Abstract
Background
Prior studies in the civilian population have reported racial disparities in lung cancer outcomes following surgical treatment, including inferior quality of care and worse survival. It is unclear if racial disparities exist in the Veterans Health Administration (VHA), the largest integrated health care system in the United States.
Research Question
Do racial disparities affect early-stage non-small cell lung cancer (NSCLC) outcomes following surgical treatment within the VHA?
Study Design and Methods
This retrospective cohort study was conducted in veterans with clinical stage I NSCLC undergoing surgical treatment in the VHA system. Demographic characteristics, access to care, surgical quality measures, and short- and long-term oncologic outcomes between White and Black veterans were evaluated.
Results
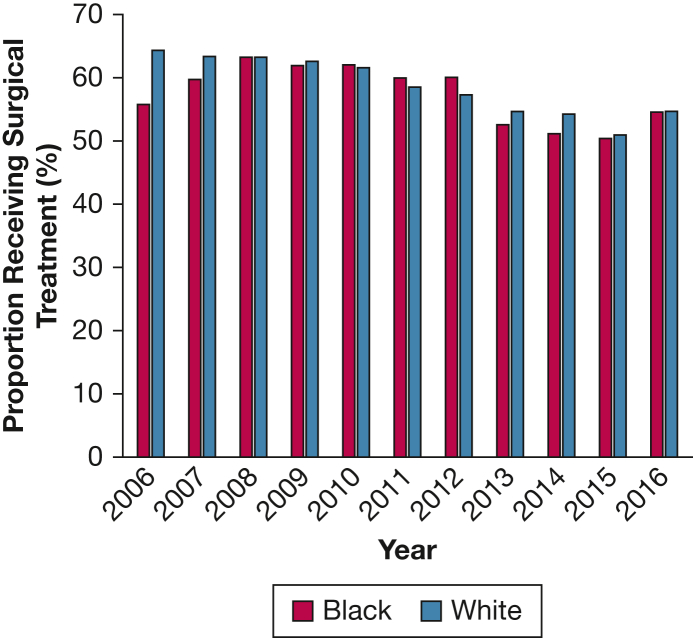
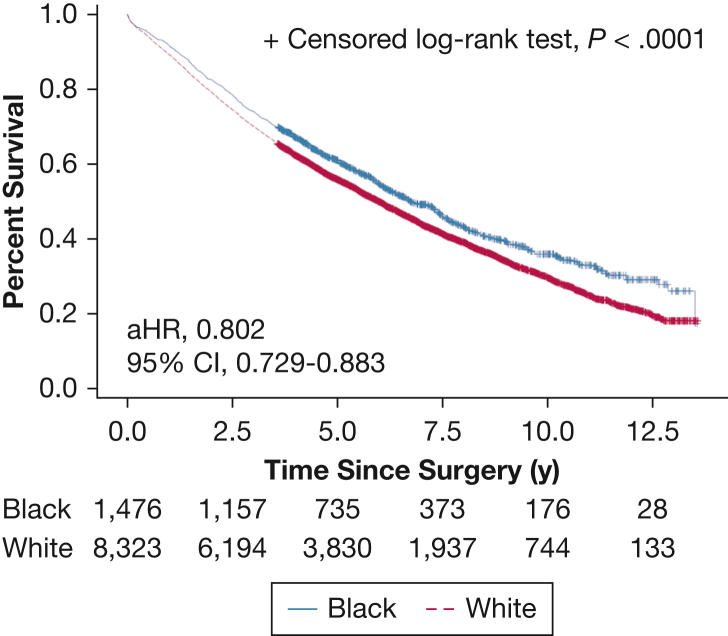
From 2006 to 2016, a total of 18,800 veterans with clinical stage I NSCLC were included. The rates of definitive surgical treatment were similar between Black (57.3%) and White (58.1%) veterans (P = .42). The final study cohort included 9,842 patients receiving surgical treatment, of whom 8,356 (84.9%) were White and 1,486 (15.1%) were Black. Black patients were younger and more likely to smoke, although comorbidities were similar between the two groups. Black patients were somewhat less likely to receive adequate lymph node sampling (30.6% vs 33.3%; P = .050); however, other access-to-care metrics and surgical quality measures, including rates of anatomic lobectomy (71.9% vs 69.4%; P = .189) and positive margins (3.2% vs 3.1%; P = .955), were similar between the two groups. Although Black veterans were less likely to experience major postoperative complications, there was no difference in 30-day readmission, 30-day mortality, or disease-free survival between the two groups. Black patients had significantly better risk-adjusted overall survival (hazard ratio, 0.802; 95% CI, 0.729-0.883; P < .001).
Interpretation
Among veterans with NSCLC undergoing surgical treatment through the VHA, Black patients received comparable care with equivalent if not superior outcomes compared with White patients.
Key Words: lung cancer, racial disparities, thoracic surgery, Veterans Health Administration
Abbreviations: CDW, Corporate Data Warehouse; DFS, disease-free survival; ICD, International Classification of Diseases; NSCLC, non-small cell lung cancer; OS, overall survival; VHA, Veterans Health Administration
FOR EDITORIAL COMMENT, SEE PAGE 742
Take-home Points.
Study Question: Do racial disparities affect early-stage NSCLC outcomes following surgical treatment within the VHA?
Results: In this retrospective cohort study of 18,800 veterans with clinical stage I NSCLC, rates of surgical treatment were similar between Black and White veterans. Furthermore, among patients who underwent surgical treatment, Black Veterans received similar quality care with superior OS compared with White Veterans.
Interpretation: Black Veterans with stage I lung cancer seeking surgical treatment through the VHA receive comparable, if not superior, care compared with their White counterparts. Harnessing strategies from the VHA may help to mitigate racial disparities in lung cancer care in the United States.
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death in the United States, including among veterans.1 The gold standard treatment for early-stage NSCLC is surgery.2 Significant racial disparities exist in access to care and treatment outcomes for NSCLC. Prior studies in the civilian population have shown that Black patients with early-stage lung cancer are less likely to undergo surgical treatement.3,4 Even when Black patients receive surgical treatment, they experience worse outcomes, including significantly shorter overall survival (OS), compared with White patients.5, 6, 7 Factors that could contribute to these racial disparities in lung cancer management and outcomes are multilevel and complex, and they include barriers at the policy level (eg, inadequate insurance coverage), organizational level4 (eg, inadequate access to care8,9), provider level (eg, provider bias and discrimination,10,11 poor communication12), and patient level (eg, distrust of physicians,13 lack of cultural sensitivity14,15).
Veterans receiving care through the Veterans Health Administration (VHA) are a unique cohort of patients who have more equitable access to care, including medicines, surgeries, inpatient and outpatient visits, and rehabilitation services with little or no copayments.8,16 In addition to medical care, the VHA also addresses social determinants of health by providing patients with services such as transportation, housing and employment assistance, substance abuse counseling, and mental health care, all of which are typically less available to patients outside the VHA.17 Theoretically, if these social determinants of health (ie, environmental conditions that affect health, functioning, and quality of life18) are adequately addressed by the VHA, these services should result in more equitable care, including the potential mitigation of racial disparities. Unfortunately, racial disparities have nevertheless been described in the VHA across several different cancers and treatment modalities.19 Although some studies have broadly examined racial disparities in lung cancer care, few recent studies have focused specifically on racial disparities and surgical outcomes in stage I disease.20,21
Several factors are important when evaluating lung cancer management. Traditional, objective biomedical outcomes such as short- and long-term morbidity and mortality are relevant outcome measures.22 Another process-based metric is receiving a high-quality cancer operation. Several quality metrics have been proposed for lung cancer resection, including receiving timely surgery,23 use of a minimally invasive approach,24 and adequate lymph node sampling.25 Adherence to these process measures is associated with improved OS in patients with stage I disease. Some studies have found that Black patients are less likely to receive high-quality surgeries in the civilian population.23,26 It is unclear, however, if racial disparities influence the quality of surgical care delivered within the VHA.
In this retrospective cohort study, we examined veterans with clinical stage I NSCLC undergoing surgical treatment in the VHA (2006-2016). Various treatment-related characteristics and access-to-care measures were compared between White and Black veterans, as well as short- and long-term outcomes between these two groups. We hypothesized that Black veterans receiving treatment through the VHA have similar access to care and equivalent outcomes compared with White veterans.
Study Design and Methods
Study Population
The United States Veterans Administration Informatics and Computing Infrastructure (VINCI) system, which contains clinical and administrative data from multiple platforms in the Corporate Data Warehouse (CDW), was used for this retrospective cohort study.27 Data abstraction was performed and analyzed by a dedicated team of researchers (including two data coordinators, one data analyst, two biostatisticians, and three physicians) over a period of more than 18 months. This rigorous process ensured minimal missing data with greater accuracy and validity than what is typically maintained in the various VHA CDW data sets.
All adults with clinical stage I NSCLC in the VHA from 2006 to 2016 were identified by using International Classification of Diseases for Oncology, 3rd Edition, codes. Surgical procedures were then determined by using International Classification of Diseases (ICD) procedure and Current Procedural Terminology codes. Further analysis was performed on the cohort of patients receiving definitive surgical treatment. We excluded patients who received neoadjuvant chemotherapy, had surgery for recurrent disease, or with unknown date of diagnosis. Only patients of White or Black race were included in this study as the relatively small proportion of other racial groups precluded meaningful analysis. The study protocol was approved by the St. Louis VHA Research and Development Committee and, given the deidentified nature of the analysis, a waiver for consent was granted by the Institutional Review Board. Data were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Exposure
The primary exposure of interest was race, categorized as “White” or “Black.” These racial groups, which reflect socially defined constructs,28 were coded according to the CDW Oncology file and Facility Oncology Registry Data Standards manual.29
Covariates
Several covariates were abstracted for further analysis, including age, sex, BMI, smoking status (assessed 2 weeks prior to surgery),30,31 and comorbidities. Comorbidities (measured from 5 years prior to surgery to 1 month following surgery) were determined by using ICD 9/10 codes to calculate a composite Charlson/Deyo score.32 Patients’ residential ZIP codes were also abstracted and used to calculate straight-line distance from facility (measured from the center of the patient’s ZIP code to the facility address) and metropolitan status (urban/rural). Median household income and education level were estimated based on county of residence. Oncologic and treatment-related variables were extracted, including tumor size, histology (adenocarcinoma, squamous cell carcinoma, other), grade, annual hospital volume (number of patients with lung cancer treated at the facility in the year prior), and year of operation. FEV, which was available for a subset of patients, was included as a covariate in various sensitivity analyses.
Access-to-Care Metrics
To explore differences in access to care between racial groups, we examined several markers of access to care that we defined a priori. First, wait time to surgery was assessed as described previously.23 Second, we extracted whether a patient had any of the following medical procedures prior to their operation: PET scanning, endobronchial ultrasound, or mediastinoscopy. Third, we investigated whether each patient had an established oncology visit prior to their operation.
Operative Quality Metrics and Short-term Outcomes
Several quality measures were assessed, including wait time to surgery (described earlier), surgical approach (video-assisted thoracic surgery or thoracotomy), type of resection (lobectomy, segmentectomy, wedge resection, or pneumonectomy), and the number of lymph nodes examined. Sampling adequacy was defined as at least 10 lymph nodes, commensurate with prior guidelines.25,33 Surgical outcomes of interest included length of stay, final pathologic stage, surgical margin status, major postoperative complications, 30-day readmission, 30-day mortality, and 90-day mortality. Major postoperative complications were assessed individually and as a composite measure as defined by the Society of Thoracic Surgery (postoperative pneumonia, empyema, myocardial infarction, respiratory failure, renal failure, or stroke).34
Long-term Outcomes
The study’s primary, long-term outcome of interest was OS. The secondary outcome of interest was cancer recurrence (ie, disease-free survival [DFS]). Episodes of recurrence were identified via several mechanisms, as described previously by our group and others within the VHA.23,35 First, the VHA CDW Oncology data files were queried for documented recurrence. Second, patients with any of the following occurrences based on ICD codes were also deemed to have recurrences: additional chemotherapy or radiation therapy (in the absence of another cancer diagnosis), additional lung resection, malignant pleural effusion, a secondary malignant neoplasm from lung cancer, or lung biopsy (that was followed by additional chemotherapy or radiation therapy). OS was censored at the end of study follow-up (May 1, 2020) by using the VHA Vital Status Files. DFS was censored at date of last follow-up or death. Patients with pathologic stage IV disease were excluded from the OS and DFS analyses.
Statistical Analysis
Cohort descriptive statistics are reported. For continuous variables, mean ± SDs are presented with two-tailed, Student t tests. Medians are presented for non-normally distributed continuous variables. For categorical variables, frequencies (proportions) are presented with χ2 tests. Short-term outcomes were analyzed by using hierarchical multivariable logistic regression (clustering at hospital level). The association between race and DFS was examined by using a hierarchical multivariable competing risk model (cause-specific model, clustering at hospital level) with recurrence as the outcome and death as a competing event. The association between race and OS was examined by using a hierarchical multivariable Cox proportional hazards model (clustering at hospital level). OS and DFS (ie, freedom from recurrence) were displayed by using the Kaplan-Meier method. Missing data were handled via complete case analysis in the models. P values < .05 were considered significant. All analyses were performed by using SAS version 9.3 (SAS Institute, Inc.).
Results
From 2006 to 2016, a total of 18,800 veterans were diagnosed with clinical stage I NSCLC. The rate of surgical treatment was similar between racial groups, with 57.3% of Black veterans and 58.1% of White veterans receiving definitive surgery (P = .42) (Fig 1). After exclusion criteria were applied, 9,842 veterans receiving surgical treatment were included in the analytical cohort. Of these patients, 8,356 (84.9%) were White and 1,486 (15.1%) were Black. A comparison of demographic characteristics is presented in Table 1. Black patients were younger, had lower BMI, had lower levels of education, and were more likely to live in urban areas closer to the hospital compared with their White counterparts. Income levels were similar between the two groups. Black patients were more likely to be current smokers but had similar composite Charlson comorbidity scores. Black patients were more likely to have diabetes, renal disease, and hypertension but less likely to have coronary artery disease, COPD, or cerebrovascular disease.
Figure 1.
Rates of surgical treatment for clinical stage I non-small cell lung cancer in Black vs White veterans.
Table 1.
Baseline Demographic Characteristics Among White and Black Veterans With Clinical Stage I NSCLC Undergoing Resection in the VHA
| Demographic | White (n = 8,356) | Black (n = 1,486) | P Value |
|---|---|---|---|
| Age, y | 68.1 ± 7.9 | 65.5 ± 8.1 | < .001 |
| Sex | .163 | ||
| Male | 8,063 (96.5) | 1,423 (95.8) | |
| Female | 293 (3.5) | 63 (4.2) | |
| BMI, kg/m2 | 27.38 ± 5.4 | 25.95 ± 5.4 | < .001 |
| Income, $ | .119 | ||
| < 30,000 | 204 (2.5) | 44 (3.0) | |
| 30,000-34,999 | 486 (5.8) | 66 (4.4) | |
| 35,000-45,999 | 3,028 (36.2) | 536 (36.1) | |
| ≥ 46,000 | 4,638 (55.5) | 840 (56.5) | |
| High School graduation rate, county | < .001 | ||
| ≤ 71% | 1,416 (16.9) | 472 (31.8) | |
| 71.1%-80% | 2,927 (35.0) | 598 (40.2) | |
| 80.1%-86% | 2,087 (25.0) | 265 (17.8) | |
| > 86% | 1,830 (21.9) | 138 (9.3) | |
| Missing | 96 (1.15) | 13 (0.87) | |
| Metropolitan status | < .001 | ||
| Urban | 6,227 (74.5) | 1,330 (89.5) | |
| Rural | 2,069 (24.8) | 147 (10.5) | |
| Missing | 60 (0.72) | 9 (0.61) | |
| Distance to hospital (quintiles), miles | < .001 | ||
| < 9.07 | 1,352 (16.2) | 612 (41.2) | |
| 9.08-22.52 | 1,671 (20.0) | 280 (18.8) | |
| 22.53-45.65 | 1,765 (21.1) | 205 (13.8) | |
| 45.66-84.04 | 1,741 (20.8) | 191 (12.9) | |
| > 84.04 | 1,821 (21.8) | 196 (13.2) | |
| Missing | 6 (0.1) | 2 (0.13) | |
| Annual hospital volume | 115.6 ± 57.0 | 112.8 ± 56.3 | .086 |
| Smoking status | < .001 | ||
| Never | 61 (0.7) | 10 (0.7) | |
| Former | 4,133 (49.5) | 632 (42.5) | |
| Current | 4,112 (49.2) | 840 (56.5) | |
| Missing | 50 (0.6) | 4 (0.3) | |
| Charlson/Deyo score | 6.92 ± 2.2 | 6.84 ± 2.5 | .251 |
| Comorbidities | |||
| CHF | 921 (11.0) | 177 (11.9) | .316 |
| COPD | 5,368 (64.2) | 872 (58.7) | < .001 |
| Cerebrovascular disease | 1,576 (18.9) | 216 (14.5) | < .001 |
| Diabetes | 2,389 (28.6) | 483 (32.5) | .002 |
| HIV | 41 (0.5) | 42 (2.8) | < .001 |
| HTN | 5,613 (67.1) | 1,067 (71.8) | < .001 |
| CAD | 3,249 (38.9) | 439 (29.5) | < .001 |
| PVD | 3,884 (46.5) | 540 (36.3) | < .001 |
| Peptic ulcer | 388 (4.6) | 89 (6.0) | .026 |
| Renal disease | 793 (9.5) | 210 (14.1) | < .001 |
| Renal failure | 11 (0.1) | 14 (0.9) | < .001 |
| Rheumatic disease | 290 (3.5) | 37 (2.5) | .052 |
| Severe liver disease | 79 (0.9) | 13 (1.0) | .794 |
| Mild liver disease | 487 (5.8) | 160 (10.8) | < .001 |
Data are expressed as mean ± SD or No. (%). CAD = coronary artery disease; CHF = congestive heart failure; HTN = hypertension; NSCLC = non-small cell lung cancer; PVD = peripheral vascular disease; VHA = Veterans Health Administration.
Black patients experienced slightly longer (although statistically insignificant) wait times between diagnosis and surgery (70 days vs 64 days; P = .090) (Table 2). Other access-to-care metrics, including the proportion of patients undergoing PET scan, endobronchial ultrasound, or mediastinoscopy or establishing care with an oncologist prior to surgery, did not differ according to race. There was no difference in tumor size or tumor grade between the groups, although Black patients were more likely to have adenocarcinoma histology. Quality measures, including the rate of anatomic lobectomy (71.9% vs 69.4%; P = .189), minimally invasive operations (38.2% vs 38.0%; P = .480), resections with positive margins (3.2% vs 3.1%; P = .955), and final pathologic upstaging (12.4% vs 11.8%; P = .644), were similar between the two groups. However, Black patients were slightly less likely to receive ideal lymph node sampling (30.6% vs 33.3%; P = .050).
Table 2.
Access to Care and Treatment Characteristics Among White and Black Veterans With Clinical Stage I NSCLC Undergoing Resection in the VHA
| Access to Care and Treatment Characteristics | White (n = 8,356) | Black (n = 1,486) | P Value |
|---|---|---|---|
| Wait time to surgery, median (IQR), d | 64 (63) | 70 (74) | .090 |
| PET scan | 6,872 (82.2) | 1,234 (83.0) | .455 |
| EBUS | 899 (10.8) | 184 (12.4) | .065 |
| Mediastinoscopy | 640 (7.7) | 108 (7.3) | .600 |
| EBUS or mediastinoscopy | 1,599 (19.1) | 296 (19.9) | .480 |
| Oncology appointment | 2,174 (26.0) | 420 (28.3) | .069 |
| Tumor size, mean ± SD, mm | 23.30 ± 10.6 | 23.5 ± 10.9 | .460 |
| Resection | .189 | ||
| Lobectomy | 5,795 (69.4) | 1,069 (71.9) | |
| Wedge | 1,846 (22.1) | 299 (20.1) | |
| Segment | 523 (6.3) | 93 (6.3) | |
| Pneumonectomy | 135 (1.6) | 18 (1.2) | |
| Unknown | 57 (0.7) | 7 (0.5) | |
| Surgical approach | .480 | ||
| VATS | 3,188 (38.2) | 564 (38.0) | |
| Thoracotomy | 4,614 (55.2) | 851 (57.3) | |
| Unknown | 544 (6.5) | 71 (4.8) | |
| Grade | .208 | ||
| I | 1,008 (12.1) | 186 (12.5) | |
| II | 4,084 (48.9) | 688 (46.3) | |
| III | 2,516 (30.1) | 484 (32.6) | |
| IV | 113 (1.4) | 22 (1.5) | |
| Unknown | 635 (7.6) | 106 (7.1) | |
| Histology | < .001 | ||
| Adenocarcinoma | 4,309 (51.6) | 895 (60.2) | |
| Squamous cell carcinoma | 2,940 (35.2) | 396 (26.6) | |
| Other | 1,106 (13.2) | 193 (13.0) | |
| Unknown | 1 (0.0) | 2 (0.1) | |
| Lymph node sampling | |||
| Median (IQR) | 6 (2,12) | 6 (2,11) | .002 |
| ≥ 10 | 2,599 (33.3) | 425 (30.6) | .050 |
| Pathological STAGE | .468 | ||
| I | 7,004 (83.8) | 1,260 (84.8) | |
| II | 640 (7.7) | 113 (7.6) | |
| III | 310 (3.7) | 61 (4.1) | |
| IV | 33 (0.4) | 10 (0.7) | |
| Unknown | 369 (4.4) | 42 (2.8) |
Data are expressed as No. (%) unless otherwise indicated. EBUS = endobronchial ultrasound; IQR = interquartile range; NSCLC = non-small cell lung cancer; VATS = video-assisted thoracic surgery; VHA = Veterans Health Administration.
Among the short-term outcomes (e-Table 1, Table 3), length of stay (8.1 vs 8.4 days; P = .122) was similar between White and Black patients, although Black veterans were less likely to experience prolonged length of stay (≥ 14 days; adjusted OR, 0.712; 95% CI, 0.567-0.895; P = .004). Similarly, Black veterans were less likely to experience a major complication within 30 days (adjusted OR, 0.746; 95% CI, 0.593-0.937; P = .012). Black and White veterans had similar risk-adjusted rates of 30-day readmission (adjusted OR, 1.008; 95% CI, 0.764-1.331; P = .95), 30-day mortality (adjusted OR, 1.143; 95% CI, 0.696-1.877; P = .60), and 90-day mortality (adjusted OR, 0.833; 95% CI, 0.562-1.235; P = .36).
Table 3.
Short-Term Outcomes Among White and Black Veterans With Clinical Stage I NSCLC Undergoing Resection in the VHA
| Outcome | White (n = 8,360) | Black (n = 1,486) | P Value |
|---|---|---|---|
| Length of stay, mean ± SD, d | 8.4 ± 6.9 | 8.1 ± 6.9 | .122 |
| Pathologic upstage | 983 (11.8) | 184 (12.4) | .644 |
| Positive surgical margins | 260 (3.1) | 47 (3.2) | .955 |
| Major complication | 956 (11.4) | 150 (10.1) | .130 |
| Complications | |||
| Pneumonia | 537 (6.4) | 81 (5.5) | .153 |
| Myocardial infarction | 90 (1.1) | 9 (0.6) | .094 |
| Empyema | 83 (1.0) | 7 (0.5) | .072 |
| Renal failure | 114 (1.4) | 36 (2.4) | .002 |
| Respiratory/cardiac failure | 458 (5.5) | 74 (5.0) | .431 |
| Stroke | 31 (0.4) | 5 (0.3) | .840 |
| 30-Day readmission | 651 (7.8) | 153 (10.3) | .001 |
| 30-Day mortality | 175 (2.1) | 32 (2.2) | .884 |
| 90-Day mortality | 340 (4.1) | 54 (3.6) | .433 |
| Adjuvant chemotherapy | 966 (11.6) | 218 (14.8) | < .001 |
Data are expressed as No. (%) unless otherwise indicated. NSCLC = non-small cell lung cancer; VHA = Veterans Health Administration.
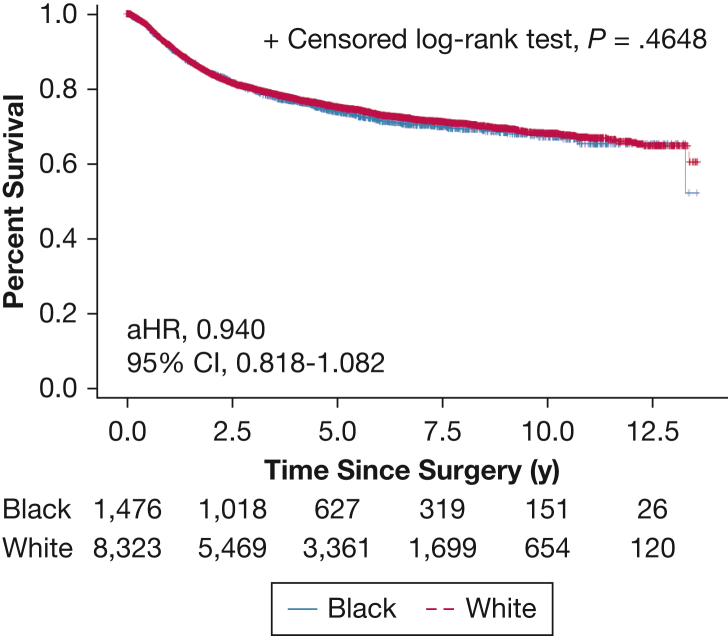
The median (interquartile range) follow-up was 6.1 (2.5-11.3) years. DFS was equivalent between White and Black patients (adjusted hazard ratio, 0.940; 95% CI, 0.818-1.082; P = .39) (e-Table 2; Fig 2); however, OS was significantly better among Black patients (adjusted hazard ratio, 0.802; 95% CI, 0.729-0.883; P < .001) (e-Tables 3, 4; Fig 3).
Figure 2.
Disease-free survival curve for clinical stage I non-small cell lung cancer following surgical treatment. aHR = adjusted hazard ratio.
Figure 3.
Overall survival curve for clinical stage I non-small cell lung cancer following surgical treatment. aHR = adjusted hazard ratio.
Discussion
This study explored racial disparities in the surgical management of early-stage lung cancer within the VHA. We found that, compared with White veterans, Black veterans seeking care through the VHA had similar rates of curative-intent surgical treatment. Furthermore, among patients receiving surgery, quality-of-care metrics, including rates of anatomic resections and minimally invasive surgeries, were similar between White and Black veterans. Additionally, although short-term outcomes and risk of cancer recurrence were similar between the groups, Black veterans had significantly longer risk-adjusted OS. In aggregate, this suggests that Black patients with stage I lung cancer seeking care through the VHA receive comparable, if not superior, care compared with their White counterparts.
The VHA is often perceived as delivering inferior, low-quality care to veterans.36,37 Although several studies have disproved this perception,37,38 a logical concern could still exist if Black veterans are more susceptible to suboptimal care within the system, especially given the disparities and worse outcomes that have been observed among Black patients with NSCLC in the civilian population and the long history of discrimination in health care systems.3 However, we did not observe worse outcomes among Black veterans. To the contrary, we found that Black veterans with clinical stage I NSCLC undergoing resection in the VHA had equivalent, if not superior, short- and long-term outcomes.
The current study builds on several prior studies in the VHA that have examined access to care and outcomes among Black veterans with early-stage lung cancer. Williams et al39 performed an analysis of 1,314 veterans (2006-2007) with pathologic stage I to II NSCLC receiving care in the VHA and showed that Black Veterans, despite similar comorbidity burden, were less likely to receive surgery than their White counterparts. Similarly, Samuel et al19 used a VHA Medicare-linked data set (2001-2004) and found that Black veterans with stage I to II NSCLC were less likely to receive curative-intent treatment. Williams et al20 compared racial disparities in the VHA vs those observed in the Surveillance, Epidemiology, and End Results-Medicare database (2001-2009) for patients with stage I NSCLC. They found that Black veterans were less likely to receive surgery, but the difference was not as pronounced as in the general US population and disappeared by the end of the study period.21 Our study expands on these findings by showing a satisfying mitigation of racial disparities in the rate of surgical treatment for stage I NSCLC in the VHA over the last decade. Furthermore, our study is among the first, to our knowledge, to report superior risk-adjusted survival rates in Black veterans compared with White veterans.
The current study further highlights that Black veterans receive surgeries that are similar in process-based quality metrics compared with the general population. For example, Black patients in the VHA have similar rates of anatomic lobectomies and resections with negative margins. These quality metrics have been associated with improved outcomes in early-stage lung cancer.25 However, not all quality metrics were similar across these two races. For example, Black patients had less extensive lymph node sampling. Inadequate lymph node sampling may be associated with worse survival following resection, likely due to unrecognized occult mediastinal disease in a group of patients who would benefit from adjuvant therapy.40 Lymph node sampling is a quality measure disparity that the VHA should target through future policy endeavors.
Another notable finding is that readmissions were more common among Black veterans, even though this difference was not significant in risk-adjusted hierarchical models. In general, readmission rates are higher in VHA medical centers compared with civilian hospitals.41 The reasons for this are unclear but could be related to better social support within the VHA and a higher propensity to readmit veterans with fewer financial penalties.41 Although it is unclear if the readmissions observed in this study were directly related to surgery, it is important to keep in mind other limitations. For example, White patients lived farther from the hospital. Owing to this distance, it is a possible that an equal proportion of White veterans were readmitted to nonindex facilities (ie, non-VHA facilities closer to home) and therefore were not captured in this data set.42 Further research on this matter is warranted.
Black veterans also waited slightly longer for surgery. Prior studies have shown that VHA patients, regardless of race, wait longer for cancer operations compared with the civilian population.43 We found that Black patients waited approximately 10 weeks for surgery from the date of radiologic diagnosis, 1 week longer than White patients. Prior work from our group has shown that operative wait times as long as 12 weeks are not associated with worse oncologic outcomes,23 implying that the vast majority of veterans still receive timely surgeries, regardless of race. In addition, although Black patients waited longer for surgery, delay-associated oncologic outcomes such as recurrence, upstaging, and positive margins were similar between the two groups. Furthermore, OS was superior among Black patients, after controlling for patient demographic, clinical, contextual, and treatment factors. This suggests that there is only a modest biologic penalty, if any, associated with slightly longer wait times for surgery. However, further efforts are needed to address the potential disparity in surgical wait times between White and Black veterans.
Our findings signal an important aspect related to equity in health care access and quality and their effect on health outcomes.44 Different from the disparities and worse outcomes that have been observed among Black patients with NSCLC in the civilian population,3 we found that Black veterans with clinical stage I NSCLC undergoing resection in the VHA had equivalent, if not superior, short- and long-term outcomes. We can hypothesize that the universal health insurance afforded by the VHA combined with its efforts to assist in other areas, including housing, employment, transportation, and food security, may mitigate disparities, including in the screening and surgical management of lung cancer.8,45, 46, 47 Further studies are needed to investigate the facilitators of equitable lung cancer care in the VHA, including conducting mixed method studies, to examine the unique policies and interventions that facilitate lung cancer equity compared with other diseases in which disparities persist in the system.48 Furthermore, because Black individuals comprise nearly 25,000 newly diagnosed lung cancer cases annually in this country, we must acknowledge that an equitable medical infrastructure needs to include policy measures that expand insurance coverage and better address social determinants of health in the civilian population if we are to improve lung cancer outcomes in good faith.49
The current study has several limitations. First, although we carefully controlled for all relevant and available variables (eg, age, comorbidities, income), residual confounding may have biased the identified racial differences. Nonetheless, the included covariates were already more comprehensive than those in other studies.8,19 Second, we were limited in terms of our inclusion criteria to White and Black patients only. Although several other racial groups receive treatment in the VHA, these numbers were small, preventing further analysis. Finally, we focused on racial disparities in terms of important perioperative and long-term outcomes; this is a relatively narrow view of what constitutes comprehensive cancer care. Further research is needed to determine if there are racial differences in other areas of surgical management, including the quality of long-term follow-up (ie, surveillance, adjuvant therapy).50
Interpretation
Although prior studies have shown inferior outcomes among Black patients undergoing lung cancer resection in the civilian population, the current study found that Black veterans receive similar quality care with equivalent if not superior outcomes through the VHA. Efforts to minimize racial disparities in lung cancer care are essential to improve treatment outcomes.
Acknowledgments
Author contributions: B. T. H., D. B. E., and V. P. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: B. T. H. has funding through a cardiothoracic surgery National Institutes of Health grant [5T32HL007776-25]. V. P., S.-H. C., Y. Y., and D. B. E. have funding through a VHA grant [1I01HX002475-01A2]. None declared (A. A. B., M. W. S., M. R. P., D. K., R. G. N., B. F. M., B. D. K.).
Funding/support: This work was supported by Merit Award # 1I01HX002475-01A2 from the United States (U.S.) Department of Veterans Affairs (V. P., S. H. C., Y. Y., D. B. E.) and 5T32HL007776- 25 from the National Institutes of Health (B. T. H.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Supplementary Data
References
- 1.Zullig L.L., Sims K.J., McNeil R., et al. Cancer incidence among patients of the U.S. veterans affairs health care system: 2010 update. Mil Med. 2017;182:e1883–e1891. doi: 10.7205/MILMED-D-16-00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puri V., Crabtree T.D., Bell J.M., et al. Treatment outcomes in stage I lung cancer: a comparison of surgery and stereotactic body radiation therapy. J Thorac Oncol. 2015;10:1776–1784. doi: 10.1097/JTO.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach P.B., Cramer L.D., Warren J.L., et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 4.Farjah F., Wood D.E., Yanez N.D., et al. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg. 2009;144:14–18. doi: 10.1001/archsurg.2008.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azin A., Hirpara D.H., Doshi S., et al. Racial disparities in surgery: a cross-specialty matched comparison between Black and White patients. Ann Surg Open. 2020;1:e023. doi: 10.1097/AS9.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soneji S., Tanner N.T., Silvestri G.A., et al. Racial and ethnic disparities in early-stage lung cancer survival. Chest. 2017;152:587–597. doi: 10.1016/j.chest.2017.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris A.M., Rhoads K.F., Stain S.C., et al. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211:105–113. doi: 10.1016/j.jamcollsurg.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 8.Mulligan C.R., Meram A.D., Proctor C.D., et al. Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiology Biomarkers and Prevention. 2006;15:25–31. doi: 10.1158/1055-9965.EPI-05-0537. [DOI] [PubMed] [Google Scholar]
- 9.Osarogiagbon R.U., Sineshaw H.M., Unger J.M., Acuña-Villaorduña A., Goel S. Immune-based cancer treatment: addressing disparities in access and outcomes. Am Soc Clin Oncol Educ Book. 2021;41:1–13. doi: 10.1200/EDBK_323523. [DOI] [PubMed] [Google Scholar]
- 10.Vyas D.A., Eisenstein L.G., Jones D.S. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383:874–882. doi: 10.1056/NEJMms2004740. [DOI] [PubMed] [Google Scholar]
- 11.Williams D.R., Kontos E.Z., Viswanath K., et al. Integrating multiple social statuses in health disparities research: the case of lung cancer. Health Serv Res. 2012;47:1255–1277. doi: 10.1111/j.1475-6773.2012.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon H.S., Street R.L., Sharf B.F., et al. Racial differences in doctors’ information-giving and patients’ participation. Cancer. 2006;107:1313–1320. doi: 10.1002/cncr.22122. [DOI] [PubMed] [Google Scholar]
- 13.Gordon H.S., Street R.L., Sharf B.F., et al. Racial differences in trust and lung cancer patients’ perceptions of physician communication. J Clin Oncol. 2006;24:904–909. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 14.Woodward E.N., Singh R.S., Ndebele-Ngwenya P., et al. A more practical guide to incorporating health equity domains in implementation determinant frameworks. Implement Sci Commun. 2021;2:61. doi: 10.1186/s43058-021-00146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorin S.S., Badr H., Krebs P., et al. Multilevel interventions and racial/ethnic health disparities. JNCI Monogr. 2012;2012:100–111. doi: 10.1093/jncimonographs/lgs015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo K., Low A., Everson T., et al. Department of Veterans Affairs; 2017. Prevalence of and Interventions to Reduce Health Disparities in Vulnerable Veteran Populations: A Map of the Evidence. Washington, DC. [PubMed] [Google Scholar]
- 17.Blosnich J.R., Dichter M.E., Gurewich D., et al. Health services research and social determinants of health in the nation’s largest integrated health care system: steps and leaps in the Veterans Health Administration. Mil Med. 2020;185:e1353–e1356. doi: 10.1093/milmed/usaa067. [DOI] [PubMed] [Google Scholar]
- 18.Social Determinants of Health Healthy People 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health
- 19.Samuel C.A., Landrum M.B., McNeil B.J., et al. Racial disparities in cancer care in the Veterans Affairs health care system and the role of site of care. Am J Public Health. 2014;104:S562. doi: 10.2105/AJPH.2014.302079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams C.D., Alpert N., Redding T.S.I.V., et al. Racial differences in treatment and survival among veterans and non-veterans with stage I NSCLC: an evaluation of Veterans Affairs and SEER-Medicare populations. Cancer Epidemiol Biomarkers Prev. 2020;29(1):112–118. doi: 10.1158/1055-9965.EPI-19-0245. [DOI] [PubMed] [Google Scholar]
- 21.Williams C.D., Salama J.K., Moghanaki D., et al. Impact of race on treatment and survival among U.S. veterans with early-stage lung cancer. J Thorac Oncol. 2016;11:1672–1681. doi: 10.1016/j.jtho.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian M., Kozower B.D., Brown L.M., et al. Patient-reported outcomes in cardiothoracic surgery. Ann Thorac Surg. 2019;107:294–301. doi: 10.1016/j.athoracsur.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heiden B.T., Eaton D.B., Jr., Engelhardt K.E., et al. Analysis of delayed surgical treatment and oncologic outcomes in clinical stage I non-small cell lung cancer. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falcoz P.E., Puyraveau M., Thomas P.A., et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardio-thoracic Surg. 2016;49:602–609. doi: 10.1093/ejcts/ezv154. [DOI] [PubMed] [Google Scholar]
- 25.Samson P., Crabtree T., Broderick S., et al. Quality measures in clinical stage I non-small cell lung cancer: improved performance is associated with improved survival. Ann Thorac Surg. 2017;103(1):303–311. doi: 10.1016/j.athoracsur.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haider A.H., Scott V.K., Rehman K.A., et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216:482. doi: 10.1016/j.jamcollsurg.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zullig L.L., Smith V.A., Jackson G.L., et al. Colorectal cancer statistics from the Veterans Affairs Central Cancer Registry. Clin Colorectal Cancer. 2016;15:e199–e204. doi: 10.1016/j.clcc.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deyrup A., Graves J.L. Racial biology and medical misconceptions. N Engl J Med. 2022;386:501–503. doi: 10.1056/NEJMp2116224. [DOI] [PubMed] [Google Scholar]
- 29.American College of Surgeons National Cancer Database. Past facility oncology registry data standards. https://www.facs.org/quality-programs/cancer/ncdb/call-for-data/fordsolder
- 30.Heiden BT, Eaton Jr DB, Chang S-H, et al. Assessment of duration of smoking cessation prior to surgical treatment of non-small cell lung cancer [published online ahead of print November 18, 2021]. Ann Surg. https://doi.org/10.1097/SLA.0000000000005312. [DOI] [PMC free article] [PubMed]
- 31.Heiden B.T., Eaton D.B., Jr, Chang S.-H., et al. The impact of persistent smoking after surgery on long-term outcomes following stage I non-small cell lung cancer resection. Chest. 2022;161(6):1687–1696. doi: 10.1016/j.chest.2021.12.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quan H., Sundararajan V., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 33.American College of Surgeons National Cancer Database. CoC Quality of Care Measures 2020 Surveys. https://www.facs.org/Quality-Programs/Cancer/NCDB/qualitymeasurescocweb
- 34.Wong M.L., McMurry T.L., Stukenborg G.J., et al. Impact of age and comorbidity on treatment of non-small cell lung cancer recurrence following complete resection: a nationally representative cohort study. Lung Cancer. 2016;102:108–117. doi: 10.1016/j.lungcan.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarlov E., Lee T.A., Weichle T.W., et al. Reduced overall and event-free survival among colon cancer patients using dual system care. Cancer Epidemiol Biomarkers Prev. 2012;21:2231–2241. doi: 10.1158/1055-9965.EPI-12-0548. [DOI] [PubMed] [Google Scholar]
- 36.Oliver A. The Veterans Health Administration: an American success story? Milbank Q. 2007;85(1):5–35. doi: 10.1111/j.1468-0009.2007.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heiden BT, Eaton DB Jr, Chang S-H, et al. Comparison between veteran and non-veteran populations with clinical stage I non-small cell lung cancer undergoing surgery [published online ahead of print May 11, 2021]. Ann Surg. https://doi.org/10.1097/SLA.0000000000004928. [DOI] [PMC free article] [PubMed]
- 38.George E.L., Massarweh N.N., Youk A., et al. Comparing Veterans Affairs and private sector perioperative outcomes after noncardiac surgery. JAMA Surg. 2022;157(3):231–239. doi: 10.1001/jamasurg.2021.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams C.D., Stechuchak K.M., Zullig L.L., et al. Influence of comorbidity on racial differences in receipt of surgery among US veterans with early-stage non-small-cell lung cancer. J Clin Oncol. 2013;31:475–481. doi: 10.1200/JCO.2012.44.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bott M.J., Patel A.P., Crabtree T.D., et al. Pathologic upstaging in patients undergoing resection for stage I non-small cell lung cancer: are there modifiable predictors? Ann Thorac Surg. 2015;100(6):2048–2053. doi: 10.1016/j.athoracsur.2015.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nuti S.V., Qin L., Rumsfeld J.S., et al. Association of admission to Veterans Affairs hospitals vs non-Veterans Affairs hospitals with mortality and readmission rates among older men hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2016;315:582–592. doi: 10.1001/jama.2016.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu Y., McMurry T.L., Isbell J.M., et al. Readmission after lung cancer resection is associated with a 6-fold increase in 90-day postoperative mortality. J Thorac Cardiovasc Surg. 2014;148:2261–2267.e1. doi: 10.1016/j.jtcvs.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bilimoria K.Y., Ko C.Y., Tomlinson J.S., et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253:779–785. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 44.Yearby R. Race based medicine, colorblind disease: how racism in medicine harms us all. Am J Bioeth. 2021;21:19–27. doi: 10.1080/15265161.2020.1851811. [DOI] [PubMed] [Google Scholar]
- 45.Bryant A.S., Cerfolio R.J. Impact of race on outcomes of patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:711–715. doi: 10.1097/JTO.0b013e31817c60c7. [DOI] [PubMed] [Google Scholar]
- 46.Lin J., Kamamia C., Brown D., et al. Survival among lung cancer patients in the U.S. military health system: a comparison with the SEER population. Cancer Epidemiol Biomarkers Prev. 2018;27(6):673–679. doi: 10.1158/1055-9965.EPI-17-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reihani A.R., Ahari J., Manning E.P., et al. Barriers and facilitators to lung cancer screening in the United States: a systematic review of the qualitative literature. J Heal Soc Sci. 2021;6:333–348. [Google Scholar]
- 48.Koehlmoos TP, Korona-Bailey J, Janvrin ML, et al. Racial disparities in the military health system: a framework synthesis [published online ahead of print December 15, 2021]. Mil Med. https://doi.org/10.1093/milmed/usab506. [DOI] [PubMed]
- 49.Erhunmwunsee L., Seewaldt V.L., Rebbeck T.R., et al. From COVID-19 to cancer, watching social determinants decide life: when will we stop spectating? J Natl Med Assoc. 2021;113(4):436–439. doi: 10.1016/j.jnma.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Heiden B.T., Subramanian M.P., Puri V., et al. Striking a balance: surveillance of non-small cell lung cancer after resection. J Thorac Cardiovasc Surg. 2021;162(3):680–684. doi: 10.1016/j.jtcvs.2020.10.166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.