Abstract
Several countries mandate informed or shared decision-making for low-dose CT (LDCT) lung cancer screening, but knowledge is limited about the type of information and presentation techniques used to support decision-making in practice. This review aimed to characterize the content, format, mode, and presentation methods of decision support tools (DSTs) for LDCT lung cancer screening. DSTs reported within peer-reviewed articles (January 2000-April 2021) were identified systematically from PubMed, PsycInfo, EMBASE, and CINAHL Plus. Inclusion criteria revolved around the development or evaluation of a resource or tool intended to support individual or shared decision-making for LDCT lung cancer screening. The data-charting and extraction framework was based on the International Patient Decision Aids Standards instrument and Template for Intervention Description and Reporting. Extracted data were organized within two categories: (1) study characteristics and context, format, and mode of DST use and (2) DST content and presentation methods. This review identified 22 DSTs in paper, video, or electronic formats across 26 articles. Most DSTs (n = 13) focused on knowledge exchange, whereas seven used interactive techniques to support values clarification (eg, Likert scales) and nine DSTs guided deliberation (eg, suggested discussion topics). The DSTs addressed similar topics, but the detail, quantification of probability, and presentation methods varied considerably. None described all the potential screening harms and results. The heterogeneity in DST design may affect the quality of decision-making, particularly for participants with lower literacy and numeracy. Evidence-based consensus guidelines for DST content and presentation methods should be developed collaboratively with screening-eligible adults.
Key Words: decision support tools, health inequality, informed decision-making, lung cancer screening, shared decision-making
Abbreviations: DST, decision support tool; LDCT, low-dose CT
Lung cancer remains the leading cause of cancer mortality globally, with incidence highest within socioeconomically deprived communities.1 Consequently, several countries have introduced screening programs for asymptomatic, early-stage lung cancer using low-dose CT (LDCT) imaging. Eligibility is based on risk (primarily age and smoking history) because increased risk increases the likelihood of benefit. In the National Lung Screening Trial,2 LDCT screening reduced lung cancer mortality among high-risk adults by 20% and 24% (men only) in the Nederlands-Leuvens Longkanker Screenings Onderzoek trial.3
However, the potential risks should be considered by eligible screening candidates, including radiation exposure, overdiagnosis, and the potential harms of further tests.4,5 Individuals should, therefore, be supported in making an informed decision about participating in the context of their values and preferences, a shared decision-making process that is required for US government insurers to reimburse screening.6 However, this is difficult to achieve. Risks are inherently challenging to communicate because of the uncertainty they pose for the individual.7 Primary health-care providers may lack specific knowledge of LDCT screening, preventing them from endorsing screening or being able to offer advice.8 People with lower numeracy are particularly likely to be deterred by and distrust ambiguous information, to overestimate probabilities, and to rely on heuristics (eg, emotions) when making decisions, rather than numerical estimates.9 Perceived information burden also can cause some aspects of information to be missed or ignored and may discourage individuals with lower health literacy from participating in LDCT screening, leading to uninformed nonparticipation.10 This is particularly important within lung cancer screening because screening-eligible candidates are overrepresented within socioeconomically deprived communities where literacy11 and numeracy12 are lower. However, early evidence from the United States suggests that individuals are being referred for screening in the community without any information about, or discussion of, the trade-offs.13 This is particularly problematic when many tend to overestimate benefit from screening interventions, potentially leading to uninformed participation.14
Therefore, the presentation techniques and format of information are critical to ensuring that decision support tools (DSTs) are equitable, accessible, engaging, and comprehensible. For example, highlighting numerical risk information using simple visual displays such as icon arrays can support those with lower numeracy by drawing and sustaining attention to numerical information, helping the recipient interpret the absolute figures (thereby reducing the cognitive burden) and minimizing biases in comprehension because of framing effects and denominator neglect.15 Similarly, using time-framed natural frequencies with a consistent denominator for multiple outcomes (the smaller the better) can aid interpretation of the absolute increment in probability relative to alternative outcomes and can avoid overestimation or underestimation of probabilities. Both methods of communicating risk were recently recommended as best practice when probabilities are known.16 However, this depends on the size of the probability and the numeracy and graph literacy of the target audience.15 Furthermore, the evidence base for presenting probabilities that are unknown is still evolving, with mixed findings concerning the use of verbal techniques such as evaluative labels.16 Several studies have designed DSTs to be used by potential lung cancer screening participants and clinicians. Fukunaga and colleagues’17 systematic review of DSTs used in the shared decision-making context found that they were highly acceptable, improved knowledge, and reduced decisional conflict.17 Although this review investigated the impact of shared decision-making DSTs on knowledge, little is known about the design, information content, and presentation techniques used by DSTs in individual as well as shared decision-making contexts. Heterogeneity in design choice is important to understand because this may affect their comprehension and equity, either positively or negatively. To our knowledge, for the first time, this scoping review aimed to identify the content, format, mode, and presentation characteristics of DSTs for LDCT lung cancer screening.
Methods
A scoping review was undertaken using the stages defined by Arksey and O’Malley18 to map how information about LDCT lung cancer screening is presented within resources designed to support individual or shared decision-making, referred to herein as DSTs. Scoping review methodologies provide a way of mapping broad fields of research with diverse study designs,19 rather than aggregating or synthesizing outcomes. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews.
Eligibility Criteria
Peer-reviewed articles were included if (1) they were published between January 1, 2000, and April 15, 2021; (2) they were written in English; and (3) they reported the development, testing, or evaluation of a resource intended to support individual or shared decision-making for LDCT lung cancer screening. Articles were excluded if (1) they did not concern lung cancer screening using LDCT imaging; (2) they included an information resource designed for a purpose other than decision-making (eg, uptake, awareness); and (3) they were review articles or conference abstracts.
Information Sources and Data Extraction
Articles were retrieved from PubMed, PsycInfo, EMBASE, and CINAHL Plus. The search strategy was devised (by S. B. and S. L. Q.) using the Sample, Phenomenon of Interest, Design, Evaluation, Research Type framework. The search strategy was refined iteratively (by S. B.) by running the search terms in the databases to establish the contributions of individual terms and to ensure that key articles were included. Details of the final search strategy are in e-Table 1.
Duplicate results were removed automatically and manually. Eligibility screening was conducted independently by two researchers (C. K. and S. B.). Article titles and abstracts were screened first, followed by full-text reviews. Disagreements were resolved by a third researcher (M. J.), who also assessed all full-text articles. Reference lists from eligible articles and review articles were searched manually. Authors of included studies were contacted to request access to the DSTs.
A data-charting and extraction framework was formulated (by M. J. and S. L. Q.) and presented for consideration by all authors (e-Tables 2A-2C). M. J. independently extracted and charted the data with input from S. L. Q.
The extracted data comprised two categories: (1) the contextual information, derived from the Template for Intervention Description and Reporting framework20 which included study characteristics, target population characteristics, and DST characteristics (including format and mode); and (2) the DST content and methods of information presentation, informed by the International Patient Decision Aids Standards instrument,21 the Template for Intervention Description and Reporting framework,20 and a systematic review of communicative aspects of decision aids.22
Results
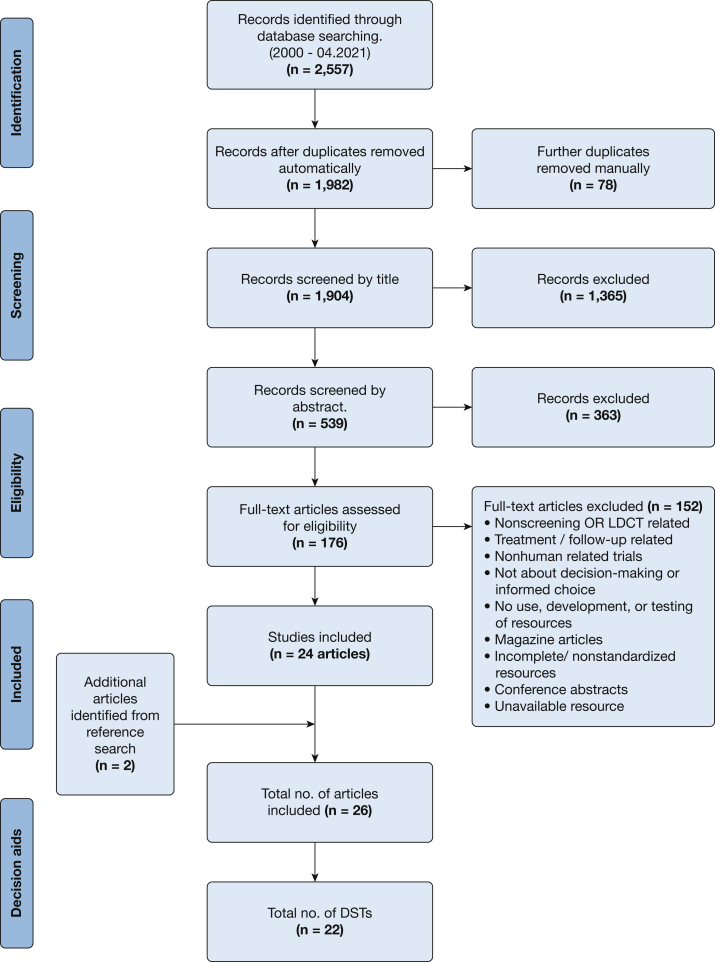
The search yielded 2,557 articles, of which 1,982 remained after removing duplicates (Fig 1). After full-text review of 176 articles, 29 initially met the eligibility criteria in addition to two identified from reference searches. However, three were excluded because it was not possible to access the DSTs described. This resulted in 26 eligible articles, including 22 distinct DSTs.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews flowchart of the search and inclusion process. LDCT = low-dose CT.
Contextual Information
Studies were largely conducted in the United States (n = 24) and were published in 2019 (n = 9) (Table 1). Their designs were heterogeneous, with randomized controlled trials (n = 6) most frequently used. Most (n = 22) studies used samples representative of characteristics that confer likely eligibility for LDCT screening, including age (range, 55-75 or 80 years; mean, 63.1 years) and current or former smoking status. Predominantly, participants were male, current smokers, and of White ethnicity. Only one study23 targeted individuals from low-income and racially diverse populations.
Table 1.
Peer-Reviewed Article Characteristics (N = 26)
| Article Characteristic | |
|---|---|
| Year of publication, No. (%) | |
| 2014 | 2 (7.7) |
| 2015 | 1 (3.8) |
| 2016 | 2 (7.7) |
| 2017 | 3 (11.5) |
| 2018 | 4 (15.4) |
| 2019 | 9 (34.6) |
| 2020 | 2 (7.7) |
| 2021 | 3 (11.5) |
| Country of origin, No. (%) | |
| United States | 24 (92.3) |
| United Kingdom | 1 (3.8) |
| Australia | 1 (3.8) |
| Study design, No. (%) | |
| RCT | 6 (23.1) |
| Development evaluation | 2 (7.7) |
| Qualitative (focus group/interviews) | 4 (15.4) |
| Evaluation only (quantitative survey) | 3 (11.5) |
| Quasiexperimental design: before-after study | 4 (15.4) |
| Mixed methods | 2 (7.7) |
| Pragmatic/feasibility | 3 (11.5) |
| Observational survey | 2 (7.7) |
| Sample characteristicsa | |
| Age, mean (range) | 63.2 (55-80) |
| Current smokers, No. (%) | 1,906 (46) |
| Former smokers, No. (%) | 2,238 (54) |
Data are presented as No. (%) or mean (range). RCT = randomized controlled trial.
Based on 20 studies.
Most (n = 17) DSTs were designed to be used by the individual considering LDCT screening, whereas the remaining five DSTs were designed either for individuals considering screening or health-care professionals offering screening (Table 2). Of these, 13 were intended to support shared decision-making, four were intended to support individual decision-making, and five were intended for shared and individual decision-making.
Table 2.
Characteristics of DSTs (N = 22)
| DST Characteristic | No. (%) |
|---|---|
| Target population | |
| Individual only | 17 (77.3) |
| Either individual or health-care professional | 5 (22.7) |
| Method of use | |
| Paper (only: information sheet/brochure/leaflet) | 4 (18.2) |
| Paper (face-to-face with health-care professional) | 3 (13.6) |
| Paper (telephone coaching) | 2 (9.1) |
| Paper (face-to-face and telephone) | 1 (4.5) |
| Classroom presentation | 1 (4.5) |
| Video (only) | 4 (18.2) |
| Video (face-to-face with health-care professional) | 2 (9.1) |
| Web-based interactive tool (only) | 3 (13.6) |
| Web-based interactive tool and video (only) | 2 (9.1) |
| Context for use | |
| Individual decision-making | 4 (18.2) |
| Shared decision-making | 13 (59.1) |
| Both (individual or shared) | 5 (22.7) |
| Timing in studya | |
| Before consultation | 9 (34.6) |
| During consultation | 9 (34.6) |
| Before and during consultation | 2 (7.7) |
| During study | 6 (23.1) |
| Guidelines or standards usedb | |
| IPDAS | 5 (22.7) |
| IPDAS and National Quality Forum certification criteria for PDAs | 2 (7.7) |
| Not specified | 19 (73.1) |
| Used theoretical frameworksb | |
| Health Belief Model | 1 (3.8) |
| Theories in cognitive psychology and decision-making | 1 (3.8) |
| Not specified | 24 (92.3) |
See e-Table 3 for further details about each individual decision support tool. DST = decision support tool; IPDAS = International Patient Decision Aids Standards.
Based on the timing of administration within the 26 studies for which some used the same DST.
Referenced in article (n = 26).
The DSTs were predominantly paper-based (n = 10), although six used video and five were web-based interactive tools. The timing of DSTs varied by study design. Most (n = 20) were used before or during a health consultation, or both. e-Table 3 presents detailed information on individual DSTs, including name, purpose, and citing studies.
DST Content and Methods of Information Presentation
e-Table 4 summarizes the components included within each individual DST, which are described in turn herein.
Risk-Based Eligibility Criteria
Most (n = 20) DSTs described the eligibility criteria for screening using written text alone or accompanied by verbal narration. All DSTs stated the eligibility criteria for screening as age (range, 55-80 years), smoking status (current or former smoker quit < 15 years earlier), and smoking history (≥ 30 pack-years). Seven DSTs referred to national guidelines (eg, US Preventive Services Task Force24) as the basis of these criteria; five explained that eligibility was based on individual risk of lung cancer or chance of benefit, whereas the remaining provided no justification. Some of the web-based DSTs also included interactive tools such as a smoking pack-year calculator (n = 5) or a lung cancer risk calculator (n = 3). These were electronic questionnaires that produced an individualized summary of personal risk estimates (eg, percentage risk score), binary eligibility status, or both.
Benefits and Harms of LDCT Lung Cancer Screening
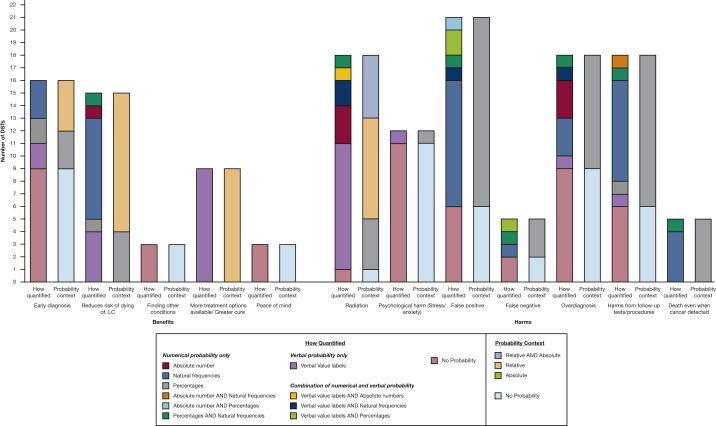
All DSTs described some form of benefit from screening (Fig 2), with a mean average of 2.3 types of benefit (range, 1-4) presented in total by each DST. Those most frequently included were early diagnosis (n = 16) and a reduced risk of dying from lung cancer (n = 15). The benefit of early diagnosis was commonly described with no probability or quantification of its size or likelihood (n = 9). However, seven DSTs quantified this benefit, using either verbal value labels (n = 2; eg, “higher chance”), percentages (n = 2), or natural frequencies (n = 3). The context of these probabilities were presented in either absolute terms (n = 3) or relative terms (n = 4) to not attending screening. One DST also used a quotation from an individual who had participated in lung cancer screening describing how it had been life-saving.,25
Figure 2.
Bar graph showing the methods used to quantify the probability of each benefit and harm of low-dose CT scan LC screening. DST = decision support tool; LC = lung cancer.
Each DST also described at least one harm of screening, with a mean average of 4.5 types of harm (range, 1-6) mentioned in total across all DSTs. Seven different harms were defined across the different DSTS, but no DST included all of these, with the frequency of inclusion varying markedly by the type of harm (Fig 2). For example, radiation exposure (n = 18), false-positive results (n = 21), and harms resulting from follow-up procedures (n = 18) were presented most frequently, whereas fewer mentioned false-negative results (n = 5) or the possibility of dying of lung cancer, even though lung cancer was detected through screening (n = 6).
Harms were also presented differently across the DSTs. Although risk of radiation was usually quantified, either using verbal value labels (n = 10; eg, “small doses”) or numbers (n = 7), nine DSTs provided no information with which to quantify the frequency of overdiagnosis. Those that did (n = 9) did so in absolute terms using numbers (n = 8), verbal value labels (n = 1; eg, “sometimes”), or a combination. In contrast, the probability of radiation risk was presented more commonly in relative terms (n = 13) when compared with other sources of radiation exposure (eg, standard CT scans, radiography, or natural radiation from the environment).
False-positive results were mainly quantified numerically using natural frequencies (n = 10), and the context of this probability was always presented in absolute terms. However, six of the DSTs used only verbal descriptions, with no quantification of their probability. Five DSTs mentioned the chance of experiencing a false-negative result, with two giving no quantification of this likelihood. Few (n = 5) described the possibility of death when lung cancer is detected through screening, which was presented as natural frequencies. Six DSTs compared the incidence of lung cancer death in both screened and nonscreened groups.
Most (n = 18) DSTs described the risk of experiencing physical harm during follow-up or diagnostic procedures for abnormal screening results, whereas two-thirds (n = 12) cautioned of potential psychological distress. The absolute risk of physical harm was predominantly quantified using natural frequencies (n = 8), although six gave no information to quantify the likelihood. Nearly all those describing the possibility of psychological distress (termed stress, worry, or anxiety) did so without quantifying its likelihood, intensity, or duration.
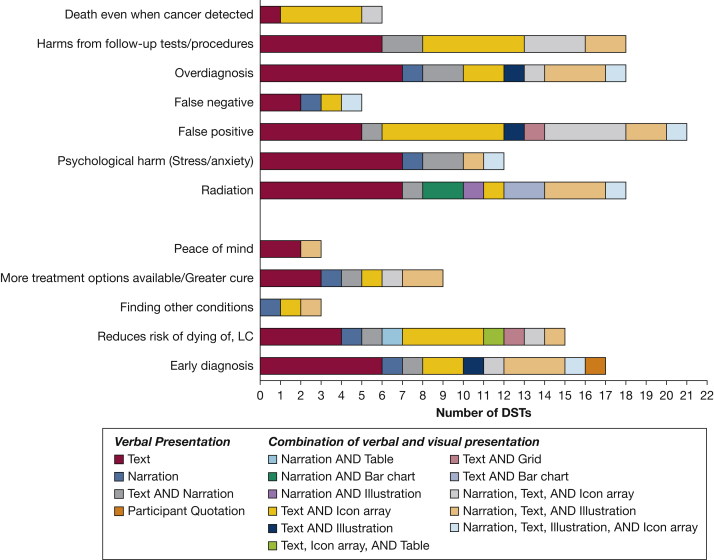
Although all DSTs used written or narrated text as a minimum to present the harms and benefits, a small proportion also included visual illustrations, bar charts, icon arrays, grids, or tables to aid comprehension or attentional engagement (Fig 3). For example, five DSTs used an illustration (eg, CT scan of the lungs), icon array, or table to illustrate their written descriptions of early diagnosis, whereas others used an illustration or animation of a balancing scale to illustrate the overall benefits and harms (n = 5). Others used icon arrays to explain the relative rates of the different harms and benefits within a screened population using a common denominator. Most presented the probability of outcomes in the screening population in isolation in an absolute context, although five DSTs included a separate icon array to show outcomes within a nonscreened population for comparison. To demonstrate the level of radiation exposure from LDCT scans, four DSTs used bar charts with written text to compare different sources of radiation, whereas five also used an animation of a CT scanner (alongside written text or verbal narrations).
Figure 3.
Bar graph showing the methods used to present each benefit and harm of low-dose CT scan LC screening. DST = decision support tool; LC = lung cancer.
Lung Cancer Information
Approximately one-third (n = 9) of the DSTs briefly described the causes of lung cancer, with most (n = 14) citing tobacco smoking as the main cause. Others (n = 5) included additional risk factors such as age, asbestos exposure, COPD, and family history. National incidence and prevalence were cited in 14 DSTs, but fewer (n = 5) mentioned the survival rate. Those that did largely presented this information in absolute terms and numerically, using natural frequencies or percentages. One-quarter (n = 6) of DSTs gave information about lung cancer symptoms, most commonly chronic cough, loss of weight, and coughing blood, with a modal average of five symptoms described. It was also emphasized that early-stage lung cancer may not cause any symptoms.
Smoking Cessation Information
Smoking cessation was promoted by 18 DSTs, which broadly stated the benefits (eg, “the best way to improve health”) and reduced risks (eg, “lowering the chances of dying from a variety of diseases”). These were primarily presented using written text or verbal narration with no information given about the magnitude of benefit or risk reduction. Eleven DSTs signposted individuals to support, and five gave detailed information on how to access support and medication.
LDCT Lung Cancer Screening Procedure and Results
Most (n = 16) of the DSTs described the experience of a LDCT scan in varying detail, with most of the video DSTs showing a person undergoing a scan, and three of the written DSTs using illustrations of the scanner to demonstrate the process. Information about the scanning process included practical details such as the time taken (n = 8), the procedure of laying on a table and raising arms (n = 5), an emphasis that no special preparation is required, and reassurance about the scan being painless and needle free and the scanner being an open ring, rather than an enclosed tunnel (n = 5). One DST also used a personal story of an individual’s experience of the screening process.26
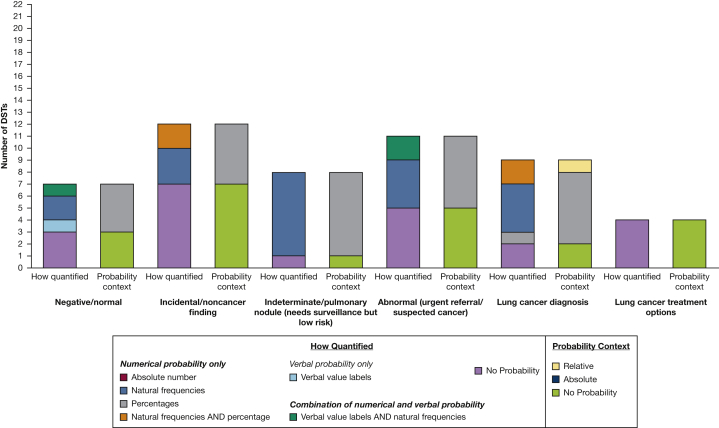
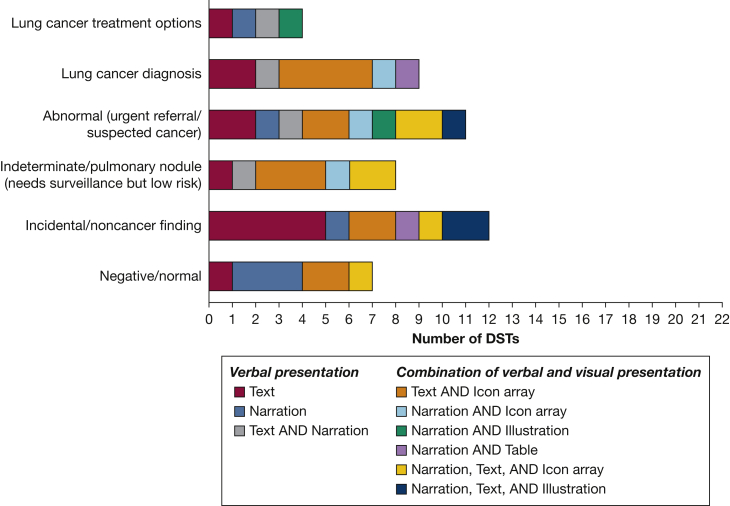
Many (n = 16) of the DSTs described at least one type of LDCT screening result (range, 1-5), but the frequency of inclusion varied by type of result, with only four DSTs describing all five possible results (Fig 4). Incidental findings (n = 12) and urgent or suspicious for cancer findings (n = 11) were mentioned most frequently, with negative results described the least frequently (n = 7). DSTs primarily explained what these results meant, but some also quantified their probability, typically using natural frequencies in the context of absolute risk within written or narrated text alone or in combination with icon arrays to illustrate their relative rates within the screening population (Fig 5). Some described the process after screening, including recommendations to repeat the scan every year for those with negative screening results, and further appointments, follow-up, and possible biopsy for those with abnormal results. Four DSTs gave information about the possible treatment options for lung cancer.
Figure 4.
Bar graph showing the methods used to quantify the probability of each type of low-dose CT scan LC screening results. DST = decision support tool; LC = lung cancer.
Figure 5.
Bar graph showing the methods used to present each type of low-dose CT scan lung cancer screening result. DST = decision support tool.
Values Clarification, Guidance in Deliberation, and Decision Prompts
More than half (n = 14) of the DSTs prompted individuals to consider their values in relation to screening. This was usually suggested as something individuals should do (eg, “think about what’s important to you”), rather than offering any guidance on how to do so. Seven DSTs included brief, structured, and interactive values clarification exercises (eg, Likert scales for ranking or rating values). Some asked individuals to list their reasons for and against attending, and others provided specific questions to guide thinking through the decision.
Fourteen of the DSTs provided prompts and reminders to consult health-care professionals, including cutouts to facilitate shared decision-making. Of these, nine offered a list of topics and points for discussions or questions. Three included spaces for noting down questions in advance of their consultation.
Reading Level or Other Strategies to Help Understanding
Only two studies designed DSTs specifically for those with lower literacy (fifth- and eighth-grade reading level), and two DSTs were available in languages other than English.
Discussion
This is the first scoping review to characterize current practices in the information content, format, and presentation methods of DSTs for LDCT lung cancer screening via in-depth review of individual DSTs. The scoping review identified and accessed 22 DSTs derived from 26 primarily US-based studies. Most were intended for use by individuals considering LDCT lung cancer screening to promote shared decision-making with a health-care professional and primarily served to convey information. However, some also included values clarification exercises designed to prompt individuals to consider their personal values and preferences.
Although the type of content was broadly similar, the detail varied considerably. Based on the totality of risks and screening results described across the different DSTs, no single DST was comprehensive in describing all these potential harms or types of results from LDCT screening. This could lead to prospective screening candidates being ill-informed, particularly if the DST is used as the only available avenue for information. However, briefer DSTs implemented in combination with other resources and discussion with a health-care professional may provide a process of decision-making that supports comprehension while mitigating any potential to feel overwhelmed by too much information in a single episode. The methods of presenting information were also heterogeneous, both between different DSTs and within the same DSTs, depending on the topic of information, with some using best practice techniques for supporting comprehension and others giving no indication of frequency or quantity, either verbally or numerically.
This variation in the information content and presentation techniques of the DSTs is important to highlight because these design choices may positively or negatively affect the extent to which individuals engage with and comprehend the information.16 Most DSTs covered similar topics of information, including eligibility criteria, the screening procedure, smoking cessation, lung cancer, and the benefits and harms of screening. They also dedicated relatively more space to the harms of screening rather than the benefits, consistent with other cancer screening decision aids,22 hence, the same ratio of space in presentation within the results section of this review. Indeed, a particular focus was found on the harms of radiation, false-positive results, and harms of follow-up across DSTs. However, the detail of information content varied substantially, as did the way in which information was quantified, making some information less accessible to individuals with lower literacy and numeracy. No DST described every possible harm, with psychological consequences and false-negative results among those least frequently mentioned.
Similarly, although the types of LDCT screening results were described by 16 of the DSTs, these were typically restricted to incidental, abnormal, and suspicious findings. Few DSTs either raised the possibility of negative results or quantified the likelihood of abnormal findings. Research suggests that a participant’s understanding of the different LDCT scan results may be important not only in supporting informed decision-making, but also in psychologically preparing individuals to manage the uncertainty associated with indeterminate findings and to mitigate over reassurance after negative screening results.27,28 The observed heterogeneity in the detail of the information provided may be driven by different perspectives on importance, the availability of research evidence with which DST designers can quantify their frequency, and concern that too much information about harm could discourage engagement. Future research should seek to establish consensus on the type, detail, and timing of information through innovative methods that seek expert and public perspectives, such as deliberative citizen jury approaches29 or Delphi consensus techniques.30
Among those DSTs that did quantify information, the use of best practice techniques for supporting comprehension among lower-literacy participants varied considerably, particularly those important for presenting and framing frequencies and risk estimates.16 For example, although all DSTs used written or narrated text as a minimum, this was sometimes accompanied by verbal evaluative terms (eg, rarely) and natural frequencies with consistent denominators or visual illustrations (eg, icon arrays) to facilitate comprehension. Furthermore, absolute (rather than relative) risk was used almost exclusively to quantify the risks, benefits, and different possible results from LDCT lung cancer screening. The two exceptions were the benefits of early diagnosis and the risk resulting from radiation, which were presented more commonly in relative terms, either solely or in combination with absolute risk.
Although the primary purpose of the DSTs was to inform individuals and support decision-making, some moved beyond didactic knowledge exchange to engage individuals in deliberation and values clarification. Examples of strategies to encourage deliberation included interpersonal method of use (eg, in-person or telephone), demonstration of the screening procedure (eg, video), or specially designed interactive exercises (eg, ranking scales). Our review showed the use of these design techniques were in the minority, yet research31 suggests that standard didactic-style cancer screening information may disadvantage individuals with a lower socioeconomic status. The relative appeal and effectiveness of these design techniques by reading age is crucial to understand, because LDCT screening DSTs must be designed explicitly for those with lower literacy. Future studies should test these approaches to improve the equity of DSTs.
A comprehensive search strategy across multiple international databases strengthened this study, and independent reviews by three researchers minimized potential biases. However, the DSTs were identified for inclusion via English language, peer-reviewed, published studies only, excluding DSTs published in other languages and those used in clinical practice outside the research context. Furthermore, we did not critically appraise the studies or individual DSTs in line with scoping review methodology to accommodate the rapid timeline for completing the review and because the focus of the review was the content of the DSTs themselves. Future research will investigate the quality of study designs and DSTs as well as adding further to understanding about the context of information content and presentation and the evidence behind specific design choices.
Future research could use cognitive testing and experimental designs with high-risk screening-eligible samples to understand how individuals respond to, interpret, and comprehend different types of information and presentation methods, as well as any variation and individual differences. Although some evidence underpins best practice presentation techniques for decision aids, this is not yet mature, particularly when probabilities are uncertain.16 Such research should use purposive sampling methods to ensure a more diverse representation of demographic groups, including different ethnicities and those from lower socioeconomic groups than those included within the present review. These findings may usefully inform variation in, and tailoring of, the chosen information presentation methods according to the target population.
DSTs are crucial for informed and shared decision-making for individuals considering LDCT screening as part of a range of resources including practitioner advice. Variety in the inclusion, detail, quantification, and presentation methods of key information could affect engagement, comprehension, and decision-making positively or negatively, particularly for those with lower literacy and numeracy. The type, detail, and timing of information included in DSTs should be underpinned by evidence-based consensus guidelines specific to the LDCT lung cancer screening context and should be developed collaboratively with screening-eligible adults. The choice of presentation methods and any variation should be based on existing and evolving evidence for decision aid design for lower-literacy groups and risk communication, for which further research is needed. Doing so could improve and quality assure the effectiveness and equity of decision-making practices.
Acknowledgments
Author contributions: M. J. contributed to formal analysis, investigation, data collection, visualization, writing the original draft, and project administration. S. B. contributed to methodology, investigation, and writing the original draft. C. K. contributed to investigation and reviewing and editing the manuscript. K. E. B., D. R. B., G. B., M. D., G. M., K. A. R., S. V. O., S. M. J., and G M. contributed to conceptualization, methodology, reviewing and editing the manuscript, and funding acquisition; S. L. Q. contributed to conceptualization, methodology, formal analysis, investigation, writing the original draft, supervision, and funding acquisition.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. M. J. is the principal investigator of an academic study (SUMMIT) that is sponsored and conducted by University College London and funded by GRAIL, Inc., through a research grant awarded to S. M. J. S. L. Q. collaborates on the SUMMIT Study and M. R. was previously supported as a researcher by funding for this study. M. R. received travel funding for a conference from Takeda and an honorarium for planning and speaking at educational meetings from Astra Zeneca. S. M. J. has been paid by Astra Zeneca, BARD1 Bioscience, Jansen, and Achilles Therapeutics for being an Advisory Board Expert and travel to one US conference. S. M. J. receives grant funding from Owlstone for a separate research study. D. R. B. has been paid by Asta Zeneca, BMS, MSD, and Roche for educational events and advice. All authors perceive that these disclosures pose no academic conflict for this study. All authors declare no other relationships or activities that could appear to have influenced the submitted work. None declared (M. J., S. B., C. K., G. B., K. E. B., M. D., G. M., K. A. R., S. V. O.).
Role of sponsors: The funding agreements ensured the authors’ independence in designing the study, interpreting the data, and writing and publishing the report.
Other contributions: The authors thank Judith Cass and Tom Haswell, the patient representatives, for their support and insight, which informed the design and conduct of this study, and all those who generously shared their decision support tools for auditing in this review.
Additional information: The e-Tables are available online under “Supplementary Data.”
Footnotes
FUNDING/SUPPORT: This study was funded by Cancer Research UK [Grant C50664/A30770]. S. L. Q. is supported by a Cancer Research UK fellowship [Grant C50664/A24460] and Barts Charity [Grant MRC&U0036].
Supplementary Data
References
- 1.https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#heading-Four
- 2.Aberle D.R., Adams A.M., Berg C.D., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Koning H., van Der Aalst C., de Jong P.A., Scholten E.T. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 4.Bach P.B., Mirkin J.N., Oliver T.K., et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones D.E., Reuland D.S., Reddy S.M., et al. Screening for lung cancer with low-dose computed tomography. Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(10):971–987. doi: 10.1001/jama.2021.0377. [DOI] [PubMed] [Google Scholar]
- 6.Moyer V.A. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 7.Han P. Conceptual, methodological, and ethical problems in communicating uncertainty in clinical evidence. Med Care Res Rev. 2013;70(suppl 1):14S–36S. doi: 10.1177/1077558712459361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis J.A., Chen H., Weaver K.E., et al. Low provider knowledge is associated with less evidence-based lung cancer screening. J Natl Compr Canc Netw. 2019;17:339–346. doi: 10.6004/jnccn.2018.7101. [DOI] [PubMed] [Google Scholar]
- 9.Peters E. Beyond comprehension: the role of numeracy in judgements and decisions. Curr Dir Psychol Sci. 2012;21:31–35. [Google Scholar]
- 10.von Wagner C., Semmler C., Good A., Wardle J. Health literacy and self-efficacy for participating in colorectal cancer screening: the role of information processing. Patient Educ Couns. 2009;75(3):352–357. doi: 10.1016/j.pec.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Riaz S.P., Horton M., Kang J., Mak V., Lüchtenborg M., Møller H. Lung cancer incidence and survival in England: an analysis by socioeconomic deprivation and urbanization. J Thorac Oncol. 2011;6(12):2005–2010. doi: 10.1097/JTO.0b013e31822b02db. [DOI] [PubMed] [Google Scholar]
- 12.Durand M.A., Yen R.W., O’Malley J., Elwyn G., Mancini J. Graph literacy matters: examining the association between graph literacy, health literacy, and numeracy in a Medicaid eligible population. PloS One. 2020;15(11) doi: 10.1371/journal.pone.0241844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner A.T., Malo T.L., Margolis M., et al. Evaluating shared decision making for lung cancer screening. JAMA Intern Med. 2018;178:1311–1316. doi: 10.1001/jamainternmed.2018.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann T.C., Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests. JAMA Intern Med. 2015;175(2):274. doi: 10.1001/jamainternmed.2014.6016. [DOI] [PubMed] [Google Scholar]
- 15.Trevena L.J., Bonner C., Okan Y., et al. Current challenges when using numbers in patient decision aids: advanced concepts. Med Decis Making. 2021;41:834–847. doi: 10.1177/0272989X21996342. [DOI] [PubMed] [Google Scholar]
- 16.Bonner C., Trevena L.J., Gaissmaier W., et al. Current best practice for presenting probabilities in patient decision aids: fundamental principles. Med Decis Making. 2021;41:821–833. doi: 10.1177/0272989X21996328. [DOI] [PubMed] [Google Scholar]
- 17.Fukunaga M.I., Halligan K., Kodela J., et al. Tools to promote shared decision-making in lung cancer screening using low-dose CT scanning: a systematic review. Chest. 2020;158(6):2646–2657. doi: 10.1016/j.chest.2020.05.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arksey H., O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 19.Levac D., Colquhoun H., O’Brien K.K. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann T.C., Glasziou P.P., Boutron I., et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;7:348. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 21.Elwyn G., O’Connor A.M., Bennett C., et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi) PloS One. 2009;4(3) doi: 10.1371/journal.pone.0004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vromans R.D., van Eenbergen M.C., Pauws S.C., et al. Communicative aspects of decision aids for localized prostate cancer treatment—a systematic review. Urol Oncol. 2019;37(7):409–429. doi: 10.1016/j.urolonc.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Crothers K., Kross E.K., Reisch L.M., et al. Patients’ attitudes regarding lung cancer screening and decision aids. A survey and focus group study. Ann Am Thorac Soc. 2016;13(11):1992–2001. doi: 10.1513/AnnalsATS.201604-289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyer V.A. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(8):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 25.Sharma A., O’Connor R., Celestino P., Killion S., Griswold-Krupski L., Bansal-Travers M. Focus groups and in-depth interviews to guide the development of lung cancer screening informational materials. J Cancer Educ. 2019;34(4):712–718. doi: 10.1007/s13187-018-1362-4. [DOI] [PubMed] [Google Scholar]
- 26.Ruparel M., Quaife S.L., Ghimire B., et al. Impact of a lung cancer screening information film on informed decision-making: a randomized trial. Ann Am Thorac Soc. 2019;16(6):744–751. doi: 10.1513/AnnalsATS.201811-841OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kummer S., Waller J., Ruparel M., Cass J., Janes S.M., Quaife S.L. Mapping the spectrum of psychological and behavioural responses to low-dose CT lung cancer screening offered within a Lung Health Check. Health Expect. 2020;23(2):433–441. doi: 10.1111/hex.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slatore C.G., Wiener R.S. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004–1015. doi: 10.1016/j.chest.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rychetnik L., Doust J., Thomas R., Gardiner R., MacKenzie G., Glasziou P. A community jury on PSA screening: what do well-informed men want the government to do about prostate cancer screening—a qualitative analysis. BMJ Open. 2014;4(4) doi: 10.1136/bmjopen-2013-004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elwyn G., O’Connor A., Stacey D., et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robb K.A., Gatting L.P., von Wagner C., McGregor L.M. Preference for deliberation and perceived usefulness of standard-and narrative-style leaflet designs: Implications for equitable cancer-screening communication. Ann Behav Med. 2020;54(3):193–201. doi: 10.1093/abm/kaz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.