVO and BRVO developing in patients who underwent cataract surgery compared with matched control participants who did not during the first year after either cataract surgery or baseline visit were 1.26 [95% confidence interval [CI], 1.16–1.38; P < 0.001] and 1.27 [95% CI, 1.19–1.36; P < 0.001], respectively, after controlling for age, sex, race, insurance, and history of DR, glaucoma, and narrow angles. Diabetic retinopathy was the strongest predictor associated with CRVO (2.79 [95% CI, 2.43–3.20; P < 0.001]) and BRVO (2.35 [95% CI, 2.09–2.64; P < 0.001]) development after cataract surgery.
Conclusions
Cataract surgery is associated with a small increase in risk of retinal vein occlusions within the first year; however, the incidence is low and likely not clinically significant.
Keywords: Branch retinal vein occlusion, Cataract surgery, Central retinal vein occlusion
Abbreviations and Acronyms: BRVO, branch retinal vein occlusion; CI, confidence interval; CRVO, central retinal vein occlusion; DR, diabetic retinopathy; HR, hazard ratio; ICD, International Classification of Diseases; IOP, intraocular pressure; IRIS, Intelligent Research in Sight; RVO, retinal vein occlusion
Retinal vein occlusion (RVO), which includes central RVO (CRVO) and branch RVO (BRVO), is the second most common vision-threatening retinal vascular disorder. Pooled data from population studies report a prevalence rate of 3.77 per 1000 adults for BRVO and 0.65 per 1000 adults for CRVO.1 Known risk factors of CRVO and BRVO include increasing age, systemic hypertension, smoking, diabetes, glaucoma, and intraocular pressure (IOP), although with varying consistency.2,3
Most of known RVO risk factors are systemic and outside the scope of ophthalmology. However, glaucoma and IOP are potential ophthalmic risk factors that can be targeted easily by ophthalmologists. Cataract surgery is the most commonly performed ophthalmic surgery in the United States, and data from Medicare Part B suggest that more than 3 million procedures are performed each year.4,5 It has been shown to reduce IOP and the number of required ocular hypotensive medications for glaucoma patients.6, 7, 8, 9, 10, 11 Furthermore, reports of RVO during the postoperative period after cataract surgery are rare.12, 13, 14, 15 However, the effects of cataract extraction on glaucoma and IOP and their influence on the incidence of RVO are not currently known.
The American Academy of Ophthalmology Intelligent Registry in Sight (IRIS) Registry is an enormous clinical dataset that includes 60 million unique patients and is well positioned to answer clinical questions at a population level.16 It has been used to report epidemiologic features, to identify subtle biomarkers, to analyze national practice patterns, and to compare results in a clinical setting with those of randomized controlled trials.17, 18, 19, 20, 21 The registry provides a unique opportunity to investigate retrospectively the effects of an intervention on the development of an uncommon disease compared with a carefully selected and matched control group, similar to how a randomized clinical trial would be designed. This type of research methodology and analysis was not possible previously with epidemiologic studies in RVO.3,22,23 In this study, we use the IRIS Registry to study the effects of cataract extraction on RVO compared with machine learning–matched control participants.
Methods
This study was conducted in accordance with the tenets Declaration of Helsinki. Given the use of de-identified patient data, the review was deemed exempted by the University of Washington Institutional Review Board. The methods of data collection and aggregation of the IRIS Registry database have been described previously.17,24 Version 2020_07_28 of the IRIS Registry, which was last modified on October 23, 2020, was used for this study.
Study Patient and Control Participant Selection Using Machine Learning Approaches
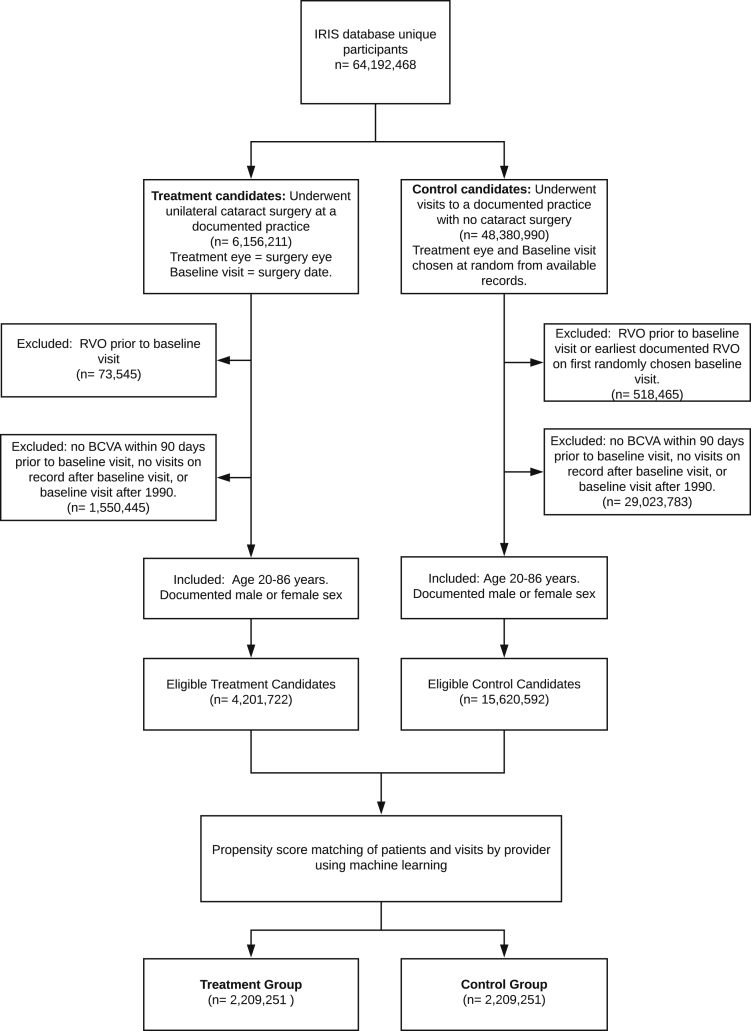
Patients in the IRIS Registry with a history of uncomplicated cataract surgery, defined by Current Procedural Terminology code 66984, were considered as candidates for the treatment group. All other patients were considered as candidates for the control group. For the treatment group, the patient’s first surgery eye and surgery date were selected as the baseline visit. For control participants, the observed eye and baseline visit were chosen at random from the available records. Any patients with a history of previous RVO before the baseline visit or cataract surgery were excluded from either group. Initial pools for the treatment and control groups were selected based on the age of 20 to 86 years, documented best-corrected visual acuity within 90 days before baseline visit, and available follow-up data after the baseline visit (Fig 1).
Figure 1.
Flowchart showing the selection process of treatment (cataract surgery) and control groups from the Intelligent Research in Sight (IRIS) Registry database. After data preparation and cleaning, approximately half of the eligible treatment candidates were matched with control participants using the propensity matching algorithm. These patients are used in the survival analysis for central retinal vein occlusion (RVO) and branch RVO outcomes. BCVA = best-corrected visual acuity.
After the patients were grouped into treatment versus control groups, we performed 1:1 matching using machine learning models as propensity models. Whereas many different models can be used to assign propensity (most commonly logistic regression), machine learning models such as tree-based classifiers are nonlinear, interoperable, and have been used for propensity models before.25,26 The following variables were provided as potential features for matching in the models: best-corrected visual acuity, age, sex, race (White, Black, Asian, other, or missing), prior history of smoking, prior surgery in the study eye, and previous diagnosis of diabetic retinopathy (DR) or uveitis in the surgical eye. A total of 4 models were evaluated to select the optimal matching model: a single decision tree, a random forest, extra trees, and gradient boosting, where all except the single decision tree are ensemble methods. The various hyperparameters of the 4 models were tuned using a 5-fold cross-validation grid search on a balanced sample of 140 000 control participants and surgery patients using the Cohen κ metric as a performance measure. After testing the performance of each model in a subset of samples, we chose the single decision tree for final matching. Further details of the model selection and matching results are shown in the Supplemental Appendix. All treatment patients and control participants were matched further on the provider and the visit window of 90 days.
Outcome Variables and Covariates
Diagnoses of CRVO and BRVO were based on International Classification of Diseases (ICD), Ninth and Tenth Revisions, Clinical Modification, codes. The following demographic and clinical variables were extracted: age at surgery, sex, race, insurance, history of comorbid ophthalmic disease diagnoses such as DR, glaucoma, narrow angles occurring before surgery based on ICD, Ninth and Tenth Revisions, Clinical Modification, codes (Supplemental Table 1), and laterality of first surgery.
If patients had more than 1 form of insurance, a hierarchical heuristic was used to prioritize insurers in the following order: commercial, Medicare or Medicare Advantage, Medicaid, or other, following methods used in previously published articles.17 Given that date of birth is redacted in the IRIS Registry for patients 87 years of age or older at the time of data release, patients older than 86 years were excluded from the patient and control group selection and subsequent analyses. Laterality was matched using the laterality specified in the eye fields of all of the tables used in the IRIS database. Retinal vein occlusions recorded without laterality were excluded from analysis.
Statistical Analysis
The primary outcome of interest was development of CRVO or BRVO within 1 year of cataract surgery for the treatment group and after 1 year of the propensity score–matched baseline visit for the control participants. The hazard ratio (HR) of CRVO or BRVO developing was evaluated by performing a time-to-event survival analysis of the treatment–control pairs using a multivariate Cox regression. Covariates in the regression model included treatment status (cataract surgery vs. control), age (by decade), sex, race (White, Black, other, or not available), primary insurance type (commercial, Medicare, Medicaid, other, or not available), and previous diagnoses of DR, glaucoma, and narrow angles. All analyses were performed with R software (R Foundation for Statistical Computing, Vienna, Austria), Python (Python Software Foundation, http://python.org), and lifelines (version 0.25.7; https://lifelines.readthedocs.io/en/latest/#).
Results
General demographic descriptions and baseline clinical factors of both patients undergoing cataract surgery and matched control participants are shown in Table 1. A total of approximately 4.0 million patients were included in the propensity-matched pairs of patients and control participants. Among matched pairs, no significant difference in age, sex, or best-corrected visual acuity were found between the cataract surgery and control group (Supplemental Fig 4). Compared with control participants, the cataract surgery group showed a slightly higher proportion of narrow angle diagnoses (3.1% vs. 2.0%) and smoking history (36.0% vs. 34.3%). A higher percentage of control participants showed a history of prior eye surgery (10.4% vs. 7.7%), glaucoma (8.6% vs. 7.3%), and uveitis (1.1% vs. 0.7%). Intraocular pressure data were available for approximately 1.9 million patients. Average ± standard error baseline IOP (up to 90 days before) was 15.854 ± 0.005 mmHg and 15.736 ± 0.003 mmHg for the control group and surgery group, respectively. The control and cataract surgery group showed an average ± standard error IOP reduction of –0.250 ± 0.005 mmHg and –1.202 ± 0.003 mmHg, respectively (30–365 days after surgery or baseline visit).
Table 1.
Distribution of Covariates in the Eligible and Selected Study Populations
| Variable | Unmatched |
Matched |
|||
|---|---|---|---|---|---|
| Total (n = 19 822 314) | Cataract Surgery Group (n = 4 201 722) | Control Group (n = 15 620 592) | Cataract Surgery Group (n = 2 209 251) | Control Group (n = 2 209 251) | |
| Age (yrs) | 60.24 ± 15.34 | 69.39 ± 8.37 | 57.78 ± 15.85 | 69.16 ± 8.61 | 69.15 ± 8.77 |
| BCVA (logMAR) | 0.21 ± 0.4 | 0.39 ± 0.39 | 0.16 ± 0.38 | 0.33 ± 0.37 | 0.33 ± 0.39 |
| Insurance | |||||
| Commercial | 13 030 198 (65.73) | 2 627 213 (62.53) | 10 402 298 (66.6) | 1 411 023 (63.87) | 1 347 286 (60.98) |
| Medicare | 3 854 864 (19.45) | 1 232 697 (29.34) | 2 622 166 (16.79) | 618 054 (27.98) | 637 038 (28.84) |
| Other/NA | 2 492 607 (12.57) | 286 690 (6.82) | 2 205 917 (14.12) | 150 732 (6.82) | 188 641 (8.54) |
| Medicaid | 444 646 (2.24) | 55 122 (1.31) | 389 524 (2.49) | 29 442 (1.33) | 36 286 (1.64) |
| Prior history | |||||
| Smoking | 5 911 250 (29.82) | 1 548 844 (36.86) | 4 362 406 (27.93) | 795 833 (36.02) | 758 763 (34.34) |
| Eye surgery | 1 305 379 (6.59) | 322 064 (7.67) | 983 315 (6.29) | 169 721 (7.68) | 228 783 (10.36) |
| DR | 703 974 (3.55) | 178 952 (4.26) | 525 022 (3.36) | 87 597 (3.97) | 83 454 (3.78) |
| Glaucoma | 1 048 351 (5.29) | 290 292 (6.91) | 758 059 (4.85) | 161 522 (7.31) | 188 640 (8.58) |
| Uveitis | 178 273 (0.9) | 29 113 (0.69) | 149 160 (0.95) | 15 679 (0.71) | 24 962 (1.13) |
| Narrow angles | 330 875 (1.67) | 116 553 (2.77) | 214 322 (1.37) | 69 063 (3.13) | 43 497 (1.97) |
| Race | |||||
| White | 13 869 167 (69.97) | 3 200 039 (76.16) | 10 669 128 (68.3) | 1 702 53 (77.06) | 1 654 974 (74.91) |
| Black | 1 481 106 (7.47) | 260 245 (6.19) | 1 220 861 (7.82) | 135 301 (6.12) | 154 039 (6.97) |
| Asian | 583 333 (2.94) | 92 783 (2.21) | 490 550 (3.14) | 46 118 (2.09) | 50 577 (2.29) |
| Other | 186 609 (0.94) | 39 245 (0.93) | 147 364 (0.94) | 20 289 (0.92) | 21 117 (0.96) |
| Missing | 3 702 099 (18.68) | 609 410 (14.5) | 3 092 689 (19.8) | 305 040 (13.81) | 328 544 (14.87) |
| Sex | |||||
| Female | 11 559 311 (58.31) | 2 494 991 (59.38) | 9 064 320 (58.03) | 1 317 399 (59.63) | 1 284 195 (58.17) |
| Male | 8 263 003 (41.69) | 1 706 731 (40.62) | 6 556 272 (41.97) | 891 852 (40.37) | 924 056 (41.83) |
BCVA = best-corrected visual acuity; DR = diabetic retinopathy; logMAR = logarithm of the minimum angle of resolution; NA = not applicable.
Data are presented as mean ± standard deviation or no. (%).
Incidence and Risks of Central Retinal Vein Occlusion and Branch Retinal Vein Occlusion
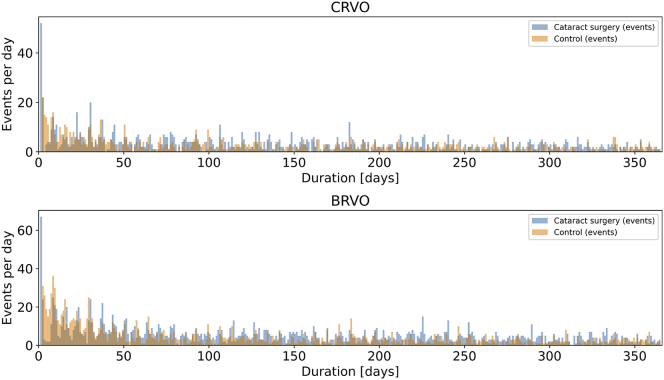
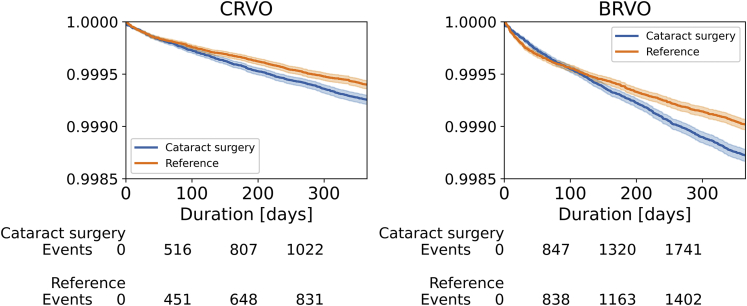
The total numbers of CRVO and BRVO events during the 1-year follow-up were 2062 and 3488, respectively, which accounted for approximately 0.1–0.2% of either group. One thousand one hundred forty-one CRVO events occurred in the cataract surgery group compared with 921 events in the control group. One thousand nine hundred forty-one BRVO events occurred in the cataract surgery group compared with 1547 in the control group. Approximately 4% of all first-year events in the treatment group (52/1141 CRVO and 67/1941 BRVO) occurred on the day of surgery. Subsequently, the number of events per day stayed relatively constant between the cataract surgery and control groups (Fig 2). The unadjusted Kaplan-Meier curves for development of CRVO or BRVO between the cataract surgery and control groups are shown in Figure 3.
Figure 2.
Bar graphs showing central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO) event rates for the cataract surgery group (blue) and matched control group (orange). The x-axis shows the follow-up duration in days after cataract surgery or baseline visit (day 1).
Figure 3.
Unadjusted Kaplan-Meier curves for central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO) stratified by cataract surgery patients (blue) and matched control participants (orange). The y-axis shows the proportion of patients without the event, and the x-axis shows follow-up duration in days after cataract surgery or baseline visit (day 1). The survival rate after 1 year is more than 99.8% in both groups. Number of events represents the number of CRVO and BRVO events at 100 days, 200 days, and 300 days. The total number of CRVO and BRVO events during the 1-year follow-up are 2062 and 3488, respectively.
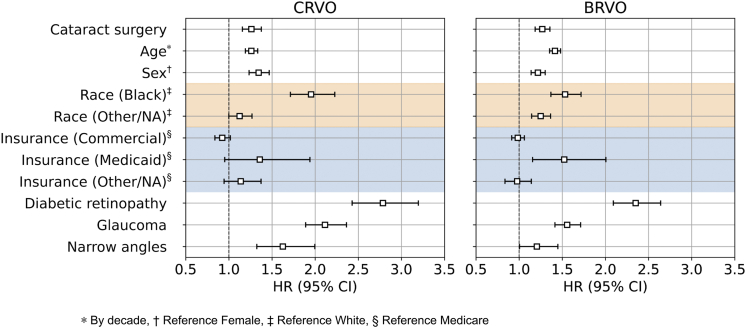
The unadjusted HR of CRVO and BRVO developing for patients who underwent cataract surgery compared with those who did not was 1.22 (95% CI, 1.12–1.33; P < 0.001 and 1.24 (95% CI, 1.16–1.32; P < 0.001), respectively. After controlling for age, sex, race, insurance, and history of DR, glaucoma, and narrow angles, the adjusted HR for CRVO and BRVO was 1.26 (95% CI, 1.16–1.38; P < 0.001) and 1.27 (95% CI, 1.19–1.36; P < 0.001), respectively (Fig 4). For the population of matched patients, the strongest predictor of CRVO or BRVO in our adjusted models was prior history of DR (HR for CRVO, 2.79 [95% CI, 2.43–3.20; P < 0.001], HR for BRVO, 2.35 [95% CI, 2.09–2.64; P < 0.001]), followed by glaucoma (CRVO, 2.11 [95% CI, 1.89–2.36; P < 0.001]; BRVO, 1.56 [95% CI, 1.42–1.71; P < 0.001]), and then Black race (CRVO, 1.95 [95% CI, 1.71–2.23; P < 0.001]; BRVO, 1.53 [95% CI, 1.37–1.72; P < 0.001]). Central retinal vein occlusion and BRVO were more common in those with older age, in men, and in patients with a history of DR, glaucoma, and narrow angles (Fig 4). Despite the increased risk of CRVO and BRVO seen for cataract surgery patients compared with control participants, the absolute risk for CRVO and BRVO remained very low. For CRVO, 5.2 cases occurred per 10 000 cataract surgery patients compared with 4.2 cases per 10 000 control patients. For BRVO, 8.8 cases occurred per 10 000 cataract surgery patients compared with 7.0 cases per 10 000 control patients.
Figure 4.
Forest plot showing hazard ratios (HRs) of central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO) developing from Cox proportional hazards regression over 1 year of follow-up involving Intelligent Research in Sight Registry database patients matched by propensity of receiving treatment (cataract surgery). Age, sex, race, insurance, and history of diabetic retinopathy, glaucoma, and narrow angles before the baseline visit are the included covariates. The adjusted HR for CRVO and BRVO developing in patients who underwent cataract surgery compared with those who did not are 1.26 (95% confidence interval [CI], 1.16–1.38) and 1.27 (95% CI, 1.19–1.36), respectively. Shading denotes the multilevel race and insurance variables. NA = not applicable.
Discussion
Based on the review of approximately 4 million pairs of patients who underwent cataract surgery versus matched control participants in the IRIS Registry, we found that the incidence of CRVO and BRVO during the first year after cataract surgery is low, ranging from 5 to 9 cases per 10 000. Compared with the matched control participants, we found no clinically significant difference in the adjusted hazard rate of CRVO and BRVO developing in patients who underwent cataract surgery after controlling for age, sex, insurance, history of DR, uveitis, glaucoma, and narrow angles. Cataract extraction and its effects on lowering IOP do not seem to be protective against RVO.
Prior studies have found associations between glaucoma and RVO.27,28 Multiple mechanisms have been proposed, including high IOP leading to decreased perfusion, compression of vein by the artery either in the lamina cribrosa or retrolaminar space for CRVOs or at the site of arteriovenous crossing in BRVOs, and degenerative changes of the venous endothelium and intima media.29, 30, 31, 32 In our study, glaucoma was a risk factor for the development of RVOs after cataract surgery, with a higher adjusted HR for CRVO compared with BRVO. Although cataract surgery lowered IOP in line with prior reports, the surgery itself did not seem to protect against the development of RVO in our study.6, 7, 8, 9, 10, 11 Intraocular pressure was not recorded consistently or at the same intervals; thus, IOP analyses were performed only on approximately half of the patients.
Limited literature exists regarding potential associations between cataract surgery and RVOs. The low absolute risk of CRVO or BRVO developing after cataract surgery seen in this study reflects the few number of cases reported in the literature.12, 13, 14, 15 Interestingly, 4% of RVOs occurred on the day of surgery in our cohort. Retinal vein occlusions that were reported on the first postoperative visit could have occurred before surgery because examinations are not usually conducted at the time of surgery. However, several perioperative factors may put patients at risk of RVOs transiently. For example, IOP during surgery can fluctuate, and spikes can occur after surgery.33,34 Additionally, cataract surgery leads to an intraocular inflammatory response, which may increase the risk of RVOs.35 Patients undergoing cataract surgery, who tend to be older and at a higher risk of cardiovascular comorbidities, commonly are instructed to hold systemic medications on the day of surgery.36,37 The higher rate of perioperative RVOs may point to a potential area where routine perioperative management may be modified to decrease the risks of RVO, particularly in patients at higher risk.
Unexpectedly, DR was associated with the greatest increase in risk of both CRVO and BRVO after cataract surgery. However, it is important to note that the distribution of DR in the analysis population may be different from that of the underlying IRIS database. The presence of end-organ damage from diabetes mellitus previously was linked to an increased risk of CRVO; however, the association of diabetes with RVOs has varied in prior studies in the literature.38 A population-based study from China did not find an association between diabetes and RVO.39 In the age-adjusted analysis of the Beaver Dam Eye Study, diabetes was a risk factor for CRVO, but not BRVO.40 A similar trend of finding associations between DR and CRVO, but not with BRVO, were seen in meta-analyses.41,42 In addition to the differences in the distribution of DR, another possible explanation for the discrepancy between our study results and previously published results is that we relied on DR codes rather than systemic diabetes mellitus codes. Thus, we would have considered only cases of diabetes severe enough to be accompanied by ocular manifestations, unlike previous studies. Furthermore, it is possible that the presence of intraocular diabetic vascular pathologic features may increase the risk of RVO development even more in the setting of cataract extraction–related perioperative risk factors.
Matching was used to derive a control group with a similar baseline risk profile as the intervention group. However, age, race, and history of DR were still found to have associations with the risk of RVO development after cataract surgery. This may stem from interactions with other confounders that were not measured in the IRIS Registry. For example, hypertension is a known risk factor for RVOs but is captured incompletely in the IRIS Registry.22 Other factors that previously were associated with RVO, but were not included, are shorter axial length, reduced ocular perfusion pressure, elevated systemic blood pressure, focal arteriovenous nicking, and lower education level.43, 44, 45, 46 Although we included as many risk factors as possible for RVO that were available within the current IRIS Registry dataset, omission of unavailable ones may affect the adjusted HR calculated in our study. Future versions of the IRIS Registry that include additional data will be beneficial.
Race and insurance status showed small associations with RVOs after cataract surgery in our study. Prior studies of RVO have reported increased risk of CRVO associated with Black race in a United States–based population study compared with White race (HR, 1.58; 95% CI, 1.25–1.99).38 In contrast, a pooled population study analysis found a higher prevalence of BRVO in Hispanic participants (5.98 per 1000; 95% CI, 4.81–7.15) compared with other ethnic groups.47 However, no difference was found in the prevalence of CRVO between races in this study.47 We found a substantial amount of missing data regarding race and insurance status; thus, the associations that we found may be an underestimate or overestimate of risks. However, our findings are consistent with previous literature and suggest that health care disparities may exist between different groups in our study population.
Strengths of this retrospective study include the use of a large national dataset collected retrospectively with extensive matching by machine learning approaches to overcome biases between the control and treatment groups. However, limitations exist. Health records are subject to coding error or missing data. Although RVO events were analyzed as having occurred at the visit they were first recorded, events could have occurred at any time up to the prior visit or before if the diagnosis was not recorded properly. Although the data are from a national dataset, certain groups still can be overrepresented or underrepresented. Additionally, our analysis included as many covariates as possible that may affect RVO outcomes; however, the effects of the residual confounding, unmeasured confounders, or both in the IRIS Registry cannot be ruled out. Finally, the risk factor findings are associations and do not necessarily imply causation. Further validation studies are warranted.
In conclusion, cataract surgery was not associated with a clinically meaningful change in the risk of RVO development. The risk factors for development of RVO after cataract surgery may differ from baseline population study risk factors.
Manuscript no. D-21-00053.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): A.Y.L.: Financial support – Santen, Carl Zeiss Meditec, Novartis, Genentech, Topcon, Verana Health, Gyroscope; Nonfinancial support – Microsoft.
Supported by the National Institutes of Health, Bethesda, Maryland (grant nos.: K23EY029246 and R01AG060942); Research to Prevent Blindness, Inc., New York, New York (unrestricted grant) and Latham Vision Innovation Award (unrestricted). The sponsors or funding organizations had no role in the design or conduct of this research.
Aaron Lee and Cecilia Lee, associate editor and editor of this journal, respectively, were recused from the peer-review process of this article and had no access to information regarding its peer review.
IRIS Registry Analytic Centers Study Group members not included in the author line: Flora Lum, MD (American Academy of Ophthalmology, San Francisco, CA), Emily Chew, MD (National Eye Institute, Bethesda, MD), Suzann Pershing, MD (Stanford University, Palo Alto, CA), Julia A. Haller, MD (Wills Eye Hospital, Philadelphia, PA), Leslie Hyman, PhD (The Vision Research Center, Wills Eye Hospital, Philadelphia, PA), Alice C. Lorch, MD, MPH (Department of Ophthalmology, Mass Eye and Ear, Harvard Medical School, Boston, MA), and Joan W. Miller, MD (Department of Ophthalmology, Mass Eye and Ear, Harvard Medical School, Boston, MA).
HUMAN SUBJECTS: Human subjects were not included in this study. Given the use of de-identified patient data, the review was deemed exempted by the human ethics committees at the University of Washington. All research adhered to the tenets of the Declaration of Helsinki.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Bagdasarova, A.Y.Lee, Maring, Wen, Lacy, C.S.Lee, Chen
Analysis and interpretation: Bagdasarova, A.Y.Lee, Maring, Wen, Lacy, C.S.Lee, Chen
Data collection: Bagdasarova, A.Y.Lee, Maring, Wen, Lacy, C.S.Lee, Chen
Obtained funding: N/A; Study was performed as part of the authors' regular employment. No additional funding was provided.
Overall responsibility: Bagdasarova, A.Y.Lee, Maring, Wen, Lacy, C.S.Lee, Chen
Contributor Information
Andrew Chen, Email: achen20@uw.edu.
IRIS Registry Analytic Centers Study Group:
Flora Lum, Emily Chew, Suzann Pershing, Julia A. Haller, Leslie Hyman, Alice C. Lorch, and Joan W. Miller
Supplementary Data
References
- 1.Rogers S.L., McIntosh R.L., Lim L., et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117:1094–1101.e5. doi: 10.1016/j.ophtha.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P., Smith W., Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243–1247. doi: 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 3.Klein R., Klein B.E., Moss S.E., Meuer S.M. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141. discussion 141–143. [PMC free article] [PubMed] [Google Scholar]
- 4.Patel S, Glasser D, Repka MX, et al. Changes in Medicare reimbursement for commonly performed ophthalmic procedures. Ophthalmology. 2021 Mar 10;S0161-6420(21)00194-9. https://doi.org/10.1016/j.ophtha.2021.02.026. Online ahead of print. [DOI] [PubMed]
- 5.Aggarwal S., Jain P., Jain A. COVID-19 and cataract surgery backlog in Medicare beneficiaries. J Cataract Refract Surg. 2020;46:1530–1533. doi: 10.1097/j.jcrs.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong J.J., Wasiuta T., Kiatos E., et al. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: a systematic review and meta-analysis of 3-year data. J Glaucoma. 2017;26:511–522. doi: 10.1097/IJG.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 7.Musch D.C., Gillespie B.W., Niziol L.M., et al. Cataract extraction in the collaborative initial glaucoma treatment study: incidence, risk factors, and the effect of cataract progression and extraction on clinical and quality-of-life outcomes. Arch Ophthalmol. 2006;124:1694–1700. doi: 10.1001/archopht.124.12.1694. [DOI] [PubMed] [Google Scholar]
- 8.Masis Solano M., Lin S.C. Cataract, phacoemulsification and intraocular pressure: is the anterior segment anatomy the missing piece of the puzzle? Prog Retin Eye Res. 2018;64:77–83. doi: 10.1016/j.preteyeres.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Poley B.J., Lindstrom R.L., Samuelson T.W., Schulze R., Jr. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. 2009;35:1946–1955. doi: 10.1016/j.jcrs.2009.05.061. [DOI] [PubMed] [Google Scholar]
- 10.Chen P.P., Lin S.C., Junk A.K., et al. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:1294–1307. doi: 10.1016/j.ophtha.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Carolan J.A., Liu L., Alexeeff S.E., et al. Intraocular pressure reduction after phacoemulsification: a matched cohort study. Ophthalmol Glaucoma. 2021;4:277–285. doi: 10.1016/j.ogla.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer C, Bruggemann A, Hager A, et al. Vascular occlusions following ocular surgical procedures: a clinical observation of vascular complications after ocular surgery. JOphthalmol. Available at: https://www.hindawi.com/journals/joph/2017/9120892/; 2017 Accessed 06.03.21. [DOI] [PMC free article] [PubMed]
- 13.Polizzi S., Barca F., Caporossi T., et al. Branch retinal vein occlusion following cataract surgery. Clin Exp Optom. 2018;101:135–136. doi: 10.1111/cxo.12525. [DOI] [PubMed] [Google Scholar]
- 14.Ting D., Hegde V. Cataract surgery and retinal vein occlusion: is there an association? Acta Ophthalmol. 2013;21(s252) [Google Scholar]
- 15.Kurc M., Arora R. Retinal vein occlusion after cataract surgery: risk factors, prognosis and 1 year outcome data. Invest Ophthalmol Vis Sci. 2019;60 3958–3958. [Google Scholar]
- 16.American Academy of Ophthalmology. IRIS Registry data analysis. Available at: https://www.aao.org/iris-registry/data-analysis/requirements; 2017 Accessed 16.03.21.
- 17.Lee C.S., Owen J.P., Yanagihara R.T., et al. Smoking is associated with higher intraocular pressure regardless of glaucoma: a retrospective study of 12.5 million patients using the Intelligent Research in Sight (IRIS®) Registry. Ophthalmol Glaucoma. 2020;3:253–261. doi: 10.1016/j.ogla.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantrell R.A., Lum F., Chia Y., et al. Treatment patterns for diabetic macular edema: an Intelligent Research in Sight (IRIS®) Registry analysis. Ophthalmology. 2020;127:427–429. doi: 10.1016/j.ophtha.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Vanner E.A., Sun C.Q., McSoley M.J., et al. The Tube Versus Trabeculectomy IRIS® Registry Study: cohort selection and follow-up and comparisons to the randomized controlled trial. Am J Ophthalmol. 2020;224:43–52. doi: 10.1016/j.ajo.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Pershing S., Lum F., Hsu S., et al. Endophthalmitis after cataract surgery in the United States: a report from the Intelligent Research in Sight Registry, 2013–2017. Ophthalmology. 2020;127:151–158. doi: 10.1016/j.ophtha.2019.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Repka M.X., Lum F., Burugapalli B. Strabismus, strabismus surgery, and reoperation rate in the United States: analysis from the IRIS Registry. Ophthalmology. 2018;125:1646–1653. doi: 10.1016/j.ophtha.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 22.The Eye Disease Case-Control Study Group Risk factors for central retinal vein occlusion. Arch Ophthalmol. 1996;114:545–554. [PubMed] [Google Scholar]
- 23.The Eye Disease Case-Control Study Group Risk Factors for branch retinal vein occlusion. Am J Ophthalmol. 1993;116:286–296. [PubMed] [Google Scholar]
- 24.Chiang M.F., Sommer A., Rich W.L., et al. The 2016 American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight) database: characteristics and methods. Ophthalmology. 2018;125:1143–1148. doi: 10.1016/j.ophtha.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Lee B.K., Lessler J., Stuart E.A. Improving propensity score weighting using machine learning. Stat Med. 2010;29:337–346. doi: 10.1002/sim.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin X., Li J., Zhang B., Lu P. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652–659. doi: 10.1111/aos.14141. [DOI] [PubMed] [Google Scholar]
- 28.Hayreh S.S., Zimmerman M.B., Beri M., Podhajsky P. Intraocular pressure abnormalities associated with central and hemicentral retinal vein occlusion. Ophthalmology. 2004;111:133–141. doi: 10.1016/j.ophtha.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Frangieh G.T., Green W.R., Barraquer-Somers E., Finkelstein D. Histopathologic study of nine branch retinal vein occlusions. Arch Ophthalmol. 1982;100:1132–1140. doi: 10.1001/archopht.1982.01030040110020. [DOI] [PubMed] [Google Scholar]
- 30.Jefferies P., Clemett R., Day T. An anatomical study of retinal arteriovenous crossings and their role in the pathogenesis of retinal branch vein occlusions. Aust N Z J Ophthalmol. 1993;21:213–217. doi: 10.1111/j.1442-9071.1993.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J., Sastry S.M., Sperduto R.D., et al. Arteriovenous crossing patterns in branch retinal vein occlusion. The Eye Disease Case-Control Study Group. Ophthalmology. 1993;100:423–428. doi: 10.1016/s0161-6420(93)31633-7. [DOI] [PubMed] [Google Scholar]
- 32.Frucht J., Shapiro A., Merin S. Intraocular pressure in retinal vein occlusion. Br J Ophthalmol. 1984;68:26–28. doi: 10.1136/bjo.68.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinbaum A., Blumenthal M., Assia E. Comparison of intraocular pressure profiles during cataract surgery by phacoemulsification and extracapsular cataract extraction. Ophthalmic Surg Lasers Imaging. 2003;34:182–186. [PubMed] [Google Scholar]
- 34.Slabaugh M.A., Bojikian K.D., Moore D.B., Chen P.P. The effect of phacoemulsification on intraocular pressure in medically controlled open-angle glaucoma patients. Am J Ophthalmol. 2014;157:26–31. doi: 10.1016/j.ajo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Juthani V.V., Clearfield E., Chuck R.S. Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery. Cochrane Database Syst Rev. 2017;7:CD010516. doi: 10.1002/14651858.CD010516.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob L., López-Sánchez G.F., Yang L., et al. Associations between cataract and multimorbidity: a cross-sectional study of 23,089 adults from Spain. Eye. 2021;35:791–798. doi: 10.1038/s41433-020-0962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pham T.Q., Wang J.J., Rochtchina E., et al. Systemic and ocular comorbidity of cataract surgical patients in a western Sydney public hospital. Clin Exp Ophthalmol. 2004;32:383–387. doi: 10.1111/j.1442-9071.2004.00842.x. [DOI] [PubMed] [Google Scholar]
- 38.Stem M.S., Talwar N., Comer G.M., Stein J.D. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology. 2013;120:362–370. doi: 10.1016/j.ophtha.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J.Q., Xu L., Wang S., et al. The 10-year incidence and risk factors of retinal vein occlusion: the Beijing Eye Study. Ophthalmology. 2013;120:803–808. doi: 10.1016/j.ophtha.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 40.Klein R., Moss S.E., Meuer S.M., Klein B.E.K. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:513–518. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Wu S., Wen F., Cao Q. Diabetes mellitus as a risk factor for retinal vein occlusion: a meta-analysis. Medicine. 2020;99 doi: 10.1097/MD.0000000000019319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Mahoney P.R.A., Wong D.T., Ray J.G. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692–699. doi: 10.1001/archopht.126.5.692. [DOI] [PubMed] [Google Scholar]
- 43.Cheung N., Klein R., Wang J.J., et al. Traditional and novel cardiovascular risk factors for retinal vein occlusion: the multiethnic study of atherosclerosis. Invest Ophthalmol Vis Sci. 2008;49:4297–4302. doi: 10.1167/iovs.08-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arakawa S., Yasuda M., Nagata M., et al. Nine-year incidence and risk factors for retinal vein occlusion in a general Japanese population: the Hisayama Study. Invest Ophthalmol Vis Sci. 2011;52:5905–5909. doi: 10.1167/iovs.11-7775. [DOI] [PubMed] [Google Scholar]
- 45.Yasuda M., Kiyohara Y., Arakawa S., et al. Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: the Hisayama study. Invest Ophthalmol Vis Sci. 2010;51:3205–3209. doi: 10.1167/iovs.09-4453. [DOI] [PubMed] [Google Scholar]
- 46.Cugati S., Wang J.J., Rochtchina E., Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726–732. doi: 10.1001/archopht.124.5.726. [DOI] [PubMed] [Google Scholar]
- 47.Rogers S., McIntosh R.L., Cheung N., et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–319.e1. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.