Abstract
Background
During surgery for spontaneous pneumothorax, parietal pleural small holes (PPSHs) are occasionally found around the apex of the intrapleural space; however, this has not been well recognized. Additionally, chest wall flatness is usually observed in patients with primary spontaneous pneumothorax (PSP) and PPSHs. This study aimed to investigate the prevalence of PPSH and evaluate the characteristics of patients with PPSH. We also investigated the degree of chest wall flatness in patients with PPSHs.
Methods
We retrospectively reviewed all patients who underwent thoracoscopic surgery for pneumothorax at our department between April 2014 and May 2021. A propensity-matched analysis was used to compare the characteristics of patients with and without PPSH.
Results
A total of 490 patients were enrolled in this study. PPSH was found in 45 of 297 (15.2%) patients with PSP and one of 193 (0.5%) patients with secondary pneumothorax. PSP was independently associated with the presence of PPSH after adjusting for age and sex [primary/secondary, odds ratio (OR) =34.3, 95% confidence interval (CI): 4.7–250.9; P<0.001]. Among patients with PSP, the flatness of the chest wall in patients with PPSH was not as severe as that in patients without PPSH {thoracic anteroposterior diameter (APDT) to transverse diameter (TDT) ratio; with PPSH: median =0.517 [interquartile range (IQR) =0.480–0.554] vs. without PPSH: median =0.487 (IQR =0.463–0.529; P=0.031)} after propensity score matching.
Conclusions
PPSH is found in a non-negligible proportion of patients with PSP, and patients with PPSHs show a relatively mild flat chest among patients with PSP. Clinicians should be aware of PPSH, and further understanding of this condition may contribute to a better understanding of PSP.
Keywords: Chest wall, flat chest, parietal pleural small holes (PPSHs), primary spontaneous pneumothorax (PSP), propensity-matched analysis
Introduction
Primary spontaneous pneumothorax (PSP) is defined as the spontaneous presence of air in the pleural space of patients without clinically apparent underlying lung disease (1). It is generally believed that PSP occurs in tall and thin males during adolescence and early adulthood (2-5). Spontaneous rupture of subpleural blebs/bullae is commonly believed to be the major cause of PSP (5); subpleural blebs have been reported to be found in 76–100% of patients during video-assisted thoracoscopic surgery (6). Recently, inflammatory cells obstructing the airways and bronchiolitis were suggested to be associated with emphysema-like changes (ELC) and PSP (7,8). Diffuse pleural porosity has also been suggested as a mechanism of PSP (9). Patients with PSP have been reported to have a flattened chest wall (10,11), and biomechanical stress caused by flattened chest wall has been suggested to contribute to the incidence of PSP (10). However, the precise etiology of PSP and mechanism of bleb/bulla formation remain unclear.
Upon careful observation, we found a parietal pleural small hole (PPSH) around the apex of the intrapleural cavity during surgery for PSP (Figure 1, Video 1). PPSH is not well recognized by clinicians. To our knowledge, PPSH was first reported in 2010 by Galetta et al. in five patients with hyperhidrosis (12). The same or similar entity has been reported by Lin et al. (13) and Yang et al. (14) in association with PSP. However, the report by Lin et al. included only a small number of patients (22 patients with PSP and nine patients with secondary spontaneous pneumothorax) (13), and the report by Yang et al. included only PSP patients under 30 years of age (14). Therefore, the prevalence and etiology of PPSH and its association with underlying diseases remain unclear.
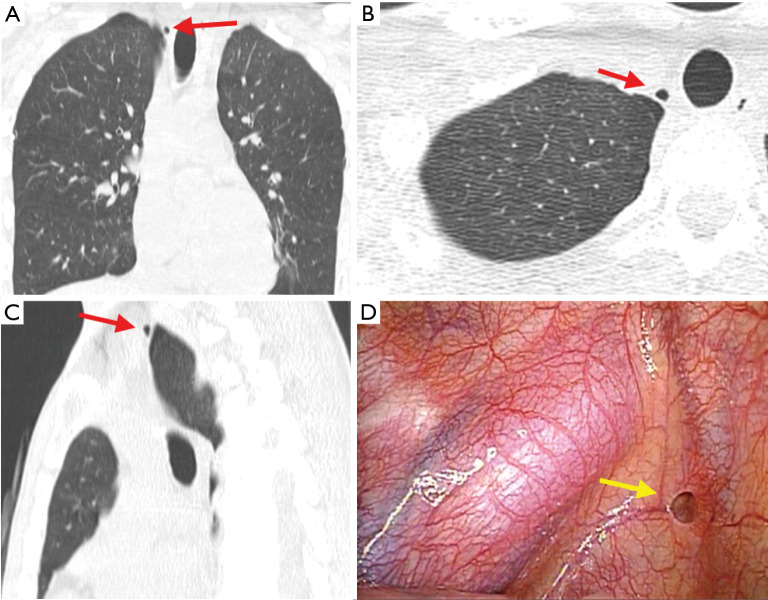
Figure 1.
PPSH. (A) Coronal section of a CT image taken after chest drainage; (B) axial section of a CT image; (C) sagittal section of a CT image; (D) intraoperative image of the same patient. Arrows indicate a PPSH. PPSH, parietal pleural small hole; CT, computed tomography.
Video 1.
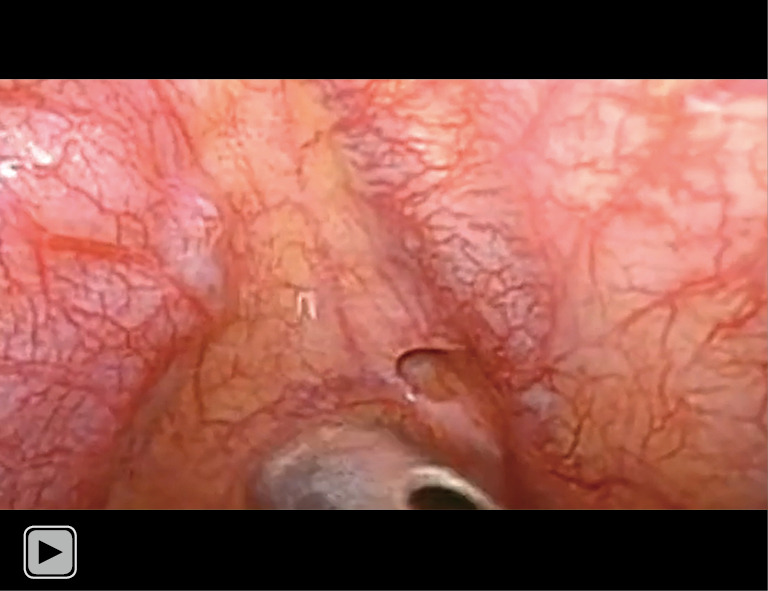
Intraoperative findings of parietal pleural small hole.
We hypothesized that PPSH is associated with underlying characteristics in patients with PSP. This study was performed to investigate the prevalence of PPSH in patients with both primary and secondary pneumothorax over a wide age range and to evaluate the characteristics of patients with PPSH. Additionally, we usually observed chest wall flatness in patients with PPSH; therefore, we also investigated and compared the degree of chest wall flatness in patients with PPSH and PSP. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-352/rc).
Methods
Study design
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Sapporo City General Hospital (approval No. R03-060-888). Considering the retrospective nature of this study, informed consent was obtained in the form of an opt-out clause on our website, and patients who rejected this option were excluded. All patients who were referred to our department between April 2014 and May 2021 and were diagnosed with pneumothorax were retrospectively reviewed. All patients who underwent thoracoscopic surgery for pneumothorax were included in the study. Patients who were treated with non-intubated surgery were excluded because fine examination of the intrapleural space is difficult. Finally, 490 patients with 523 pneumothorax sides were enrolled in this study (Figure 2).
Figure 2.
Flow chart of the 960 patients with pneumothorax. In total, 960 patients were referred to our department and diagnosed with pneumothorax. Among them, 458 patients treated without surgery and 12 patients treated with non-intubated surgery were excluded. Finally, 490 patients with pneumothorax who underwent surgery were enrolled in this study.
Data collection and definitions
From April 2014, we prospectively checked for the presence of PPSH during surgery and recorded it in an institutional database. We retrospectively reviewed and collected the number and sites of PPSH and background information (age at first pneumothorax, age at operation, sex, history of smoking, etiology, and laterality of pneumothorax, underlying disease(s), height, and body weight) from the institutional database.
In our study, PSP was defined as the accumulation of air in the pleural space, mostly due to the rupture of a subpleural bleb or bulla, without apparent underlying lung disease (1,5). Secondary pneumothorax is defined as that with an apparent underlying lung disease (1,5). Emphysema was defined as the presence of bullae and multiple low-attenuation areas, with a history of smoking. Interstitial pneumonia, lung tumor, catamenial pneumothorax, diffuse panbronchiolitis, and lymphangiomatosis were diagnosed based on pathological examinations of resected specimens. Birt-Hogg-Dube syndrome was diagnosed by DNA testing (15).
The ratio of the anteroposterior diameter (APDT) to the transverse diameter (TDT) of the thoracic wall was calculated at the tracheal bifurcation level on the axial section of the computed tomography (CT) scan taken prior to surgery (Figure 3). The APDT was measured in two ways as described in the previous studies. In this study, they were defined as mediastinal APDT (16) and thoracic APDT (17). Mediastinal APDT was defined as the maximum diameter between the posterior rim of the sternum and anterior rim of the vertebra. The thoracic APDT was defined as the maximum APDT of the thoracic cage. TDT was defined as the maximum TDT of the thoracic cage.
Figure 3.
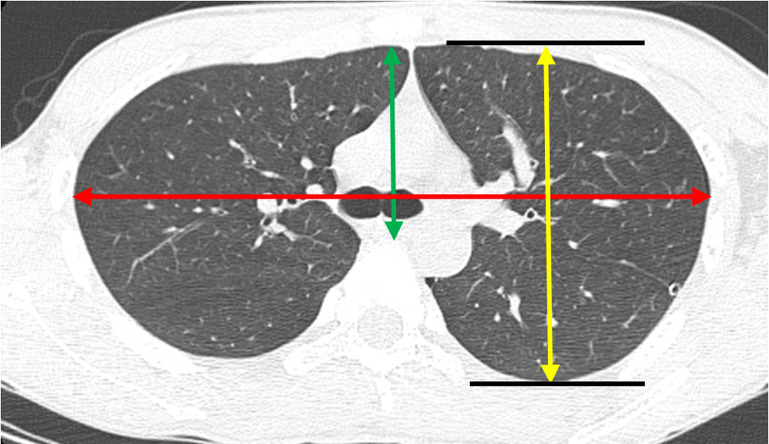
The methods for calculating APDT and TDT. The mediastinal APDT was defined as the maximum diameter between posterior rim of the sternum and anterior rim of the vertebra (green arrow). The thoracic APDT was defined as the maximum anteroposterior diameter of the thoracic cage (yellow arrow). The TDT was defined as the maximum diameter of the transverse diameter of the thoracic cage (red arrow). APDT, anteroposterior diameter; TDT, transverse diameter.
Statistical analysis
Statistical analyses were performed using JMP® 16 software (SAS Institute Inc., Cary, NC, USA). Continuous data are presented as median and interquartile range (IQR). Categorical data were presented as numbers and proportions. Continuous values in the two groups were compared using the Mann-Whitney U-test. Fisher’s exact test for categorical variables was used to compare frequencies. A logistic regression model was used in the univariable and multivariable analyses to identify factors associated with the presence of PPSH. Variables were selected based on their confounding and potential effects on the incidence of pneumothorax, as well as multicollinearity (variance inflation factor <10). Age at first pneumothorax (<20/≥20 years), sex, and etiology of pneumothorax (primary/secondary) were included, and simultaneous entry was used in the multivariable analysis. In another analysis among patients with PSP, age at operation (<20/≥20 years), body mass index (BMI) (<22/≥22), sex, and mediastinal APDT to TDT ratio divided by mean were included in a logistic regression model to predict the presence of PPSH. When comparing height, body weight, BMI, and APDT to TDT ratio between patients with PPSH and those without PPSH, a propensity-matched analysis was applied to minimize the potential confounding factors including age and sex. We calculated the propensity score for each patient using a logistic regression model including age at operation and sex, and patients were matched one-to-one without replacement using a nearest-neighbor approach with a caliper restriction of 0.2. All analyses were two-tailed, and statistical significance was set at P<0.05.
Results
Baseline characteristics
Among the 490 patients, 297 had PSP, and 193 had secondary pneumothorax. The underlying diseases of secondary pneumothorax included emphysema in 153 patients (79.3%), Birt-Hogg-Dube syndrome in 14 (7.3%), interstitial pneumonia in 11 (5.8%), catamenial pneumothorax in 6 (3.1%), lung tumor in 3 (1.6%), lymphangiomatosis in 2 (1.0%), 1 (0.5%) with diffuse panbronchiolitis, and traumatic pneumothorax in 3 (1.6%).
The baseline characteristics of the patients with and without PPSH are summarized in Table 1. In total, PPSHs were found in 46 patients, including 45 patients (97.8%) with PSP and 1 (2.2%) with secondary pneumothorax. The patient with a secondary pneumothorax was a 47-year-old man with lung emphysema. The PPSH data are summarized in Table 2. PPSHs were round, approximately 1 to 10 mm in diameter, clear-shaped and appeared to be covered by pleura. Up to four PPSHs were found on one side, and a total of 85 PPSHs were observed. In more than half of the cases, there was only one PPSH found on one side. Approximately half (55.3%) of PPSHs were found at the chest wall above the first rib, 36 (42.4%) of PPSHs were found at the first intercostal space, and only 2 (2.4%) PPSHs were found at the second intercostal space. No PPSH was found below the second intercostal space. All PPSHs were blind-ended and did not communicate with the other organs or surrounding structures. In more than half of the cases, the PPSH was single on one side. There were no apparent local infections, tumors, or hemangiomas near the PPSH. Capillary vascularization around PPSH was occasionally found around PPSH. A bleb/ELC was not always present adjacent to PPSH. Local adhesions between the PPSHs and the lung surface were not observed in our cases. Not all patients with surgically observed PPSH showed apparent findings in preoperative CT.
Table 1. Baseline characteristics of the patients.
| Baseline characteristics | All (N=490) | Patients with PPSH (N=46) | Patients without PPSH (N=444) |
|---|---|---|---|
| Age† (years), median [IQR] | 26.5 [18–55] | 19 [16.8–21.3] | 30 [19–58] |
| Sex, n (%) | |||
| Male | 415 (84.7) | 44 (95.7) | 371 (83.6) |
| Female | 75 (15.3) | 2 (4.4) | 73 (16.4) |
| History of smoking, n (%) | 256 (52.2) | 9 (19.6) | 247 (55.8) |
| Etiology, n (%) | |||
| Primary spontaneous | 297 (60.6) | 45 (97.8) | 252 (56.8) |
| Secondary | 193 (39.4) | 1 (2.2) | 192 (43.2) |
| Laterality of pneumothorax, n (%) | |||
| Right | 232 (47.3) | 11 (23.9) | 221 (49.8) |
| Left | 225 (45.9) | 24 (52.2) | 201 (45.3) |
| Bilateral‡ | 33 (6.7) | 11 (23.9) | 22 (4.6) |
†, age at the time of first pneumothorax; ‡, bilateral pneumothorax included both simultaneous and metachronous bilateral pneumothorax. PPSH, parietal pleural small hole; IQR, interquartile range.
Table 2. Laterality, numbers in one side, and site of PPSH.
| Characteristics of PPSH | Numbers (%) |
|---|---|
| Laterality of PPSH | |
| Right | 11 (23.9) |
| Left | 30 (65.2) |
| Bilateral | 5 (10.9) |
| Number of PPSH | |
| Right (1/2/3/4) | 11/3/2/0 |
| Left (1/2/3/4) | 19/7/7/2 |
| Site of PPSH (N=85) | |
| Upper than 1st rib | 47 (55.3) |
| 1st intercostal space | 36 (42.4) |
| 2nd intercostal space | 2 (2.4) |
PPSH, parietal pleural small hole.
Factors associated with the presence of PPSH
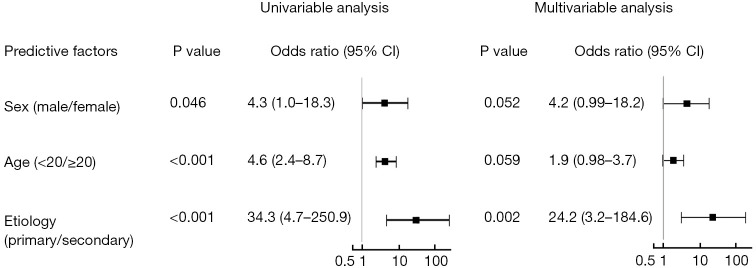
Univariable analysis indicated that primary spontaneous etiology was a predictor of the presence of PPSH [primary/secondary, odds ratio (OR) =34.3; 95% confidence interval (CI): 4.7–250.9; P<0.001; Figure 4]. In the multivariable analysis, primary spontaneous etiology was still independently associated with the presence of PPSH after adjusting for age (<20/≥20 years) and sex (OR =24.2; 95% CI: 3.2–184.6; P=0.002; Figure 4).
Figure 4.
Univariable and multivariable analyses for predicting the presence of a parietal pleural small hole. Etiology of pneumothorax (primary/secondary) was independently associated with the presence of a parietal pleural small hole after adjusting for age and sex (primary/secondary, OR =34.3; 95% CI: 4.7–250.9; P<0.001). CI, confidence interval; OR, odds ratio.
Comparisons of baseline characteristics in patients with PSP
The baseline characteristics of the PSP patients with and without PPSH are summarized in Table 3. Among patients with PSP, patients with PPSH were significantly younger than those without PPSH (Table 3).
Table 3. Comparison in patients with PSP.
| Characteristics at first PSP | Patients with PPSH (N=45) | Patients without PPSH (N=252) | P value |
|---|---|---|---|
| Age (years), median [IQR] | 19 [16.5–21] | 20 [17–26] | 0.046† |
| Sex, n (%) | 0.039‡ | ||
| Male | 43 (95.6) | 209 (82.9) | |
| Female | 2 (4.4) | 43 (17.1) | |
| History of smoking, n (%) | 8 (17.8) | 74 (29.4) | 0.147‡ |
†, continuous variables were compared using the Mann-Whitney U-test; ‡, frequencies were compared using Fisher’s exact test for categorical variables. PSP, primary spontaneous pneumothorax; IQR, interquartile range; PPSH, parietal pleural small hole.
Univariable and multivariable analyses for factors associated with the presence of PPSH among patients with PSP
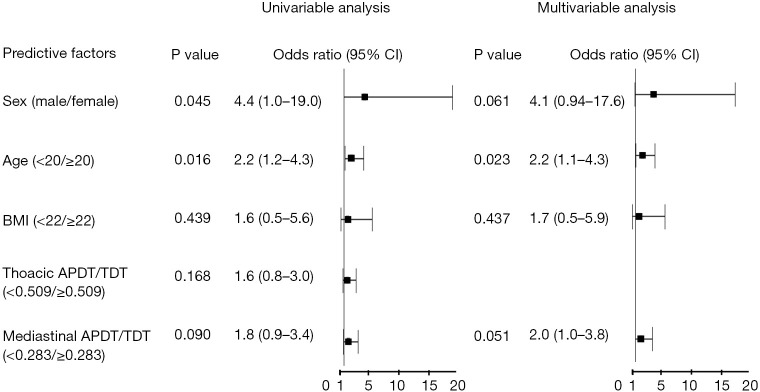
Univariable analysis indicated that age was a predictor of the presence of PPSH (<20/≥20), OR =2.2; 95% CI: 1.2–4.3; P=0.016; Figure 5). In the multivariable analysis, age was an independent predictor of PPSH (<20/≥20, OR =2.2; 95% CI: 1.1–4.3; P=0.023; Figure 5). Higher mediastinal APDT to TDT ratio was associated with the presence of PPSH, although it was not statistically significant (<0.283/≥0.283, OR =2.0; 95% CI: 1.0–3.8; P=0.051; Figure 5).
Figure 5.
Univariable and multivariable analyses for factors associated with the presence of PPSH among patients with PSP. Higher mediastinal APDT to TDT ratio was associated with the presence of PPSH, although it was not statistically significant (<0.283/≥0.283, OR =2.0; 95% CI: 1.0–3.8; P=0.051). OR, odds ratio; CI, confidence interval; BMI, body mass index; APDT, anteroposterior diameter; TDT, transverse diameter; PPSH, parietal pleural small hole; PSP, primary spontaneous pneumothorax.
Propensity score-matched analysis for physical characteristics
One-to-one propensity score matching was performed in 297 patients with PSP, including 45 patients with PPSH and 252 without PPSH. Consequently, 45 pairs were matched. Comparisons of the characteristics at surgery are summarized in Table 4. Age (P=1.000), sex (P=1.000), height (P=0.875), body weight (P=0.884), and BMI (P=0.821) were not significantly different between the two groups. However, both thoracic APDT and TDT ratios [with PPSH, 0.517 (0.480–0.554) vs. without PPSH, 0.487 (0.463–0.529); P=0.031] and mediastinal APDT to TDT ratio [with PPSH, 0.306 (0.262–0.333) vs. without PPSH, 0.270 (0.228–0.309); P=0.008] were significantly higher in patients with PPSH than in those without PPSH.
Table 4. Propensity-matched comparison in patients with PSP.
| Characteristics at operation | Patients with PPSH (N=45) | Patients without PPSH (N=45) | P value |
|---|---|---|---|
| Age (years), median [IQR] | 19 (16.5–21) | 19 (16.5–21) | 1.000† |
| Sex, n (%) | 1.000‡ | ||
| Male | 43 (95.6) | 43 (95.6) | |
| Female | 2 (4.4) | 2 (4.4) | |
| Height (cm), median (IQR) | 174.0 (169.5–177.5) | 173.0 (168.5–178) | 0.875† |
| Body weight (kg), median (IQR) | 56.0 (51.5–61.0) | 55.0 (50.5–61.5) | 0.884† |
| BMI (kg/m2), median (IQR) | 19.1 (17.3–20.3) | 18.9 (17.3–20.7) | 0.821† |
| Thoracic APDT/TDT ratio, median (IQR) | 0.517 (0.480–0.554) | 0.487 (0.463–0.529) | 0.031† |
| Mediastinal APDT/TDT ratio, median (IQR) | 0.306 (0.262–0.333) | 0.270 (0.228–0.309) | 0.008† |
†, continuous variables were compared using the Mann-Whitney U-test; ‡, frequencies were compared using Fisher’s exact test for categorical variables. PSP, primary spontaneous pneumothorax; PPSH, parietal pleural small hole; IQR, interquartile range; BMI, body mass index; APDT, anteroposterior diameter; TDT, transverse diameter.
Discussion
In the present study, PPSHs were found in 45 of 297 (15.2%) patients with PSP, whereas only 1 of 193 (0.5%) patients with secondary pneumothorax showed PPSH. Multivariable analysis demonstrated that PSP was an independent predictor of PPSH in patients with pneumothorax after adjusting for age and sex. These results suggest that PPSH is associated with PSP and is not a rare entity.
Galetta et al. hypothesized that PPSH is a precursor to cervical lung hernias (12). Approximately 80% of lung hernias are considered acquired, mainly traumatic in origin (18), whereas approximately 20% of cases are congenital (19). The thoracic outlet is covered by Sibson’s fascia and the underlying cervical parietal pleura (19-21). Sibson’s fascia, also called the deep cervical fascia or suprapleural membrane, is a thickened portion of the endothoracic fascia. The fascia is attached to the inner border of the whole length of the first rib and to the transverse process of the seventh cervical vertebra (19-21). When the fascia is weakened or torn, cervical hernia may occur through a weak area. Galetta et al. reported that PPSHs were located lateral to the subclavian artery (12), and Lin et al. reported that PPSHs were found exclusively on the chest wall within the first ribs (13). However, in the present study, approximately half of the PPSHs were found to be lower than the first rib, which was outside Sibson’s fascia. Furthermore, cervical lung hernia through a defect in Sibson’s fascia was reported to occur most often in the first three years of life, and in the majority of cases, these protrusions resolve spontaneously in the first years of life (21). Therefore, PPSH may not always be a precursor of cervical lung hernia, and Sibson’s fascia may not always be associated with the origin of PPSH.
Lin et al. hypothesized that PPSH trapped the visceral pleura and caused abrasion and inflammatory changes, resulting in ELC formation (13). In general, intrathoracic inflammation results in adhesion between the lung and the thoracic wall. However, in this study, localized adhesion between PPSH and bleb/ELC was not observed. In contrast, Yang et al. hypothesized that the preformed bleb causes mechanical compression of the parietal pleura, resulting in the formation of PPSH (14). However, although the presence of blebs or a history of pneumothorax was not mentioned, Galetta et al. reported that 5 of 750 (0.67%) patients with hyperhidrosis showed PPSH during the operation (12); given that the mechanical compression induced by a bleb results in PPSH formation, a bleb should pre-exist. Furthermore, in the present study, a PPSH was not always adjacent to a bleb/ELC; therefore, the association between PPSH and bleb/ELC should be carefully determined in chronological order in future studies.
Yamada et al. reported a fistulous hole communicating with bilateral pleural spaces, which were closed during surgery for bilateral pneumothorax (22). The appearance of this fistulous hole looks similar to PPSH. In our study, however, PPSH were blind-ended and were not communicating with other structures. Hence, PPSH may not be the same entity with the fistulous hole reported by Yamada et al. (22). Furthermore, PPSHs were not always adjacent to blebs or ELC, suggesting that PPSH did not directly cause bleb formation and subsequent occurrence of PSP. Therefore, there is currently no evidence to support surgical intervention for PPSH.
There are two hypotheses regarding the etiology of PPSH: acquired or congenital. If PPSH is a completely acquired condition, it should also be found in patients with secondary pneumothorax. The present study showed that PPSH was exclusively found in patients with PSP, especially juvenile subjects; therefore, some congenital or genetic factors, which are associated with PSP, may be involved in the etiology of PPSH. Patients with PSP were reported to have a flattened chest wall, and biomechanical stress caused by thoracic cage deformity has been suggested to contribute to the development of blebs and incidence of PSP (10,11). In the present study, the flatness of the chest wall in patients with PPSH was not as severe as that in patients without PPSH. Ideally, the flatness of the chest wall in patients with PPSH needs to be compared with that in healthy controls; nevertheless, these findings suggest that patients with PPSH may have some distinct characteristics that leads to pneumothorax, which are different from other patients with PSP. As the shape of the chest wall is genetically determined, genetic factors may also be involved in the development of PPSH. Further studies are warranted to investigate the underlying features in patients with PPSH. Other chest wall deformity, including the asymmetry (11) and the wide TDT of the thoracic cage (10), are worth further investigation. Given that patients with PPSH have some underlying characteristics and different mechanisms of occurrence of pneumothorax, the prevalence and recurrence rate might be different from patients without PPSH. Possible clinical significance of PPSH including the prevalence and recurrence rate of pneumothorax should also be investigated in future studies.
In our study, not all patients with surgically observed PPSH showed apparent findings in preoperative CT, and even if PPSH-like findings were recognized in preoperative CT, they were not always identified as PPSHs during operation. In some cases, a fold of the thoracic cavity could be mistaken for PPSH. Another possibility is that only when the hole is filled with air can any PPSH be identified on CT. Therefore, a CT-based diagnosis of PPSH could include a high number of cases resulting in false positives and negatives. In addition, although some cases with PPSH have apparent findings on chest CT, they usually disappear once pneumothorax was treated. Further, not all non-surgically treated patients underwent CT scanning. Therefore, we did not examine PPSH on CT and only investigated surgically proven cases with PPSH.
Our study had several limitations. First, this was a retrospective single-center study. There may be potential bias should the study include surgical cases only. Further studies are required to confirm these results. Second, we did not include all potential confounding factors in our multivariable model and propensity score matching. Although PSP was an independent predictor of PPSH, other possible confounders may exist. In particular, we only included patients with pneumothorax; therefore, PPSH may also found in young patients with other diseases. Further studies are necessary to confirm our results and genetic analysis may contribute to confirm the relevance between PPSH and PSP. Ideally, the presence of PPSH in healthy controls or in young patients with other diseases should be investigated. Young patients with congenital heart or lung diseases may be potential control subjects. In addition, surgical intervention for a healthy subject is generally impossible. Furthermore, PPSH can be recognized in CT images only when the patient has pneumothorax. As a replacement, we included patients with secondary pneumothorax with various underlying diseases over a wide age range. Third, preoperative CT was not always performed after chest drainage, which may have affected the APDT/TDT ratio. However, we calculated the APDT/TDT ratio in two ways to minimize the influence of the timing of the examination. In addition, further studies should investigate whether patients with PPSH have a flatter chest wall than those who undergo thoracic surgery for other underlying diseases. The flatness of chest wall in patients with PPSH still seems flatter than normal controls reported in previous studies, although the methods of measuring the chest wall were slightly different from ours (11,23). Fourth, we could not perform tissue analysis of the PPSH. Further studies are needed to investigate the pathological features of PPSH. Finally, although we checked the thoracic wall carefully and PPSH was usually found easily, the presence of PPSH might have been missed in some cases. Nevertheless, the present study indicates that PPSH is not rare.
Conclusions
This study showed that PPSH was found in a substantial proportion of patients with PSP, and that patients with PPSH showed a characteristic feature among patients with PSP. To our knowledge, this is the first study to investigate patients with pneumothorax over a wide range of ages. This study raises the need for further understanding of PPSH, which may contribute to the understanding of the underlying mechanism of PSP. Therefore, further studies are warranted to confirm the clinical relevance between PPSH and PSP and to investigate PPSH and its associated characteristics, including flattened chest walls, in patients with PSP.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Sapporo City General Hospital (approval No. R03-060-888). Considering the retrospective nature of this study, informed consent was obtained in the form of an opt-out clause on our website, and patients who rejected this option were excluded.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-352/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-352/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-352/coif). The authors have no conflicts of interest to declare.
References
- 1.Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev 2010;19:217-9. 10.1183/09059180.00005310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Withers JN, Fishback ME, Kiehl PV, et al. Spontaneous pneumothorax. Suggested etiology and comparison of treatment methods. Am J Surg 1964;108:772-6. 10.1016/0002-9610(64)90030-3 [DOI] [PubMed] [Google Scholar]
- 3.Shih CH, Yu HW, Tseng YC, et al. Clinical manifestations of primary spontaneous pneumothorax in pediatric patients: an analysis of 78 patients. Pediatr Neonatol 2011;52:150-4. 10.1016/j.pedneo.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 4.Ghisalberti M, Guerrera F, De Vico A, et al. Age and Clinical Presentation for Primary Spontaneous Pneumothorax. Heart Lung Circ 2020;29:1648-55. 10.1016/j.hlc.2020.05.107 [DOI] [PubMed] [Google Scholar]
- 5.Wilson PM, Rymeski B, Xu X, et al. An evidence-based review of primary spontaneous pneumothorax in the adolescent population. J Am Coll Emerg Physicians Open 2021;2:e12449. 10.1002/emp2.12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. 10.1056/NEJM200003233421207 [DOI] [PubMed] [Google Scholar]
- 7.Cottin V, Streichenberger N, Gamondès JP, et al. Respiratory bronchiolitis in smokers with spontaneous pneumothorax. Eur Respir J 1998;12:702-4. 10.1183/09031936.98.12030702 [DOI] [PubMed] [Google Scholar]
- 8.Plojoux J, Froudarakis M, Janssens JP, et al. New insights and improved strategies for the management of primary spontaneous pneumothorax. Clin Respir J 2019;13:195-201. 10.1111/crj.12990 [DOI] [PubMed] [Google Scholar]
- 9.Noppen M, Dekeukeleire T, Hanon S, et al. Fluorescein-enhanced autofluorescence thoracoscopy in patients with primary spontaneous pneumothorax and normal subjects. Am J Respir Crit Care Med 2006;174:26-30. 10.1164/rccm.200602-259OC [DOI] [PubMed] [Google Scholar]
- 10.Casha AR, Manché A, Gatt R, et al. Is there a biomechanical cause for spontaneous pneumothorax? Eur J Cardiothorac Surg 2014;45:1011-6. 10.1093/ejcts/ezt659 [DOI] [PubMed] [Google Scholar]
- 11.Saita K, Murakawa T, Kawano H, et al. Chest wall deformity found in patients with primary spontaneous pneumothorax. Asian Cardiovasc Thorac Ann 2013;21:582-7. 10.1177/0218492312467174 [DOI] [PubMed] [Google Scholar]
- 12.Galetta D, Serra M, Gossot D. Apical parietal pleural holes: what are they? Thorac Cardiovasc Surg 2010;58:237-8. 10.1055/s-0029-1240830 [DOI] [PubMed] [Google Scholar]
- 13.Lin FC, Chou MC, Jeng KC, et al. Vascular-penetration defect detected in parietal pleura of primary spontaneous pneumothorax. Interact Cardiovasc Thorac Surg 2014;19:861-3. 10.1093/icvts/ivu229 [DOI] [PubMed] [Google Scholar]
- 14.Yang HC, Jung S. Bullae formation hypothesis in primary spontaneous pneumothorax. J Thorac Dis 2020;12:2833-7. 10.21037/jtd.2020.03.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menko FH, van Steensel MA, Giraud S, et al. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol 2009;10:1199-206. 10.1016/S1470-2045(09)70188-3 [DOI] [PubMed] [Google Scholar]
- 16.Miyahara S, Chen-Yoshikawa TF, Motoyama H, et al. Impact of flat chest on cadaveric lung transplantation: postoperative pulmonary function and survival. Eur J Cardiothorac Surg 2019;55:316-22. 10.1093/ejcts/ezy248 [DOI] [PubMed] [Google Scholar]
- 17.Harada T, Yoshida Y, Kitasato Y, et al. The thoracic cage becomes flattened in the progression of pleuroparenchymal fibroelastosis. Eur Respir Rev 2014;23:263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moncada R, Vade A, Gimenez C, et al. Congenital and acquired lung hernias. J Thorac Imaging 1996;11:75-82. 10.1097/00005382-199601110-00008 [DOI] [PubMed] [Google Scholar]
- 19.Galetta D, Spaggiari L. Beyond the boundaries: Surgical repair of apical parietal pleural hernia. J Thorac Cardiovasc Surg 2019;157:e189-90. 10.1016/j.jtcvs.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 20.Grunebaum M, Griscom NT. Protrusion of the lung apex through Sibson’s fascia in infancy. Thorax 1978;33:290-4. 10.1136/thx.33.3.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Currarino G. Cervical lung protrusions in children. Pediatr Radiol 1998;28:533-8. 10.1007/s002470050405 [DOI] [PubMed] [Google Scholar]
- 22.Yamada S, Yoshino K, Inoue H. Simultaneous bilateral spontaneous pneumothorax with pleural window communicating with bilateral pleural spaces. Ann Thorac Surg 2008;85:1434-6. 10.1016/j.athoracsur.2007.10.033 [DOI] [PubMed] [Google Scholar]
- 23.Kılıçgün A, Yakşi O, Ünal M. Is Pectus Excavatum a Risk Factor for Spontaneous Pneumothorax? “haller Index Measurements in Patients with Primary Spontaneous Pneumothorax.” Can Respir J 2019;2019:3291628. 10.1155/2019/3291628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as