Abstract
Interleukin-6 (IL-6) is a pleotropic cytokine implicated in the pathogenesis of local inflammation during viral upper respiratory infections. This study determined if experimental influenza A virus infection causes local IL-6 production. Seventeen healthy, adult subjects were intranasally inoculated, by course drops, with a safety-tested strain of influenza A/Kawasaki/86 (H1N1) virus. Nasal lavage samples were collected, symptoms were recorded, and expelled nasal secretions were weighed once before and then daily for 8 days after the virus inoculation. Lavage samples were submitted for virus culture and were examined for IL-6 and IL-4 by enzyme-linked immunosorbent assay. The IL-6, but not IL-4, levels were significantly increased in the nasal lavage samples of the 12 subjects who shed virus but not in those of the 5 subjects who did not shed virus. Moreover, the elevations in IL-6 levels were related temporally to the development of nasal symptoms and secretions but not to systemic symptoms. These results suggest a role for locally produced IL-6 in the pathogenesis and expressed symptomatology of influenza A virus infection.
Respiratory virus infection causes upper airway inflammation which is expressed as signs and symptoms, responses that are believed to be orchestrated by the sequential elaboration of various cytokines. Interleukin-6 (IL-6) is a proinflammatory cytokine with pleotropic expressions consistent with a primary role in the pathogenesis of local inflammation. IL-6 release in vitro by cell lines was observed to be increased after rhinovirus, influenza A virus, and respiratory syncytial virus infections (1, 9, 14), and the IL-6 level was reported to be elevated in nasal lavage samples recovered from otherwise healthy subjects during naturally occurring and experimental upper respiratory virus infections (10, 16). More recently, Hayden and colleagues reported elevations of cytokines (including IL-6) in adults experimentally infected with influenza A/Texas (H1N1) virus and related those elevations to the development of signs and symptoms (6).
In this study, nasal lavage sample IL-6 and IL-4 concentrations, symptom scores, and nasal secretion weights were measured for adult subjects experimentally exposed to a different strain of influenza A (H1N1) virus. These measures were compared for subjects who shed virus and, as a control for the effects of the cloistered environment, subjects who did not shed virus. The kinetics of cytokine elaboration and symptom and sign expression were examined for evidence of causality. Also, because epidemiological studies reported an association between upper respiratory virus infections and the expression of allergic disorders (5, 8), and because differences in the immune and inflammatory responses of allergic and nonallergic subjects to experimental viral respiratory infections were reported previously (12, 13), the data analysis was stratified for the allergy status of the subjects. The hypotheses tested were as follows: (i) experimental influenza A virus infection of adult volunteers provokes the local elaboration of IL-6 but not IL-4, (ii) the kinetics of this response is similar to that of certain symptoms and signs, and (iii) the various responses of allergic subjects differ in magnitude from those of nonallergic subjects.
MATERIALS AND METHODS
Subjects.
This study was approved by the Human Rights Committee at the Children’s Hospital of Pittsburgh. Seventeen adult subjects were enrolled after providing written informed consent. All were in good health as evidenced by medical history, physical examination, normal blood and urine chemistry profiles, and a negative human immunodeficiency virus antibody assay. By methods previously described, seven subjects were diagnosed as having allergic rhinitis (12, 13). None had experienced allergic or upper respiratory infection symptoms within 30 days prior to the study.
Study protocol.
Subjects were cloistered in a local hotel from study day 0 through day 8. At the end of study day 0, each subject was intranasally inoculated with, as course drops, approximately 107 50% tissue culture infective doses of a safety-tested clinical isolate of influenza A/Kawasaki/86 (H1N1) virus (wild type, lot E-262) obtained from Brian R. Murphy, Respiratory Viruses Section, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Daily during the cloister period, nasal lavage samples were collected, symptoms were scored, and nasal secretion weights were measured. Lavage fluid samples were submitted to the virology laboratories at the University of Virginia for viral culture (15) and then to the Immunologic Monitoring and Diagnostic Laboratory at the Pittsburgh Cancer Institute for the measurement of cytokine levels. Enzyme-linked immunosorbent assays for IL-4 and IL-6 were obtained from R & D Systems (Minneapolis, Minn.) and had lower limits of detectability of 3.0 and 5.0 pg/ml, respectively.
Sneezing, nasal discharge, nasal congestion, malaise, headache, chilliness, muscle ache, joint pain, sweats, and fever were each rated by the subject on a four-point scale (scores from 0 to 3, corresponding to no, mild, moderate, and severe, respectively). Also, all nasal secretions were expelled into preweighed tissues, which were sealed within plastic bags. Daily secretion weights were calculated by subtraction of the tissue weights. Blood collected at baseline and at day 21 after virus exposure was assayed for hemagglutination inhibition (HI) antibodies to influenza A/Kawasaki/86 virus by standard methods (4).
Statistical analysis.
The duration of viral shedding was defined as the number of days between the first and last day of virus isolation. Seroconversion was defined as a fourfold or greater rise in specific serum HI antibody between the prechallenge and convalescent-phase samples. For each subject and study day, two influenza-related summary symptom scores were constructed, corresponding to nasal symptoms (sum of scores for rhinorrhea, nasal congestion, and sneezing) and systemic symptoms (sum of scores for malaise, headache, chilliness, muscle ache, joint pain, sweats, and fever).
Prior to formal analysis, the data for each subject were reviewed. Both the symptomatic and cytokine responses were shown to be discriminated on the basis of whether the subject shed challenge virus (on any postinoculation day), and this information was used to stratify the population prior to analysis. The data for the daily nasal symptom score, systemic symptom score, secretion weight, and cytokine level were analyzed for each subgroup (shedding and nonshedding subjects) by repeated-measure analysis of variance (ANOVA), with variance partitioned for effects of study day (virus infection) and allergy status. Also, for each of those measures, the sum of the values for all postexposure days of cloister for each subject was calculated to estimate the area under the response-time curve (AUC) for that measure. Differences between subgroups in the AUCs for nasal and systemic symptom scores, in the AUCs for secretion weight, in the logs of the AUCs for IL-6 concentration, and in the logs of the prechallenge and postchallenge HI antibody titers were analyzed for statistical significance with a two-tailed Mann-Whitney U test (see Table 1). Correlations between daily cytokine levels and measures of illness expression were calculated by the method of least squares and are reported as the Pearson product-moment correlation coefficients. Throughout, the data are expressed as means ± standard deviations consistently.
TABLE 1.
Statistical comparisons for the groups of HI antibody titers and AUCs for the four outcome measuresa
| Group | Log HI antibody titer
|
AUC for:
|
||||
|---|---|---|---|---|---|---|
| Preexposure | Postexposure | Log IL-6 concn (pg/ml) | Nasal secretion wt (g) | Nasal symptom score | Systemic symptom score | |
| Subjects who did not shed virus (n = 5) | 1.5 ± 0.4 | 1.6 ± 0.3 | 0.9 ± 1.2 | 1.9 ± 2.6 | 6.6 ± 6.8 | 4.2 ± 5.3 |
| Subjects who shed virus (n = 12) | 0.7 ± 0.2 (<0.01) | 1.6 ± 0.2 (NSb) | 2.2 ± 0.5 (<0.05) | 32.5 ± 23.6 (<0.01) | 15.3 ± 8.1 (<0.05) | 11.3 ± 8.4 (<0.06) |
Data are means ± standard deviations. P values, in parentheses, are versus the data for the subjects who did not shed virus.
NS, not significant.
RESULTS
Twelve subjects (eight allergic and four nonallergic) shed virus on at least one day. The preexposure HI antibody titers were 4 for 10 of these subjects, and the titers were 8 and 16 for the other 2 subjects (average log titer of 0.7 ± 0.2). All subjects in this subgroup seroconverted (average log convalescent-phase titer of 1.6 ± 0.2). In the five subjects who did not shed virus (three allergic and two non-allergic), the preexposure titers were 8, 16, 32, 64, and 64 (average log titer of 1.5 ± 0.4) and the convalescent-phase titers were 32, 16, 32, 64, and 128 (average log titer of 1.6 ± 0.03). The difference between subgroups in preexposure, but not postexposure, HI antibody titer was statistically significant.
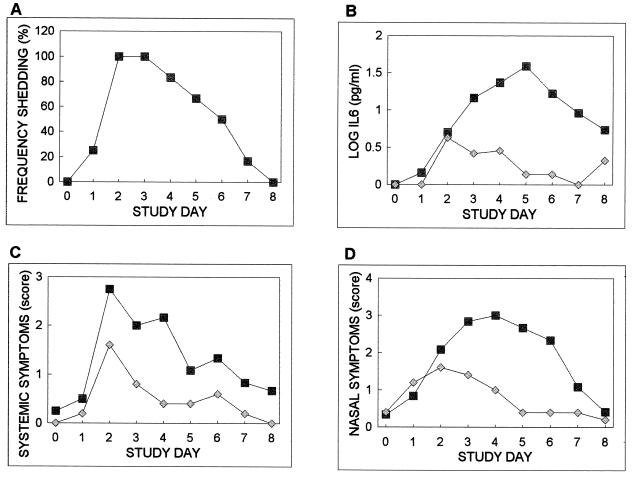
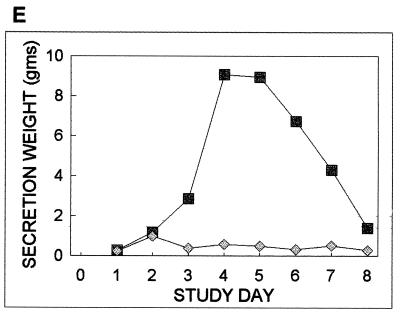
Figure 1 shows for each subgroup the percentage of subjects shedding virus, the average log IL-6 concentration, and the average systemic symptom score, nasal symptom score, and secretion weight as a function of time. The percentage of subjects shedding virus in that subgroup increased to 100% on days 2 and 3 and then showed a uniform decrease to 0% by day 8. All of the subjects who shed virus had IL-6 elevations on at least one postexposure study day. In contrast, only one of the five subjects who did not shed virus had detectable IL-6 levels after virus exposure. In that subject, the IL-6 level peaked on day 2 and decreased to baseline levels by day 5. For the group that shed virus, IL-6 levels peaked on day 5. ANOVA documented a significant effect of study day, but not allergy status, on IL-6 concentrations in the group that shed virus.
FIG. 1.
The percentage of subjects who shed virus and the average daily log IL-6 concentrations, systemic symptom scores, nasal symptom scores, and nasal secretion weights as a function of time for the 12 subjects who shed virus (squares) and for the 5 subjects (diamonds) who did not shed virus.
For systemic symptom scores, nasal symptom scores, and secretion weights, the curves for the nonshedding subgroup were characterized by a shallow, transient increase to peak on day 2 and then a decrease to approach baseline by day 4 or 5. Of these subjects, four had postexposure patterned increases in nasal symptoms, three had increases in systemic symptoms, and one had increases in secretion weight. Interestingly, the individual with the greatest-magnitude response for these measures did not have IL-6 elevations, and conversely, the single individual with patterned IL-6 elevations had relatively minor elevations in nasal symptoms, no systemic symptoms, and no increase in secretion weight.
The average values of those measures for the subgroup that shed virus showed greater magnitudes of increases and more prolonged elevations than those responses for the subgroup that did not shed virus (Fig. 1C to E). Two different kinetic patterns characterize these data. While systemic symptoms showed an early increase, a peak on day 2, and a uniform decrease to baseline, nasal symptoms and secretion weights increased to peak on day 4 or 5 and then decreased to baseline by day 8. The systemic symptoms had a kinetic pattern similar to that documented for the percentage of persons shedding virus, while the secretion weight and nasal symptoms had overall kinetic patterns more similar to that of IL-6. ANOVA identified a significant effect of study day, but not allergy status, on all three measures in this subgroup.
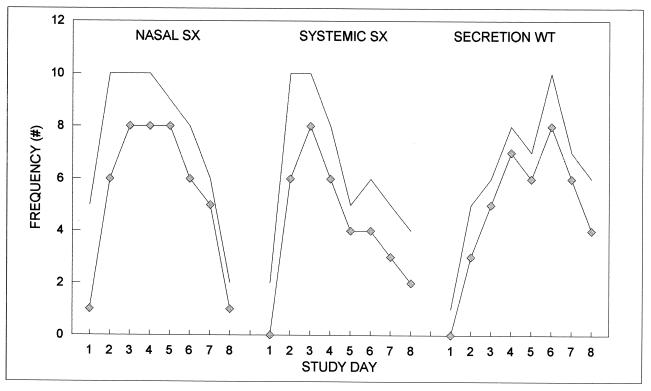
For the subgroup that shed virus, Fig. 2 shows the numbers of subjects on each day who reported any nasal symptoms, reported any systemic symptoms, and had secretion weights greater than 1 g. Also shown are the numbers of subjects with those symptoms and signs who had concurrently measurable IL-6 concentrations. For all three measures, most notably nasal symptoms on day 1, the presence of a response was not a sole and sufficient signal of measurable levels of IL-6. These data suggest that while the presence of IL-6 and symptoms or signs covary in time, IL-6 alone does not drive those responses. To determine if the magnitude of IL-6 value and that of symptom and sign expression are temporally related, the temporally varying values of the three response measures were correlated to that of IL-6 for each subject. Of the 12 subjects who shed virus, 9 had definable (nonzero variances in comparison variables) correlation coefficients ranging from 0.29 to 0.79 (average, 0.5 ± 0.19) for nasal symptoms, 0.07 to 0.83 (average, 0.42 ± 0.23) for systemic symptoms, and 0.10 to 0.83 (average, 0.55 ± 0.23) for secretion weights.
FIG. 2.
The numbers of subjects on each day who shed virus and reported any nasal symptom, reported any systemic symptom, and had greater than 1 g of nasal secretion (solid lines) are shown along with the numbers of those subjects with concurrently measurable IL-6 concentrations (diamonds). SX, symptoms; WT, weight.
Table 1 presents for the two subgroups the average AUCs for the four outcome measures and the levels of significance of the intersubgroup differences. With the exception of the systemic symptom score, all measures were significantly greater in the subgroup that shed virus than in the subgroup that did not. Within the subgroup that shed virus, there were no differences between allergic (n = 8) and nonallergic (n = 4) subjects in the average AUCs for IL-6 production (2.0 ± 0.2 and 1.8 ± 0.6, respectively), secretion weight (32.5 ± 21.8 and 32.6 ± 24, respectively), nasal symptom score (16 ± 9 and 14 ± 3, respectively), and systemic symptom score (10 ± 8 and 15 ± 7, respectively).
There was no significant effect of influenza A virus inoculation on lavage fluid IL-4 concentration. That cytokine was not detected in the recovered nasal lavage fluid of any subject at baseline. Only one subject (who shed virus and was allergic) had a progressive increase in IL-4 concentrations during the postexposure study period, and its pattern was similar to that of the subject’s own IL-6 elevations.
DISCUSSION
In this study, the IL-6, but not IL-4, concentrations were increased in the nasal lavage fluids of all subjects who were infected with influenza A virus and did shed virus. For the group of subjects exposed to the virus but without evidence of viral shedding, the IL-6 response was significantly lower, with only 20% of the subjects exhibiting a response. Because all subjects were exposed to the same virus and were cloistered under identical conditions, these results strongly support a role for active viral replication in the production of IL-6. Also, as expected, high preexisting HI antibody titers were associated with resistance to infection, with only one of five subjects with titers of 16 or greater shedding virus after exposure. Interestingly, the kinetic pattern of IL-6 production documented in this study was identical to that reported by Hayden and colleagues for experimental infection with a different strain of influenza A virus (6). While those investigators did not report data for a control group of uninfected subjects, their observation of correlations between quantitative virus titers (which were not measured in the present experiment) and cytokine levels is consistent with the relationship between virus shedding and IL-6 levels reported for this experiment.
These results for influenza A virus infection complement those of earlier studies that documented elevated IL-6 levels in nasal lavage fluids of pediatric patients during naturally occurring upper respiratory tract infections of unknown etiologies and increased IL-6 levels in nasal lavage fluids of healthy adults during experimental rhinovirus infection (10, 16). Thus, local IL-6 production appears to be a prominent feature of a number of, if not all, upper respiratory virus infections.
Neither this nor the earlier study defined the cellular source of IL-6 produced during influenza A virus infection. Increased levels of IL-6 mRNA and its protein product were demonstrated in epithelial cell cultures after virus infection (1, 14, 16), and other potential cell sources include granulocytes, lymphocytes, monocytes, and macrophages (2, 3). Also not known is the triggering mechanism for IL-6 production, but recent studies demonstrated activation of IL-6 transcription factors in epithelial cell lines after infection with influenza A virus, respiratory syncytial virus, or rhinovirus (7, 11, 16). While it is possible that the IL-6 elevations documented for influenza A virus-infected subjects represented a nonspecific elaboration of all cytokines, the lack of a concomitant rise in IL-4 level in the present study contradicts that hypothesis.
In this study, local IL-6 levels, nasal symptoms, and secretion weights had similar kinetics, which could support a role for that cytokine in the pathogenesis of acute local inflammation. A similar temporal relationship between local IL-6 levels and nasal symptoms was reported by Hayden and colleagues (6) for experimental influenza A virus infections of adult volunteers. Interestingly, those investigators also reported an earlier, day 2, peak in the serum IL-6 level for infected subjects and related this to the observed early development of systemic symptoms and signs. In the present study, the systemic symptom score also peaked on day 2 and was related kinetically to the temporal pattern of viral shedding. Unfortunately, IL-6 concentrations in serum were not measured in this study and the relationship suggested by Hayden and colleagues (6) could not be evaluated.
However, two lines of evidence contradict a direct role of local IL-6 in promoting the development of nasal symptoms and signs during influenza A virus infection. First, in the nonshedding subjects, nasal symptoms and secretion weight were not related to IL-6 level. Specifically, the subject that had IL-6 elevations had no increase in secretion weights and only minor symptoms, while the subject that manifested the greatest responses did not have measurable IL-6. Second, as the analysis presented in Fig. 2 shows, the initial presentation of nasal symptoms (and also systemic symptoms and secretion production) occurs prior to documented IL-6 elevations and the presence of local IL-6 is not a sole and sufficient delimiter of the population with concurrent symptoms and signs. These results suggest that while the presence of IL-6 and symptoms and signs covary in time, IL-6 alone does not drive those responses.
Previous studies demonstrated a link between viral infections of the upper respiratory tract and the expression of allergic disorders in genetically susceptible individuals (5, 8). However, no effect of allergy status on influenza A virus-induced increases in nasal lavage fluid IL-6 levels was demonstrated. This negative result may reflect limitations associated with the small sample size of the present study or the inclusion of only asymptomatic allergic subjects. Based on the averages and standard deviations for the IL-6 AUC data available in the present study, a sample size of eight subjects per group has an 80% power of detecting two-tailed differences at α = 0.05 of approximately 15%, and this value can be considered to be a reasonable limit for between-group differences in IL-6 levels. Thus, the expected effect of allergy status on elaboration of that cytokine is rather small and of magnitude order similar to that of the standard deviation of the measure.
In summary, this study documented that the local IL-6 levels closely parallel nasal symptoms during influenza A virus infection. This association suggests that IL-6 may be involved in promoting the expression of illness. Modulation of IL-6 production or activity in controlled clinical trials using experimental virus infections of adult volunteers could lead to a better understanding of the role of this cytokine in the pathogenesis of upper respiratory virus infections.
ACKNOWLEDGMENTS
This study was supported in part by grants AI19262 and DC02833 from the National Institutes of Health.
REFERENCES
- 1.Arnold R, Humbert B, Werchau H, Gallati H, Konig W. Interleukin-8, interleukin-6, and soluble necrosis factor receptor type I release from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology. 1994;82:126–133. [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold R, Konig B, Galatti H, Werchau H, Konig W. Cytokine (IL-8, IL-6, TNF-alpha) and TNF receptor-I release from peripheral blood mononuclear cells after respiratory syncytial virus infection. Immunology. 1995;85:364–372. [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold R, Werner F, Humbert B, Werchau H, Konig W. Effect of respiratory syncytial virus-antibody complexes on cytokine (IL-8, IL-6, TNF-alpha) release and respiratory burst in human granulocytes. Immunology. 1994;82:184–191. [PMC free article] [PubMed] [Google Scholar]
- 4.Dowdle W, Kendal A, Noble G. Influenza viruses. In: Lennette E, Schmidt N, editors. Procedures for viral, rickettsial and chlamydial infections. 5th ed. Washington, D.C: American Public Health Association; 1979. pp. 585–609. [Google Scholar]
- 5.Fraenkel D, Bardin P, Sanderson G, Lampe F, Johnston S, Holgate S. Lower airway inflammatory response during rhinovirus colds in normal and asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 6.Hayden F G, Fritz R S, Lobo M C, et al. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Investig. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamaluddin M, Garofalo R, Ogra P L, Brasier A R. Inducible translational regulation of the NF-IL6 transcription factor by respiratory syncytial virus infection in pulmonary epithelial cells. J Virol. 1996;70:1554–1563. doi: 10.1128/jvi.70.3.1554-1563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston S, Pattemore P, Sanderson G, et al. Community study of role of virus infections in exacerbations of asthma in 9-11 year old children. Br Med J. 1995;310:1225–1228. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsukura S, Kokubu F, Noda H, Tokunaga H, Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J Allergy Clin Immunol. 1996;98:1080–1087. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 10.Noah T, Henderson F, Wortman I, et al. Nasal cytokine production in viral acute upper respiratory infection of childhood. J Infect Dis. 1995;171:584–592. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 11.Pahl H L, Baeuerle P A. Expression of influenza virus hemagglutinin activates transcription factor NF-κB. J Virol. 1995;69:1480–1484. doi: 10.1128/jvi.69.3.1480-1484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skoner D, Doyle W, Tanner E, Kiss J, Fireman P. Effect of rhinovirus 39 (RV-39) infection on immune and inflammatory parameters in allergic and non-allergic subjects. Clin Exp Allergy. 1995;24:561–567. doi: 10.1111/j.1365-2222.1995.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 13.Skoner D, Whiteside T, Wilson J, Doyle W, Herberman R, Fireman P. Effect of rhinovirus 39 infection on cellular immune parameters in allergic and nonallergic subjects. J Allergy Clin Immunol. 1993;92:732–743. doi: 10.1016/0091-6749(93)90017-a. [DOI] [PubMed] [Google Scholar]
- 14.Subauste M, Jacoby D, Richards S, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus; induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Investig. 1995;96:549–557. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobita K, Sugiura A, Enomoto C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975;162:9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Z, Tang W, Ray A, et al. Rhinovirus stimulation of interleukin-6 in vivo and in vitro; evidence for nuclear factor KB-dependent transcriptional activation. J Clin Investig. 1996;97:421–430. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]