Laboratory surrogate markers of human immunodeficiency virus (HIV) infection are needed to provide information about the stages and course of disease, especially in the years prior to the development of clinical signs and symptoms of immune disorder, e.g., opportunistic infections or neoplasia. Changes in immune parameters also reflects damage to the host caused by HIV infection and indicate pathogenic mechanisms of disease.
The distinction between markers and surrogate markers is relevant to judgment of the usefulness of laboratory tests. Many measurable abnormalities have been identified in HIV infection, but only a few have been shown to relate closely to disease course. In cross-sectional studies, laboratory markers may be found to be more abnormal in advanced disease than in less advanced and asymptomatic infection. This general association, however, does not qualify abnormalities as surrogate markers. The CD8 T-cell increase in HIV infection is a good example of a marker change that does not relate closely to prognosis. In the clinical context, “surrogate” implies some responsibility, e.g., an ability to contribute substantial information about clinical prognosis or an evaluation of therapy.
Validation of laboratory markers as surrogate markers of disease requires extensive collaboration between laboratory and clinical investigators, biostatisticians, and HIV-infected study participants. Longitudinal observations are required, and suitable biostatistical analyses need to be employed. Furthermore, valuable information is obtained if new markers are compared with already validated markers in the same study. Major considerations for clinically relevant surrogate markers in HIV infection are listed in Table 1 and are addressed in this article.
TABLE 1.
Major considerations for clinically relevant surrogate markers in HIV infection
| Classification of surrogate markers based on disease pathogenesis |
| Immune activation and dysregulation in HIV pathogenesis |
| Cytokine changes |
| Soluble markers of activation in plasma and serum |
| Lymphocyte phenotypic changes in HIV infection |
| Neoplasia |
| Response to therapy |
| Surrogate markers for cytokines: serum (soluble) markers of immune activation as measures of cytokine activity |
| Comparing the value of surrogate markers: POP graphs |
| Test availability, quality control, and cost |
(This work was presented in part as the Abbott Lecture, 95th General Meeting of the American Society for Microbiology, Washington, D.C., 21 to 25 May 1995.)
CLASSIFICATION OF SURROGATE MARKERS BASED ON DISEASE PATHOGENESIS
A pathogenesis-based classification system of surrogate markers which emphasizes the distinct mechanisms that are involved in AIDS pathogenesis is proposed. This classification system is based on the major features of HIV and AIDS: the pathogenic virus, the protective host resistance systems and specific immune responses of the host, and the major pathologies induced in the immune system, e.g., CD4 T-cell reduction, immune activation and dysregulation, and functional immune defects. Recognizing the existence of different categories of HIV disease markers is important both for selection and assessment of individual markers and for development of plans for combining markers to achieve better staging of disease.
Several laboratory markers, such as CD4 T-cell reduction, viral load, and increased soluble immune activation markers, have been shown in many studies to be definitely predictive of the course of illness (23, 37, 46, 49, 55, 57, 64, 81, 84, 85). Since all of these markers relate to pathogenesis, a classification of surrogate markers based on disease pathogenesis is described below. The CD4 T-cell level has been the principal laboratory surrogate marker utilized for clinical evaluations of disease. Measurements of plasma HIV load have also been shown to be clinically relevant and are being incorporated into therapeutic evaluations.
A separate system for classifying the stages of surrogate marker evaluation has been proposed by Mildvan et al. (58). This involves progress in staging from detection of HIV infection, to relation to prognosis, and finally to relevance to therapeutic benefit. Such a system is useful in indicating the readiness for clinical application of particular markers. However, it does not indicate marker groups or pathology.
Viral features.
Many characteristics of HIV have been identified and investigated for relevance to disease course. Initially, plasma (free) p24 antigen levels (17) and, later, total immune-complexed dissociated p24 antigen (63) were used to assess viral load. Subsequently, measures of plasma HIV content by RNA PCR (69) and B-DNA hybridization techniques (8) and of infectious virus levels (17) have been added. As expected, higher viral load is associated with adverse prognosis.
The syncytium-inducing capacity of viral strains, as demonstrated in a specific tissue culture system (47), has been shown to be associated with more-rapid disease progression in some but not all patients. The development of viral resistance to nucleoside analog therapies and other agents is highly relevant to the duration of effectiveness of antiretrovirus therapies (73).
Host protective responses and host resistance systems.
Nonspecific factors are likely to contribute to resistance to HIV infection. Most health care workers who are exposed to the virus fail to become infected (9). Furthermore, activated CD8 T cells have been shown to produce a soluble factor which inhibits HIV replication in CD4 T cells (88). A factor from macrophages (MDF) appears to be an identical or similar factor (65). Since CD8 T cells and macrophages are activated in HIV infection, such factors may help to control viral production and disease activity.
Chemokines such as RANTES, MIP-1α, and MIP-1β have been shown to block HIV-1 entry into target cells and influence HIV replication (15, 16). Host factors involved in the pathogenesis of HIV disease also include the two distinct chemokine receptors CXCR4 and CCR5. They have recently been identified as coreceptors for T-cell-tropic and macrophage-tropic HIV isolates, respectively (20, 29), and are involved in suppression of HIV entry. The transition from macrophage-tropic, non-syncytium-inducing to T-tropic, syncytium-inducing HIV strains in vivo is often associated with rapid disease progression (74). Furthermore, a mutant CCR5 phenotype has been reported to correlate with slow progression (19).
Specific immunity to HIV.
Both humoral and cellular immune mechanisms are likely to contribute to host containment of HIV infection. Antibodies to the p24, gp120, gp41, p17, nef, gag, and pol components of HIV have been described (84). The levels of specific p24 antibodies are widely variable between individuals but are stable over time (81). Low levels, in general, are associated with adverse prognosis (81, 84), and reductions in the levels of these antibodies later in the disease course indicate advanced disease.
Neutralizing antibodies, on in vitro testing, reduce the infectivity of HIV for susceptible cell lines in tissue culture. Neutralizing antibodies are fairly easily demonstrated. Unfortunately, the levels of neutralizing antibody do not have a strong relationship to the course of disease (16, 70).
Enhancing antibodies have effects opposite those of neutralizing antibodies (44). They increase the infectivity of virus for target cells in vitro and can be demonstrated in most patients. Unfortunately, enhancing antibodies are rarely measured and their full significance in relation to disease course is not settled.
Substantial attention is devoted to measurements of cell-mediated immunity (CMI), as this is a principal means of controlling many viral infections. Specific cellular immunity to HIV has been identified where HIV infection is not advanced. CMI, however, is usually detected at low levels in later stages of disease (57, 75, 87). Furthermore, CMI has been found in some people who have had substantial exposure to HIV infection but who have not become infected (12, 77). This supports the possibility that CMI in some individuals is significant in the prevention of HIV infection.
Measurements of cellular immunity have focused on a variety of HIV gene products, including Env, Gag, Nef, and other proteins. The methodologies, however, are expensive and require skilled technical personnel. Furthermore, testing of each person is individualized in order to assure that testing is specifically directed to HIV antigens. As a result, this testing is restricted to a few research locations.
CD4 T-cell decrease.
When AIDS was first identified, CD4 T-cell reduction was shown to be a major feature of the disease. Subsequent studies demonstrated that it is a useful indicator of the likelihood of HIV infection progressing to AIDS or to death in those with AIDS. Since CD4 T cells are key helper cells for many immune functions, a marked reduction in numbers is associated with immune deficiency. Progressive reductions in CD4 T-cell levels are regarded as indications of disease progression. CD4 T-cell levels of 200/mm3 or less were shown to be associated with AIDS-defining opportunistic infections (68) and are used as indicators for prophylactic antibiotics. Confirmed CD4 levels below 200/mm3 are designated by the Centers for Disease Control and Prevention to be sufficient for AIDS diagnosis (10). Finally, CD4 T-cell levels generally fall below 50/mm3 before death (91).
CD4 T-cell levels are, however, incomplete markers of HIV disease. This is seen in the evidence that markers detecting other aspects of disease, such as plasma immune activation markers, can add to the prognostic significance of CD4 T-cell measurements when they are combined (18, 22). Also, CD4 T-cell increases after zidovudine administration were found to be only a partial indicator of clinical benefits (11).
This limitation in CD4 usefulness occurs for several reasons. CD4 reduction is only a part of disease pathogenesis, as discussed below. Another factor is the variability in CD4 measurements, which is caused by biological factors (such as diurnal variations) and by methodological issues. Variability occurs both within and between laboratories (43). Substantial variations in serial measurements of CD4 T-cell levels also occur because of problems in hematological measurements of leukocytes and the percentage of lymphocytes. Quality control programs, such as that instituted by the Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), which involve periodic overnight shipments of normal and abnormal blood samples to participating laboratories have helped to reduce flow cytometry problems (67).
Also, the availability of stabilized blood samples (28) has permitted the establishment of the International Program for Quality Assurance and Standardization of Immunologic Measurements relevant to HIV and AIDS (the QASI program), which provides blood samples for CD4, CD8, and CD3 proficiency testing, especially for developing countries throughout the world.
New methods of measuring CD4 levels directly by simplified flow-cytometric procedures and by non-flow-cytometric techniques such as enzyme-linked immunosorbent assay and bead-based CD4 identification offer possibilities for improvement and extension of CD4 measurements (53). Nevertheless, regardless of how much CD4 measurements are improved, there will still be a need to have additional surrogate markers that reflect other aspects of disease pathogenesis.
Immune system activation.
Initial studies of immune system changes in AIDS concentrated on defining the immune deficiency. However, early reports on AIDS showed that serum immunoglobulin G (IgG) and IgA levels were elevated (80), and increases in CD38 expression (then known by the identifying antibody T10) on T lymphocytes were reported (24). Subsequently, immune activation received increased attention as a major feature of HIV pathogenesis. The level of immune activation was found to relate closely to the course of illness (prognosis) and to contribute in major ways to the pathology of HIV infection, e.g., HIV production, neoplasia, immune malfunction, and susceptibility to infection. Current evidence for immune activation is outlined below and elsewhere (1, 23, 27, 30, 64, 66, 84).
Functional impairment.
Reduced lymphocyte proliferative responses to soluble microbial antigens, allo antigens, and mitogens are reduced with advancing HIV infection (14, 40). Impaired interleukin 2 (IL-2) cytokine production (50) as well as reduced gamma interferon (IFN-γ) release (61) have been documented. Loss of delayed-type hypersensitivity skin test responses also characterizes HIV disease.
Reduced proliferation in response to stimulation reflects disordered function and may occur before CD4 cell numbers are markedly reduced. The reduced responses of lymphoid cells could be due to advanced differentiation. Alternatively, functional fatigue or exhaustion or elevated suppressor systems because of chronic stimulation could account for the impairment of immune functions in HIV infection. The lymphocyte proliferation assay is important in clinical research. However, functional tests are not likely to be routinely used for clinical evaluation of HIV-infected individuals because of restricted availability, substantial variability, cost, and the absence of a national quality assurance program.
IMMUNE ACTIVATION AND DYSREGULATION IN HIV PATHOGENESIS
Cytokine changes.
Many cytokines are altered in HIV infection (Table 2). The mode of testing for cytokines, however, has influenced the interpretation of HIV effects. Three categories of tests are used to evaluate the cytokine changes in HIV infection. The first is measurement of circulating levels in serum or plasma. The second measures increased cytokine gene expression in peripheral blood mononuclear cells (PBMC) or lymphoid subsets. These two evaluations indicate the effect that HIV has on the operating status of the patient’s immune system. The third category of tests measures the capacity of the immune system to respond to new stimuli. New cytokine production is measured in vitro after stimulation. However, for some cytokines, such as IFN-γ, the first two methods have indicated increased immune cell activity in patients with HIV infection. In contrast, the third technique may indicate decreased capacity to respond to new stimuli. Interpretations of these several types of tests differ and should not be confused.
TABLE 2.
Cytokine changes in HIV infection
| Cytokine | Levels in plasma/seruma | In vivo cytokine gene expressionb | In vitro response to stimulation |
|---|---|---|---|
| IL-2 | NM | ↓PBMC | ↓ |
| N CD4 | |||
| IFN-γ | ↑ | ↑PBMC | ↓ |
| ↑CD8 | |||
| IL-4 | Low | N PBMC | ? |
| IL-10 | NM | N PBMC | ↑ |
| TNF-α | ↑ | ↑PBMC and Mφ | ↑ |
| IL-6 | ↑ | ↑PBMC and Mφ | ↑ |
| IL-12 | NM | ? | ↓ |
NM, Not measurable (or uncertain).
Specific cytokine mRNA levels in lymphoid cells and monocytes. N, normal range; Mφ, macrophage.
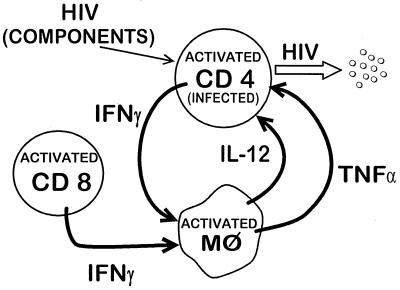
Widespread activation of CD4 and CD8 T cells, NK and B cells, and macrophages is characteristic of HIV infection (27). Activation of T cells and macrophages is schematically diagrammed in Fig. 1. Special emphasis is on IFN-γ and tumor necrosis factor alpha (TNF-α), which are substantially increased in HIV infection and contribute to immune pathogenesis. Immune cell activation results in increased production of many cytokines and increased expression of cytokine receptors and soluble products of activation.
FIG. 1.
Schematic representation of some of the significant cytokine-mediated interactions between T cells and macrophages (Mφ) during HIV infection.
Levels of the proinflammatory cytokines TNF-α and IL-6 in plasma are increased, especially in patients with more advanced disease. IFN-γ, a proactive lymphokine with many functions including macrophage activation, is elevated progressively with more advanced disease. IL-2 levels cannot be measured accurately in most serum samples, but IL-2 is known to stimulate IL-2 receptor (IL-2R) production, and elevated serum IL-2R levels are presumed to reflect increased IL-2 production in lymphoid tissues. Regulatory cytokines, such as IL-4, have not been found to be elevated in plasma. It should be remembered that these plasma and serum cytokine levels do not simply reflect the production of cytokines but are also modified by removal by cell-bound receptors, neutralization by soluble receptors, metabolism, and excretion.
Cytokine gene expression occurs during activation and can be evaluated by the measurement of cytokine-specific mRNA in cells of the immune system. Directional changes have been documented, including increases in TNF-α, IL-6, and IFN-γ mRNA, decreased IL-2 mRNA, and approximately normal IL-4 mRNA levels in PBMC (7, 26, 78). Examination of purified cell populations has demonstrated that the IL-2 mRNA decrease is attributable to substantial reduction in the number of CD4 cells, which are the principal source of IL-2 (26). The increase in IFN-γ appears to be due to increased production in CD8 and NK cells (7, 21). Multiple cellular sources also are likely to contribute to the increases in TNF-α and IL-6. These observations on cytokine gene expression confirm and extend the plasma cytokine changes noted above.
A Th1 and Th2 switch has been hypothesized on the basis of in vitro responses to stimulation and extrapolation from findings in several non-HIV infections (13). Subsequent HIV studies have not confirmed the switch hypothesis (26, 38, 76). At present, it is clear that progression of HIV disease is associated with increasing dysregulation of Th1 lymphocytes and cytokines, with diverse changes in Th2 cytokines.
Soluble markers of immune activation in plasma and serum.
Activation occurs in all categories of immune cells during HIV infection. This activation can be identified and quantified by measurements of appropriate soluble markers which are detectable in plasma and serum. These include neopterin for macrophages, soluble IL-2R (sIL-2R) and sCD4 antigens for CD4 T cells, sCD8 antigen for CD8 T cells, and serum IgA and IgG for B cells.
The serum markers of activation, such as the well-characterized neopterin and β2-microglobulin (β2M) increases, have been shown to be largely independent of the CD4 T-cell changes in HIV infection (23, 31, 42, 56, 60, 84). They provide prognostic information distinctive and separate from that obtainable by CD4 T-cell measurements. This has been documented in patients with CD4 levels over 450/mm3, from 450 to 250/mm3, and under 250/mm3 (56). Additional markers with confirmed relevance to prognosis include serum IFN-γ, TNF-α, sTNF-α–RII, and sCD8 (2, 35, 62). Serum levels of sIL-2R correlate less well and presumably are under somewhat different regulation (41). Increased serum IgA levels are also prognostic in HIV infection (23).
Neopterin and β2M increases, for the most part, correlate well during HIV infection. However, they may be under different controls, and there is evidence that neopterin correlates better than β2M with HIV prognosis in intravenous drug abusers (83, 92) and infants (89). Furthermore, serum neopterin is increased in other infections, such as tuberculosis, where immune activation also occurs (86). Tuberculosis and HIV infection are mutually disadvantageous, presumably because both stimulate the immune system. Thus, the elevated neopterin levels are likely to reflect the true hazard of disease progression when HIV and tuberculosis occur together.
In contrast to the poor correlation with CD4 levels, recent studies have indicated that many soluble markers of activation correlate moderately well with plasma viral load (25, 78). However, the plasma immune activation markers correlate more strongly with each other than with viral load, indicating that they reflect significant host factors.
Lymphocyte phenotypic changes in HIV infection.
Many changes in phenotypic antigens on peripheral blood lymphocytes in HIV infection have been described (5, 32, 71). Some increases, such as CD38 and HLA-DR, are associated with activation of CD8 and CD4 T cells. A CD71 increase is seen on T and B cells. These changes are generally described as “associated with HIV infection,” and, for the most part, they have not been subjected to quantitative measures such as the relative hazard of disease progression or their correlation with other markers of disease. An exception is the reports of Giorgi et al. (34, 52), which indicate a strong relationship of the relative expression of CD38 antigen on CD8 T cells to progression of HIV infection. CD38 expression is also known to correlate well with serum neopterin increases (5). Increased HLA-DR expression appears to have somewhat different significance. Although increased by activation, HLA-DR increases do not correlate closely with CD38 changes. Indeed, they appear to be elevated more in HIV disease-stable individuals and may be related to a state of protective immunity (33).
The association of phenotypic antigens with naive and memory (or committed) lymphocytes has been explored. Naive (CD45RA+) CD8 cell levels decrease, and memory (CD45RO+) cell levels increase with advancing HIV infection (6). This concept has been further developed by the use of three-color testing, adding CD62L to CD45RA as markers for naive CD8 cells (72). One of the implications of this shift from naive to memory or activated cells is the impairment of immune functions. A decrease in CD25 (IL-2R alpha chain) levels also occurs on all lymphoid subpopulations in HIV infection (41). This decrease has been shown to correlate with impaired proliferative functions (4). Other phenotypic changes on CD8 T cells have been explored with the hope that some phenotypic change would be closely associated with the appearance of (or loss of) protective cellular immunity against HIV infection. The goal of substituting phenotypic for functional measurement, however, has not yet been achieved.
Neoplasia.
Increased cytokine production is likely to be a factor in the development of Kaposi’s sarcoma and B-cell lymphoma. IL-6 and oncostatin M are autocrine growth factors for Kaposi’s sarcoma cells (59). IL-4 and sCD23 increases are related to B-cell lymphoma (90). It is clear that cytokine dysregulation contributes to the immune disorders of HIV infection, susceptibility to opportunistic infection, neoplasia, and wasting associated with HIV infection.
Response to therapy.
The levels of immune activation markers in plasma respond to therapy. The nucleoside analogues cause transient reduction levels in neopterin, β2M, and other plasma markers (3, 51). Transient increases in plasma immune activation markers also reflect the stimulation secondary to IL-2 administration (48, 79).
SURROGATE MARKERS FOR CYTOKINES: SERUM (SOLUBLE) MARKERS OF IMMUNE ACTIVATION AS MEASURES OF CYTOKINE ACTIVITY
The levels of most cytokines in serum or plasma cannot be detected in the normal state and are often not measurable in HIV infection. Difficulties in detecting circulating cytokines arise for several reasons. Most cytokine activity occurs in cells in close proximity to the sites of cytokine formation. Receptors for cytokines exist on many tissues and assure rapid removal. Thus, the cytokines appearing in the circulation are in excess of those utilized at local sites. Furthermore, the soluble receptors that are present for many (if not all) cytokines may accelerate their removal or block their detection. However, an alternative to measuring excess cytokines in the circulation is the measurement of specific cytokine actions, e.g., the induction of soluble products in plasma or serum which reflect specific actions by cytokines. These soluble molecules, which are markers of cytokine activities, thus become surrogate markers for cytokines.
The principal surrogate markers for IFN-γ, TNF-α, IL-4, IL-6, and IL-2 are neopterin, TNF-α–RII, CD23, C-reactive protein, and sIL-2R, respectively. IFN-γ has long been known to be a major macrophage-activating factor. IFN-γ increases the cycloxygenase within macrophages which, in turn, contributes to the breakdown of GTP to pteridine molecules and neopterin. These are released into the circulation from activated macrophages (30, 45). Thus, the neopterin level is an indication of both IFN-γ activity and macrophage activation.
Activation of T cells is reflected by increased sIL-2R alpha chain production which has been stimulated by IL-2 (82). Thus, sIL-2R is a surrogate for IL-2. Similarly, sTNF-α–RII expression reflects increased production of TNF-α (93). A high correlation exists between elevated levels of TNF-α and elevated sTNF-α–RII levels in HIV infection (2). Because this marker (TNF-α–RII) appears to be more stable than TNF-α after collection of blood, it is likely to be a useful surrogate marker for TNF-α levels and activity. IL-6 activity is reflected in the levels of circulating C-reactive protein (54). Soluble CD23 in the circulation reflects increased IL-4 activity (36). β2M reflects activation of several cell types expressing major histocompatibility complex type 1 receptor and has not been attributed to a specific cytokine (39).
PRECISION OF PREDICTION GRAPHS: COMPARING THE VALUE OF SURROGATE MARKERS
Many methods have been used to compare the ability of different assays to assess prognosis or response to therapy. These have included Kaplan-Meier plots, log-likelihood calculations, and relative hazard, etc. Some graphs are difficult to compare. Other data end up in long tables with values that are not in common parlance outside of biostatistical circles. Other analyses reveal which tests are better than others but not by how much. Recently, J. M. G. Taylor suggested a method (25) which allows ready perception of how much individual surrogate markers or combinations add to prognostic capability (and how far from perfect each is) (Fig. 2). The calculation is based on the spread or standard deviation of the various times from sample date to an end point, such as the occurrence of AIDS. Additional information is included in the legend to Fig. 2. In addition to demonstrating that surrogate markers do advance prognostic capability, the precision of prediction (POP) method allows ready appreciation of several facts, e.g., (i) many markers have very similar prognostic values, (ii) the “best” markers may not be much better than several others, and (iii) combinations of two selected markers may be substantially better than either alone.
FIG. 2.
POP for occurrence of AIDS. This graphic method allows ready comprehension and comparison of several surrogate markers. Calculations are based on survival analysis regression methods with Gaussian error distribution, as described in detail in reference 25. Lower values are better; a perfect predictor would be one that had zero deviation and could predict future occurrence of AIDS to a precise day. This would have a scale value of zero. (A) Illustrative diagram of three hypothetical surrogate markers, X, Y, and Z. In this example, assay Y is not as good as X but is better than Z. (B) Graphic presentation of several surrogate markers. A group of 659 seropositive men with mean CD4 T-cell levels of 514/mm3 were evaluated for prediction of AIDS occurrence within 3 years (25). SP, prognostic precision for the study population as a whole without surrogate markers; T, plasma sTNF-α–RII levels; 4, CD4 T-cell levels; H, plasma HIV concentration; C1, combined value for CD4 T cells plus HIV load; C2, combined value for CD4 T cells plus sTNF-α–RII.
The POP graphic method also allows markers to be evaluated in the light of relative cost and ease of performance. For example, a relatively inexpensive test (plasma activation marker, such as sTNF-α–RII or neopterin) that is almost as good as one that costs 10 times as much (plasma viral load) might be entirely suitable when serial testing is needed. Furthermore, combination of a relatively inexpensive test with an established test when the combination has better prognostic capability (e.g., plasma activation marker plus CD4 T-cell level) may be preferable to other expensive tests.
TEST AVAILABILITY, QUALITY CONTROL, AND COST
Surrogate markers should be easily quantifiable, reliable, clinically available, and affordable, as pointed out by Tsoukas and Bernard (84). However, many of the immunologic evaluations have practical limitations. Generally, tests become widely available if there is sufficient demand and if test reliability is easily controlled. Thus, the need for CD4 testing brought flow cytometry out of the research laboratory and into the clinical immunology laboratory. On the other hand, extension of phenotypic testing to activation or naive cell markers, whether by percent positive cells or relative fluorescence intensity, requires fresh blood samples, a good flow cytometer, suitable computer programs for data analyses, and technical specialists in flow cytometry. All in all, this is a relatively expensive process.
The HIV viral load test requires proper plasma collection and storage, specially trained technical staff, and expensive reagents and/or equipment. Furthermore, the HIV levels in patients vary substantially on serial testing.
Functional testing of the immune system also demonstrates substantial test and intrasubject variability. Freshly obtained blood is preferred, and testing requires several days, is labor intensive, and is now feasible only in research settings.
Cytokine measurements in plasma and serum have focused on TNF-α, since it is the most readily quantified of all cytokines. Other cytokine levels are often at or below the levels of detection in many healthy seronegative and seropositive individuals.
There are many advantages in measuring the levels of soluble markers of activation in plasma or serum. They can be readily quantified and can serve as surrogates for specific cytokine activity. Neopterin, β2M, TNF-α–RII, sIL-2R, sCD8 antigen, and many other products of cytokine activity can be readily tested by enzyme-linked immunosorbent assays. Automated technologies are being developed. Plasma or serum factors are generally stable, and samples can be stored in a refrigerator or freezer for future and batch testing. Cost is substantially lower than for viral load, phenotypic, or functional testing. When data have been obtained on the value of plasma activation marker measurements in assessing antiretroviral therapeutic regimens, perhaps the cheaper test will become routine for assessing treatment success as well as the need to modify antiretroviral regimens.
Quality assurance procedures are being established for each group of analyses, with the goal of comparability of analytic data between different laboratories. The quality control programs for CD4 testing inaugurated by the Division of AIDS, NIAID, and by the College of American Pathologists have had considerable positive influence on laboratory performance. Furthermore, the view that poor performance of CD4 testing yields misleading conclusions has been validated (11). An international CD4 proficiency testing and quality assurance program (the QASI program) has been established and is available worldwide for countries that lack these resources (contact the author for more information). A proficiency testing program for research laboratories measuring cytokines and soluble markers of activation is being established under NIAID auspices.
Hopefully, resources will be made available worldwide to assure quality control of other laboratory tests relevant to prognosis and therapeutic response. Ultimately, tests such as plasma immune activation markers may be “good enough” for clinical purposes, especially if other tests are more expensive, less readily available, unfamiliar, and/or not hugely better.
ACKNOWLEDGMENTS
Many individuals have contributed to the concepts and data referred to in this article. I acknowledge especially the contributions of Bo Hofmann, who died early in 1997. I also thank Pari Nishanian, Najib Aziz, Susan Plaeger, Oto Martinez-Maza, Hong Bass, Elizabeth Breen, Susan Stehn, Jeremy Taylor, Roger Detels, and the many others who have contributed to studies of HIV and AIDS in the past 17 years.
This work was supported by NIH grants AI 36086, AI 27660, AI 95040, and TW 00003.
REFERENCES
- 1.Ascher M S, Sheppard H W. AIDS as immune system activation. II. The panergic imnesia hypothesis. J Acquired Immune Defic Syndr. 1990;3:177–191. [PubMed] [Google Scholar]
- 2.Aukrust P, Liabakk N B, Müller F, Lien E, Espevik T, Froland S S. Serum levels of tumor necrosis factor-α (TNF-α) and soluble TNF receptors in human immunodeficiency virus type 1 infection. Correlations to clinical immunologic and virologic parameters. J Infect Dis. 1994;169:420–424. doi: 10.1093/infdis/169.2.420. [DOI] [PubMed] [Google Scholar]
- 3.Bass H Z, Hardy D W, Mitsuyasu R T, Taylor J M, Wang Y X, Fischl M A, Spector S A, Richman D D, Fahey J L. The effect of zidovudine treatment on serum neopterin and β2-microglobulin levels in mildly symptomatic human immunodeficiency virus type-1 (HIV-1) seropositive individuals. J Acquired Immune Defic Syndr. 1992;5:215–221. [PubMed] [Google Scholar]
- 4.Bass H Z, Fahey J L, Nishanian P, Detels R, Cumberland W, Kemeny M, Plaeger S. Relation of impaired lymphocyte proliferative function to other major human immunodeficiency virus type 1-induced immunological changes. Clin Diagn Lab Immunol. 1997;4:64–69. doi: 10.1128/cdli.4.1.64-69.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bass H Z, Nishanian P, Hardy W D, Mitsuyasu R T, Esmail E, Cumberland W, Fahey J L. Immune changes in HIV infection: significant correlations and differences in serum markers and lymphoid phenotypic antigens. Clin Immunol Immunopathol. 1992;64:63–70. doi: 10.1016/0090-1229(92)90060-2. [DOI] [PubMed] [Google Scholar]
- 6.Benito J M, Zabay J M, Gil J, Bermejo M, Escudero A, Sanchez E, Fernandez Cruz E. Quantitative alterations of the functionally distinct subsets of CD4 and CD8 T lymphocytes in asymptomatic HIV infection: changes in the expression of CD45RO, CD45RA, CD11b, CD38, HLA-DR and CD25 antigens. J Acquired Immune Defic Syndr. 1997;14:128–135. doi: 10.1097/00042560-199702010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Breen E C, Salazar-Gonzalez J F, Shen L P, Kolberg J A, Urdea M S, Martinez-Maza O, Fahey J L. Circulating CD8 T cells show increased interferon-γ mRNA expression in HIV infection. Cell Immunol. 1997;178:91–98. doi: 10.1006/cimm.1997.1115. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Ho D D, Todd J, Kokka R, Urdea M, Lifson J D, Piatak M, Jr, Chen S, Hahn B H, Saag M S. Clinical evaluation of branched DNA signal amplification for quantifying HIV type 1 in human plasma. AIDS Res Hum Retroviruses. 1995;11:353–361. doi: 10.1089/aid.1995.11.353. [DOI] [PubMed] [Google Scholar]
- 9.Cardo D M, Culver D H, Ciesielski C A, Srivastava P U, Marcus R, Abiteboul D, Heptonstall J, Ippolito G, Lot F, McKibben P S. A case-control study of HIV seroconversion in health care workers after precutaneous exposure. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbid Mortal Weekly Rep. 1992;41:961. [PubMed] [Google Scholar]
- 11.Choi S, Lagakos S W, Schooley R T, Volberding P A. CD4 lymphocytes are an incomplete surrogate marker for clinical progression in persons with asymptomatic HIV infection taking zidovudine. Ann Intern Med. 1993;118:674–680. doi: 10.7326/0003-4819-118-9-199305010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Clerici M, Giorgi J V, Chou C C, Gudeman V K, Zack J A, Gupta P, Ho H N, Nishanian P G, Berzofsky J A, Shearer G M. Cell-mediated immune response to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure to HIV-1. J Infect Dis. 1992;165:1012–1019. doi: 10.1093/infdis/165.6.1012. [DOI] [PubMed] [Google Scholar]
- 13.Clerici M, Shearer G M. A Th1 and Th2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 14.Clerici M, Stocks N, Zajac R A, Boswell R N, Lucey D R, Via C S, Shearer G M. Detection of three different patterns of T helper cell dysfunction in asymptomatic human HIV+ patients. J Clin Investig. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocchi F, DeVico A, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α and MIP-1β as the major HIV suppressive factor produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 16.Cohen O J, Kinter A, Fauci A S. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997;159:31–48. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 17.Coombs R W, Collier A C, Allain J P, Nikora B, Leuther M, Gjerset G F, Corey L. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989;321:1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- 18.Crocchiolo P R, Lizioli A, Bedarida D R, Panzeri M P. CD4:neopterin ratio significantly improves correlation with the Walter Reed staging system if compared with CD4 and neopterin levels separately. AIDS. 1988;2:481–486. [PubMed] [Google Scholar]
- 19.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E. Genetic restriction of HIV-1 infection and progression to AIDS by deletion allele of the CKR-5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 20.Deng H, Liu R, Ellmeier W, Chou S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 21.Emilie D, Fior R, Llorente L, Marfaing-Koka A, Peuchmaur M, Devergne O, Jarrousse B, Wijdenes J, Boue F, Galanaud P. Cytokines from lymphoid organs of HIV-infected patients: production and role in the immune dysequilibrium of the disease. Immunol Rev. 1994;140:5–34. doi: 10.1111/j.1600-065x.1994.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 22.Fahey J L, Taylor J M G, Detels R. Proceedings of the IXth International Conference on AIDS, Berlin, Germany. 1993. Is there a better index of HIV disease status than just CD4 T cell levels? [Google Scholar]
- 23.Fahey J L, Taylor J M G, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi J V. The prognostic value of cellular and serologic markers in HIV infection. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 24.Fahey J L, Taylor J M G, Kozns E, Nishanian P. Diagnostic and prognostic factors in AIDS. Mt Sinai J Med. 1986;53:657–663. [PubMed] [Google Scholar]
- 25.Fahey, J. L., J. M. G. Taylor, B. Manna, P. Nishanian, N. Aziz, J. V. Giorgi, and R. Detels. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T cell measurements. AIDS, in press. [DOI] [PubMed]
- 26.Fan J, Bass H Z, Fahey J L. Elevated IFNγ and decreased IL-2 expression are associated with HIV infection. J Immunol. 1993;151:5031–5037. [PubMed] [Google Scholar]
- 27.Fauci A S. Multifactorial nature of HIV disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 28.Fay S P, Barnett B, Granger V, Mercolino T. Absolute control-full-process leukocyte immunophenotyping quality control using a stabilized whole blood preparation. Cytom Commun Clin Cytom. 1994;18(3):177. [Google Scholar]
- 29.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry co-factor: functional cDNA cloning of a seven-transmembrane domain, G-protein coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs D, Jager H, Popescu M, Reibnegger G, Werner E R, Dierich M P, Kaboth W, Tilz G P, Wachter H. Immune activation markers to predict AIDS and survival in HIV-1 seropositives. Immunol Lett. 1990;26:75–80. doi: 10.1016/0165-2478(90)90178-s. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs D, Hausen A, Reibnegger G, Werner E R, Dierich M P, Wachter H. Neopterin as a marker for activated cell mediated immunity: application to HIV infection. Immunol Today. 1988;9:150–155. doi: 10.1016/0167-5699(88)91203-0. [DOI] [PubMed] [Google Scholar]
- 32.Giorgi J V. Characterization of T lymphocyte subset alterations by flow cytometry in HIV disease. Ann N Y Acad Sci. 1993;677:126–137. doi: 10.1111/j.1749-6632.1993.tb38771.x. [DOI] [PubMed] [Google Scholar]
- 33.Giorgi J V, Ho H N, Hirji K, Chou C C, Hultin L E, O’Rourke S, Park L, Margolick J B, Ferbas J, Phair J P. CD8+ lymphocyte activation of HIV type 1 seroconversion: development of HLA-DR+ CD38− CD8+ cells is associated with subsequent stable CD4+ cell levels. J Infect Dis. 1994;170:775–781. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 34.Giorgi J V, Liu Z, Hultin L E, Cumberland W G, Hennessey K, Detels R. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. J Acquired Immune Defic Syndr. 1993;6:904–912. [PubMed] [Google Scholar]
- 35.Godfried M H, van der Poll T, Weverling G J, Mulder J W, Jansen J, van Deventer S J H, Sauerwein H P. Soluble receptors for tumor necrosis factor as predictors of progression to AIDS in asymptomatic human immunodeficiency virus type 1 infection. J Infect Dis. 1994;169:739–745. doi: 10.1093/infdis/169.4.739. [DOI] [PubMed] [Google Scholar]
- 36.Gordon J, Flores-Romo L, Cairns J A, Millsum M J, Lane P J, Johnson G D, MacLennan I C. CD23: a multi-functional receptor/lymphokine? Immunol Today. 1989;10:153–157. doi: 10.1016/0167-5699(89)90171-0. [DOI] [PubMed] [Google Scholar]
- 37.Graham N M H. The role of immunologic and viral markers in predicting clinical outcome in HIV infection. AIDS. 1996;10:S21–S25. doi: 10.1097/00002030-199612005-00004. [DOI] [PubMed] [Google Scholar]
- 38.Graziosi G, Pantaleo G, Gantt K R, Fortin J P, Demarest J F, Cohen O J, Sekaly R P, Fauci A S. Lack of evidence for the dichotomy of Th1 and Th2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann B, Bass H, Nishanian P, Faisal M, Figlin R A, Sarna G P, Fahey J L. Different lymphoid cell populations produce varied levels of neopterin, beta-2 microglobulin and sIL-2R when stimulated with IL-2, IFNγ and TNFα. Clin Exp Immunol. 1992;88:548–554. doi: 10.1111/j.1365-2249.1992.tb06485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann B, Bygbjerg I, Dickmeiss E, Faber V, Frederiksen B, Gaub J, Gerstoft J, Jakobsen B K, Jacobsen K D, Lindharadt B O. Prognostic value of immunologic abnormalities and HIV antigenemia in asymptomatic HIV-infected individuals: proposal for immunologic staging. Scand J Infect Dis. 1989;21:633–643. doi: 10.3109/00365548909021691. [DOI] [PubMed] [Google Scholar]
- 41.Hofmann B, Nishanian P, Fahey J L, Esmail I, Jackson A L, Detels R, Cumberland W. Serum increases and lymphoid cell surface losses of IL-2 receptor CD25 in HIV-1 infection: distinctive parameters of HIV-1 induced changes. Clin Immunol Immunopathol. 1991;61:212–224. doi: 10.1016/s0090-1229(05)80025-x. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann B, Wang Y, Cumberland W G, Detels R, Bozorgmehri M, Fahey J L. Serum β2M level increases in HIV infection: relation to seroconversion, CD4 T cell fall and prognosis. AIDS. 1990;4:207–214. [PubMed] [Google Scholar]
- 43.Homburger H A, Rosenstock W, Paxton H, Paton M L, Landay A L. Assessment of interlaboratory variability of immunophenotyping. Results of the College of American Pathologists survey. Ann N Y Acad Sci. 1993;677:43–49. doi: 10.1111/j.1749-6632.1993.tb38762.x. [DOI] [PubMed] [Google Scholar]
- 44.Homsy I, Meyer M, Levy J A. Serum enhancement of human immunodeficiency virus (HIV) infection correlates with disease in HIV-infected individuals. J Virol. 1990;64:1437–1440. doi: 10.1128/jvi.64.4.1437-1440.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huber C, Batchelor J R, Fuchs D, Hausen A, Lang A, Niederwieser D, Reibnegger G, Swetly P, Troppmair J, Wachter H. Immune response-associated production of neopterin. Release from macrophages is primarily under control of interferon-gamma. J Exp Med. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jurriaans S, Van Gemen B, Weverling G J, Van Strijp D, Nara P, Coutinho R, Koot M, Schuitemaker H, Goudsmit Y. The natural history of HIV-1 infection: virus load and virus phenotype independent determinants of clinical course? Virology. 1994;204:223–233. doi: 10.1006/viro.1994.1526. [DOI] [PubMed] [Google Scholar]
- 47.Koot M, Keet I P M, Vos A H V, de Goede R E, Roos M T, Coutinho R A, Miedema F, Schellekens P T, Tersmette M. Prognostic value of HIV-1 biological phenotype for rate of CD4 cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 48.Kovacs J A, Vogel S, Albert J M, Falloon J, Davey R T, Walker R E, Polis M A, Spooner K, Metcalf J A, Baseler M. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996;335:1350–1356. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 49.Kramer A, Biggar R J, Hampl H, Friedman R M, Fuchs D, Wachter H, Goedert J J. Immunological markers of progression to AIDS are time-dependent and illness-specific. Am J Epidemiol. 1992;136:71–80. doi: 10.1093/oxfordjournals.aje.a116422. [DOI] [PubMed] [Google Scholar]
- 50.Lane H C, Depper J M, Greene W C, Whalen G, Waldmann T A, Fauci A S. Qualitative analysis of immune function in patients with acquired immune deficiency syndrome. N Engl J Med. 1985;313:79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- 51.Liu M, Fahey J L, Aziz N, Cumberland W G, Skowron G, Merigan T. Zidovudine and dideoxycytidine differ in their effects on human immunodeficiency virus-induced pathologic activation of the immune system. J Infect Dis. 1994;170:1165–1171. doi: 10.1093/infdis/170.5.1165. [DOI] [PubMed] [Google Scholar]
- 52.Liu Z, Cumberland W G, Hultin L E, Prince H E, Detels R, Giorgi J V. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquired Immune Defic Syndr. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 53.Lyamuya E F, Kagona C, Mbena E C, Urassa W K, Pallangyo K, Mhalu F S, Biberfeld G. Evaluation of the FACScount, TRAx CD4 and Dynabeads methods for CD4 lymphocyte determination. J Immunol Methods. 1996;195:103–112. doi: 10.1016/0022-1759(96)00094-4. [DOI] [PubMed] [Google Scholar]
- 54.Marinkovic S, Jahreis G P, Wong G G, Baumann H. IL-6 modulates the synthesis of a specific set of acute phase plasma proteins in vivo. J Immunol. 1989;142:808–812. [PubMed] [Google Scholar]
- 55.Mellors J W, Muñoz A, Giorgi J V, Margolick J B, Tassoni C J, Gupta P, Kingsley L A, Todd J A, Saah A J, Detels R, Phair J P, Rinaldo C R., Jr Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 56.Melmed R N, Taylor J M G, Detels R, Bozorgmehri M, Fahey J L. Serum neopterin changes in HIV-infected subjects: indicator of significant pathology, CD4 T cell changes, and the development of AIDS. J Acquired Immune Defic Syndr. 1989;2:70–76. [PubMed] [Google Scholar]
- 57.Miedema F, Meyaard L, Koot M, Klein M R, Roos M T, Groenink M, Fouchier R A, Van’t Wout A B, Tersmette M, Schellekens P T. Changing virus-host interactions in the course of HIV infection. Immunol Rev. 1994;140:35–72. doi: 10.1111/j.1600-065x.1994.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 58.Mildvan D, Landay A, DeGruttola V, Machado S G, Kagan J. An approach to the validation of markers for use in AIDS clinical trials. Clin Infect Dis. 1997;24:764–774. doi: 10.1093/clinids/24.5.764. [DOI] [PubMed] [Google Scholar]
- 59.Miles S, Martinez-Maza O, Rezai A, Magpantay L, Kishimoto T, Nakamura S, Radka S F, Linsley P S. Oncostatin M as a potent mitogen for AIDS Kaposi sarcoma cells. Science. 1992;255:1432–1434. doi: 10.1126/science.1542793. [DOI] [PubMed] [Google Scholar]
- 60.Moss A R, Bacchetti P, Osmond D, Krampf W, Chaisson R E, Stites D, Wilber J, Alain J P, Carlson J. Seropositivity for HIV and development of AIDS: 3 year follow up. Br Med J. 1988;296:745–750. doi: 10.1136/bmj.296.6624.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray H W, Rubin B Y, Masur H, Roberts R B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immune deficiency syndrome. N Engl J Med. 1984;310:883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- 62.Nishanian P, Hofmann B, Wang Y, Jackson A L, Detels R, Fahey J L. Serum soluble CD8 molecule is a marker of CD8 T-cell activation in HIV-1 disease. AIDS. 1991;5:805–812. doi: 10.1097/00002030-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Nishanian P, Huskins R, Stehn S, Detels R, Fahey J L. A simple method for improved assay demonstrates that HIV p24 antigen is present as immune complexes in most sera from HIV-infected individuals. J Infect Dis. 1990;162:21–28. doi: 10.1093/infdis/162.1.21. [DOI] [PubMed] [Google Scholar]
- 64.Osmond D H, Shiboski S, Bacchetti P, Winger E E, Moss A R. Immune activation markers and AIDS prognosis. AIDS. 1991;5:505–511. doi: 10.1097/00002030-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Pal R, Garzino-Demo A, Markham P D, Burns J, Brown M, Gallo R C, DeVico A L. Inhibition of HIV-1 infection by the β-chemokine MDC. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 66.Pantaleo C, Fauci A S. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;15:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 67.Peddecord K M, Berenson A S, Hofherr L K, Francis D P, Garfein R S, Cross G D, Schalla W O. Variability of reporting and lack of adherence to consensus guidelines in human T-lymphocyte immunophenotyping reports. J Acquired Immune Defic Syndr. 1993;6:823–830. [PubMed] [Google Scholar]
- 68.Phair J, Muñoz A, Detels R, Kaslow R, Rinaldo C, Saah A. The risk of pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. N Engl J Med. 1990;322:161–165. doi: 10.1056/NEJM199001183220304. [DOI] [PubMed] [Google Scholar]
- 69.Piatak M, Jr, Saag M, Yang L, Clark S J, Kappes J C, Luk K C, Hahn B H, Shaw G M, Lifson J D. High levels of HIV-1 in plasma at all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 70.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 71.Plaeger, S., H. Z. Bass, P. Nishanian, J. Thomas, N. Aziz, R. Detels, J. King, W. Cumberland, M. Kemeny, and J. L. Fahey. The prognostic significance in HIV infection of immune activation represented by cell surface antigen and plasma activation markers changes. Clin. Immunol. Immunopathol., in press. [DOI] [PubMed]
- 72.Rabin R L, Roederer M, Maldonado Y, Petru A, Herzenberg L A. Altered representation of naive and memory CD8 T cell subsets in HIV infected children. J Clin Investig. 1995;95:2054–2060. doi: 10.1172/JCI117891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richman D D. Antiretroviral drug resistance: mechanisms, pathogenesis, clinical significance. Adv Exp Med Biol. 1996;394:383–395. doi: 10.1007/978-1-4757-9209-6_35. [DOI] [PubMed] [Google Scholar]
- 74.Richman D D, Bozzette S A. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J Infect Dis. 1994;169:968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 75.Rinaldo C R, Beltz L A, Huang X L, Gupta P, Fan Z, Torpey D J. Anti-HIV type 1 cytotoxic T lymphocyte effector activity and disease progression in the first 8 years of HIV type 1 infection of homosexual men. AIDS Res Hum Retroviruses. 1995;11:481–489. doi: 10.1089/aid.1995.11.481. [DOI] [PubMed] [Google Scholar]
- 76.Romagnani S, del Prete G, Manetti R, Ravina A, Annunziato F, De Carli M, Mazzetti M, Piccini M P, D’Elios M M, Parronchi P. Role of Th1/Th2 cytokines in HIV infection. Immunol Rev. 1994;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 77.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T. HIV-specific cytotoxic T cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 78.Salazar-Gonzalez J F, Martinez-Maza O, Aziz N, Kolberg J A, Yeghiazarian T, Shen L P, Fahey J L. Relationship of plasma HIV-RNA levels and levels of TNFα and immune activation products in HIV infection. Clin Immunol Immunopathol. 1997;84:36–45. doi: 10.1006/clin.1997.4364. [DOI] [PubMed] [Google Scholar]
- 79.Sarna G, Machleder H, Collins J, Bonavida B, Jacobs E, Hawkins R, Golub S, Shau H, Fahey J L, Popow J. A comparative study of intravenous versus intralymphatic interleukin-2 with assessment of effects of interleukin-2 on both peripheral blood and thoracic-duct lymph. J Immunother. 1994;15:140–146. doi: 10.1097/00002371-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 80.Schroff R H, Gottlieb M S, Prince H E, Chai L L, Fahey J L. Immunological studies of homosexual men with immunodeficiency and Kaposi’s sarcoma. Clin Immunol Immunopathol. 1983;27:308–314. doi: 10.1016/0090-1229(83)90083-1. [DOI] [PubMed] [Google Scholar]
- 81.Sheppard H W, Ascher M S, McRae B, Anderson R E, Lang W, Allain J P. The initial immune response to HIV and immune system activation determine the outcome of HIV disease. J Acquired Immune Defic Syndr. 1991;4:704–712. [PubMed] [Google Scholar]
- 82.Smith K, Cantrell D A. IL-2 regulates its own receptors. Proc Natl Acad Sci USA. 1995;82:864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strickler H D, Blanchard J F, Vlahov D, Taylor E, Munoz A, Nelson K E, Margolick J B. Elevated serum levels of neopterin but not beta 2-microglobulin in HIV-1 seronegative injecting drug users. AIDS. 1993;7:361–367. doi: 10.1097/00002030-199303000-00009. [DOI] [PubMed] [Google Scholar]
- 84.Tsoukas C M, Bernard N F. Markers predicting progression of HIV-related disease. Clin Microbiol Rev. 1994;7:14–28. doi: 10.1128/cmr.7.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valentine F T, Jacobson M A. Immunological and virological surrogate markers in the evaluation of therapies for HIV infection. AIDS. 1990;405:201–207. [PubMed] [Google Scholar]
- 86.Vanham G, Edmonds K, Qing L, Hom D, Toossi Z, Jones B, Daley C L, Huebner B, Kestens L, Gigase P. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immunol. 1996;103:30–34. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walker B D, Chakrabarti S, Moss M, Paradis T J, Flynn T, Durno A G, Blumberg R S, Kaplan J C, Hirsch M S, Schooley R T. HIV-specific cytotoxic lymphocytes in seropositive individuals. Nature. 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 88.Walker C M, Moody D J, Stites D P, Levy J A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 89.Watchi R, Stiehm E R, Fahey J L, Stone M, Deveikis A, Frenkel L, Aldrovandi G, O’Rourke S, Wei L, Bryson Y J. Neopterin is a better predictor of disease severity than b2M in pediatric HIV infection. Pediatr AIDS HIV Infect Fetus Adolesc. 1994;5(4):226–231. [Google Scholar]
- 90.Yametz S, Cumberland W G, van der Meyden M, Martinez-Maza O. Elevated serum levels of soluble CD23 (sCD23) precede the appearance of acquired immunodeficiency syndrome-associated non-Hodgkin’s lymphoma. Blood. 1995;85:1843–1849. [PubMed] [Google Scholar]
- 91.Yarchoan R, Venzon D J, Pluda J M, Lietzau J, Wyvill K M, Tsiatis A A, Steinberg S M, Broder S. CD4 counts and the risk for death in patients infected with HIV receiving antiretroviral therapy. Ann Intern Med. 1991;115:184–189. doi: 10.7326/0003-4819-115-3-184. [DOI] [PubMed] [Google Scholar]
- 92.Zangerle R, Fuchs D, Reibnegger G, Fritsch P, Wachter H. Markers for disease progression in intravenous drug users infected with HIV-1. AIDS. 1991;5:985–991. doi: 10.1097/00002030-199108000-00010. [DOI] [PubMed] [Google Scholar]
- 93.Zangerle R, Gallati H, Sarcletti M, Weiss G, Denz H, Wachter H, Fuchs D. Increased serum concentrations of soluble tumor necrosis factor receptors in HIV infected individuals are associated with immune activation. J Acquired Immune Defic Syndr. 1994;7:79–85. [PubMed] [Google Scholar]