Abstract
Objective
Pediatric laboratory medicine is a unique practice serving a vulnerable group of patients including highly specialized testing aiming to detect and treat inherited conditions early to avoid adverse outcomes. Data on the actual impact of COVID-19 pandemic on this speciality is lacking.
Methods
A survey was conducted by the IFCC Committee on Emerging Technologies in Pediatric Laboratory Medicine in partnership with the Society for the Study of Inborn Errors of Metabolism and International Society for Neonatal Screening, to assess the impact on the clinical service provision during the initial wave (January to July 2020) of the COVID-19 pandemic and to gather experiences learned in order to improve laboratory preparedness for future outbreaks.
Results
217 survey responses were received from 69 regions. Sixty-three laboratories (29%) reported a restriction or suspension of service for a median period of four months. The common tests/ services suspended were new-born screening program, body fluids and sweat testing. The reasons for the suspension were related to bio-safety risks of COVID-19 transmission, manpower constraints and supplies disruption. A minority (9-10%) of laboratories did observe delayed/missed diagnoses or a more severe presentation of a clinical disorder. The critical operational decisions that helped manage the initial wave of COVID-19 included modifying work shift patterns, split-teams arrangement, use of personal protection equipment and social distancing.
Conclusion
The provision and delivery of pediatric laboratories services were affected during the initial wave of the COVID-19 pandemic. Manpower preparedness for future potential disruptions to pediatric laboratory services is a key finding and recommendation from this survey.
Key words: quality management, hospital, metabolic, pediatrics, newborn screening, COVID-19
INTRODUCTION
The coronavirus disease-2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a global pandemic first detected in December 2019. Global healthcare priorities have needed to primarily focus on the adult population who were clinically compromised or exhibited symptoms. During this pandemic, laboratory medicine has also been under considerable pressure and needed to change its operations to manage its services, including reorganizing laboratory operations, change management, diverting resources, deferring services, and overall ensuring business continuity of services defined as essential (1, 2). Reviewing the success or otherwise of these changes is important for future planning.
The global stress caused by the COVID-19 pandemic on healthcare services is unprecedented. Whilst there have been restrictions, there has also been a reluctance to seek pediatric care during the pandemic (3). With limited or reluctant access to normal healthcare brings the potential for later presentations of common childhood conditions (such as diabetes and appendicitis), delayed or stopped vaccination or screening programs, resulting in potentially worse prognosis (4-6). In addition, unique presentations of acutely sick children post-viral infection, mimicking the previously described Kawasaki disease (e.g., pediatric multisystem inflammatory syndrome temporally associated with COVID-19), also presented a new challenge for pediatric laboratories.
Pediatric laboratory medicine is a unique practice serving a vulnerable group of patients (7). It includes highly specialized testing that aims to detect and treat inherited conditions early to avoid adverse outcomes. Anecdotes of diversion or limitation of pediatric laboratory testing, particularly those related to inborn errors, have been discussed in various online laboratory medicine professional fora. Whilst a reduction in pediatric health care access has been reported, the direct influence of the initial wave of the COVID-19 pandemic on pediatric laboratory medicine services is less clear (8-10).
It is important to understand the impact globally of COVID-19 on pediatric laboratory service delivery, especially through the initial wave when the greatest changes and hesitancy were seen. Understanding the changes enforced or initiated due to COVID-19 during the initial impact and response will allow pediatric laboratories and relevant authorities to learn from their past experiences and implement appropriate mitigation measures. Additionally, it can serve to provide important comparisons between laboratories to support future planning.
This survey by the IFCC Committee on Emerging Technologies in Pediatric Laboratory Medicine (C-ETPLM), in partnership with the Society for the Study of Inborn Errors of Metabolism (SSIEM) and International Society for Neonatal Screening (ISNS), was conducted to:
understand how laboratories serving the pediatric population changed their clinical service delivery in response to the initial wave (January to July 2020) of the COVID-19 pandemic;
gather experiences learned from managing the initial wave(s) of the pandemic to improve laboratory preparedness for future outbreaks.
METHODS
Questionnaire
A descriptive electronic survey comprising 17 questions in English was constructed using SurveyMonkey software (Momentive Inc, San Mateo, California, United States). The survey questions were developed by four co-authors (TPL, TL, RG, CMM) from Singapore, the United Kingdom, Australia and Hong Kong, respectively on behalf of the IFCC Committee for Emerging Technologies in Pediatric Laboratory Medicine (C-ETPLM; https://www.ifcc.org/ifcc-emerging-technologies-division/etd-committees/c-et-plm/). The survey comprised a variety of question formats including multiple-choice and open-ended questions. Where relevant, the participants were invited to elaborate on their responses using free text. The survey was organized into the following areas:
general information (4 questions);
change of practice - delivery of laboratory services (6 questions);
effect - impact on clinical care or pathology of pediatric diseases during the initial wave of COVID-19 (3 questions);
retrospective - lessons learned from the initial wave of COVID-19 (4 questions).
For this survey, the “initial wave” was defined as the period between January 2020 and July 2020. The draft of the survey was piloted and reviewed by laboratories in Singapore, the United Kingdom and Australia before further refinements. No individually identifiable data was collected for this survey. This survey was exempted from Ethics Board review. A copy of the complete questionnaire is provided in Supplement 1.
DISTRIBUTION
This survey was distributed between February and April 2021. Participation was entirely voluntary, and participants were assured that complete anonymity would be preserved. The survey was publicized through multiple channels including email alerts, electronic news alerts, electronic newsletter and social media of the IFCC C-ETLM, SSIEM, ISNS and national societies. This network distribution approach provided a mechanism for the broadest reach. However, it did not allow for the calculation of the response rate as the distribution channels overlapped significantly.
DATA ANALYSIS
The electronic survey responses were reviewed in Survey Monkey and then exported into an Excel (Microsoft Excel, Microsoft, Seattle, WA, USA) spreadsheet for additional analysis. Question 2 of the survey was designed as an exclusion criterion if the participants only answered to the option: “We do not serve pediatric patients” or only “other” for Question 2 (laboratory testing type).
The survey responses contained both quantitative and qualitative data, together with some free-text comments. To quantify the numerical data, results are presented as the percentage (%) of participants providing a particular response (numerator), compared to the total number of participants who responded to the question (denominator). As some survey questions allowed for multiple responses, the denominator reflects the addition of all responses made in these instances. That is, where multiple responses were solicited, the denominator may show a total response greater than the actual number of survey participants. Qualitative responses were reviewed and summarized categorically.
RESULTS
In total, the survey generated 238 responses. Responses from 21 laboratories were excluded as they did not indicate that they were involved in pediatric laboratory medicine in Question 2. These excluded laboratories either indicated that they serviced only adult patients or other settings. This left 217 valid responses with 96 (44%) of these deemed to be “complete” by Survey Monkey. The most frequent question avoided was number 15 relating to “operational decisions you wish your laboratory had avoided when managing the initial wave of COVID-19”. Following examination of the complete versus incomplete response group, it was decided to include all responses obtained for the quantitative data. These responses were received from 69 geographical regions (Figure 1).
Figure 1.
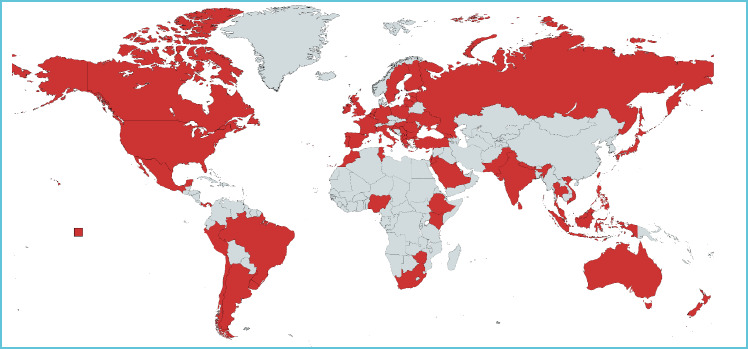
World map showing valid responses from 69 region for the global survey
GENERAL INFORMATION (QUESTIONS 1-4)
The characteristics of the participating laboratories are summarized in Table 1.
Table 1.
Characteristics of the laboratories participating in the survey
| Frequency | Percentage | |
|---|---|---|
| Laboratory services provided | ||
| Newborn bloodspot screening | 59 | 27.2 |
| Inherited metabolic diseases testing | 63 | 29.0 |
| Sweat testing | 41 | 18.9 |
| Genetic / molecular testing | 54 | 24.9 |
| Specialist pediatric endocrinology testing | 36 | 16.6 |
| Pediatric blood sciences - general biochemistry / hematology / endocrinology / immunology | 92 | 42.4 |
| General adult laboratory serving neonatal/ pediatric population | 116 | 53.5 |
| Number of patient samples processed per day | ||
| <200 | 91 | 41.9 |
| 200-500 | 48 | 22.1 |
| >500 | 78 | 35.9 |
| Pediatric specialty supported by laboratory | ||
| General pediatric medicine | 164 | 75.6 |
| Community pediatrics | 73 | 33.6 |
| Neonatal unit | 133 | 61.3 |
| Specialist children’s hospital serving multiple subspecialties | 96 | 44.2 |
| Maternity | 102 | 47.0 |
DELIVERY OF LABORATORY SERVICES (QUESTIONS 5-10)
The ten clinical laboratory tests and services that were most restricted or suspended during the initial wave of the COVID-19 pandemic are summarized in Table 2. The restriction or suspension was in force for a median of four months. The most common reasons for suspension were the following: concern over the risk of COVID-19 transmission (41%, n = 37/90), manpower diversion to other areas (20%, n = 18), insufficient manpower (e.g., due to split team arrangement; 20%, n = 18), and disruption of reagent/consumable delivery due to COVID-19 (19%, n = 17). A variety of additional responses were recorded by 35% of participants. One laboratory reported diversion of funding to COVID-19 testing as the reason for service restriction/suspension. At the same time, the participating laboratories reported a significant decrease in workload for 15 clinical tests which are summarized in Table 3.
Table 2.
Laboratory tests and services that were restricted or suspended during the initial wave of COVID-19 pandemic (Jan to July 2020)
| Frequency | |
|---|---|
| Clinical test / clinical laboratory service restricted/ suspended | |
| Newborn screening program (including congenital hypothyroidism, inborn errors of metabolism) | 12 |
| Body fluids | 6 |
| Sweat testing | 5 |
| Fecal elastase, calprotectin, copro-parasitology | 5 |
| Saliva, sputum tests | 4 |
| Endocrine tests | 4 |
| Procalcitonin | 3 |
| Outpatient department services | 3 |
| Urine tests | 2 |
| Therapeutic drug monitoring | 2 |
| Autoimmune, allergy markers | 2 |
| D-dimer | 2 |
| IL-6 | 2 |
| Vitamin testing | 2 |
| Duration of service restriction or suspension (months) | |
| Median (interquartile range) | 4 (5.5) |
| Min | 1 |
| Max | 12 |
Table 3.
Laboratory tests with a decrease in workload compared to pre-COVID-19 in your laboratory during the initial wave of COVID-19 (Jan to July 2020)
| Laboratory tests | Response frequency | Workload reduction (%) | ||
|---|---|---|---|---|
| Median | Min | Max | ||
| General chemistry | 19% | 50 | 5 | 90 |
| Diabetes testing (glucose, HbA1c) | 5% | 50 | 25 | 90 |
| Endocrinology and lipids | 6% | 50 | 15 | 80 |
| Vitamin testing (folate, B12, others) | 3% | 65 | 15 | 80 |
| Inflammatory markers (CRP, procalcitonin) | 2% | 25 | 15 | 40 |
| Tumour markers | 1% | 17 | 5 | 30 |
| Special chemistry (markers, metals, drugs) | 6% | 55 | 5 | 100 |
| Sweat test | 2% | 100 | 90 | 100 |
| Inherited metabolic testing | 18% | 60 | 20 | 100 |
| Newborn screening | 3% | 30 | 5 | 50 |
| Haematology | 10% | 50 | 5 | 100 |
| Histopathology and cytology | 3% | 80 | 50 | 90 |
| Allergy and immunology testing | 10% | 55 | 15 | 95 |
| Genetic and genomic testing | 2% | 50 | 40 | 100 |
| Clinical microbiology, virology and parasitology | 13% | 60 | 10 | 100 |
Quality standards related to national, or accreditation standards were reported by 15 % (n = 19/124) of laboratories. The laboratories reporting difficulty meeting national standards for newborn/pediatric screening programmes in their local setting with delayed turnaround time (n=3), suspension of confirmatory testing for newborn screening owing to maternal COVID-19 concerns (n=2), delay in sample delivery (n=2), insufficient reagent (n=1) and manpower constraints (n=1). This was consistent with the cited difficulty of meeting laboratory accreditation standards due to delayed turnaround time (n=2), delayed laboratory audits (n=2), non-availability of instrument technical support (n=1), manpower constraints in following quality issues (n=1), and delay in laboratory testing or diagnosis of pediatric conditions (n=1).
IMPACT ON CLINICAL CARE OR PATHOLOGY OF PEDIATRIC DISEASES (QUESTIONS 11-13)
Most participants reported no impact from COVID-19 on clinical care. However, an increase in missed detection of pediatric diseases related to the clinical laboratory tests/services stopped or restricted due to COVID-19 was noted by 10% (n = 12/121) of participants. Similarly, 9% (10/114) of participants observed an increase in later (more severe) presentation of pediatric diseases related to the tests/services stopped, restricted due to COVID-19. Clinical conditions associated with increased missed detections were neonatal jaundice, congenital hypothyroidism, urosepsis, non-COVID-19 viral infections, phenylketonuria, anaemia, endocrine disorders, leukaemia and lysosomal disease; each was reported by one participant. Clinical conditions associated with delayed or more severe presentation were Crohn’s disease, phenylketonuria, neonatal jaundice, and inborn errors of metabolism with one participant reporting each.
By contrast, almost a quarter (23%, n = 27/116) of participants observed positive effects of COVID-19 on their laboratory services or clinical conditions that can be detected via laboratory tests.
LESSONS LEARNED FROM THE INITIAL WAVE (QUESTIONS 14-17)
The most important operational decision made that helped the management of the initial wave of COVID-19 was changing the normal working patterns of their staff either by modifying the shift patterns or splitting the staff into teams to prevent further infections (34%, n = 30/91 participants).
The next ranked change (18%) was implementing measures to protect their staff from potential infection including provision of personal protection equipment, social distancing where possible, restricting access and reviewing risk assessments.
Fifty-one participants described at least one operational decision that they would wish to avoid when managing the initial wave of COVID-19. The most important operational decisions were problems in routine service interruption (49%, n = 25/51), inadequate safety measures and education to the staff (43%, n = 22), reagent shortages (20%, n = 10), and lack of alternating teams, long working hours and staff deployment (20%, n = 10).
Only 8% of laboratories introduced SARS-CoV-2 PCR testing to support the active management of suspected patients. Workload strategies were also implemented to ensure essential services such as neonatal and maternal screening were maintained, and COVID-19 patients were processed quickly. Some laboratories changed their inventory practices to reduce the number of deliveries by holding or ordering extra stock of essential consumables. A range of technologies (e.g., new laboratory platforms, automation) were employed by 44% (n = 41/93) of participants to help manage business continuity.
Similarly, there were 65 participants who responded the significant operational areas to prepare the laboratory for subsequent COVID-19 waves were staffing, analytical testing phase, business continuity planning, and occupational health and safety were given the highest priority by participants (Figure 2).
Figure 2.
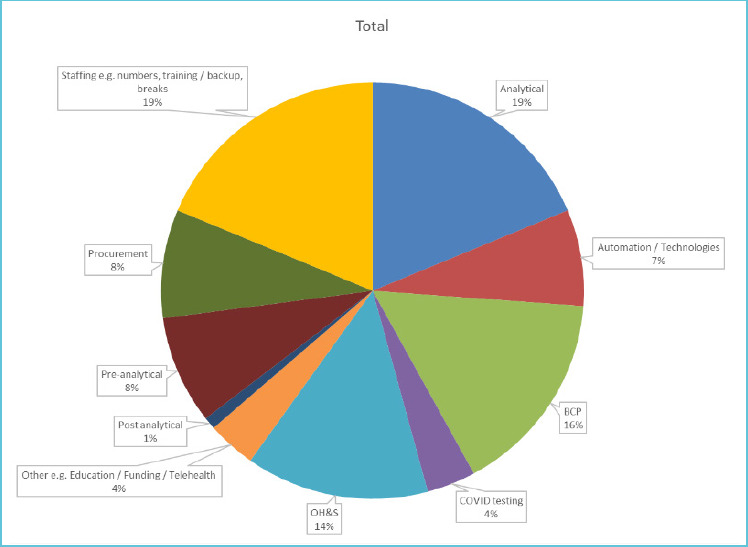
Significant operational areas were identified to prepare the laboratory for subsequent COVID-19 waves*
DISCUSSION
The result of this global survey showed that COVID-19 has significantly impacted the clinical laboratories serving the pediatric population during the initial wave of the pandemic. It was necessary to restrict or suspend certain clinical laboratory services. Among the most frequent restricted laboratory tests were newborn screening and sweat test programs that are designed to detect early hereditary abnormalities to avoid long-term adverse consequences. While the median duration of reduced access was relatively short at four months, a longer restriction (e.g., up to 12 months) may lead to suboptimal patient care. Restriction or suspension of certain laboratory tests such as newborn screening is highly undesirable as it may be associated with severe consequences to infants in case of late diagnosis. If such a decision is unavoidable, such as the case of the unprecedented biosafety and resource challenges brought on by the pandemic, its duration should be kept to a minimum.
Importantly, the concerns over risk of COVID-19 transmission through laboratory procedures calls for early availability of risk-based biosafety/biohazard risk assessment (1). Nonetheless, the lack of sufficient personal protective equipment and biosafety facility impeded the ability of the laboratory to provide a safe environment for its staff. At the same time, the lack of manpower required improved and more consistent funding to build pandemic-preparedness and cross-training of staff to allow more dynamic deployment of resources. These approaches were thought to reduce the impact of a pandemic supporting the continuity of clinical services.
Split teams and triaging of activities have been employed in new born screening laboratories to mitigate the risk to continuity of service delivery during the initial wave of COVID-19(2). In this survey, 12 (20%) of NBS laboratories had to restrict their services. This was similar to those reported by the COVID-19-NBS ISNS global network, which approximated the number of laboratories reporting moderate effects from COVID-19 on NBS laboratories (11). The magnitude of decrease in newborn screening activities reported by the survey participants corroborated with a recent study that examined 16 specialized medical centers treating inborn error of metabolism patients in Europe, Asia and Africa and reported a decrease of 60-80% in service activity compared to the pre-pandemic period (12).
At the same time, many clinical laboratory services saw a significant decrease in workload compared to the pre-pandemic period, with some services experiencing a near-complete reduction. Whilst not mentioning a specific reduction in workload, the ISNS global network quoted that up to 83.7% of NBS laboratories were affected by COVID-19(11). Another study summarizing two surveys performed by the European Reference Network for Hereditary Metabolic Diseases have similarly found major disruption to clinical care delivery for patients with metabolic disorders (10). Besides the reduced clinical service delivery, the laboratory survey participants also reported difficulty meeting local national standards for newborn screening programmes as well as relevant accreditation standards, which may lead to sub-optimal service quality.
Testing for other hereditary conditions, such as cystic fibrosis and hereditary angioedema, were also reduced. The reduction in the test volume for the other general laboratory tests may be related to the general reduction in patient visits to healthcare facilities. Moreover, the reduced laboratory testing for chronic conditions such as allergy, diabetes, dyslipidaemia, chronic renal conditions and tumour markers may lead to suboptimal care of these groups of patients. The reduced laboratory testing activity may be due to a combination of restricted laboratory services as well as reduced healthcare attendance during the initial wave of the pandemic.
The reduced laboratory testing, reduced clinical access and a deterioration in laboratory standards risked missed opportunity for early diagnosis and intervention, leading to delayed and potentially more severe clinical presentation. Such adverse consequences were reported by a dozen or fewer laboratories with the clinical conditions closely associated with laboratory tests that had reduced testing activity. Nevertheless, these survey responses should be considered anecdotal evidence as the information provided was not accompanied by curated objective data. A national study in Scotland on general pediatric healthcare use found that while the rate of clinical consultations in primary care and secondary care fell during the lockdown, they were not associated with increased clinical severity scores or mortality (9). More studies are required that examine the journey of pediatric patients during the pandemic to provide more definitive evidence.
This study has some limitations. The number of laboratories participating in this study is relatively small, although pediatric laboratory medicine is a specialized area within the discipline. Many laboratories served mixed adult-pediatric populations, which may somewhat dilute the focus of the survey response. Additionally, this survey was designed with many open-ended, qualitative questions to best capture the response of the participants. Consequently, the summarization of the data involved subjective categorization and some fields were left unanswered, leading to suboptimal response rates.
There were various lessons learned from the first wave of COVID-19. Technology solutions are important to support business continuity but needs to be tailored according to local needs. With hindsight, several operational decisions made around manpower would not be repeated and this also was the focus for prioritization of future business continuity planning.
Manpower considerations ranged from staff wellbeing, minimizing the spread of COVID-19 between team members, the agility of team members, redeployment of team members to other areas and the maintaining a minimum number of staff available to run services. Some guidance on manpower arrangement in the laboratory setting has since been made available (12). Hence, manpower preparedness for future potential disruptions to pediatric laboratory services is a key finding and recommendation from this survey.
CONCLUSIONS
This survey supports our understanding of how laboratories serving the pediatric population changed their clinical service delivery in response to the initial wave of the COVID-19 pandemic. Staffing decisions were highlighted as both retrospective decisions that would have been done differently and as the priority for future business continuity planning. Overall, these gathered experiences learned from managing the initial wave(s) of the pandemic should improve laboratory preparedness for future outbreaks.
IFCC C-ETPLM, SSIEM, ISNS SURVEY ON IMPACT OF COVID-19 ON PEDIATRIC LABORATORY MEDICINE
Background
The coronavirus disease-2019 (COVID-19) pandemic has spread globally since its first detection in December 2019. In many regions, the COVID-19 is now entering a second and subsequent waves of outbreak. A recent survey by The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Task Force on COVID-19 has revealed that laboratories have had to change their operations to manage the pandemic, including diverting resources and deferring services.
Pediatric laboratory medicine is a unique practice serving a vulnerable group of patients. It includes highly specialized testing that aims to detect and treat inherited conditions early to avoid adverse outcomes. This survey by the IFCC Committee on Emerging Technologies in Pediatric Laboratory Medicine (C-ETLM), in partnership with the Society for the Study of Inborn Errors of Metabolism (SSIEM) and International Society for Neonatal Screening (ISNS), is conducted to:
Understand how laboratories serving the pediatric population have changed their clinical service delivery in response to the initial wave (January to July 2020) of the COVID-19 pandemic
Evaluate how these changes are affecting clinical care
Gather experiences learned from managing the initial wave(s) of the pandemic to improve laboratory preparedness for future outbreaks
This 17-item survey is completely voluntary and should take approximately 15 minutes to complete. The results of this survey will be analyzed in an aggregated manner and published to inform the larger clinical community. No individually identifiable information will be made public. By proceeding with this survey, you have provided an implied consent to the above. Thank you for your participation.
For queries about the survey, please contact Dr. Tim Lang (tim.lang@nhs.net).
General Information
1. Please indicate your country/ region of practice
-
2. Which of the following best describes your laboratory? Please select all that apply.
Newborn Bloodspot Screening
Inherited Metabolic Diseases Testing
Sweat Testing
Genetic / Molecular Testing
Specialist Pediatric Endocrinology Testing
Pediatric Blood Sciences - General Biochemistry / Haematology / Endocrinology / Immunology
General adult laboratory serving neonatal/ pediatric population
Others, please specify:___________________
We do not serve pediatric patients (this is an exclusion question)
-
3. Please indicate the number of patient samples your laboratory processes per day.
<200 samples
200-500 samples
>500 samples
-
4. Which of the following pediatric specialities are supported by your laboratory? Please select all that apply.
General Pediatric Medicine
Community Pediatrics
Neonatal Unit
Specialist Children’s Hospital serving multiple subspecialties
Maternity
Others, please specify:___________________
Delivery of Laboratory Services (to be mirrored for all laboratory areas)
5. What clinical test / clinical laboratory service did your laboratory stop OR restrict during the initial wave of COVID-19 pandemic (Jan to July 2020)? Please specify. Free text
6. How long was the laboratory test / laboratory service suspended for? Months
-
7. What were the two main reasons for the suspension?
Manpower diversion to other areas
Insufficient manpower (e.g. due to split team arrangement)
Concern over risk of COVID-19 transmission
Disruption of reagent/ consumable delivery due to COVID-19
Others, please specify:___________________
8. What are the top 5 tests with a decrease in workload compared to pre-COVID-19 in your laboratory during the initial wave of COVID-19 (Jan to July 2020)? Please indicate the estimated percentage decrease during the worst month.
-
9. Please indicate if the COVID-19 pandemic affected your laboratory’s ability to meet any National Standards for newborn/ pediatric screening programmes available in your country?
Yes/ No. If yes, please elaborate:____________
-
10. Please indicate if the COVID-19 pandemic affected your laboratory’s ability to meet any Accreditation Standards (e.g. ISO 15189) available in your country?
Yes/ No. If yes, please elaborate:____________
Impact on Clinical Care or Pathology of Pediatric Diseases During the Initial Wave of COVID-19 (Jan to July 2020)
11. Did your laboratory observe any increase in missed detection of pediatric diseases related to the clinical laboratory tests / services stopped or restricted due to COVID-19? Please specify the condition and related laboratory tests.
12. Did your laboratory observe any increase in later (more severe) presentation of pediatric diseases related to the tests / services stopped, restricted due to COVID-19? Please specify the condition and related laboratory tests.
13. Did your laboratory observe any positive effect of COVID-19 on laboratory services (or those that can be detected via laboratory tests)?
14. Lessons learned from the Initial Wave of COVID-19 (Jan to July 2020)
15. What are the three most important operational decisions your laboratory has made that positively helped the management of the initial wave of COVID-19? Please specify.
16. What are the three operational decisions you wish your laboratory has avoided when managing the initial wave of COVID-19? Please specify.
17. Was there any technology that your laboratory employed that were helpful in managing the COVID-19? Please specify.
18. What are the two operational areas you consider most important for preparing your laboratory for subsequent waves of COVID-19? Please specify.
This is the end of the survey. Thank you very much for your participation.
Acknowledgements
We wish to acknowledge and thank Mrs. Silvia Collie-Lanzi from the IFCC Office for her support in building of the questionnaire in Survey Monkey and her attention to detail in the delivery of support services to the C-ETPLM, as well as Mr. Nick Law from SSIEM for the assistance in disseminating the survey questionnaire.
REFERENCES
- 1.Loh TP, Horvath AR, Wang CB, Koch D, Lippi G, Mancini N, et al. Laboratory practices to mitigate biohazard risks during the COVID-19 outbreak: an IFCC global survey. Clin Chem Lab Med. 2020;58(9):1433-1440. 10.1515/cclm-2020-0711. [DOI] [PubMed] [Google Scholar]
- 2.Greaves RF, Pitt J, McGregor C, Wall M, Christodoulou J. Newborn bloodspot screening in the time of COVID-19. Genet Med. 2021;23(6):1143-1150. 10.1038/s41436-020-01086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciacchini B, Tonioli F, Marciano C, Faticato MG, Borali E, Pini Prato A, et al. Reluctance to seek pediatric care during the COVID-19 pandemic and the risks of delayed diagnosis. Italian Journal of Pediatrics. 2020;46(1):87. 10.1186/s13052-020-00849-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerall CD, DeFazio JR, Kahan AM, Fan W, Fallon EM, Middlesworth W, et al. Delayed presentation and sub-optimal outcomes of pediatric patients with acute appendicitis during the COVID-19 pandemic. J Pediatr Surg. 2021;56(5): 905-910. 10.1016/j.jpedsurg.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen DEMC, Illy KE. Delayed presentation to regular Dutch paediatric care in COVID-19 times: a national survey. BMJ Paediatrics Open. 2020;4(1):e000834. 10.1136/bmjpo-2020-000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erlichman M, Zalut T, Schwartz S, Weiser G. The ongoing indirect effect of the COVID-19 pandemic on a pediatric emergency department. PLOS ONE. 2021;16(5): e0251003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grey VL, Loh TP, Metz M, Lang T, Hersberger M. Paediatric Laboratory Medicine - Some reflections on the sub-specialty. Clin Biochem. 2017;50(12):648-650. 10.1371/journal.pone.0251003. [DOI] [PubMed] [Google Scholar]
- 8.Conlon C, McDonnell T, Barrett M, Cummins F, Deasy C, Hensey C, et al. The impact of the COVID-19 pandemic on child health and the provision of Care in Paediatric Emergency Departments: a qualitative study of frontline emergency care staff. BMC Health Serv Res. 2021;21(1):279. 10.1186/s12913-021-06284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams TC, MacRae C, Swann OV, Haseeb H, Cunningham S, Davies P, et al. Indirect effects of the COVID-19 pandemic on paediatric healthcare use and severe disease: a retrospective national cohort study. Archives of disease in childhood. 2021;106(9):911-917. 10.1136/archdischild-2020-321008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lampe C, Dionisi-Vici C, Bellettato CM, Paneghetti L, van Lingen C, Bond S, et al. The impact of COVID-19 on rare metabolic patients and healthcare providers: results from two MetabERN surveys. Orphanet journal of rare diseases. 2020;15(1):341. 10.1186/s13023-020-01619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koracin V, Loeber JG, Mlinaric M, Battelino T, Bonham JR, Groselj U. Global impact of COVID-19 on newborn screening programmes. BMJ Global Health. 2022;7(3): e007780. 10.1136/bmjgh-2021-007780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmonem MA, Belanger-Quintana A, Bordugo A, Boruah R, Cortès-Saladelafont E, Endrakanti M, et al. The impact of COVID-19 pandemic on the diagnosis and management of inborn errors of metabolism: A global perspective. Mol Genet Metab. 2020. Nov;131(3):285-288. 10.1016/j.ymgme.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IFCC C-ETPLM, SSIEM, ISNS SURVEY ON IMPACT OF COVID-19 ON PEDIATRIC LABORATORY MEDICINE
Background
The coronavirus disease-2019 (COVID-19) pandemic has spread globally since its first detection in December 2019. In many regions, the COVID-19 is now entering a second and subsequent waves of outbreak. A recent survey by The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Task Force on COVID-19 has revealed that laboratories have had to change their operations to manage the pandemic, including diverting resources and deferring services.
Pediatric laboratory medicine is a unique practice serving a vulnerable group of patients. It includes highly specialized testing that aims to detect and treat inherited conditions early to avoid adverse outcomes. This survey by the IFCC Committee on Emerging Technologies in Pediatric Laboratory Medicine (C-ETLM), in partnership with the Society for the Study of Inborn Errors of Metabolism (SSIEM) and International Society for Neonatal Screening (ISNS), is conducted to:
Understand how laboratories serving the pediatric population have changed their clinical service delivery in response to the initial wave (January to July 2020) of the COVID-19 pandemic
Evaluate how these changes are affecting clinical care
Gather experiences learned from managing the initial wave(s) of the pandemic to improve laboratory preparedness for future outbreaks
This 17-item survey is completely voluntary and should take approximately 15 minutes to complete. The results of this survey will be analyzed in an aggregated manner and published to inform the larger clinical community. No individually identifiable information will be made public. By proceeding with this survey, you have provided an implied consent to the above. Thank you for your participation.
For queries about the survey, please contact Dr. Tim Lang (tim.lang@nhs.net).
General Information
1. Please indicate your country/ region of practice
-
2. Which of the following best describes your laboratory? Please select all that apply.
Newborn Bloodspot Screening
Inherited Metabolic Diseases Testing
Sweat Testing
Genetic / Molecular Testing
Specialist Pediatric Endocrinology Testing
Pediatric Blood Sciences - General Biochemistry / Haematology / Endocrinology / Immunology
General adult laboratory serving neonatal/ pediatric population
Others, please specify:___________________
We do not serve pediatric patients (this is an exclusion question)
-
3. Please indicate the number of patient samples your laboratory processes per day.
<200 samples
200-500 samples
>500 samples
-
4. Which of the following pediatric specialities are supported by your laboratory? Please select all that apply.
General Pediatric Medicine
Community Pediatrics
Neonatal Unit
Specialist Children’s Hospital serving multiple subspecialties
Maternity
Others, please specify:___________________
Delivery of Laboratory Services (to be mirrored for all laboratory areas)
5. What clinical test / clinical laboratory service did your laboratory stop OR restrict during the initial wave of COVID-19 pandemic (Jan to July 2020)? Please specify. Free text
6. How long was the laboratory test / laboratory service suspended for? Months
-
7. What were the two main reasons for the suspension?
Manpower diversion to other areas
Insufficient manpower (e.g. due to split team arrangement)
Concern over risk of COVID-19 transmission
Disruption of reagent/ consumable delivery due to COVID-19
Others, please specify:___________________
8. What are the top 5 tests with a decrease in workload compared to pre-COVID-19 in your laboratory during the initial wave of COVID-19 (Jan to July 2020)? Please indicate the estimated percentage decrease during the worst month.
-
9. Please indicate if the COVID-19 pandemic affected your laboratory’s ability to meet any National Standards for newborn/ pediatric screening programmes available in your country?
Yes/ No. If yes, please elaborate:____________
-
10. Please indicate if the COVID-19 pandemic affected your laboratory’s ability to meet any Accreditation Standards (e.g. ISO 15189) available in your country?
Yes/ No. If yes, please elaborate:____________
Impact on Clinical Care or Pathology of Pediatric Diseases During the Initial Wave of COVID-19 (Jan to July 2020)
11. Did your laboratory observe any increase in missed detection of pediatric diseases related to the clinical laboratory tests / services stopped or restricted due to COVID-19? Please specify the condition and related laboratory tests.
12. Did your laboratory observe any increase in later (more severe) presentation of pediatric diseases related to the tests / services stopped, restricted due to COVID-19? Please specify the condition and related laboratory tests.
13. Did your laboratory observe any positive effect of COVID-19 on laboratory services (or those that can be detected via laboratory tests)?
14. Lessons learned from the Initial Wave of COVID-19 (Jan to July 2020)
15. What are the three most important operational decisions your laboratory has made that positively helped the management of the initial wave of COVID-19? Please specify.
16. What are the three operational decisions you wish your laboratory has avoided when managing the initial wave of COVID-19? Please specify.
17. Was there any technology that your laboratory employed that were helpful in managing the COVID-19? Please specify.
18. What are the two operational areas you consider most important for preparing your laboratory for subsequent waves of COVID-19? Please specify.
This is the end of the survey. Thank you very much for your participation.


