Abstract
Background and Objective
Non-tuberculous mycobacterial lung disease (NTM-LD) manifests with bronchiectasis, inflammatory bronchiolitis, nodules, and/or cavitation. Bronchiectasis is characterized by permanently dilated airways wherein mucus accumulates, creating a vicious cycle of chronic injurious inflammation and recurrent infections. While antibiotics are an important part of the treatment of NTM-LD, airway clearance techniques to mitigate this pathogenic mechanism of bronchiectasis as well as other ancillary measures are also important components of NTM-LD treatment. The objective of this contemporaneous Narrative Review is to emphasize the importance of such ancillary measures.
Methods
We searched PubMed for the key words of “airway clearance”, “pulmonary rehabilitation”, “nutrition”, “swallowing dysfunction”, “gastroesophageal reflux”, “vestibular dysfunction”, or “cochlear dysfunction” with that of “non-tuberculous mycobacterial lung disease”, “bronchiectasis”, or “respiratory disease”. The bibliographies of identified articles were further searched for relevant articles not previously identified. Each relevant article was reviewed by one or more of the authors and a narrative review was composed.
Key Content and Findings
Herein, we discuss five ancillary treatment measures that are pertinent to patients with bronchiectasis and NTM-LD: (I) airway clearance; (II) physical and pulmonary rehabilitation; (III) nutrition; (IV) diagnosis and mitigation of swallowing dysfunction and of gastroesophageal reflux disease (GERD); and (V) minimization of vestibular and cochlear dysfunction associated with some anti-NTM drugs.
Conclusions
While antibiotics is often the central focus of treatment of NTM-LD, given its propensity for recurrent and recalcitrant infection, other ancillary measures to break the vicious cycle of injurious inflammation and infection should also be emphasized to optimize treatment success.
Keywords: Airway clearance, aspiration, bronchiectasis, gastroesophageal reflux, nutrition, vestibular dysfunction
Introduction
Brief overview of non-tuberculous mycobacterial lung disease (NTM-LD)
Non-tuberculous mycobacteria (NTM) are ubiquitous environmental organisms of the home and natural habitats. NTM-LD is likely the most common manifestation of clinically relevant NTM infections (1). While NTM-LD is often a complication of pre-existing structural lung disease such as chronic obstructive pulmonary disease (COPD) or bronchiectasis, NTM may also be a primary cause of bronchiectasis.
Rationale for emphasizing ancillary treatment measures for NTM-LD
Though much of the discussion of NTM-LD treatment has focused on the efficacy of specific antibiotics and cocktail regimens, ancillary treatments with favorable benefit-to-risk ratio are intuitively important. Since bronchiectasis is irreversible and relapses or re-infections with NTM are relatively common, long-term ancillary treatment measures such as airway clearance become critically important. Moreover, airway clearance alone may result in the conversion of NTM sputum culture to negative in a minority of NTM-LD patients with relatively mild bronchiectasis (2,3). The objective of this review is to focus on the following ancillary measures that are relevant for NTM-LD: (I) airway clearance; (II) physical and pulmonary rehabilitation; (III) nutrition; (IV) diagnosis and mitigation of swallowing dysfunction and gastroesophageal reflux disease (GERD); and (V) minimization of vestibular and cochlear dysfunction associated with some of the anti-NTM drugs. In a publication written by an individual with bronchiectasis, similar topics are discussed from a patient’s perspective, denoted by the acronym BE CLEARTM: “Breathing, Exercise, Clearance of airways, Laughter, Eating and drinking, Alternative therapies, and Relaxation, rest, and sleep” (4).
A thorough patient history and examination encompassing cough history (e.g., precipitators, time-of-day), sputum production (e.g., amount, color, consistency), degree of shortness of breath, and symptoms of GERD help funnel what specific ancillary treatment measures should be emphasized. For example: (I) if coughing worsens while eating, exposure to cold air, or while lying down, then swallowing dysfunction, reactive airway disease, and GERD should be considered, respectively, as possible exacerbators of NTM-LD; (II) having a constellation of dental erosion, hoarseness due to laryngitis, chronic cough, and asthma/wheezing may indicate extraesophageal manifestations of GERD (5); and (III) the presence of a localized rhonchi or wheeze helps target specific areas of the lungs for airway clearance. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-410/rc).
Methods
We searched PubMed for relevant articles using the key words of “airway clearance”, “pulmonary rehabilitation”, “nutrition”, “swallowing dysfunction”, “gastroesophageal reflux”, “vestibular dysfunction”, or “cochlear dysfunction” with that of “non-tuberculous mycobacterial lung disease”, “bronchiectasis”, or “respiratory disease”. Citations from 2000 to 2022 were preferentially referenced whenever possible. Further details of the literature search are described in Table 1. The bibliographies of identified articles were further perused for relevant articles not previously identified. Each relevant article was reviewed by one or more of the authors and a narrative review was composed. Since most of the references on treatments such as hypertonic saline (HS) and nutrition pertain to bronchiectasis in general or to those who are malnourished, respectively, and not necessarily to individuals with NTM-associated bronchiectasis, we have used some of these published papers in this article but noted the limitations. While other topics such as control of sinusitis and optimization of sleep and mental health may indirectly impact respiratory health, they are not discussed in this narrative review.
Table 1. The search strategy summary.
| Items | Specification |
|---|---|
| Date of search | March 1, 2020 to June 15, 2022 |
| Databases and other sources searched | PubMed; up to June 15, 2022 |
| Search terms used | “Airway clearance”, “pulmonary rehabilitation”, “nutrition”, “swallowing dysfunction”, “gastroesophageal reflux”, “vestibular dysfunction”, or “cochlear dysfunction” with that of “non-tuberculous mycobacterial lung disease”, “bronchiectasis”, or “respiratory disease” |
| Timeframe | 2000–2022 |
| Inclusion and exclusion criteria | Inclusion: English language only |
| Selection process | EDC did the initial literature search with subsequent help from all authors |
Discussion
Airway clearance
Healthy individuals produce 10–100 mL of airway secretions per day. Normally, these secretions are cleared by the mucociliary escalator as well as transient increases in expiratory airflow during more forceful exhalation or coughing. Individuals with COPD (emphysema) and bronchiectasis often have excessive sputum production, which may be thick, tenacious, and difficult to expectorate. For patients with bronchiectasis, changes in rheologic properties of mucus, defects in mucociliary clearance, and ineffective cough all contribute to a vicious cycle of infection, inflammation, and airway damage (Figure 1). Chronic sputum production in patients with NTM-LD is a marker of more severe disease, reduced lung function, and worse health-related quality-of-life (HR-QoL) (6).
Figure 1.
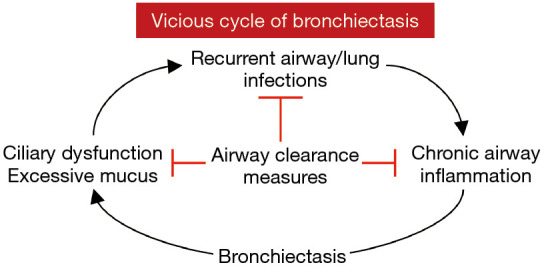
Vicious cycle of bronchiectasis. Independent of the underlying risk factor for bronchiectasis, once it is established, bronchiectasis tends to beget more bronchiectasis. The excessive mucus and ciliary dysfunction in the bronchiectatic airway predispose to recurrent infections, which exacerbates airway inflammation, resulting in more mucus accumulation, ciliary dysfunction, and progression of bronchiectasis. In addition to treatment of infections, airway clearance measures can help mitigate these pathogenic mechanisms that drive bronchiectasis.
Chest physical therapy and airway clearance therapy (chest PT-ACT) are important components of treatment for all causes of bronchiectasis. However, a study of ~1,800 bronchiectatic patients from the U.S. Bronchiectasis and Non-TB Mycobacteria Research Registry showed that only 56% of the subjects were employing such bronchial hygiene. Those with NTM-LD had greater use of bronchial hygiene (59% vs. 50%), chest percussion (19% vs. 12%), and use of positive expiratory pressure (PEP) valves (52% vs. 40%) than those without NTM-LD (7). The same registry also showed that bronchiectatic patients with productive cough were more likely to use ACT if they experienced a prior exacerbation, hospitalization for pulmonary illness, or had Pseudomonas aeruginosa cultured (8). Unfortunately, the use of ACT was substantially lower at one-year follow up (8). In a retrospective analysis at enrollment and at one-year follow-up of bronchiectatic patients in the same registry, ACT use at baseline and follow-up was associated with greater odds of experiencing exacerbations at follow-up compared to those who did not use ACT; the authors interpreted this counterintuitive finding as ACT use perhaps identified a more severe bronchiectasis population (8). Similarly, a prospective study of bronchiectasis patients showed that adherence to ACT was associated with lower (i.e., worse) scores in both the QoL-bronchiectasis Treatment Burden (i.e., spending longer time on treatment) and QoL-bronchiectasis Respiratory Symptoms domains (i.e., having more problematic respiratory symptoms) (9). Since airway clearance is low risk and intuitively helpful in patients with NTM-LD, it seems unlikely that randomized studies without an ACT arm will be conducted to determine its efficacy. Furthermore, there is indirect evidence for its beneficial effect in that in individuals with mild NTM-LD who are not treated with antibiotics but continued on ACT, 30–50% spontaneously convert their sputa to negative (3,10). Chest PT-ACT is comprised of a combination of: (I) breathing and cough techniques; (II) airway clearance devices; (III) skilled manual techniques and postural drainage; and (IV) pharmacologic agents to help mobilize airway secretions. The goals of chest PT-ACT are to enhance mucociliary clearance, improve ventilation and oxygenation, and reduce both breathlessness and cough frequency. Chest PT-ACT is usually performed twice a day. When possible, patients should initially be coached by a physical therapist trained in outpatient airway care.
Breathing and cough techniques
Several breathing and cough techniques are available. All aim to facilitate secretion clearance and mobilize impacted sputum. The most common techniques utilized in patients with bronchiectasis and NTM-LD are the autogenic drainage (AD), pursed-lip breathing and huff cough (Forced Expiratory Technique), and Active Cycle of Breathing Technique (ACBT) (11).
AD technique
AD is an ACT comprised of a series of controlled breathing exercises aimed at achieving the highest possible expiratory airflow while minimizing bronchospasm and dynamic airway collapse (12). This technique loosens and mobilizes secretions from distal airways toward the proximal and larger central airways (13). The three phases of AD are comprised of escalating breath volumes (small, medium, and large tidal volumes) and active exhalation with mouth open. The exhalation should not be forceful as this may collapse the airways, obstructing mucus movement.
Pursed-lip breathing and huff cough
Pursed-lip breathing is an expiration technique wherein exhalation is performed through the mouth with the lips loosely apposed together, with the resistance created akin to blowing into a highly compliant balloon (Figure 2A). In essence, pursed-lip breathing creates PEP to stent airways open during exhalation. A huff cough (also known as the “forced expiratory technique”) is performed by forcibly exhaling slowly once or twice through an open mouth with the abdomen moving in, creating the “HAA ” sound as if fogging a mirror (Figure 2B). A 51 seconds video shows how a huff cough is done (14). Huff coughing optimizes shearing forces along the airway walls and mobilizes secretions to the proximal airways with less airway collapse than the active cough. Huff cough from low lung volumes mobilizes secretions from the peripheral to the central airways whereas from high lung volumes expectorates the secretions.
Figure 2.
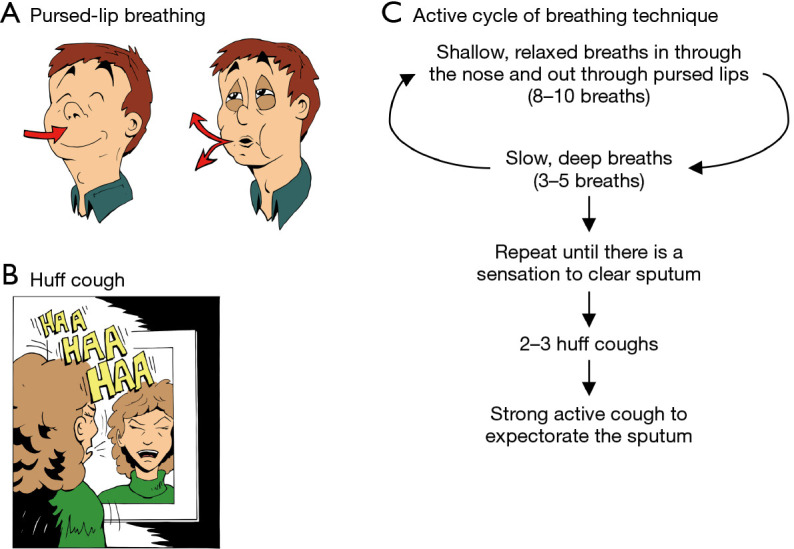
Basic breathing and cough techniques to help expectorate sputum. (A) Pursed-lip breathing: following inhalation, bring the lips loosely together and exhaled through the mouth against resistance. (B) Huff cough: with mouth open, inhale either a normal or deep breath, hold for 2–3 seconds, and then exhale using your abdomen, making a “HAA” sound, as in fogging a mirror. (C) ACBT is comprised of cycles of shallow and deep breathing, followed by huff and strong, active coughs. There are three steps to ACBT. Step 1: take 8 to 10 slow, shallow, relaxed breaths (also known as Breathing Control) in through the nose and out through pursed lips. Step 2: take 3 to 5 slow deep breaths, expanding the lung bases. If coughing is triggered by deep breathing, return to Step 1. Repeat Steps 1 and 2 until there is a sensation of secretions in the chest or throat, then proceed to the next step. Step 3: perform 2 to 3 huff coughs followed by strong, active coughs to expectorate the sputum. ACBT, Active Cycle of Breathing Technique.
ACBT
ACBT is a cycle of 8 to 10 relaxed breaths, followed by 3 to 5 deep breaths (Figure 2C). In contrast to the AD technique of exhalation through an open mouth, the exhalation in ACBT is performed through pursed lips. Once secretions are sensed or heard, then huff coughs followed by strong active coughs are performed. ACBT can be done sitting or in other postural drainage positions. If no secretions are sensed in the throat or chest after performing the relaxed and deep breaths for at least 5 to 7 cycles, the patient should proceed to huff and active coughs, and then re-start the cycle of relaxed followed by deep breaths. Each session may be repeated two to four times per day.
Airway clearance devices
PEP device
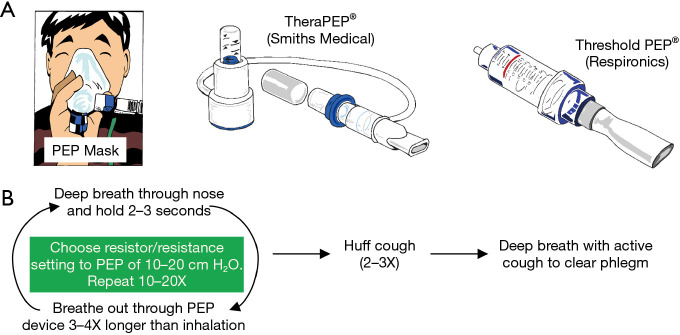
A PEP device requires the patient to perform exhalation against a set resistance, a maneuver that stents airways open and enhances mobilization of secretions, similar to pursed-lip breathing. Several different types of PEP devices are available, including a PEP face mask with different color-coded resistor options or a PEP mouthpiece with variable resistance determined by lumen size or a dial (Thera PEP® and Threshold PEP®) (Figure 3A). The general use of the PEP devices is summarized according to manufacturers’ instructions (Figure 3B). The resistance of the device is set to achieve an expiratory pressure of 10 to 20 cmH2O. There are no absolute contraindications to their use. Relative contraindications include untreated pneumothorax, increased intracranial pressure, and active hemoptysis. Currently, PEP devices are used principally to treat atelectasis since oscillatory PEP devices are superior for secretion clearance.
Figure 3.
Positive expiratory pressure mucus clearing devices and their use. (A) PEP devices may be used with a mask or mouthpiece. (B) Algorithm of PEP use. PEP, positive expiratory pressure.
Oscillatory positive expiratory pressure (OPEP) therapy devices
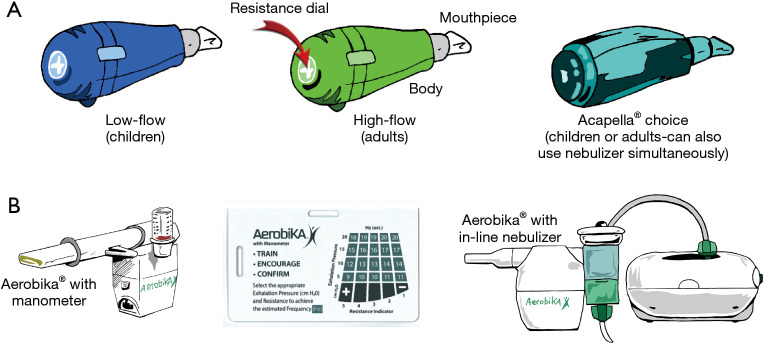
OPEP therapy devices combine PEP with vibrations of the airway walls during active exhalation. The airflow oscillations created decrease the viscosity of sputum, facilitating mobilization of secretions (15). Two commonly used OPEP devices are the Acapella® and Aerobika® (Figure 4A,4B). Both can be used in most postural drainage positions. Directions for the use of the Acapella® and Aerobika® devices are shown in Tables 2,3, respectively. A video for use of the Acapella® is also available on-line (16).
Figure 4.
Various OPEP devices. (A) Acapella® OPEP devices. Acapella® blue is for individuals who are only able to maintain expiratory flows of <15 liters/minute for 3 seconds and thus for patients with suboptimal inspiratory capacity such as children; and Acapella® green is for those who are able to maintain expiratory flows of >15 liters/minute for 3 seconds (most adults); Acapella Choice® can be used by all patients. (B) Aerobika® OPEP device with a manometer; a manometer guide correlating exhalation pressure with resistance indicator and number of beats per second (Hz) achievable; and the device attached to a nebulizer. OPEP, oscillatory positive expiratory pressure.
Table 2. Use of the Acapella®.
| Use |
| Assure proper setting of the resistance dial on the end of the Acapella® valve. Start at the mid-resistance point and rotate toward + or − to increase or decrease resistance as tolerated. Adjust resistance so that you can exhale for at least 3 seconds |
| Sit upright although various positions may be required for optimal drainage of secretions |
| Take in a bigger than normal breath through the nose and hold for 2 to 3 seconds |
| Place the mouthpiece in the mouth and seal lips tightly around it |
| Exhale actively (NOT forcefully and keeping the cheeks flat and firm) for at least 3 times longer than it took to inhale and until the flutter sound ceases |
| Repeat 10 times, then perform 3 huff coughs, followed by a big cough to bring out the sputum. These 10 exhalations and 3 huff coughs is one set |
| Repeat the above set 2 to 4 times per day |
| Cleaning |
| Clean the mouthpiece at the end of the day in water and liquid dish detergent, rinse, and dry thoroughly overnight |
| Disinfect once weekly by removing the mouthpiece and soak in 70% rubbing (isopropyl) alcohol for 5 minutes or 3% hydrogen peroxide for 30 minutes. Rinse thoroughly with water and drain/dry in a vertical position |
Table 3. Use of the Aerobika®.
| Use |
| Inhale bigger than normal breath and hold 2 to 3 seconds |
| Place mouthpiece in mouth |
| Exhale for 3 to 4 times longer than inspiration, if able |
| Do 10 to 20 breaths as tolerated |
| Perform 2 to 3 huff coughs, followed by a deep cough |
| Repeat 2 to 4 times per day or as tolerated |
| Use with manometer |
| Aerobika® may come with a manometer, which gauges whether the expiratory blow is adequate. The manometer contains a green zone (5 to 20 cmH2O), yellow zone (20 to 40 cmH2O), and red zone (40 to 60 cmH2O). It is recommended to stay within the GREEN ZONE; e.g., 10 to 15 cmH2O pressure |
| Based on the chart below, at a resistance setting of “3”, if one exhales to 10 to 15 cmH2O pressure, this results in an airway beating frequency of 13 to 16 hertz |
| Use with nebulizer |
| Aerobika® may be used in-line with a nebulizer that contains a bronchodilator or hypertonic saline |
| There is an adaptor called AeroEclipse® specially designed to be use with the Aerobika®. It is a breath-actuated small volume nebulizer. Aerosolized medication is only produced when patients inhale through the device. This means that between breaths or during breaks in treatment, prescribed medication is contained in the cup and there is less medication waste |
Daily cleaning of the devices consists of disassembling parts as recommended by the manufacturer, soaking in warm, soapy water for 15 minutes, rinsing in cooled previously boiled water, and air drying. Weekly sterilizing options—if manufacturer allows—include placing the device: (I) on a colander and boiling in water for 10 minutes; (II) in 70% rubbing alcohol for 10 minutes and rinsing with cooled, boiled water; (III) in 3% hydrogen peroxide for 30 minutes and rinsing; (IV) in microwave-safe steam bags, or (V) in a self-contained steam or ultraviolet light sterilizer, followed by thorough air drying before reassembling the device.
High frequency chest wall oscillation (HFCWO)
A HFCWO device is comprised of a wearable thoracic vest connected to an air-pulse generator capable of supplying various frequencies. The generator produces pressures of about 50 cmH2O, delivered at a frequency of around 525 Hz, with the air-pulse oscillation (rapid cycle of inflation-deflation) felt as vibrations. The rapid oscillations increase expiratory flow, increase air-liquid interactions in the airways, decrease mucus viscosity, and facilitate mucus clearance from the peripheral to the central airways.
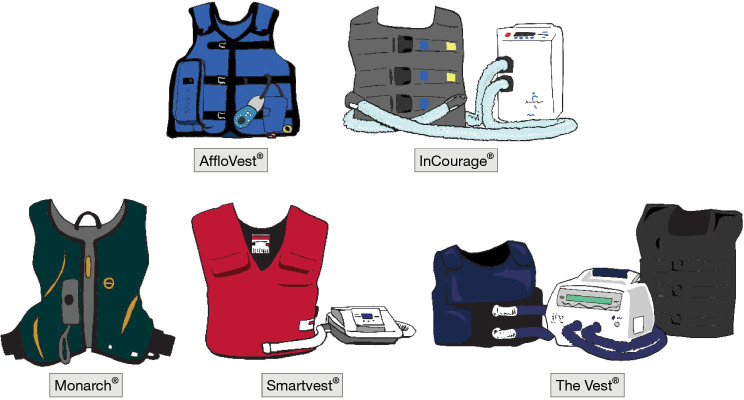
Several HFCWO devices are available, with each having different features, including how the vests are secured (Figure 5). Typically, HFCWO devices are used at intervals of 5, 7, or 10 minutes, followed by the ACBT and/or huff coughs. This cycle is repeated for 15 to 30 minutes for each session, with two sessions performed daily. HFCWO devices may be used simultaneously with nebulized HS. The use of HFCWO device in those with small airway disease may be associated with greater propensity for airway collapse (11).
Figure 5.
The HFCWO devices. Several different manufactured HFCWO devices are shown. Basic instructions for set up and use are usually provided by the manufacturer technician, the use time and frequency by the pulmonologist, and incorporation of breathing techniques and/or OPEP devices with the HFCWO equipment by the physical therapists who specialize in secretion clearance techniques. See relevant text on additional discussions on its use. HFCWO, high frequency chest wall oscillation; OPEP, oscillatory positive expiratory pressure.
Skilled manual techniques and postural drainage
Skilled manual techniques
With the introduction of OPEP and HFCWO devices, manual techniques to mobilize secretions are used less frequently. Still, some patients prefer them alone or in conjunction with OPEP and/or HFCWO devices, especially if there is a family member with technical expertise. Skilled manual techniques include percussion, shaking, and vibration. Percussion is performed over the chest wall using cupped hands (“clapping”) (Figure 6A). Shaking consists of larger and slower movements, performed with a downward and inward motion of both hands over the chest wall (Figure 6B). Vibrating uses smaller and more rapid manual movements over the chest wall (Figure 6C). While percussion is performed during normal breathing or during a deep breath hold, shaking and vibration are performed during expiration.
Figure 6.
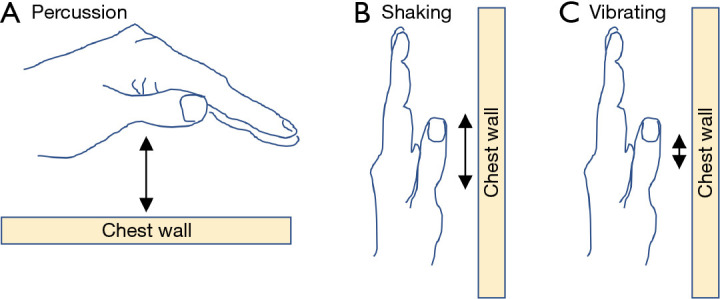
Manual techniques. (A) Cupped hand used for “clapping” chest physiotherapy to help disrupt airway mucus. This “cupping-and-clapping” technique can be performed with one or both hands and is applied relatively rapidly to the chest wall in a patient who is supine, prone, sitting up, or in another position. (B) Position of the hand used for the shaking technique (the larger movements depicted by the longer double-arrow) or (C) the vibrating technique (the smaller movements depicted by the shorter double-arrow) typically performed on and parallel to the chest wall. The orientation of the hand depicted is for shaking or vibrating the chest wall in a patient who is sitting upright.
Postural drainage
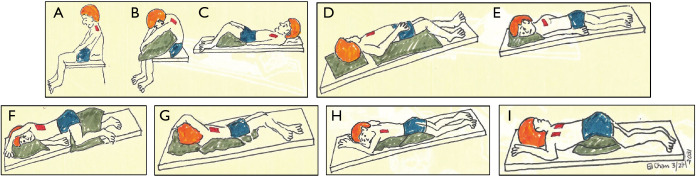
Postural drainage utilizes body positioning and gravity to facilitate mobilization and expectoration of airway secretions. Patients position themselves to target specific lung lobes (Figure 7A-7I), followed by skilled manual techniques (or use of an OPEP device and/or ACBT/huff coughs) to disrupt airway secretions and to facilitate expectoration. If GERD is present, certain postural drainage positions—such as prone and head down positions—should either be modified or avoided.
Figure 7.
Postural drainage positions. The different positions used are aimed to target drainage from specific lung lobes and segments. (A) Upper lobes, apical segments: patient sits and leans back 30 degrees. Cupping and clapping over area between the clavicle and top of scapula on both sides. (B) Upper lobes, posterior segments: patient leans forward 30 degrees. Cupping and clapping over upper back on both sides. (C) Upper lobes, anterior segments: patient lies supine with knees bent. Cupping and clapping between clavicles and nipples on both sides. (D) Right middle lobe: patient lies in Trendelenburg position and rotate to left 1/4 turn with knees flexed. In males, cupping and clapping over right nipple area. In women, raise right arm over the head and perform cupping and clapping at mid-axillary line. (E) Lingula: patient lies in Trendelenburg position and rotate to right 1/4 turn with knees flexed. In males, cupping and clapping over left nipple area. In women, raise left arm over the head and perform cupping and clapping at mid-axillary line. (F) Lower lobes, anterior basal segments: patient lies in Trendelenburg position on contralateral side with pillow under knees. Cupping and clapping over lower ribs at mid-axillary line. (G) Lower lobes, lateral basal segments: patient lies in Trendelenburg prone position and rotate 1/4 turn upward ipsilaterally with legs between pillow and upper legs flexed. Cupping and clapping over upper part of lower ribs. (H) Lower lobes, posterior basal segments: patient lies in Trendelenburg prone position with pillows under hips. Cupping and clapping over lower ribs just lateral to the spine. (I) Lower lobes, superior segments: patient lies prone with two pillows under hips. Cupping and clapping over the middle of the back at the scapula tips next to the spine.
Pharmacologic agents to help mobilize airway secretions
HS solution
While HS is commonly used in patients with cystic fibrosis (CF) and non-CF bronchiectasis including those with NTM-LD (17), the available studies are relatively small and most often compared to isotonic (0.9%) saline. In CF patients with bronchiectasis, nebulized HS modestly improves lung function [forced expiratory volume in the first second (FEV1) increased by 3–4% at four weeks of treatment but not at 48 weeks], reduces the frequency of pulmonary exacerbations, and enhances sputum clearance (18).
Four studies have analyzed the efficacy of HS in non-CF subjects and one with NTM-LD. In a four-week trial of 24 subjects with non-CF bronchiectasis of isotonic vs. 7% HS, use of HS resulted in greater sputum weight and ease of expectoration, and less viscosity compared to isotonic saline use (19). In a follow-up, randomized cross-over study of 30 patients of daily isotonic saline vs. 7% HS for three months for each of the two phases, 7% HS was superior to isotonic saline with regards to increase in FEV1 and ease of expectoration as well as significant decrease in respiratory symptoms, antibiotic usage, and emergency room visits (20). In contrast to these salubrious effects of HS, a study of 40 subjects with non-CF bronchiectasis showed no difference in exacerbation frequency, QoL parameters, sputum colonization, or FEV1 after one year of treatment when randomized to either isotonic saline or 6% HS (21). Similarly, in 22 patients with primary ciliary dyskinesia who underwent a randomized, cross-over study of isotonic saline vs. 7% HS for 12 weeks each, there were no differences in the FEV1, forced vital capacity (FVC), St George’s Respiratory Questionnaire score, number of exacerbations, or inflammatory markers (22). Only the QoL-Bronchiectasis Health Perception score improved more with HS than isotonic saline (22). In a retrospective study of 25 NTM-LD patients who were treated for at least three months with HS without antibiotics, 36% reported symptomatic improvement, 28% reported stability, and 20% noted deterioration (2). There was no change in lung function measurements with the HS. While six of the 12 patients who continued the HS without antibiotics beyond three months converted their respiratory culture to negative, the lack of a control arm limited more definitive interpretation (2).
The mechanism of action of HS is considered to be due to increase hydration of mucus (reducing its stickiness), increase volume of the epithelial lining fluid (improving mucociliary clearance), and stimulation of cough, ultimately enhancing sputum expectoration, reducing exacerbations, increasing lung function, and improving QoL. In addition, 3% or 7% HS may have some antimicrobial activity against pyogenic bacteria and NTM; i.e., while NTM grows well in 1–2% saline, it does not grow in the 3.5% saline present in ocean water (23). The recommended dose is 3% or 7% HS once or twice daily, either before use of or simultaneously with an OPEP device. Side effects include throat irritation, uncontrolled cough, and bronchoconstriction.
DNAse
Dying neutrophils and other cells in the airways release DNA, a highly viscous macromolecule. Breaking down extracellular DNA present in the biofilm matrix and tenacious sputum with DNAse I helps reduce their viscosity (24). Dornase alfa is a recombinant pancreatic human DNAse used for CF patients with bronchiectasis and shown to decrease viscosity and elasticity of the airway mucus (25). However, dornase alfa was found not to be helpful in patients with non-CF bronchiectasis and generally not recommended in them (26).
Mannitol
Mannitol is a naturally occurring sugar alcohol that is neither metabolized, absorbed, nor transported across the epithelium. Consequently, mannitol works as an osmotic agent by drawing water into the airway lumina, hydrating, thinning, and enhancing clearance of viscous mucus (27,28). Mannitol also improves mucociliary clearance by increasing the osmotic pressure of the fluid lining and mucosal surfaces (29).
In patients with bronchiectasis, inhaled dry-powder mannitol augments mucus clearance in a dose-response manner both acutely and over 24 hours (30). Inhaled mannitol in CF patients has been shown to increase lung function as well as decrease pulmonary exacerbations, need for additional antibiotics, and hospitalizations (28,31). The dry powder formulation of mannitol is more convenient and easier to use than that delivered by a nebulizer. The recommended dose of mannitol is 400 mg twice daily by oral inhalation. Side effects include throat irritation, cough, and bronchospasm.
Oral dipeptidyl dipeptidase-1 inhibitor
Extracellular neutrophil elastase is a key pathogenic mediator of bronchiectasis by causing ciliary dysfunction, mucous gland hyperplasia, increased mucus secretion as well as cleavage of Fcγ receptors and complement receptor-1 from cell surfaces (32). The latter two activities may impair host-defense against microbes, perpetuating the vicious cycle of inflammation and infection observed with bronchiectasis. In a phase 2, double-blind, placebo-controlled trial of brensocatib (an inhibitor of dipeptidyl peptidase-1, a protease that activates elastase by removing the N-terminal dipeptide of elastase during neutrophil maturation), in patients with bronchiectasis showed that the time to first exacerbation was prolonged compared to placebo (33).
Physical and pulmonary rehabilitation
Patients with chronic lung disease may exhibit a vicious cycle of physical inactivity, deconditioning, decreased strength, and increased breathlessness, resulting in further diminished activity (34). This cycle may also result in less participation in social activities and increased vulnerability to social isolation and depression (35).
The main goal of pulmonary rehabilitation for patients with NTM-LD is to minimize this cycle of deconditioning, breathlessness, and decreased activity (35,36). Pulmonary rehabilitation encompasses several elements including: (I) exercise training; (II) education about proper breathing strategies, medication usage, disease self-management, and appropriate use of supplemental oxygen therapy; and (III) behavioral modifications such as smoking cessation and nutritional counseling (37). This section will only focus on the first of these.
In patients with COPD, pulmonary rehabilitation with exercise training has been shown to increase the oxidative capacity of skeletal muscle, augment physical strength and improve breathing mechanics and function, leading to decreased dyspnea and increased exercise capacity (38,39); these benefits likely apply to patients with NTM-LD although this has not been formally studied (40). In patients with bronchiectasis, pulmonary rehabilitation decreases depression and anxiety (41) and improves QoL, as measured by dyspnea, fatigue, emotional well-being, and/or exercise capacity (34,42-44). A retrospective study of 108 patients with non-CF bronchiectasis showed that three weeks of pulmonary rehabilitation significantly decreased dyspnea (a change in the Baseline Dyspnea Index of ≥1 unit in 90%), improved HR-QoL, and increased the six-minute walk test (6MWT) distance by ~36 meters, with the lattermost element quantifying the distance covered with six minutes of walking at a normal pace (45). Those with airflow limitation showed an even greater improvement in 6MWT distance and HR-QoL with such intervention (45). A review of four studies (164 participants) demonstrated that eight weeks of outpatient pulmonary rehabilitation or exercise training in patients with non-CF bronchiectasis significantly increased walk distance by ~67 meters (95% CI: 52–82 meters) and improved HR-QoL for up to six months (34). Blakney and co-workers (46) recently showed that in patients with the nodular-bronchiectasis form of NTM-LD, the distance walked in the 6MWT was inversely related to mortality. Thus, if pulmonary rehabilitation can increase 6MWT distance walked (34,45), one could infer that it can also potentially improve mortality although this remains to be determined in NTM-LD patients. We elaborate below how the benefits of pulmonary rehabilitation can be objectively evaluated, the types of exercise training, and the schedule prescribed.
Evaluating the benefits of pulmonary rehabilitation
Objective evaluation to determine if pulmonary rehabilitation improved physical performance may be accomplished with either cardiopulmonary exercise stress testing (CPET) and/or 6MWT. CPET is performed on treadmill or bicycle ergometer and measures several physiologic variables at baseline and response to exercise—such as maximum oxygen consumption, oxygen pulse, anaerobic threshold, tidal volume, dead space ventilation, and minute ventilation as well as maximum heart rate, blood pressure, and electrocardiography (47,48). Thus, a collective analysis of these CPET variables can implicate broadly whether the exercise limitation is due to cardiovascular, pulmonary, and/or deconditioning in nature. The 6MWT provides practical and functional information to a walking challenge, is well validated in those with cardiopulmonary dysfunction, and is simple to perform (49).
Types of exercise training
The types of exercise training include aerobic exercise, resistance training, flexibility exercise, balance training, and breathing exercises. Aerobic exercise, which should include both upper and lower extremities, progressively challenges skeletal muscles and, with training, decreases ventilatory demands over time (38,50). Lower ventilatory requirement and lesser dynamic hyperinflation may reduce dyspnea at rest or with activity (38). Upper extremity aerobic exercise can also reduce arm fatigue, thus improving daily function (35,50).
Resistance training improves muscle mass, strength, and function, resulting in lower oxygen consumption, minute ventilation, and dyspnea (51). Flexibility exercises (e.g., shoulder rolls, upper back stretch, scapular retractions, cervical spine stretches, pectoral muscle stretches) and thoracic mobility exercises (e.g., upper chest stretches, rib cage mobility and stretches, thoracic extension, child pose position) assist to improve breathing mechanics. Balance training may decrease fall risk and increase confidence in functional activities. Breathing exercises include pursed-lip breathing, diaphragmatic breathing, and paced breathing (a controlled breathing technique that coordinates a deep breath with physical exertion).
Schedule of exercise training
Exercise prescription for pulmonary rehabilitation should use the FITT (Frequency, Intensity, Time and Type) formula (48,52). A reasonable target frequency of exercise is three to five times per week. The intensity and duration time of exercise should be increased gradually and dictated by (I) symptoms; (II) metabolic equivalents (METs) from the CPET or distance achieved from the 6MWT; (III) target heart rate range; or (IV) a patient self-reported rate of perceived exertion scale. Periods of gradual warm up and cool down should be incorporated to all exercise types.
Nutrition
Low body weight adversely affects outcome of NTM-LD
A low body mass index (BMI) in individuals with NTM-LD has been associated with increased number of diseased lung segments, greater disease progression, worse outcome, and greater NTM-LD-specific mortality (41,53-56). Many individuals with NTM-LD and no known predisposing conditions possess a life-long slender body habitus with greater than expected frequency of thoracic cage abnormalities such as pectus excavatum and scoliosis (57-63). NTM-LD subjects often demonstrate reduced visceral (and likely subcutaneous) fat (64). Those with purging eating disorders may be at increased risk for NTM-LD (65). Hypotheses proposed for the possible increased susceptibility to NTM-LD in fat-depleted individuals include: (I) a reduction in leptin with a secondary reduction in both interferon-gamma producing TH1 cells (61,62,66,67) and phagocytic cell and NK cell function (60); (II) the asthenic body habitus being a component of a underlying connective tissue disorder such as forme fruste Marfan syndrome resulting in both compromised airway wall integrity (due to fibrillin-1 defect) and increased expression of the immunosuppressive cytokine transforming growth factor-beta (57,62,68); and (III) aspiration into the lungs during the self-induced vomiting in patients with bulimia (which may be occult) (65). While optimizing nutrition improves outcomes in individuals with caloric-restrictive eating disorders (69), it is not known whether active weight gain improves NTM-LD outcomes due to a lack of prospective studies. Nevertheless, until such data are available, it is prudent to optimize nutrition.
Malnutrition and immunity
Malnutrition (protein-energy malnutrition and/or micronutrient deficiencies) impairs the ability of the immune cells to mount an effective inflammatory and killing response against microbial pathogens (70,71). Certain micronutrients (e.g., vitamin C, vitamin E, iron, zinc, copper, selenium) are important antioxidants that protect immune cells against oxidative injury created during the destruction of the microbes. Others (e.g., vitamin A, vitamin D, vitamin B6, vitamin B12, folate, iron, zinc) are critical for developing new immune cells or enhancing their function against mycobacteria (72,73). Additionally, the omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid are incorporated into immune cell membranes and shown to increase phagocytosis (74). Because these fatty acids induce anti-inflammatory compounds and polarize macrophages to the M2 phenotype, they may be important in limiting tissue damage once the infection is under control (74). Similarly, in those with eating disorders and malnutrition, the increased pro-inflammatory state and a dysregulated immune system may promote increased oxidative stress and predispose to NTM infections (75).
Albumin is not an accurate biomarker of nutritional status
Since inflammation decreases plasma albumin level, blood albumin concentration is not a reliable biomarker for malnutrition (76). Furthermore, a normal albumin does not necessarily indicate adequate nutrition as evinced by the fact that individuals with anorexia nervosa often have normal albumin levels (77). The implication is that achieving a normal plasma albumin level requires both optimizing caloric intake and controlling inflammation. In contrast, prealbumin has greater validity as a measure of nutritional status in individuals with acute, chronic, or critical illnesses, due, in part, to its shorter half-life than albumin (2 vs. 20 days), it being less affected by liver disease, and not affected by hydration (78). While prealbumin may be a better biomarker of nutritional status than albumin, its level has also been found to be inversely related to acute inflammation associated with critical illness (79); thus, its use as a marker of malnutrition may be limited. Although preoperative prealbumin level <20 mg/dL is a risk factor for post-operative infection after elective spinal surgery (80) and longer mechanical ventilation after cardiac surgery (81), comparable data are unavailable for surgical lung resection for NTM-LD.
Optimizing nutrition in NTM-LD
Restoring weight and maintaining a healthy weight may be difficult in patients with NTM-LD due to numerous factors. NTM-LD is often characterized by factors that impair weight gain such as fever, increased work of breathing, muscle and/or fat breakdown, and barriers to adequate intake—such as excessive cough, early satiety, anorexia, fatigue, shortness of breath, and gastrointestinal/taste disturbance due to medication adverse effects. In some NTM-LD patients with overt or occult eating disorders (65), body image distortions with or without purging actively prevents adequate caloric intake (82).
Nutritional therapy in individuals with NTM-LD is focused on restoring weight and meeting increased needs for calories, protein, and micronutrients. A registered dietitian can identify those NTM-LD patients who are at nutritional risk. Some weight restoration suggestions are shown in Table 4. General types of foods recommended or not endorsed are shown in Table 5. Because of the large number of calories required to restore weight, there may be a need to supplement oral calories with enteral tube feeds (continuous or nocturnal). Those at risk of refeeding syndrome have been identified to possess at least one of the major risk factors (BMI <16 kg/m2, unintentional weight loss >15% in the past 3–6 months, little or no nutritional intake >10 days, low baseline levels of potassium, phosphorus, magnesium) or at least two of the minor risk factors (BMI <18.5 kg/m2, unintentional weight loss >10% in the past 3–6 months, little or no nutritional intake >5 days) (83). Such at-risk individuals should be supervised by a provider with expertise in refeeding of the severely underweight (83).
Table 4. Nutritional tips for weight restoration.
| Food tips | Behavioral tips for food intake |
|---|---|
| Limit non-calorie beverages such as water, coffee, tea, and diet soda. Avoid non-fat, low-fat, diet items | Schedule 4–6 small, frequent meals/snacks daily |
| Add drinks that have higher content of calories and/or protein: milk, non-citrus juice. Blend homemade, high-calorie smoothies with added protein powder | Rely on family and friends to prepare meals or frozen, restaurant and take-out foods as needed |
| Eat more calories per meal with higher calorie but nutritious foods, aiming for ~500 calories per meal. Examples of high-calorie, healthy foods: avocados, canned tuna, cottage cheese, dried fruits, unsweetened yogurt, nuts, nut butters (peanut or almond), seeds, and healthy fats such as olive oil, canola oil, and fish fat | Ask your doctor about ways to relieve gastrointestinal symptoms such as nausea, vomiting, and constipation, which can negatively impact appetite |
| Choose regular, high-calorie snacks. Aim for 250 calories per snack | If experiencing a metallic taste, try using wooden utensils instead of metal |
| Calorie boosters: item [calories] | If your sense of taste is diminished, try adding spices and condiments to foods to make them more appealing. Sweet additives like maple syrup stimulate the palate. Fats like olive oil enhance flavor Try light exercise, such as a 20-minute walk, about an hour before meals to stimulate your appetite. Consult your physician before starting an exercise program Meet with an RD for additional advice on meal planning and for individualized recommendations Consider recording everything you eat and drink for a three-day period to assess opportunities for improving your caloric intake. This journal can also be reviewed with your physician or dietitian |
| 1 cup whole milk [150] | |
| 1 cup apple juice [125] | |
| 1 avocado [210] | |
| 1/4 cup granola [130] | |
| 1/4 cup nuts [200] | |
| 1 Tbsp. nut butter [peanut, almond] [90] | |
| 1 Tbsp. olive oil [120] | |
| 1/4 cup raisins [100] |
RD, registered dietitian; Tbsp., tablespoon.
Table 5. General types of foods recommend or to avoid.
| Food types | Examples |
|---|---|
| Healthy carbohydrates: deliver vitamins, minerals, fiber, phytochemicals. Try to pair with a protein when able | Starchy vegetables: sweet potato, potatoes, winter squash, corn, green peas |
| Whole fruits: berries, banana, apple, pear, stone fruit, citrus fruit, tropical fruit | |
| Pulses: lentils, beans, mung beans | |
| Whole grains: oats, quinoa, brown rice, black rice, bulgur, barley, whole grain breads/products | |
| Other: popcorn, whole grain 3 grams fiber breakfast cereal, sourdough bread | |
| Starchy vegetables: sweet potato, potatoes, winter squash, corn, green peas | |
| Whole fruits: berries, banana, apple, pear, stone fruit, citrus fruit, tropical fruit | |
| Pulses: lentils, beans, mung beans | |
| Whole grains: oats, quinoa, brown rice, black rice, bulgur, barley, whole grain breads/products | |
| Other: popcorn, whole grain breakfast cereal, sourdough bread | |
| Less healthy carbohydrates to avoid or limit: delivers starches, additives, fillers, preservatives, little to no nutrition value | Sugary drinks: fruit juice, cola, vitamin water, sweetened teas |
| Refined flour: pastries, muffins, bagels, rolls, tortillas, white bread, white pasta, white rice, cookies, and cakes. Products made with whole grain flours are better choices | |
| Many gluten-free products are laden with gluten free starches like rice and tapioca flours that are devoid of nutrition | |
| Sweets: limit candies and chocolates | |
| Junk: French fries, potato chips, highly processed packaged foods | |
| ‘Health’ or diet products: sugar sweetened yogurts, nutrition bars | |
| Healthy fats: eat fat to get healthy calories and help absorb nutrients | Olive oil, grapeseed, or avocado oil to cook with, cold-pressed preferred |
| Use an avocado as a spread, dip, or base for a creamy dressing | |
| Fatty fish like arctic char, sardines, herring, mackerel, anchovies | |
| Nuts and seeds like almonds, pistachios, pecans, macadamia, pine nuts, walnuts, cashews, pumpkin seeds, flax, and chia | |
| Eggs, 3–4 per week if cholesterol sensitive | |
| Butter from grass fed cows if able, coconut oil in moderation | |
| Probiotic foods: Fermented foods promote healthy microbiome, healthy immunity, controlled by the food we eat | Pickled vegetables, sauerkraut, kimchi |
| Yogurt, coconut yogurt, kefir, buttermilk | |
| Miso, tempeh, natto, kombucha | |
| Supplements: if choosing over the counter probiotics, look for one with multiple strains of bacteria with a dose in the billions. Time around antibiotics by ±3 hours. Always discuss and disclose with physician | |
| Prebiotic foods: essentially, high fiber foods feed the bacteria that create a healthy microbiome | Leafy greens like dandelion greens, kale, collards, chard, arugula, spinach, mustard greens |
| Asparagus, jicama, Jerusalem artichokes, fennel, bok choy, watercress, lettuces | |
| Garlic, onions, leek, scallions, shallots | |
| Bananas, plantains, apples, pears | |
| Fresh herbs like parsley, dill, mint | |
| Healthy proteins to meet increased needs with acute or chronic infection | Chicken, fish, meat (>3 oz serving size), eggs, dairy foods like cottage cheese, Greek yogurt, and legumes/pulses and nuts/seeds |
Diagnosis and mitigation of swallowing dysfunction and of GERD
Oropharyngeal dysphagia and GERD increase the risk of aspiration. In turn, microaspiration is likely to exacerbate the lung inflammation associated with NTM-LD (56). Esophageal disorders complicated by lipoid pneumonia, a sign of aspiration, are also associated with NTM-LD (84). While the prevalence of swallowing dysfunction is unknown for patients with NTM-LD, the prevalence of dysphagia in those over 50 years old (the age group at greatest risk for NTM-LD) is estimated to be 15–22%, higher than the 6–9% prevalence for the general population (85). In three separate studies, GER was present in 26–44% of NTM-LD subjects versus 12–28% in non-NTM infected controls (86-88). The prevalence of GERD in NTM-LD subjects may even be greater as some may not experience typical reflux symptoms. Whether mitigation of swallowing dysfunction or of GER impacts outcomes of NTM-LD is not known. However, based on our collective clinical experience, it is prudent to formally evaluate for GERD and/or swallowing dysfunction, particularly for those with recurrent and/or recalcitrant NTM-LD.
Oropharyngeal dysphagia
Clinical assessment of dysphagia by a trained speech therapist can identify heightened aspiration risk but in itself is insufficient to guide treatment of swallowing disorders (89). Therefore, testing with Modified Barium Swallow (MBS) fluoroscopic study or by Fiberoptic Endoscopic Evaluation of Swallowing (FEES) should be considered in NTM-LD patients in whom there is suspicion for microaspiration, as identified clinically and/or radiographically. MBS is a structured procedure performed fluoroscopically where the patient consumes barium-containing boluses of different consistencies (90). The goals of a MBS are to: (I) determine appropriate function of the oropharyngeal structures and (II) if abnormal swallowing is detected, to evaluate the effect of compensatory strategies on decreasing aspiration risk (90).
An alternative to MBS study is a FEES. During a FEES, a fiberoptic scope is passed to the level of the pharynx, followed by self-fed boluses (91). Velar (soft palate) function can also be assessed fiberoptically. The goals of FEES are to: (I) observe the integrity, function, and sensory response of the pharyngeal structures and (II) evaluate the effect of compensatory strategies on decreasing aspiration risk (90,91). Based on identified oropharyngeal dysfunction and successful mitigating strategies that are tried during these exams, appropriate treatment plans can be developed.
Speech therapy is the cornerstone of treatment for oropharyngeal dysphagia (92). Management is targeted toward (I) swallowing safely via compensatory strategies and dietary modifications, and, when appropriate (II) swallow strengthening. Behavioral and compensatory strategies to decrease aspiration risk include adjustments in posturing, bolus size, or corrective swallowing maneuvers, such as swallowing with effort or with the chin tucked. Training on coordinating breathing with swallowing has been shown to be another effective treatment strategy to decrease aspiration in patients with head and neck cancer (92). Modifications to food or liquid consistencies (e.g., thickening of liquids) may reduce aspiration risk during drinking or eating for the dysphagic individual (93). Successful swallowing techniques tried during an MBS or FEES procedure are useful to formulate the recommended behavioral and dietary modifications to limit swallowing-induced aspiration. Strengthening exercises to target swallowing muscles may be introduced in speech therapy.
GERD
GERD is caused by a combination of increased frequency of transient relaxation of the lower esophageal sphincter (LES), decreased basal LES tone, and poor esophageal motility (94,95). Laryngopharyngeal reflux (LPR), where stomach contents flow toward the pharynx, raises the risk of aspiration of gastric contents into the lungs (96). Common symptoms of GERD or LPR include chest pain (heartburn), regurgitation (“water brash”), and/or a chronic cough. Accuracy of diagnosing GERD or LPR solely on reported clinical symptoms is unclear (95). Since many individuals with NTM-LD do not experience typical reflux symptoms or may be asymptomatic (86), formal diagnostic testing may be recommended.
A 24-hour pH impedance probe has high sensitivity and specificity for diagnosing GERD and is considered the gold standard (95) as compared to tests for esophageal function (esophagram, manometry) or mucosal injury (endoscopy). A catheter with ring-shaped electrodes to measure electro-conductance is placed transnasally and remains for 24 hours. Symptoms, mealtimes, medications, and changes in position are recorded by the patient (97). A summary of the correlation between acidic or nonacidic reflux events to the symptoms is assembled. Similarly, a Bravo probe (Bravo pH Monitoring System®, Medtronic, Minneapolis, MN, USA) is a wireless pH recording capsule that is placed into the esophagus and records data over a 48-hour period (95).
The cornerstone in the management of GERD includes lifestyle changes to reduce episodes of reflux. These measures include dietary changes that avoid acidic foods or foods which reduce LES pressure (Table 6), or behavioral changes such as elevating head of bed (30–45 degrees with knees slightly elevated), weight loss, smoking cessation, remaining upright after eating, no food or drink within three hours of sleeping, and sleeping on the left side or supine (94). Certain drugs can also lower the LES tone and worsen reflux (Table 7). Antacid medications such as proton pump inhibitors or H2 blockers are used mainly to control the heartburn symptoms of reflux and to prevent esophagitis (94). However, in the context of NTM-LD, acid reduction may prevent the suppression of NTM growth in the stomach as the optimal growth pH is ~6.0 for slow-growing NTM and Mycobacterium chelonae and is ~7–7.4 for other rapidly growing mycobacteria (98-100). This pH hypothesis is supported by a study that analyzed for the presence of NTM in both the gastric juice and gastrostomy tubes in 16 CF patients who were receiving gastric tube feedings (101). In 7 of 16 patients, live NTM (M. abscessus complex) were isolated in the gastric juices in three subjects and in the gastrostomy tube in two individuals (101). Interestingly, all 5 patients in whom NTM was isolated from gastric juice or gastrostomy tube had gastric pH ≥3 (range, 3 to 6) with a mean pH of 5.0; in other words, all with NTM isolated had gastric pH greater than the normal gastric pH of 0.3–2.9 (102). Furthermore, of the 11 patients without NTM isolated from either the gastric juice or gastrostomy tube, five had gastric pH of two and six had gastric pH ≥3 with a mean pH of 3.4 (101). While not definitive, these findings bolster the notion that a higher gastric pH may be more likely to support NTM viability in the upper gastrointestinal tract.
Table 6. Foods that decrease the tone of the lower esophageal sphincter.
| Alcohol (especially red wine) |
| Caffeine |
| Carbonated beverages |
| Chocolate |
| Citrus fruits |
| Coffee—caffeinated or non-caffeinated |
| Fatty-spicy foods |
| Garlic |
| Onions |
| Peppermint |
| Tomatoes |
Table 7. Drugs that can worsen gastroesophageal reflux.
| Albuterol |
| Benzodiazepines |
| Calcium channel blockers |
| Diphenhydramine |
| Nitrates |
| Opioids |
| Oxybutynin |
| Progesterone |
| Quinidine |
| Theophylline |
| Tricyclic antidepressant |
Surgery specific to anti-GERD management is the Nissen fundoplication, where the proximal stomach is wrapped around the distal esophagus to create a barrier to prevent reflux (94). Fundoplication can better improve control over reflux symptoms as compared to antacid therapy when medical and behavioral management is insufficient (103). The LINX Reflux Management System is an alternative, less-invasive surgical approach that preserves the gastric anatomy and may be reversed if needed (104). In this laparoscopic procedure, a circular chain of magnetic titanium beads is placed around the esophagus inferior to the diaphragm. The magnetic ring prevents GERD but allows swallowed contents to enter the stomach. This procedure may be appropriate for individuals whose reflux is sub-optimally managed with lifestyle changes with and without pharmaceuticals, but who may not be candidates for surgical fundoplication (104).
Minimization of vestibular and cochlear dysfunction associated with some anti-NTM drugs
Ototoxicity and risk factors for vestibular and cochlear dysfunction
Ototoxicity is defined as the cellular degeneration of cochlear and/or vestibular tissues leading to functional deterioration as a result of exposure to certain drugs or chemicals (105). Treatment of NTM-LD often involves medication(s) that are potentially toxic to the vestibulo-cochlear (or VIII cranial) nerve, resulting in bilateral hearing loss, tinnitus, and/or loss of balance. These offending medications include macrolides (azithromycin or clarithromycin) and aminoglycosides (amikacin or streptomycin). Ototoxicity was observed in 42% of patients treated with streptomycin and 27% of patients treated with amikacin for macrolide-resistant Mycobacterium avium complex pulmonary disease (106).
Occurrence of ototoxicity increases when multiple risk factors are present (Table 8). The risk of ototoxicity is typically greater with intravenous administration of the offending agent than with the inhaled or oral routes, when the drug is in the body for longer durations, and with increased lifetime dose of the drug (107). In the presence of concurrent renal insufficiency, the risk for ototoxicity with these antibiotics may increase further (105,108). In addition, a genetic susceptibility to aminoglycoside-induced ototoxicity has been identified (109).
Table 8. Risk factors for ototoxicity.
| Age: >60 years old |
| Co-morbid conditions: congestive heart failure, renal failure, hypertension, and dehydration leading to accumulation of ototoxic drugs |
| Genetic susceptibility: two mutations in the mitochondrial 12s rRNA gene have been implicated to place predisposed carriers at risk for aminoglycoside ototoxicity |
| Drugs: examples include non-steroidal anti-inflammatory drugs, quinine-based derivatives, loop diuretics, aminoglycoside antibiotics, macrolide antibiotics, platinum-based chemotherapies like cisplatin |
| Dosing: greater dose, lifetime dose, and duration of administration of otoxic drug(s) as well as prior and/or concurrent administration of other ototoxic drugs |
| Route and rate of administration: faster, intravenous administration at greater risk |
| History of noise exposure/pre-existing sensorineural hearing loss |
Clinical manifestations of vestibular ototoxicity
As ototoxic medications are not selective to one ear, bilateral hearing and/or vestibular loss is the typical presentation. Toxicity specific to cochlear nerve results in the onset or worsening of hearing loss and/or complaints of tinnitus. With vestibular toxicity, patients may complain of dizziness, vertigo, oscillopsia (a disturbing bouncing sensation of the surroundings with head or whole body movements resulting in an inability to see clearly during such movements), and decreased balance especially with the eyes closed (resulting in limitations when walking in the dark or on uneven surfaces) (110,108). Oscillopsia often results in reduced physical activity and greater social isolation. Due to these limitations, vestibular dysfunction places patients at an increased risk for falling, especially when compounded by muscle disuse due to respiratory compromise. As respiratory issues may dominate the symptomatology, patients may under-report or not appreciate symptoms related to ototoxicity. Thus, a thorough history and examination to elicit vestibular dysfunction becomes essential.
Diagnostic laboratory tests for ototoxicity
Audiology screening is used to monitor for cochlear nerve toxicity. High frequency audiogram is a standardized and sensitive tool to detect early hearing loss. In contrast, testing for vestibulotoxicity is not standardized. Some of the diagnostic tests used to assess vestibular function include electro/video-nystagmography (111), caloric testing (112), rotary chair test (113), and video head impulse test (114) (Table 9). Bilateral vestibular loss is present when there is reduced or absent function of the vestibulo-ocular reflex in both ears. The diagnostic criteria for bilateral vestibular loss are listed in Table 10 (107). Diagnosis is primarily based on the history of exposure to ototoxic drugs, risk factors, symptoms, and degree of impairment quantified by the aforementioned tests. A clinical vestibular evaluation may help determine impaired functional status and risk for falling.
Table 9. Diagnostics tests for vestibular function.
| ENG or VNG |
| ENG refers to a series of clinical tests that utilize small surface electrodes positioned around the eyes to monitor eye movements when visual fixation is present or removed. VNG utilize goggles with an infrared video camera to track the eyes. Eye movements are monitored during visual tracking, saccade testing, positional testing and during vestibular stimulation via caloric stimulation (see below). Both clinical tests can be used to evaluate signs of nystagmus, central or peripheral vestibular dysfunction or neurological problems. These tests are commonly administered to people with symptoms of dizziness, vertigo, and/or imbalance and offer valuable information in order to diagnose vestibular disorders. VNG is the preferred method between the two |
| Caloric testing |
| Considered the “gold standard” vestibular test to diagnose hypofunction, the caloric test is a component of the VNG test. Warm or cold temperature air or water is introduced into the auditory canal to provoke a stimulus of either excitation or inhibition of the horizontal semicircular canal of the inner ear. In the intact ear, nystagmus is generated and measured by VNG |
| Rotary chair test |
| Rotary Chair test has been utilized as a diagnostic tool to primarily evaluate bilateral vestibular function. The test is performed with patients seated in a special chair while wearing VNG goggles or ENG. The chair is rotated at physiologic frequencies that stimulate bilateral horizontal semicircular canals. The VOR response from the chair motion is simultaneously measured via surface EMG electrodes or, more commonly, eye movements are measured via infrared video camera with goggles VOR eye movement responses are then compared to the chair rotational velocities to determine if the response is within normal ranges. Normative data for rotational chair testing has been established and stratified by age from 6 to >50 years old. When compared to caloric tests, the rotary chair test is more sensitive and ENG testing more specific when diagnosing peripheral vestibulopathy |
| vHIT |
| vHIT utilizes eye tracking technology with head velocity transducers affixed to glasses or goggles to determine how well a client’s eyes stay fixed on a stable target when the head is passively rotated with an unpredictable, high velocity, small amplitude head movement. vHIT measures the VOR response to the head movement to determine if gaze is stable or altered. It can be used to determine unilateral or bilateral vestibular hypofunction related to dysfunction of the vestibular ocular reflex by measuring head/eye movement gain and overt/covert abnormal saccadic eye movements |
ENG, electronystagmography; VNG, videonystagmography; EMG, electromyography; VOR, vestibular-ocular reflex; vHIT, video head impulse test.
Table 10. Diagnostic criteria for bilateral vestibular loss.
| A. Chronic vestibular syndrome with at least three of the following symptoms: |
| Postural imbalance |
| Unsteadiness of gait |
| Movement-induced blurred vision or oscillopsia during walking or quick head/body movements |
| Worsening of postural imbalance or unsteadiness of gait in darkness and/or on uneven ground |
| B. No symptoms while sitting or lying down under static conditions |
| C. Bilaterally reduced or absent angular VOR function documented by bilaterally pathological horizontal angular VOR gain <0.6, measured by the video-head impulse test or scleral-coil technique and/or reduced caloric response on VNG and/or reduced horizontal angular VOR gain <0.1 upon sinusoidal stimulation on a rotary chair |
| D. Not better accounted for by another disease |
VOR, vestibular-ocular reflex; VNG, Videonystagmography.
Vestibular evaluation
Patient symptom history is a critical component when there is suspected bilateral vestibular loss. As the vestibular system works in conjunction with vision and proprioception to optimize postural control and gaze stability during movements, a complete evaluation would screen for abnormalities in these other sensory systems. A thorough history should include: (I) patient complaints; (II) abnormal signs noted by caretakers or those close to the patient; (III) functional and participation limitations; (IV) previous and current medical problems; and (V) lifetime history of ototoxin exposure and other risk factors.
Standardized screening questionnaires should be utilized to determine a patient’s perception of his or her handicap as well as to identify risk for falling. The Dizziness Handicap Inventory is a validated measure intended to assess a patient’s perceived degree of limitations due to dizziness. The Activities-Specific Balance Confidence (ABC) Scale is a self-report measure that assesses patient confidence in performing various activities without falling
Clinical bedside tests to assess vestibular function include: ocular tracking, saccades, rapid head thrust, post head shaking nystagmus (requires infrared goggles), dynamic visual acuity, modified Clinical Test of Sensory Interaction on Balance, and assessment of gait speed (108).
Treatment of vestibular dysfunction
Once ototoxicity is diagnosed or strongly suspected, discontinue the offending drug promptly—if it can be done safely—as nerve damage may become irreversible with continued use of the ototoxic medication. While such discontinuation may deprive individuals of an effective antibiotic against the offending NTM, there is optimism in the near future that aminoglycoside-induced ototoxicity may be prevented pharmacologically (115-117).
Vestibular rehabilitation is an exercise-based program designed by a licensed physical therapist to reduce problems related to dizziness and to improve balance by promoting gaze stability and postural control. This type of therapy involves head movements and balance exercises that are essential in stimulating and retraining the vestibular system.
For patients with bilateral vestibular loss, the goal of treatment is to recover lost vestibular function by promoting central compensation. This attempt at recovery is accomplished through gaze stability exercises, involving the combined use of adaptation, substitution, and imaginary target exercises that foster dynamic use of visual information (Figure 8A-8I) (110). Treatment may also include a range of tailored postural stability and gait exercises to improve static and dynamic balance, enhance gait speed, and decrease risk for falling. Patient education should include reducing further ototoxic exposure, protecting vision and proprioception, and ensuring a safe home environment to prevent falls. Properly treated, patients can improve their overall functional level with a decreased risk for falling. However, some residual deficits may persist.
Figure 8.
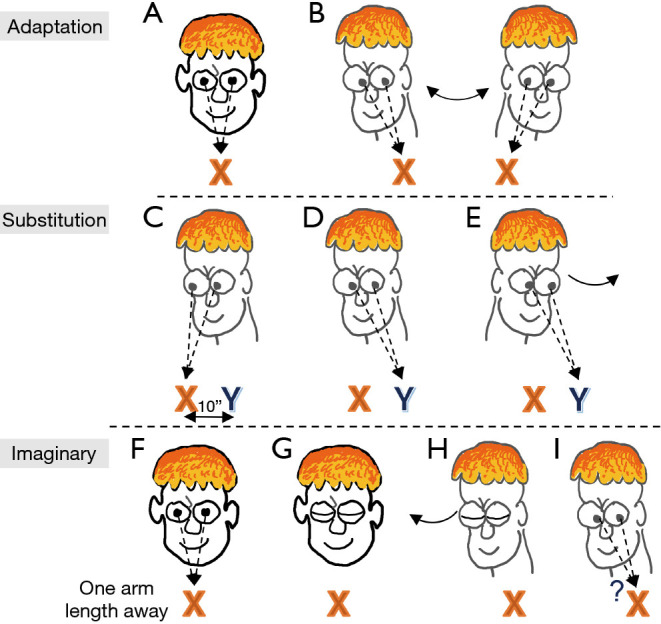
Exercises for promoting gaze stability. Adaption: (A) to test the vestibular-ocular reflex, have a target directly in front of the subject at eye level; the target may be as simple as an “X” written on a sheet of paper taped to a wall. (B) Sit or stand at one arm length from the target. Move the head quickly from side-to-side as if shaking the head for “no” while maintaining direct eye contact with the target. Repeat while shaking the head up and down. Substitution: To perform this exercise, place two targets (“X” and “Y”) at eye level approximately 10 inches apart (close enough so when the subject is looking at one target, the second one is in the peripheral vision). (C) Look at one target with the head facing in the same direction as the target. (D) Then move only the eyes to the second target. (E) Then move the head to face the second target. Be sure to keep the target in focus when moving the head and be as accurate as possible. Gradually increase the speed of the head turn and length of time while keeping the target in focus. Imaginary: (F) sit or stand facing the target one arm length away and focus on the target. (G) Close both eyes. (H) Turn head to the side while keeping closed eyes focused to the direction of the target. (I) Then open eyes and ask if the eyes are still fixated on the target. Repeat in opposite direction. Gradually increase the speed of the head turn. Exercise can also be performed with up and down head movements.
Conclusions
NTM-LD is often recalcitrant to treatment. Thus, in addition to reducing exposures to the NTM present in certain environmental niches and treating with antibiotics, ancillary treatment measures are an important component to treatment. Herein, we focused on five ancillary measures that should be considered to help maximize treatment outcomes: airway clearance, physical and pulmonary rehabilitation, nutrition, diagnosis and mitigation of swallowing dysfunction and of GERD, and minimization of vestibular and cochlear dysfunction associated with some of the anti-NTM drugs.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Betsy Glaeser, NTM Support Group Leader in New York City, for her dedication to the education of patients with NTM lung disease and to the health care providers. We also are grateful to our patients who continue to teach us how to better address their health needs.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-410/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-410/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-410/coif). EDC serves as an unpaid editorial board member of Journal of Thoracic Disease from February 2021 to January 2023. The other authors have no conflicts of interest to declare.
References
- 1.Griffith DE. Nontuberculous Mycobacterial Disease: A Comprehensive Approach to Diagnosis and Management. Switzerland: Springer Nature; 2019. [Google Scholar]
- 2.Huiberts A, Zweijpfenning SMH, Pennings LJ, et al. Outcomes of hypertonic saline inhalation as a treatment modality in nontuberculous mycobacterial pulmonary disease. Eur Respir J 2019;54:1802143. 10.1183/13993003.02143-2018 [DOI] [PubMed] [Google Scholar]
- 3.Moon SM, Jhun BW, Baek SY, et al. Long-term natural history of non-cavitary nodular bronchiectatic nontuberculous mycobacterial pulmonary disease. Respir Med 2019;151:1-7. 10.1016/j.rmed.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 4.Esposito L. The BE CLEAR Method to Living with Bronchiectasis. New York, NY: Independently Published; 2021. [Google Scholar]
- 5.Hom C, Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease. Gastroenterol Clin North Am 2013;42:71-91. 10.1016/j.gtc.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 6.Yamane K, Furuuchi K, Tabusadani M, et al. Influence of chronic sputum symptoms on quality of life in patients with nontuberculous mycobacterial pulmonary disease: A cross-sectional study. Respir Investig 2022;60:277-83. 10.1016/j.resinv.2021.09.011 [DOI] [PubMed] [Google Scholar]
- 7.Aksamit TR, O'Donnell AE, Barker A, et al. Adult Patients With Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017;151:982-92. 10.1016/j.chest.2016.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basavaraj A, Choate R, Addrizzo-Harris D, et al. Airway Clearance Techniques in Bronchiectasis: Analysis From the United States Bronchiectasis and Non-TB Mycobacteria Research Registry. Chest 2020;158:1376-84. 10.1016/j.chest.2020.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCullough AR, Tunney MM, Quittner AL, et al. Treatment adherence and health outcomes in patients with bronchiectasis. BMC Pulm Med 2014;14:107. 10.1186/1471-2466-14-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang JA, Kim S, Jo KW, et al. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J 2017;49:1600537. 10.1183/13993003.00537-2016 [DOI] [PubMed] [Google Scholar]
- 11.Flude LJ, Agent P, Bilton D. Chest physiotherapy techniques in bronchiectasis. Clin Chest Med 2012;33:351-61. 10.1016/j.ccm.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 12.McCormack P, Burnham P, Southern KW. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database Syst Rev 2017;10:CD009595. 10.1002/14651858.CD009595.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hough A. Physiotherapy in Respiratory Care: An evidence based approach to respiratory and cardiac management. 3rd ed. Nelson Thornes Ltd; 2001. [Google Scholar]
- 14.Available online: https://www.youtube.com/watch?v=qYStVdltzTU
- 15.Myers TR. Positive expiratory pressure and oscillatory positive expiratory pressure therapies. Respir Care 2007;52:1308-26; discussion 1327. [PubMed] [Google Scholar]
- 16.Available online: https://www.youtube.com/watch?v=b2RhtbBIi3w
- 17.Máiz Carro L, Martínez-García MA. Nebulized hypertonic saline in noncystic fibrosis bronchiectasis: a comprehensive review. Ther Adv Respir Dis 2019;13:1753466619866102. 10.1177/1753466619866102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wark P, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev 2018;9:CD001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellett F, Redfern J, Niven RM. Evaluation of nebulised hypertonic saline (7%) as an adjunct to physiotherapy in patients with stable bronchiectasis. Respir Med 2005;99:27-31. 10.1016/j.rmed.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 20.Kellett F, Robert NM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir Med 2011;105:1831-5. 10.1016/j.rmed.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 21.Nicolson CH, Stirling RG, Borg BM, et al. The long term effect of inhaled hypertonic saline 6% in non-cystic fibrosis bronchiectasis. Respir Med 2012;106:661-7. 10.1016/j.rmed.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 22.Paff T, Daniels JM, Weersink EJ, et al. A randomised controlled trial on the effect of inhaled hypertonic saline on quality of life in primary ciliary dyskinesia. Eur Respir J 2017;49:1601770. 10.1183/13993003.01770-2016 [DOI] [PubMed] [Google Scholar]
- 23.George KL, Parker BC, Gruft H, et al. Epidemiology of infection by nontuberculous mycobacteria. II. Growth and survival in natural waters. Am Rev Respir Dis 1980;122:89-94. [DOI] [PubMed] [Google Scholar]
- 24.Roy R, Tiwari M, Donelli G, et al. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018;9:522-54. 10.1080/21505594.2017.1313372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon GM, Hoover W, Sorscher EJ, et al. Cystic fibrosis: Diagnosis and management. In: Broaddus VC, Ernst JD, King TE Jr, et al., editors. Murray & Nadel’s Textbook of Respiratory Medicine. Philadelphia, PA: Elsevier; 2022:919-40. [Google Scholar]
- 26.O'Donnell AE, Barker AF, Ilowite JS, et al. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 1998;113:1329-34. 10.1378/chest.113.5.1329 [DOI] [PubMed] [Google Scholar]
- 27.Button B, Goodell HP, Atieh E, et al. Roles of mucus adhesion and cohesion in cough clearance. Proc Natl Acad Sci U S A 2018;115:12501-6. 10.1073/pnas.1811787115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nevitt SJ, Thornton J, Murray CS, et al. Inhaled mannitol for cystic fibrosis. Cochrane Database Syst Rev 2018;2:CD008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong HX, Traini D, Salama R, et al. The effects of mannitol on the transport of ciprofloxacin across respiratory epithelia. Mol Pharm 2013;10:2915-24. 10.1021/mp400030n [DOI] [PubMed] [Google Scholar]
- 30.Daviskas E, Anderson SD. Inhaled mannitol as a therapeutic medication. Clin Pulm Med 2016;23:197-202. 10.1097/CPM.0000000000000165 [DOI] [Google Scholar]
- 31.Jaeger A, Moole V, Dharmapuri S, et al. Efficacy and Safety of Inhaled Dry Powder Mannitol in Treating Cystic Fibrosis: A Meta-Analysis and Systematic Review of Randomized Trials. Austin Crit Care J 2016;3:1015. [Google Scholar]
- 32.Solomon GM, Chan ED. Bronchiectasis. In: Broaddus VC, Ernst JD, King TE Jr, et al., editors. Murray and Nadel’s Textbook of Respiratory Medicine. Philadelphia, Pennsylvannia: Elsevier; 2022:941-60. [Google Scholar]
- 33.Chalmers JD, Haworth CS, Metersky ML, et al. Phase 2 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N Engl J Med 2020;383:2127-37. 10.1056/NEJMoa2021713 [DOI] [PubMed] [Google Scholar]
- 34.Lee AL, Hill CJ, McDonald CF, et al. Pulmonary Rehabilitation in Individuals With Non-Cystic Fibrosis Bronchiectasis: A Systematic Review. Arch Phys Med Rehabil 2017;98:774-782.e1. 10.1016/j.apmr.2016.05.017 [DOI] [PubMed] [Google Scholar]
- 35.Corhay JL, Dang DN, Van Cauwenberge H, et al. Pulmonary rehabilitation and COPD: providing patients a good environment for optimizing therapy. Int J Chron Obstruct Pulmon Dis 2014;9:27-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casaburi R. Impacting patient-centered outcomes in COPD: deconditioning. Eur Respir Rev 2006;15:42-46. 10.1183/09059180.00009904 [DOI] [Google Scholar]
- 37.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. Erratum in: Am J Respir Crit Care Med 2014;189:1570. 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 38.Belman MJ. Exercise in patients with chronic obstructive pulmonary disease. Thorax 1993;48:936-46. 10.1136/thx.48.9.936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahler DA. Pulmonary rehabilitation. Chest 1998;113:263S-8S. 10.1378/chest.113.4_Supplement.263S [DOI] [PubMed] [Google Scholar]
- 40.Ali J. A multidisciplinary approach to the management of nontuberculous mycobacterial lung disease: a clinical perspective. Expert Rev Respir Med 2021;15:663-73. 10.1080/17476348.2021.1887734 [DOI] [PubMed] [Google Scholar]
- 41.Paz-Díaz H, Montes de Oca M, López JM, et al. Pulmonary rehabilitation improves depression, anxiety, dyspnea and health status in patients with COPD. Am J Phys Med Rehabil 2007;86:30-6. 10.1097/PHM.0b013e31802b8eca [DOI] [PubMed] [Google Scholar]
- 42.Lee AL, Hill CJ, Cecins N, et al. The short and long term effects of exercise training in non-cystic fibrosis bronchiectasis--a randomised controlled trial. Respir Res 2014;15:44. 10.1186/1465-9921-15-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metersky ML, ZuWallack RL. Pulmonary rehabilitation for bronchiectasis: if not now, when? Eur Respir J 2019;53:1802474. 10.1183/13993003.02474-2018 [DOI] [PubMed] [Google Scholar]
- 44.Patel S, Cole AD, Nolan CM, et al. Pulmonary rehabilitation in bronchiectasis: a propensity-matched study. Eur Respir J 2019;53:1801264. 10.1183/13993003.01264-2018 [DOI] [PubMed] [Google Scholar]
- 45.Zanini A, Aiello M, Adamo D, et al. Effects of Pulmonary Rehabilitation in Patients with Non-Cystic Fibrosis Bronchiectasis: A Retrospective Analysis of Clinical and Functional Predictors of Efficacy. Respiration 2015;89:525-33. 10.1159/000380771 [DOI] [PubMed] [Google Scholar]
- 46.Blakney RA, Ricotta EE, Follmann D, et al. The 6-minute walk test predicts mortality in a pulmonary nontuberculous mycobacteria-predominant bronchiectasis cohort. BMC Infect Dis 2022;22:75. 10.1186/s12879-022-07054-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pichurko BM. Exercising your patient: which test(s) and when? Respir Care 2012;57:100-10; discussion 110-3. 10.4187/respcare.01428 [DOI] [PubMed] [Google Scholar]
- 48.Bernard S, Riberiro F, Maltais F, et al. Prescribing exercise training in pulmonary rehab: A clinical experience. Rev Port Pneumol 2014;20:92-100. 10.1016/j.rppneu.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 49.Available online: https://www.sralab.org/rehabilitation-measures/6-minute-walk-test
- 50.de Blasio F, Polverino M. Current best practice in pulmonary rehabilitation for chronic obstructive pulmonary disease. Ther Adv Respir Dis 2012;6:221-37. 10.1177/1753465812444712 [DOI] [PubMed] [Google Scholar]
- 51.Daabis R, Hassan M, Zidan M. Endurance and strength training in pulmonary rehabilitation for COPD patients. Egyptian J Chest Dis Tuberc 2017;66:231-6. 10.1016/j.ejcdt.2016.07.003 [DOI] [Google Scholar]
- 52.Butts JF, Belfer MH, Gebke KB. Exercise for patients with COPD: an integral yet underutilized intervention. Phys Sportsmed 2013;41:49-57. 10.3810/psm.2013.02.1999 [DOI] [PubMed] [Google Scholar]
- 53.Griffith DE, Wallace RF, Jr. Pulmonary disease due to rapidly growing mycobacteria. Semin Respir Med 1988;9:505-13. 10.1055/s-2007-1012744 [DOI] [Google Scholar]
- 54.Ikegame S, Maki S, Wakamatsu K, et al. Nutritional assessment in patients with pulmonary nontuberculous mycobacteriosis. Intern Med 2011;50:2541-6. 10.2169/internalmedicine.50.5853 [DOI] [PubMed] [Google Scholar]
- 55.Hayashi M, Takayanagi N, Kanauchi T, et al. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012;185:575-83. 10.1164/rccm.201107-1203OC [DOI] [PubMed] [Google Scholar]
- 56.Sulaiman I, Wu BG, Li Y, et al. Evaluation of the airway microbiome in nontuberculous mycobacteria disease. Eur Respir J 2018;52:1800810. 10.1183/13993003.00810-2018 [DOI] [PubMed] [Google Scholar]
- 57.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis 1991;144:914-6. 10.1164/ajrccm/144.4.914 [DOI] [PubMed] [Google Scholar]
- 58.Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066-74. 10.1164/rccm.200805-686OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okumura M, Iwai K, Ogata H, et al. Clinical factors on cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease. Intern Med 2008;47:1465-72. 10.2169/internalmedicine.47.1114 [DOI] [PubMed] [Google Scholar]
- 60.Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gend Med 2010;7:5-18. 10.1016/j.genm.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 61.Tasaka S, Hasegawa N, Nishimura T, et al. Elevated serum adiponectin level in patients with Mycobacterium avium-intracellulare complex pulmonary disease. Respiration 2010;79:383-7. 10.1159/000231975 [DOI] [PubMed] [Google Scholar]
- 62.Kartalija M, Ovrutsky AR, Bryan CL, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med 2013;187:197-205. 10.1164/rccm.201206-1035OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talbert J, Chan ED. The association between body shape and nontuberculous mycobacterial lung disease. Expert Rev Respir Med 2013;7:201-4. 10.1586/ers.13.23 [DOI] [PubMed] [Google Scholar]
- 64.Wakamatsu K, Nagata N, Maki S, et al. Patients with MAC Lung Disease Have a Low Visceral Fat Area and Low Nutrient Intake. Pulm Med 2015;2015:218253. 10.1155/2015/218253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grayeb DE, Chan ED, Swanson LM, et al. Nontuberculous mycobacterial lung infections in patients with eating disorders: plausible mechanistic links in a case series. AME Case Rep 2021;5:9. 10.21037/acr-20-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lord GM, Matarese G, Howard JK, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998;394:897-901. 10.1038/29795 [DOI] [PubMed] [Google Scholar]
- 67.Ordway D, Henao-Tamayo M, et al. Animal model of Mycobacterium abscessus lung infection. J Leukoc Biol 2008;83:1502-11. 10.1189/jlb.1007696 [DOI] [PubMed] [Google Scholar]
- 68.Ovrutsky AR, Merkel PA, Schonteich E, et al. Patients with non-tuberculous mycobacterial lung disease have elevated transforming growth factor-beta following ex vivo stimulation of blood with live Mycobacterium intracellulare. Scand J Infect Dis 2013;45:711-4. 10.3109/00365548.2013.800947 [DOI] [PubMed] [Google Scholar]
- 69.Garber AK, Cheng J, Accurso EC, et al. Short-term Outcomes of the Study of Refeeding to Optimize Inpatient Gains for Patients With Anorexia Nervosa: A Multicenter Randomized Clinical Trial. JAMA Pediatr 2021;175:19-27. 10.1001/jamapediatrics.2020.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drake VJ, Angelo G, Gombart AF. Immunity in depth. Oregon State University; 2016. [Google Scholar]
- 71.Linus Pauling Institute. Immunity in brief. Oregon State University; 2020. [Google Scholar]
- 72.Kim EW, De Leon A, Jiang Z, et al. Vitamin A Metabolism by Dendritic Cells Triggers an Antimicrobial Response against Mycobacterium tuberculosis. mSphere 2019;4:e00327-19. 10.1128/mSphere.00327-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richter WJ, Sun Y, Psoter KJ, et al. Vitamin D Deficiency Is Associated with Increased Nontuberculous Mycobacteria Risk in Cystic Fibrosis. Ann Am Thorac Soc 2021;18:913-6. 10.1513/AnnalsATS.202003-216RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gutiérrez S, Svahn SL, Johansson ME. Effects of Omega-3 Fatty Acids on Immune Cells. Int J Mol Sci 2019;20:5028. 10.3390/ijms20205028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gibson D, Mehler PS. Anorexia Nervosa and the Immune System-A Narrative Review. J Clin Med 2019;8:1915. 10.3390/jcm8111915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loftus TJ, Brown MP, Slish JH, et al. Serum Levels of Prealbumin and Albumin for Preoperative Risk Stratification. Nutr Clin Pract 2019;34:340-8. 10.1002/ncp.10271 [DOI] [PubMed] [Google Scholar]
- 77.White JV, Guenter P, Jensen G, et al. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr 2012;36:275-83. 10.1177/0148607112440285 [DOI] [PubMed] [Google Scholar]
- 78.Gaudiani JL, Sabel AL, Mehler PS. Low prealbumin is a significant predictor of medical complications in severe anorexia nervosa. Int J Eat Disord 2014;47:148-56. 10.1002/eat.22233 [DOI] [PubMed] [Google Scholar]
- 79.Davis CJ, Sowa D, Keim KS, et al. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr 2012;36:197-204. 10.1177/0148607111413896 [DOI] [PubMed] [Google Scholar]
- 80.Salvetti DJ, Tempel ZJ, Gandhoke GS, et al. Preoperative prealbumin level as a risk factor for surgical site infection following elective spine surgery. Surg Neurol Int 2015;6:S500-3. 10.4103/2152-7806.166893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu PJ, Cassiere HA, Dellis SL, et al. Impact of Preoperative Prealbumin on Outcomes After Cardiac Surgery. JPEN J Parenter Enteral Nutr 2015;39:870-4. 10.1177/0148607114536735 [DOI] [PubMed] [Google Scholar]
- 82.Rabito G, Blalock DV, Beaty L, et al. Refeeding patients with moderate and severe eating disorders: A retrospective cohort study. Int J Nutritional Sci 2021;6:1. [Google Scholar]
- 83.Friedli N, Stanga Z, Culkin A, et al. Management and prevention of refeeding syndrome in medical inpatients: An evidence-based and consensus-supported algorithm. Nutrition 2018;47:13-20. 10.1016/j.nut.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 84.Griffith DE, Girard WM, Wallace RJ, Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis 1993;147:1271-8. 10.1164/ajrccm/147.5.1271 [DOI] [PubMed] [Google Scholar]
- 85.Aslam M, Vaezi MF. Dysphagia in the elderly. Gastroenterol Hepatol (N Y) 2013;9:784-95. [PMC free article] [PubMed] [Google Scholar]
- 86.Koh WJ, Lee JH, Kwon YS, et al. Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest 2007;131:1825-30. 10.1378/chest.06-2280 [DOI] [PubMed] [Google Scholar]
- 87.Thomson RM, Armstrong JG, Looke DF. Gastroesophageal reflux disease, acid suppression, and Mycobacterium avium complex pulmonary disease. Chest 2007;131:1166-72. 10.1378/chest.06-1906 [DOI] [PubMed] [Google Scholar]
- 88.Kang JB, Lee DH, Kwon SH, et al. The Prevalence of Nontuberculous Mycobacterial Lung Disease with orwithout Reflux Esophagitis. Korean J Gastroenterol 2018;71:18-23. 10.4166/kjg.2018.71.1.18 [DOI] [PubMed] [Google Scholar]
- 89.Garand KLF, McCullough G, Crary M, et al. Assessment Across the Life Span: The Clinical Swallow Evaluation. Am J Speech Lang Pathol 2020;29:919-33. 10.1044/2020_AJSLP-19-00063 [DOI] [PubMed] [Google Scholar]
- 90.Lind CD. Dysphagia: evaluation and treatment. Gastroenterol Clin North Am 2003;32:553-75. 10.1016/S0889-8553(03)00024-4 [DOI] [PubMed] [Google Scholar]
- 91.Langmore SE. History of Fiberoptic Endoscopic Evaluation of Swallowing for Evaluation and Management of Pharyngeal Dysphagia: Changes over the Years. Dysphagia 2017;32:27-38. 10.1007/s00455-016-9775-x [DOI] [PubMed] [Google Scholar]
- 92.Martin-Harris B, McFarland D, Hill EG, et al. Respiratory-swallow training in patients with head and neck cancer. Arch Phys Med Rehabil 2015;96:885-93. 10.1016/j.apmr.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thiyagalingam S, Kulinski AE, Thorsteinsdottir B, et al. Dysphagia in Older Adults. Mayo Clin Proc 2021;96:488-97. 10.1016/j.mayocp.2020.08.001 [DOI] [PubMed] [Google Scholar]
- 94.Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med 2008;359:1700-7. 10.1056/NEJMcp0804684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lacy BE, Weiser K, Chertoff J, et al. The diagnosis of gastroesophageal reflux disease. Am J Med 2010;123:583-92. 10.1016/j.amjmed.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 96.Khoma O, Burton L, Falk MG, et al. Predictors of reflux aspiration and laryngo-pharyngeal reflux. Esophagus 2020;17:355-62. 10.1007/s10388-020-00726-9 [DOI] [PubMed] [Google Scholar]
- 97.Hong SK, Vaezi MF. Gastroesophageal reflux monitoring: pH (catheter and capsule) and impedance. Gastrointest Endosc Clin N Am 2009;19:1-22, v. 10.1016/j.giec.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 98.Portaels F, Pattyn SR. Growth of mycobacteria in relation to the pH of the medium. Ann Microbiol (Paris) 1982;133:213-21. [PubMed] [Google Scholar]
- 99.Piddington DL, Kashkouli A, Buchmeier NA. Growth of Mycobacterium tuberculosis in a defined medium is very restricted by acid pH and Mg(2+) levels. Infect Immun 2000;68:4518-22. 10.1128/IAI.68.8.4518-4522.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Forbes BA, Hall GS, Miller MB, et al. Practical Guidance for Clinical Microbiology Laboratories: Mycobacteria. Clin Microbiol Rev 2018;31:e00038-17. 10.1128/CMR.00038-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Al-Momani H, Perry A, Jones R, et al. Nontuberculous mycobacteria in gastrostomy fed patients with cystic fibrosis. Sci Rep 2017;7:46546. 10.1038/srep46546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ayazi S, Leers JM, Oezcelik A, et al. Measurement of gastric pH in ambulatory esophageal pH monitoring. Surg Endosc 2009;23:1968-73. 10.1007/s00464-008-0218-0 [DOI] [PubMed] [Google Scholar]
- 103.Lundell L, Miettinen P, Myrvold HE, et al. Seven-year follow-up of a randomized clinical trial comparing proton-pump inhibition with surgical therapy for reflux oesophagitis. Br J Surg 2007;94:198-203. 10.1002/bjs.5492 [DOI] [PubMed] [Google Scholar]
- 104.Bonavina L, Saino G, Lipham JC, et al. LINX(®) Reflux Management System in chronic gastroesophageal reflux: a novel effective technology for restoring the natural barrier to reflux. Therap Adv Gastroenterol 2013;6:261-8. 10.1177/1756283X13486311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ganesan P, Schmiedge J, Manchaiah V, et al. Ototoxicity: A Challenge in Diagnosis and Treatment. J Audiol Otol 2018;22:59-68. 10.7874/jao.2017.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Ingen J, Aliberti S, Andrejak C, et al. Management of Drug Toxicity in Mycobacterium avium Complex Pulmonary Disease: An Expert Panel Survey. Clin Infect Dis 2021;73:e256-9. 10.1093/cid/ciaa1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Strupp M, Kim JS, Murofushi T, et al. Bilateral vestibulopathy: Diagnostic criteria Consensus document of the Classification Committee of the Bárány Society. J Vestib Res 2017;27:177-89. 10.3233/VES-170619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol 2018;57:S99-S107. 10.1080/14992027.2018.1468092 [DOI] [PubMed] [Google Scholar]
- 109.Nguyen T, Jeyakumar A. Genetic susceptibility to aminoglycoside ototoxicity. Int J Pediatr Otorhinolaryngol 2019;120:15-9. 10.1016/j.ijporl.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 110.Hall CD, Herdman SJ, Whitney SL, et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Evidence-Based Clinical Practice Guideline: FROM THE AMERICAN PHYSICAL THERAPY ASSOCIATION NEUROLOGY SECTION. J Neurol Phys Ther 2016;40:124-55. 10.1097/NPT.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pietkiewicz P, Pepaś R, Sułkowski WJ, et al. Electronystagmography versus videonystagmography in diagnosis of vertigo. Int J Occup Med Environ Health 2012;25:59-65. 10.2478/s13382-012-0002-1 [DOI] [PubMed] [Google Scholar]
- 112.Blakley BW, Barakat N. Reliability of caloric testing. Ir J Med Sci 2021;190:1571-5. 10.1007/s11845-020-02501-2 [DOI] [PubMed] [Google Scholar]
- 113.Chan FM, Galatioto J, Amato M, et al. Normative data for rotational chair stratified by age. Laryngoscope 2016;126:460-3. 10.1002/lary.25497 [DOI] [PubMed] [Google Scholar]
- 114.Stevens MN, Garrison DB, Kaylie DM. What is the potential clinical utility of vHIT when assessing adult patients with dizziness? Laryngoscope 2017;127:2689-90. 10.1002/lary.26774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chowdhury S, Owens KN, Herr RJ, et al. Phenotypic Optimization of Urea-Thiophene Carboxamides To Yield Potent, Well Tolerated, and Orally Active Protective Agents against Aminoglycoside-Induced Hearing Loss. J Med Chem 2018;61:84-97. 10.1021/acs.jmedchem.7b00932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kitcher SR, Kirkwood NK, Camci ED, et al. ORC-13661 protects sensory hair cells from aminoglycoside and cisplatin ototoxicity. JCI Insight 2019;4:e126764. 10.1172/jci.insight.126764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kenyon EJ, Kirkwood NK, Kitcher SR, et al. Identification of a series of hair-cell MET channel blockers that protect against aminoglycoside-induced ototoxicity. JCI Insight 2021;6:145704. 10.1172/jci.insight.145704 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as