Abstract
Background
We aimed to analyze perioperative complications, postoperative neuropathic pain, and the necessity of epidural anesthesia in uniportal video-assisted thoracoscopic surgery (U-VATS) compared to conventional multiportal VATS (M-VATS) for anatomical lung resection.
Methods
This retrospective study included all patients who underwent elective VATS lobectomy and segmentectomy between April 2016 and December 2019. The exclusion criteria were as follows: age ≤19 years, planned thoracotomy, re-operation in thoracic surgery, median sternotomy, robot-assisted thoracic surgery, simultaneous resection of extrathoracic organs, locally invasive lung tumor with bronchoplasty or angioplasty, past or current neuropathic pain, and a large tumor with a minimum diameter ≥5 cm. M-VATS had 4 ports approach. U-VATS port positions were placed by extending the thoracoscope port of M-VATS.
Results
U-VATS patients showed significant differences compared to M-VATS patients: smaller intraoperative bleeding (1 vs. 30 mL; P=0.0010), shorter operative time (141 vs. 183 min; P<0.0001), post-hospitalization (5 vs. 8 days; P=0.0002), fewer complications (23.9% vs. 40.9%; P=0.048), less acute pain, less postoperative neuropathic pain (32.4% vs. 52.1%; P=0.027) and shorter duration of neuropathic pain (30 vs. 60 days; P=0.041). For the postoperative neuropathic pain and pain score until postoperative day 5, there were no differences between the groups with and without epidural anesthesia.
Conclusions
As a single-center initial experience, U-VATS lobectomy and segmentectomy seemed safe and minimally invasive based on not only postoperative neuropathic pain and complications but also time management. U-VATS would provide better pain control, without epidural anesthesia.
Keywords: Uniportal, video-assisted thoracoscopic surgery (VATS), lung resection, neuropathic pain, time management
Introduction
Uniportal video-assisted thoracoscopic surgery (VATS) for major lung resections is a novel upcoming and promising approach that is gaining popularity worldwide (1-10). It has already been introduced not only in standard operations, but also in complex techniques such as bronchoplasty or pulmonary angioplasty (11,12).
The development of minimally invasive approaches such as uniportal VATS (U-VATS) and robotic surgery or needle scope surgery is one of the causes of postoperative neuropathic pain (13,14). Despite being asymptomatic before surgery, many thoracic surgery patients suffer from severe neuropathic pain after surgery.
Previously, we have reported on neuropathic pain after thoracic surgery (15-17). The risk factors for neuropathic pain were preoperative use of hypnotic medication, thoracotomy, and duration of surgery ≥2.5 h, and VATS was negatively associated with postoperative neuropathic pain (15). Pregabalin (50 mg/day) had a significant preventive effect on postoperative neuropathic pain after thoracic surgery, and the duration of neuropathic pain was significantly shorter, without obvious side effects (16). Nevertheless, 19.6% of patients experienced postoperative neuropathic pain after lung resection. In cases of severe adhesion, bronchoplasty, angioplasty, and large tumors with a minimum diameter ≥5 cm, VATS tended to take a long period or convert to thoracotomy. The patients who underwent VATS with operation time longer than 5 hours and converted to thoracotomy, had significantly higher postoperative neuropathic pain (71.4% vs. 22.0%; P<0.0001) (17). It was difficult to eliminate postoperative neuropathic pain, and further improvements are needed. Most thoracic surgery patients are asymptomatic before surgery. Ideally, patients should be asymptomatic after surgery. Our previous studies showed that multidimensional approaches are necessary to achieve symptom-free status, including less invasive surgical approaches, additional pain control methods, and the time effect (15-17).
Among surgical approaches, U-VATS is expected to reduce postoperative neuropathic pain compared to conventional multiportal VATS (M-VATS); therefore, we have introduced U-VATS lobectomy and segmentectomy since July 2018 (18). Many reports on U-VATS have been related to safety and feasibility (2-10), and few studies have strictly evaluated postoperative neuropathic pain, time effects, and the necessity of epidural anesthesia.
In this study, we aimed to compare postoperative neuropathic pain and perioperative complications between U-VATS and M-VATS for lobectomy and segmentectomy. We also evaluated acute pain and the effect of time under epidural anesthesia. The novelty of this study is that it analyzes not only postoperative neuropathic pain over 3 months after surgery, but also the necessity of epidural anesthesia and operating room time. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-6/rc).
Methods
Study design and patients
The study was conducted in accordance with the Declaration of Helsinki (revised in 2013). This retrospective study was approved by the Ethics Committee of Toyama University Hospital with a waiver of the need for informed consent due to its retrospective design (No. COI20200310, UMIN-R000050213). This study included all patients who were admitted to Toyama University Hospital (Toyama, Japan) for elective VATS lobectomy and segmentectomy between April 2016 and December 2019. The exclusion criteria were as follows: age ≤19 years, planned thoracotomy, re-operation in thoracic surgery, median sternotomy, robot-assisted thoracic surgery, simultaneous resection of extrathoracic organs, locally invasive lung tumor with bronchoplasty or angioplasty, past or current neuropathic pain, and a large tumor with a minimum diameter ≥5 cm (17).
Preoperative tests
In all cases, simple chest radiography, contrast-enhanced computed tomography (CT), blood tests, urine tests, electrocardiograms, and pulmonary function tests were performed. In the cases with lung cancer, cerebral magnetic resonance imaging and positron emission tomography were also performed. Patients aged over 80 years with one or more risk factors for coronary artery disease underwent preoperative cardiac echography and/or cardiac stress test.
Surgical approach
General anesthesia was induced using a single-lung ventilation technique with a double-lumen endotracheal tube. The patients were placed in the lateral decubitus position. In cases of M-VATS lobectomy and segmentectomy, patients underwent 4-port VATS with two 5-mm ports and two 10-mm ports. Segmentectomy was performed for cN0 lung cancer of 2 cm or less, with ground-glass opacity.
The surgical approach and indications were different for each phase (Table 1). Phase I involved procedures using M-VATS, and the indications were not restricted. The surgical approach in phase II (early 50 cases) and phase III (after 50 cases) used was U-VATS, and the indication in phase II was limited to clinical N0 in patients with lung cancer. There were no restrictions in phase III.
Table 1. Management for each phase.
| Characteristic | Phase I | Phase II | Phase III |
|---|---|---|---|
| Surgical approach | M-VATS | U-VATS (initial 50 cases) | U-VATS (after 50 cases) |
| Indication | No restriction | cN0 | No restriction |
| Pain management | |||
| Epidural anesthesia | Yes | Yes | No |
| Intercostal nerve block | Yes | Yes | Yes |
| Oral drug | |||
| Loxoprophen | Yes | Yes | Yes |
| Acetaminophen | Yes | Yes | Yes |
| Tramadol | – | – | Yes |
M-VATS, conventional multiportal video-assisted thoracoscopic surgery; U-VATS, uniportal video-assisted thoracoscopic surgery.
In all phases, intraoperative water sealing test was routinely performed to detect air leaks after lung resection. If air leaks were detected, fibrin glue plus a poly glycolic acid sheet was used in all cases (19,20). Free pericardial fat pad suturing was performed together when moderate to severe air leaks were detected. If a severe air leak continued after the supine position or after extubation despite these procedures, the patient was returned to the lateral position, and the above procedure was repeated to stop air leak in all phases.
Complicated or bogged procedures were performed by the chief surgeon or converted to M-VATS or thoracotomy. Guidelines for conversion to thoracotomy included terrible adhesion and unexpected hemorrhage that could not be controlled using a thoracoscopic procedure. In contrast, guidelines for conversion to M-VATS in U-VATS include controllable hemorrhage and severe adhesions that cannot be performed by U-VATS.
Surgeons
Regardless of the surgical approach and tumor location, the surgeon stood on the patient’s right side and the assistant on the left side. The assistant handled the scope and provided a better surgical view. All procedures were performed by the chief surgeon and five residents. Surgery was mainly performed by two surgeons. The two surgeons have been accredited by the Japanese Board of General Thoracic Surgery and was involved in deciding the surgical indication. The overall responsibility for the quality of all procedures for a given patient was controlled by the chief surgeon in accordance with sufficient quality requirements. The chief surgeon finalized all the treatment plans. The chief surgeon gave instructions to always go smoothly. If the chief surgeon judged that the procedure was very dangerous, the surgeon was replaced by the chief surgeon. All surgeons performed M-VATS. In uniportal VATS, only one chief surgeon performed the procedures. The chief surgeon was trained in the procedures as a visitor at Shanghai Pulmonary Hospital (SPH) (Shanghai, China) and modified it to make it easier to follow in Japan. Skin closures were performed by not only the chief surgeon but also by five residents or medical students. This study targeted patients performed by the chief surgeon.
Port placement (Figure 1)
Figure 1.
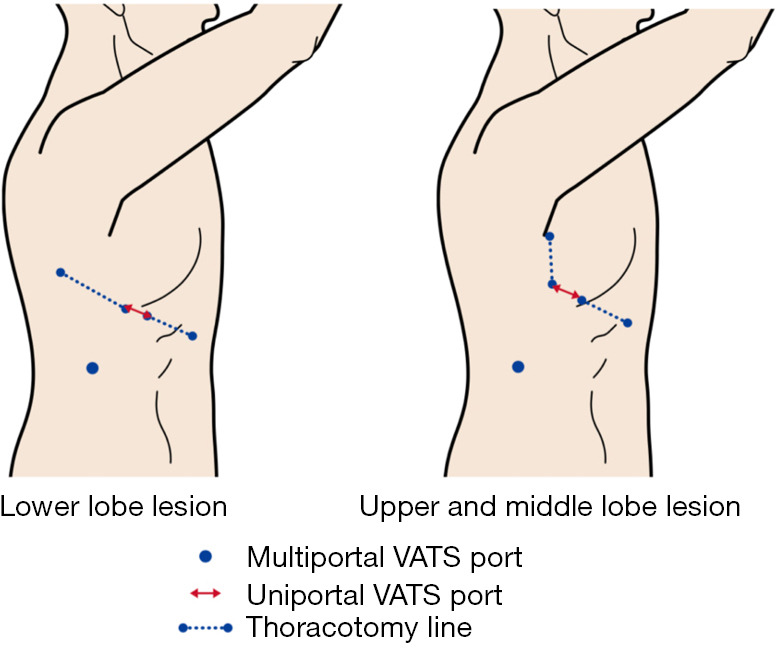
Port placements in U-VATS and M-VATS. U-VATS port positions were placed by extending thoracoscope port of M-VATS forward. U-VATS, uniportal video-assisted thoracoscopic surgery; M-VATS, conventional multiportal video-assisted thoracoscopic surgery.
The position of the M-VATS port differed depending on the lobe. In the right upper and middle lobes, a 5–10 mm port was placed at the fifth intercostal space at the mid-axillary line for the thoracoscope. A 5-mm port was placed in the third intercostal space at the mid-axillary line and in the third intercostal space at the anterior axillary line. A 10-mm port was placed in the eighth intercostal space at the inferior angle of the scapular line. An anterior axillary thoracotomy was possible by connecting the former three. The left upper lobe was similar to the right upper and middle lobes. In the right lower lobe, a 5–10 mm port was placed at the sixth intercostal space at the post-axillary line for the thoracoscope. A 5-mm port was placed in the sixth intercostal space at the medial border of the scapular line and in the sixth intercostal space at the middle axillary line. A 10-mm port was placed in the ninth intercostal space at the inferior angle of the scapular line. Posterolateral thoracotomy was possible by connecting the former three. In the left lower lobe, a 5–10 mm port was placed at the sixth intercostal space at the posterior axillary line for the thoracoscope. A 5-mm port was placed in the fourth intercostal space at the middle axillary line and in the sixth intercostal space at the medial border of the scapula line. A 10-mm port was placed in the ninth intercostal space at the posterior axillary line. Connecting the two ports between the sixth intercostals allowed for posterolateral thoracotomy. In M-VATS, a thoracoscope was used with a 30-degree, 5- or 10-mm camera. The M-VATS procedures were performed using linear surgical instruments. During specimen extraction, a one-port incision was extended to approximately 3 cm.
U-VATS port positions were placed by extending each thoracoscope port of M-VATS forward by a 3 cm incision (18). Port sites were marked before surgery in preparation for conversion to M-VATS or thoracotomy. A wound protector was inserted into the single-access incision. In U-VATS, a thoracoscope was used with a 30-degree, 5 mm camera (Endoeye, Olympus, Japan). U-VATS procedures were performed using curved surgical instruments; a curved lung grasping clamp (Foerster lung grasping clamp, Scanlan, USA), a curved suction instrument (Curved blunt tip, Wolf suction instruments, Scanlan, USA), a forceps (Pro DeBakey Grasper, Geister, Germany, and Aesculap uniport XS, B-Braun, Germany) and cotton rods (CS two-way handle, Unimedic, Japan) were essential. Energy devices mainly used were advanced bipolar devices (LigaSure, Covidien, USA and EnSeal, Ethicon, USA).
Despite the port number, a 20-Fr chest tube was inserted at the end of the procedure. In the U-VATS, a chest tube was placed through the incision and in the same intercostal space.
Conversion criteria
Based on the difficulty factor analysis in M-VATS (9), the conversion criteria were set as follows:
Conversion to the M-VATS; Situations that were difficult with U-VATS but were expected to be possible with M-VATS (bleeding, adhesions, poor isolated lung ventilation, etc.). In addition, it was assumed that the situation was stagnant if it remained the same for 30 min or more.
Conversion to thoracotomy; Critical bleeding, unexpected local invasion, intractable air leaks, and severe adhesions that were difficult to manage with VATS.
Pain management
Pain management differed for each phase (Table 1). Phase I involved procedures using M-VATS. Phase II included the early 50 cases of U-VATS, and in phase III, U-VATS was used after 50 cases.
Epidural anesthesia was performed as the standard pain management in phases I and II. Intravenous fentanyl was administered to patients with contraindications for epidural analgesia, such as prolonged coagulation time or patients who declined epidural analgesia. In phase III, neither epidural anesthesia nor intravenous fentanyl anesthesia was administered for pain management. Adverse events of epidural anesthesia were defined as epidural puncture, nausea, and vomiting, numbness and motor paralysis of the lower limbs, itching, severe pain [visual analog scale (VAS) score ≥5], puncture site pain, urinary obstruction, epidural hematoma, epidural abscess, meningitis, total spinal anesthesia, and other associated events.
Thoracic epidural catheterization is usually performed before induction at the T4–5, T5–6, or T6–7 intervertebral space using a loss-of-resistance technique. Catheter placement was confirmed using a test dose of 4 mL of 1% lidocaine. An epidural infusion was initiated preoperatively in the operating room. The drug combination was administered as continuous infusions of 2.0 mg/mL of ropivacaine and fentanyl (2.5 µg/mL). The infusion rate was adjusted to 2–4 mL/h. Epidural analgesia was discontinued the day after chest tube removal. In phase I and II patients who did not receive an epidural, intravenous fentanyl was started in the operating room. The fentanyl infusion rate was adjusted between 0.01 and 0.04 mg/h. Epidural analgesia or intravenous fentanyl infusion was stopped on the day of chest tube removal. When a chest tube could not be removed, epidural analgesia or intravenous fentanyl infusion was stopped by postoperative day 14 according to pain.
All patients received intraoperative internal intercostal nerve block (21) and loxoprofen 180 mg/day from the first postoperative day and 25 mg pregabalin twice daily from the second postoperative day through phase I–III (16). Intercostal nerve blocks were performed at port location levels using 20 mL 0.75% lopivacaine. In patients with an estimated glomerular filtration rate (eGFR) of <50 mL/min, 1,200 mg/day acetaminophen was prescribed instead of loxoprofen. Patients in phase III received tramadol (37.5 mg) twice daily from the night of the operative day. The medication was continued until a pain-free status was achieved.
Variables and assessments
The following patient, surgical characteristics, and follow-up parameters were recorded: age, sex, past medical history, smoking history, body mass index, eGFR, respiratory function as measured by spirometry, diagnosis, disease side, tumor size, clinical node disease (N0, N1/2) preoperative use of hypnotic medication (15), procedure type (lobectomy or segmentectomy), intraoperative bleeding, operative time, procedure starting time (defined as the time from entry to the start of skin incision), operating room time (defined as the time from entry to the time of leaving operation room), chest tube duration, epidural anesthesia and its side effects, conversion to M-VATS or thoracotomy, conversion reasons, complications (prolonged air leak defined as an air leak lasting >5 days), acute pain, postoperative neuropathic pain, its onset and duration, and postoperative hospitalization.
A complication was defined as any deviation from the normal postoperative course and graded according to the Clavien–Dindo classification (22).
The side effects of epidural anesthesia were measured in cases of puncture site bleeding, medicine leakage from the puncture site, headache, neurological symptoms, nausea, vomiting, low blood pressure, urinary retention, when there was no cause other than epidural anesthesia, and ineffective pain.
The pain was measured three times per day at rest and movements on a postoperative day by ward nurses during hospitalization using the VAS. A score of 0 denotes no pain, and a score of 10 denotes the worst pain imaginable. Patients were also asked questions about pain intensity during daily life activities and the location and characteristics of the pain. If pain was present during the interview, the patient was asked to describe the characteristics of the pain they experienced. In addition, we routinely used a validated neuropathic pain screening tool, including the related self-completed version of Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) to ensure consistency and reduce missing data between assessments during the acute and follow-up periods (23,24). The S-LANSS score is an evaluation method to identify the pain of predominantly neuropathic origin, as distinct from no nociceptive pain, without the need for clinical examination. Since this score is a 7-item, 2-stage evaluation questionnaire, screening for neuropathic pain is easy through daily interviews. In this study, postoperative neuropathic pain was evaluated when two or more of the seven items were applicable. Chronic pain was defined as pain that persisted for >3 months after surgery.
After discharge, patients were assessed as outpatients at postoperative 2 weeks and 1, 2, and 3 months using the same methods. During each follow-up visit, the outpatients were asked about the duration of pain and the use of analgesic drugs. Neuropathic pain, respiratory function as measured by spirometry, hospital stay, and side effects were also determined at each visit.
Data management and statistical analyses
In a previous observational study of a similar population (15-17), we aimed to detect neuropathic pain of 35% with 95% power and a 0.05 significance level (2-sided), with an allocation ratio U-VATS/M-VATS of 0.55, yielding a minimum of 240 patients (85 in U-VATS and 155 in M-VATS). Expecting a dropout rate of 20% due to conversion and missing data, we initially preplanned consecutive 100 patients in U-VATS, but dropouts and exclusions reached about 12%. The collected data included operation records, anesthesia records, surgical videos, and neuropathic pain. Patients with missing data and those who converted from U-VATS to M-VATS or thoracotomy and from M-VATS to thoracotomy were excluded from the analyses.
For univariate analysis, intergroup differences were evaluated using the non-parametric Wilcoxon test. The χ2 or Fisher’s exact test was used to compare categorical variables when necessary. Continuous variables are presented as mean ± standard deviation (SD) for normally distributed data and median with interquartile range for non-normally distributed data. Categorical variables were presented as n (%). The analyses were limited to patients operated by the chief surgeon. Logistic regression analysis was used to calculate the propensity score for the selection of patients for the M-VATS and U-VATS groups, using the preoperative variables, including sex, smoking history, interstitial pneumonia, emphysema, tumor size, clinical node disease and preoperative use of hypnotic medication, which are reported to be associated with postoperative complications after thoracic surgery (25-27). The allowable calipers used for the matching included 0.2 SD of the logit-transformed propensity score. A matched balance between the groups was assessed using standardized mean differences in the variables included in the propensity score estimation. Within the matched pairs, the differences between the preoperative values in each group were analyzed using a paired Student’s t-test or Wilcoxon test. Statistical significance was defined as P<0.05. All reported P values were two-sided. All statistical analyses were performed using JMP Pro version 15.2.0 (SAS Institute, Inc., Cary, NC, USA).
Results
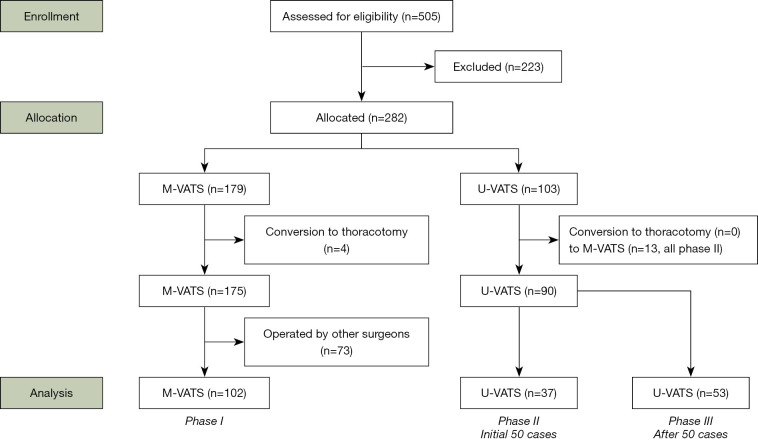
A total of 282 patients were enrolled in this study (Figure 2). In phase I using M-VATS, the procedures in 4 of 179 (2.2%) patients were converted to thoracotomy. In the early phase using U-VATS, the procedures in 13 of 50 (26.0%) patients were converted to M-VATS in phase II. In the late phase using U-VATS, none of the procedures were converted to M-VATS in phase III, and none were converted to thoracotomy in phases II and III (Table S1).
Figure 2.
Patient selection flowchart. M-VATS, conventional multiportal video-assisted thoracoscopic surgery; U-VATS, uniportal video-assisted thoracoscopic surgery.
A total of 192 patients were operated by the chief surgeon. The univariate analysis of them who underwent M-VATS and U-VATS had some significant variables (Table S2).
Analysis of propensity score matching patients
Using propensity score matching, 71 patients were selected from each group. The variables of the two groups after matching are presented in Tables 2,3. The following factors in U-VATS patients were significant compared to M-VATS patients: smaller intraoperative bleeding (1 vs. 30 mL; P=0.0010), shorter operative time (141 vs. 183 min; P<0.0001), and operation start time (63 vs. 65 min; P=0.023), and operating room time (228 vs. 279 min; P<0.0001), post-hospitalization (5 vs. 8 days; P=0.0002), and fewer complications [17 (23.9%) vs. 29 (40.9%); P=0.048]. In terms of postoperative pain, U-VATS patients had significantly less postoperative neuropathic pain [23 (32.4%) vs. 37 (52.1%); P=0.027] and shorter duration of neuropathic pain (30 vs. 60 days; P=0.041) compared to M-VATS patients. M-VATS patients required significant the day of last analgesic use was later (0 vs. 1 day; P=0.0102). Postoperative acute pain until POD 5 showed no significant differences between the groups.
Table 2. Univariate analysis of the patients after propensity score match.
| Characteristic | M-VATS (phase I) (n=71) | U-VATS (phase II and III) (n=71) | P value |
|---|---|---|---|
| Age, median [IQR], years | 72 [66–76] | 71 [65–77] | 0.34 |
| Sex, male (%) | 43 (60.6) | 42 (59.2) | 0.63 |
| BMI, median [IQR] | 23.1 [20.4–25.1] | 23.2 [21.3–25.4] | 0.67 |
| Hypertension (%) | 41 (57.8) | 35 (49.3) | 0.40 |
| Hyperlipidemia (%) | 28 (39.4) | 30 (42.3) | 0.86 |
| Diabetes (%) | 20 (28.2) | 20 (28.2) | 0.57 |
| Smoking history (%) | 45 (63.4) | 41 (57.8) | 0.60 |
| eGFR (mL/min), median [IQR] | 69 [56.4–80.3] | 70 [59.6–78.1] | 0.81 |
| Interstitial pneumonia (%) | 16 (22.5) | 9 (12.7) | 0.19 |
| Obstructive airway disorder (COPD or asthma) (%) | 25 (35.2) | 27 (38.0) | 0.86 |
| Emphysema (%) | 24 (33.8) | 20 (28.2) | 0.59 |
| Diseased side, right (%) | 44 (62.0) | 44 (62.0) | 0.57 |
| Tumor size (mm), [IQR] | 21 [18–31] | 20 [15–30] | 0.21 |
| Clinical N1/2 (%) | 9 (12.7) | 5 (7.0) | 0.40 |
| Preoperative use of hypnotic medication (%) | 24 (33.8) | 26 (36.6) | 0.86 |
| Procedure | |||
| Segmentectomy (%) | 18 (25.4) | 22 (31.0) | 0.71 |
| Lobectomy (%) | 53 (74.7) | 49 (69.0) | 0.58 |
| Intraoperative bleeding (mL), median [IQR] | 30 [1–80] | 1 [1–50] | 0.0010 |
| Operative time (min), median [IQR] | 183 [158–224] | 141 [121–161] | <0.0001 |
| Operation staring time (min), median [IQR] | 65 [59–74] | 63 [53–69] | 0.023 |
| Operating room time (min), median [IQR] | 279 [255–325] | 228 [211–250] | <0.0001 |
| Chest tube duration (day), median [IQR] | 1 [1–2] | 1 [1–2] | 0.11 |
| Complications (%) | |||
| Total | 29 (40.9) | 17 (23.9) | 0.048 |
| Prolonged air leak | 13 (18.3) | 9 (12.7) | 0.49 |
| Pneumonia | 5 (7.0) | 7 (9.8) | 0.76 |
| Arrhythmia | 7 (9.8) | 3 (4.2) | 0.33 |
| Delirium | 5 (4.9) | 0 (0) | 0.062 |
| ARDS | 0 (0) | 1 (1.4) | 0.50 |
| Others | 5 (7.0) | 3 (4.2) | 0.72 |
| Postoperative hospitalization (day), median [IQR] | 8 [6–9] | 5 [4–7] | 0.0002 |
M-VATS, conventional multiportal video-assisted thoracoscopic surgery; U-VATS, uniportal video-assisted thoracoscopic surgery; IQR, interquartile range; BMI, body mass index; eGFR, estimated glomerular filtration rate; COPD, chronic obstructive pulmonary disease; ARDS, acute respiratory distress syndrome.
Table 3. Postoperative pain in the patients after propensity score match.
| Characteristic | M-VATS (phase I) (n=71) | U-VATS (phase II and III) (n=71) | P value |
|---|---|---|---|
| VAS-POD | |||
| 0 | 4 [2–6] | 4 [2–7] | 0.72 |
| 1 | 4 [3–5] | 3 [2–5] | 0.18 |
| 2 | 3 [2–5] | 3 [2–5] | 0.66 |
| 3 | 2 [1–3] | 2 [1–3] | 0.97 |
| 4 | 2 [1–3] | 1 [1–2] | 0.43 |
| 5 | 2 [0–3] | 1 [1–2] | 0.48 |
| Additional analgesics, n (%) | 51 (71.8) | 42 (60.0) | 0.16 |
| Number of analgesic uses (times), median [IQR] | 3 [1–5] | 1 [1–4] | 0.082 |
| Last day of analgesic use (day) [IQR] | 1 [1–2] | 0 [0–1] | 0.0102 |
| Postoperative neuropathic pain (%) | 37 (52.1) | 23 (32.4) | 0.027 |
| Onset (day) [IQR] | 6 [4–9] | 5 [3–11] | 0.51 |
| Duration (day) [IQR] | 60 [45–80] | 30 [30–74] | 0.041 |
M-VATS, conventional multiportal video-assisted thoracoscopic surgery; U-VATS, uniportal video-assisted thoracoscopic surgery; VAS, visual analogue scale; POD, postoperative day; IQR, interquartile range.
Analysis of all U-VATS patients
Subgroup analysis was carried out for all U-VATS patients between phase II with epidural anesthesia and phase III without epidural anesthesia. Background and preoperative factors were not significantly different between the groups (Table S3). Phase III patients without epidural anesthesia had a significantly shorter starting time (61 vs. 69 min; P<0.001), and operating room time (225 vs. 242 min; P=0.012), lesser use of analgesics (2 vs. 3 times; P=0.023), and the day of last analgesic use was shorter (0 vs. 1 day; P=0.019). There were no significant differences in postoperative neuropathic pain and pain scores until POD 5 (Table 4).
Table 4. Postoperative pain in all U-VATS patients.
| Characteristic | Early (phase II) (n=37) | Late (phase III) (n=53) | P value |
|---|---|---|---|
| VAS-POD | |||
| 0 | 4 [1–5] | 4 [2–5] | 0.25 |
| 1 | 3 [2–5] | 3 [2–4] | 0.24 |
| 2 | 3 [2–5] | 2 [2–4] | 0.49 |
| 3 | 2 [2–3] | 1 [1–2] | 0.059 |
| 4 | 2 [1–2] | 1 [1–2] | 0.11 |
| 5 | 1 [1–2] | 1 [0–2] | 0.16 |
| Additional analgesics, n (%) | 27 (72.9) | 29 (54.7) | 0.12 |
| Number of analgesic uses (times), median [IQR] | 3 [1–4] | 2 [1–2] | 0.023 |
| Last day of analgesic use (day) [IQR] | 1 [0–2] | 0 [0–1] | 0.019 |
| Postoperative neuropathic pain (%) | 9 (24.3) | 13 (24.5) | 0.60 |
| Onset (day) [IQR] | 5 [4–6] | 5 [4–12] | 0.36 |
| Duration (day) [IQR] | 30 [22–38] | 32 [19–59] | 0.87 |
U-VATS, uniportal video-assisted thoracoscopic surgery; VAS, visual analogue scale; POD, postoperative day; IQR, interquartile range.
In phase II, 33 of 37 patients (89.2%) received epidural anesthesia. Of these, 14 (42.4%) showed epidural anesthesia-related adverse events as follows: poor effect in 4, nausea and vomiting in 3, puncture site pain in 2, puncture site leakage in 2, enuresis in 1, reversible motor disability of the lower limb in 1, and dural puncture in 1.
Discussion
In this single-center initial experience of sequential 100 cases, U-VATS anatomical lung resection seemed safer and more minimally invasive than M-VATS. U-VATS patients had significantly less intraoperative bleeding, shorter operative time, shorter operating room time, shorter chest tube duration, shorter post-hospitalization, fewer complications, less postoperative neuropathic pain, and shorter duration of neuropathic pain after propensity score matching.
Although there are differences depending on review articles (3-6), U-VATS has been reported to have reduced postoperative complications, postoperative hospitalization, intraoperative bleeding, and postoperative pain compared to M-VATS. These were almost the same this study as well. However, postoperative neuropathic pains of U-VATS have been not fully investigated (2-10). Based on our postoperative neuropathic pain studies (15-17), it is important to introduce a less invasive surgical approach and to shorten the surgical time. Because U-VATS has only one intercostal nerve damage, there was less postoperative neuropathic pain, as expected. The duration of neuropathic pain in U-VATS patients disappeared earlier than expected. The use of a wound retractor instead of a port may have an effect. In this study, the S-LANSS score was evaluated with two or more items instead of 12 points or more; therefore, the neuropathic pain rate was higher than in a previous report (23,24). This was because if 12 points or more were used as diagnostic criteria for neuropathic pain, the comparison could be inadequate. A diagnosis with an exact score of 12 or higher would further reduce the rate. Table 3 showed that no difference in acute pain until POD 5 between U-VATS and M-VATS. However, when the data were analyzed without limited to the chief surgeon, U-VATS patients had significantly less acute pain (Table S4). Postoperative acute pain may be affected by the skill of the surgeon.
Through phases II and III, U-VATS had a shorter operation time than M-VATS. Operation time is shorter for U-VATS in past reports (28,29). Some reviews showed no statistically significant difference in operation time, but operation time of U-VATS tended to be shorter than M-VATS. Depending on the surgeon’s VATS proficiency, there is a possibility that the operation time of U-VATS will be shortened. We think the reason might be the following: (I) it is not necessary to wait for the intervention of an assistant because a surgeon can make a good surgical view by himself, and (II) there were few wounds; hence, the time required for opening and closing the wound was short. A shortened operation time may also decrease postoperative neuropathic pain. In addition, less pain contributed to the few postoperative complications of U-VATS.
Another major benefit of U-VATS is the elimination of epidural anesthesia. Postoperative acute pain and postoperative neuropathic pain were not significantly different between patients with and without epidural anesthesia. Eliminating epidural anesthesia shortened the time required to start surgery, and the effect of U-VATS on reducing surgery time also contributed to the reduction of working hours for the medical staff. Furthermore, the fact that 42.4% of adverse events due to epidural anesthesia in phase II became zero in phase III could lead to a reduction in labor. Long working hours have become a social problem. For doctors, an increased mental workload is associated with inferior technical performance (30). Proper management of working time is important (31). Tramadol may have contributed to good pain control in the absence of epidural anesthesia.
Before the introduction of U-VATS, we regarded that U-VATS was very difficult, but we were able to introduce it without major difficulty by preparing special equipment. In phase II, we emphasized safety and converted it readily to M-VATS without hesitation. However, until phase III, corrections were made to overcome difficult factors, as shown in Table 2. We have modified the original procedure demonstrated at the SPH (32). The major correction is the port position. The port positions used in the surgeries conducted at SPH made us feel that the dorsal surgical view was poor. We improved this by changing the camera port in the M-VATS to the position of the U-VATS. For facilities aiming to introduce U-VATS, it would be better to start with the procedure of extending the camera port of the conventional M-VATS method. The second major change was the surgeon’s position. The surgeon stood on the ventral side similar to the SPH style in the first two cases on the right side, but after the third case, the surgeon standing side returned to the right side of the patient, as in the M-VATS approach. For a safe procedure, it was considered important not to make a major change from the conventional method, especially the field of view.
The third major change was the instrument used. A forceps (Pro DeBakey Grasper, Geister, Germany and Aesculap uniport XS, B-Braun, Germany) and cotton rods (CS two-way handle, Unimedic, Japan) made it possible to operate “off the ground” and involved a procedure that separated the targeted tissue from the deep tissue and protected the deep tissue. At SPH, tissue tension was achieved by a non-grasping technique (33), but it was considered that “off the ground” was insufficient, and it was difficult to handle the vascular sheath, which caused two cases of bleeding in phase II. These instruments made it possible to operate “off the ground” and to overcome the factors involved for the conversion to M-VATS. One of the most difficult procedures in the early stage of U-VATS introduction is lymph node dissection. The introduction of these instruments has also improved the accuracy of dissection (18).
Middle lobe lobectomy was performed in the 5th intercostal space in the first two cases but changed to the 6th intercostal space in the 3rd case. At the 5th intercostal space, the operation site was very close to the port, making it difficult to use the stapler. In addition, in cases of severe adhesion such as the vascular sheath, large tumors with a minimum diameter ≥5 cm, and locally invasive tumors, U-VATS is considered to be challenging as well as M-VATS (17).
It is important to maintain the safety and curability of minimally invasive surgery. When introducing U-VATS, safety and minimal invasiveness would be guaranteed, considering appropriate equipment, dry lab training, familiar surgical view, and easy conversion to M-VATS when U-VATS is difficult. A good indication for initial cases would be a ground-glass opacity case, which is less likely to have lymph node metastases. The learning curve has been reported to be 30 cases (34,35). By trial and error, even in complicated cases, U-VATS procedures would provide shorter operation times and are safer without conversion.
U-VATS was not only less invasive to patients than expected but was also advantageous to the medical staff considering the management of working time. While U-VATS has many advantages, long-term follow-up data are needed to eliminate drawbacks. For the chief surgeon, U-VATS has become almost the only approach. However, for inexperienced surgeons, U-VATS is technically difficult. Therefore, M-VATS may be safer and more educational for them. It is unclear how many operations should be performed with M-VATS in order to move to U-VATS, and it is also considered to be one of the problems in the future.
Limitations
This study had several limitations as follows: single-institution study, limited patient number, patient background, single surgeon in U-VATS, surgical changes in the early phase, anesthesiologists performing epidural anesthesia, and the definition of postoperative neuropathic pain and pain management in each phase. Although the number of institutions and patients is limited, it is a continuous series, and the number of cases has been statistically examined. The U-VATS operator was the only chief surgeon. However, many reports of U-VATS were basically reported by a single operator. Since M-VATS was performed by some surgeons, the analysis was limited to the operations performed by the chief surgeon. The chief surgeon was more experienced U-VATS during the time with the epidural. However, in the early stage of introduction for U-VATS, it would be unavoidable to keep safety. For accurate evaluation of U-VATS, it may be desirable to report in the future in a prospective comparison study. We introduced propensity score matching to minimize background differences. Appropriate changes in the early cases of U-VATS are required to ensure safe procedures. Epidural anesthesia was mainly performed by some anesthesiologists. This might be associated with adverse events and may affect the efficacy of epidural anesthesia. Few U-VATS patients did not meet the definition of neuropathic pain but clinically complained of symptoms of postoperative neuropathic pain. For this reason, applying the strict definition resulted in very few complications of postoperative neuropathic pain in U-VATS, which could lead to excessive comparison differences. Therefore, the diagnostic criteria were lowered and examined in this study. Because pain management differed depending on the phase, U-VATS were compared between phases II and III.
Conclusions
Based on the results of this single-center initial experience, U-VATS lobectomy and segmentectomy seemed safe and minimally invasive in terms of postoperative neuropathic pain and complications as well as working time management. U-VATS provides better pain control, without epidural anesthesia. While U-VATS has many advantages, long-term follow-up data of U-VATS are expected in the future.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank the Japanese Association for Chest Surgery and the Shanghai Pulmonary Hospital for the fellowship of U-VATS. We would also like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Ethics Committee of Toyama University Hospital with a waiver of the need for informed consent due to its retrospective design (No. COI20200310, UMIN-R000050213).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-6/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-6/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-6/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-6/coif). The authors have no conflicts of interest to declare.
References
- 1.Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. 10.1510/icvts.2010.256222 [DOI] [PubMed] [Google Scholar]
- 2.Sihoe ADL. Uniportal Lung Cancer Surgery: State of the Evidence. Ann Thorac Surg 2019;107:962-72. 10.1016/j.athoracsur.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 3.Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. 10.21037/acs.2016.03.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abouarab AA, Rahouma M, Kamel M, et al. Single Versus Multi-Incisional Video-Assisted Thoracic Surgery: A Systematic Review and Meta-analysis. J Laparoendosc Adv Surg Tech A 2018;28:174-85. 10.1089/lap.2017.0446 [DOI] [PubMed] [Google Scholar]
- 5.Yan Y, Huang Q, Han H, et al. Uniportal versus multiportal video-assisted thoracoscopic anatomical resection for NSCLC: a meta-analysis. J Cardiothorac Surg 2020;15:238. 10.1186/s13019-020-01280-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magouliotis DE, Fergadi MP, Spiliopoulos K, et al. Uniportal Versus Multiportal Video-Assisted Thoracoscopic Lobectomy for Lung Cancer: An Updated Meta-analysis. Lung 2021;199:43-53. 10.1007/s00408-020-00411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng CS, Rocco G, Wong RH, et al. Uniportal and single-incision video-assisted thoracic surgery: the state of the art. Interact Cardiovasc Thorac Surg 2014;19:661-6. 10.1093/icvts/ivu200 [DOI] [PubMed] [Google Scholar]
- 8.Ng CSH, MacDonald JK, Gilbert S, et al. Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systemic Review and Meta-Analysis. Innovations (Phila) 2019;14:90-116. 10.1177/1556984519837027 [DOI] [PubMed] [Google Scholar]
- 9.Meacci E, Nachira D, Zanfrini E, et al. Uniportal VATS approach to sub-lobar anatomic resections: literature review and personal experience. J Thorac Dis 2020;12:3376-89. 10.21037/jtd.2020.01.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulgarelli ML, Garcia-Perez A, Minasyan A, et al. Uniportal VATS for non‐small cell lung cancer. Gen Thorac Cardiovasc Surg 2020; 68: 707-15. 10.1007/s11748-019-01221-4 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Rivas D, Fieira E, de la Torre M, et al. Bronchovascular right upper lobe reconstruction by uniportal video-assisted thoracoscopic surgery. J Thorac Dis 2014;6:861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian K, Yang R, Han B. Left upper lobectomy and pulmonary angioplasty by uniportal video-assisted thoracic surgery. J Thorac Dis 2017;9:3269-71. 10.21037/jtd.2017.07.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618-25. 10.1016/S0140-6736(06)68700-X [DOI] [PubMed] [Google Scholar]
- 14.Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin 2008;26:355-67, vii. 10.1016/j.anclin.2008.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homma T, Doki Y, Yamamoto Y, et al. Risk factors of neuropathic pain after thoracic surgery. J Thorac Dis 2018;10:2898-907. 10.21037/jtd.2018.05.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homma T, Doki Y, Yamamoto Y, et al. Efficacy of 50 mg pregabalin for prevention of postoperative neuropathic pain after video-assisted thoracoscopic surgery and thoracotomy: a 3-month prospective randomized controlled trial. J Thorac Dis 2019;11:694-701. 10.21037/jtd.2019.02.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Homma T, Shimada Y, Tanabe K, et al. Adverse factors and postoperative neuropathic pain in challenging video-assisted thoracoscopic surgery. Ann Palliat Med 2021;10:2849-58. 10.21037/apm-20-1729 [DOI] [PubMed] [Google Scholar]
- 18.Homma T, Shimada Y, Tanabe K. Lymphadenectomy in the subcarinal zone using a uniportal thoracoscopic approach: a narrative review. AME Surg J 2022;2:6. 10.21037/asj-21-85 [DOI] [Google Scholar]
- 19.Kawai H, Harada K, Ohta H, et al. Prevention of alveolar air leakage after video-assisted thoracic surgery: comparison of the efficacy of methods involving the use of fibrin glue. Thorac Cardiovasc Surg 2012;60:351-5. 10.1055/s-0031-1293599 [DOI] [PubMed] [Google Scholar]
- 20.Ikeda T, Sasaki M, Yamada N, et al. Controlling air leaks using free pericardial fat pads as surgical sealant in pulmonary resection. Ann Thorac Surg 2015;99:1170-5. 10.1016/j.athoracsur.2014.11.040 [DOI] [PubMed] [Google Scholar]
- 21.Guerra-Londono CE, Privorotskiy A, Cozowicz C, et al. Assessment of Intercostal Nerve Block Analgesia for Thoracic Surgery: A Systematic Review and Meta-analysis. JAMA Netw Open 2021;4:e2133394. 10.1001/jamanetworkopen.2021.33394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001;92:147-57. 10.1016/S0304-3959(00)00482-6 [DOI] [PubMed] [Google Scholar]
- 24.Bennett MI, Smith BH, Torrance N, et al. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain 2005;6:149-58. 10.1016/j.jpain.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 25.Brown LM, Thibault DP, Kosinski AS, et al. Readmission After Lobectomy for Lung Cancer: Not All Complications Contribute Equally. Ann Surg 2021;274:e70-9. 10.1097/SLA.0000000000003561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8; discussion 1068. 10.1016/j.athoracsur.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 27.Motono N, Ishikawa M, Iwai S, et al. Individualization of risk factors for postoperative complication after lung cancer surgery: a retrospective study. BMC Surg 2021;21:311. 10.1186/s12893-021-01305-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Liu D, Lu J, et al. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer 2017;17:75. 10.1186/s12885-017-3069-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu CC, Shih CS, Pennarun N, et al. Transition from a multiport technique to a single-port technique for lung cancer surgery: is lymph node dissection inferior using the single-port technique?†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i64-72. [DOI] [PubMed] [Google Scholar]
- 30.Weigl M, Stefan P, Abhari K, et al. Intra-operative disruptions, surgeon's mental workload, and technical performance in a full-scale simulated procedure. Surg Endosc 2016;30:559-66. 10.1007/s00464-015-4239-1 [DOI] [PubMed] [Google Scholar]
- 31.Lavander P, Meriläinen M, Turkki L. Working time use and division of labour among nurses and health-care workers in hospitals - a systematic review. J Nurs Manag 2016;24:1027-40. 10.1111/jonm.12423 [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Zhou X, Xie D, et al. Uniportal video-assisted thoracic surgery-the experiences of Shanghai Pulmonary Hospital. J Vis Surg 2016;2:56. 10.21037/jovs.2016.03.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Ma L, Guo C, et al. Non-grasping en bloc mediastinal lymph node dissection through uniportal video-assisted thoracic surgery for lung cancer surgery. J Thorac Dis 2016;8:2956-9. 10.21037/jtd.2016.10.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Chen X, Shen Y, et al. Learning curve for uniportal video-assisted thoracoscopic surgery lobectomy-results from 120 consecutive patients. J Thorac Dis 2018;10:5100-7. 10.21037/jtd.2018.08.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedetti B, Bertolaccini L, Solli P, et al. Learning curve and established phase for uniportal VATS lobectomies: the Papworth experience. J Thorac Dis 2017;9:138-42. 10.21037/jtd.2017.01.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as