Abstract
Objective
The objective of this study was to evaluate how urine drug screening (UDS) frequency is associated with retention in opioid agonist treatment (OAT).
Methods
Data for this retrospective cohort study of 55 921 adults in OAT in Ontario, Canada, were derived from administrative sources between 1 January 2011 and 31 December 2015. All patient information was linked anonymously across databases using encrypted health card numbers. Descriptive statistics were calculated for comparing UDS frequency groups using standardised differences (d) where d less than 10% indicated a statistically significant difference. A logistic regression model was then used to calculate ORs adjusting for baseline covariates, including sex, age, location of residence, income quintile, mental disorders, HIV status and deep tissue infections.
Results
Over 70% of the cohort had four or more UDS tests per month (weekly or more UDS). Significant associations were observed between UDS frequency and 1-year treatment retention in OAT biweekly (adjusted OR (aOR)=3.20, 95% CI 2.75 to 3.75); weekly UDS (aOR=6.86, 95% CI 5.88 to 8.00) and more than weekly (aOR=8.03, 95% CI 6.87 to 9.38) using the monthly or less groups as the reference.
Conclusion
This study identified an association between weekly UDS and 1-year treatment retention in OAT. There is an active discussion within Canada about the utility of UDS. The lack of evidence for the impact of UDS on retention has left it open to some to argue they simply provide a barrier to patient engagement. Therefore, it is timely of this study to demonstrate that more frequent urine testing is not associated with a reduction in treatment retention.
Keywords: Substance misuse, PUBLIC HEALTH, EPIDEMIOLOGY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
There is the possibility of data entry and reporting errors associated with using administrative-level data.
There is potential for unmeasured confounding, including confounding related to polysubstance use, social and interpersonal factors and clinical characteristics, due to our study only having access to routinely collected data.
In this study, we analysed opioid agonist treatment (methadone and buprenorphine/naloxone); therefore, we did not adjust for medication type which has been shown to potentially impact retention.
Some expert opinions have suggested that routine use of urine toxicology testing reinforces a power dynamic and invites shame, stigma and judgement. We were not able to account for such factors in our analysis.
This study cannot determine whether the requirement for urine drug screening is a barrier to potential patients ever engaging in care; however, the high level of treatment engagement in Ontario compared with other jurisdictions weighs against this being a substantial factor from a public health perspective.
Introduction
An epidemic of opioid use disorder (OUD) and deaths related to opioid poisoning has emerged across Canada in the last decade.1–5 Fortunately, OUD is treatable with opioid agonist treatment (OAT), including methadone and buprenorphine/naloxone. Research has shown that OAT is the most effective treatment to reduce mortality and hospitalisation rates, decrease the use of opioids and other substances, lower the transmission of HIV, hepatitis C and other infectious diseases, and improve unemployment rates and other social factors.2 6–9 Despite its known benefits, uptake and effective use of OAT by general practitioners is relatively low. Little training is given to medical professionals about the complexity and continuum of care necessary for the successful treatment of individuals with OUD.10 Additionally, treatment discontinuation and cycling are very common7 8; and changes in opioid tolerance while on OAT11 are contributing factors that lead to an exceptionally high risk of overdose mortality following discontinuation.2 12–14 Sustained engagement in OAT, ideally for 1 year or more,15–17 is thus critical to realising the protective benefits of this vital tool to address the opioid overdose crisis.
Patients in Ontario typically start treatment in a specialised addiction clinic for observed daily dosing for both methadone and buprenorphine/naloxone. Patients can receive increasing number of take-home doses, based on the assessment of the physician in determining their level of functional stability (cessation of other opioid use, reduced problematic use of other substances, stable housing, stable physical and mental health, along with other factors). Increasing or decreasing numbers of take-home doses are linked to urine drug screening (UDS) results and frequency in an explicit contingency management schedule such that patients who are in the process of gradually increasing their level of stability, and thus number of weekly take-home doses, will have more frequent urine testing.18 19 These take-home privileges are increased based on appointment attendance and consistently negative UDS for opioids, cocaine, stimulants and other substances. In Ontario, patients enrolled in OAT at specialised addiction clinics will achieve six take-home doses after at least 8 months of negative UDS, which is equivalent to visiting the clinic once per week for a UDS and assessment. Within this general context, there is room for some variability in how this approach is applied by individual physicians. Some physicians place less emphasis on this contingency management approach or rely less on UDS to determine which patients receive increased numbers of take-home doses. Some physicians may also be concerned that frequent UDS acts as a deterrent to treatment retention which counteracts the effectiveness of contingency management in reducing other drug use and improving retention. It is important to note that not all UDS collection events are associated with a physician appointment. Many patients are attending the clinic more often than weekly and can leave samples during the visit to receive medication. So the frequency of urine collection does not add an additional burden to reintegration over and above the burden of supervised ingestion of medication.
The cost of UDS billing has been the source of debate in Ontario,17 18 resulting in recent UDS billing fee cuts20 and recommendations for less frequent screening.21 Ideal UDS frequency is therefore critical to treat OUD effectively in a specialised OAT setting. However, a recent review conducted by McEachern et al concluded that there is a critical gap in peer-reviewed evidence regarding UDS frequency and health outcomes for individuals in OAT.10 Despite this lack of evidence, that OAT guidelines in Ontario have been recently replaced with new national guidelines which recommend drug screening only once per month, even when a much higher frequency of UDS is currently being conducted. Furthermore, federal and provincial guidelines are inconsistent. They often rely on expert opinion and politically driven reasons rather than peer-reviewed evidence.22 In Ontario, there has been some variability in physician practice in terms of frequency of UDS and application of contingency management practices with respect to linking carry doses to drug-free urine. The study is meant to look at whether this variability impacts patient outcomes and in particular whether more frequent testing represents a barrier to retention in OAT in Ontario.
Methods
Study design and setting
Data for this retrospective cohort study of 55 921 adults with OUD in Ontario were derived from three databases that routinely collect publicly funded healthcare services between 1 January 2011 and 31 December 2015. These data were obtained through the Data Analytics Services Department at Institute for Clinical Evaluative Sciences (ICES). ICES is a not-for-profit research organisation that gathers population-based health and social data from Ontario’s publicly funded health services to generate knowledge.23 The study data were accessed remotely using a secure server. Patient-level information was linked anonymously across databases using encrypted 10-digit health card numbers. The linking protocol is used routinely for health system research in Ontario.24–26 The Strengthening the Reporting of Observational Studies in Epidemiology guidelines were used to write this manuscript.27
The Ontario Drug Benefit Plan (ODB) Database using drug identification numbers and the Ontario Health Insurance Plan (OHIP) Database physician billing codes including OAT monthly management codes (K682, K683 and K684), visit/consultation codes (A680 and A957) and point-of-care testing codes (G040, G041, G042 or G043) were used to define the primary study cohort. All patients who initiated OAT for the first time within the study time frame in Ontario were included. First-time OAT was defined as no history of treatment in the year before the first treatment episode. It is common for OAT patients to cycle between treatment and relapse.28 29 Studies have demonstrated that multiple treatment attempts are correlated with a higher likelihood of positive outcomes.30–32 We chose only to include first-time OAT patients to eliminate bias related to numerous treatment attempts.
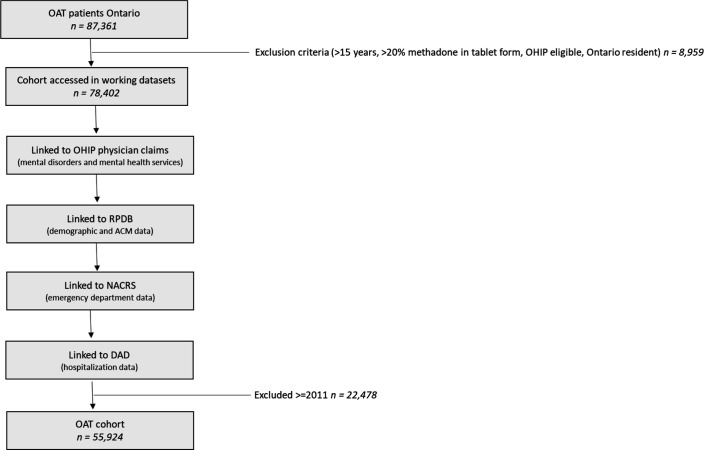
We excluded all patients under 15 years old, patients who were not eligible for OHIP, non-Ontario residents, and those with missing age, gender, and postal codes used for identification and linking across databases. We then combined patients identified from ODB, patients identified from OHIP and patients identified in both databases to create the primary study cohort (see figure 1).
Figure 1.
Flow chart outlining data build including linkages. ACM, all-cause mortality; DAD, Discharge Abstract Database; NACRS, National Ambulatory Care Reporting System; OAT, opioid agonist treatment; OHIP, Ontario Health Insurance Plan; RPDB, Registered Persons Database.
Patient and public involvement
There was no patient and public involvement involved in the design, conduct, reporting or dissemination of our research.
Study variables
Baseline statistics were used to describe the study population and included age groups (18–34, 35–64, 65+ years), sex (male vs female), income quintile (1–highest, 2, 3, 4, 5) and location of residence, missing n=3 (northern/rural, northern/urban, southern/rural, southern/urban), all extracted from the Registered Persons Database. Comorbidity variables included: HIV status (positive vs negative), deep tissue infections (yes vs no) and mental health conditions (yes vs no). We defined patients with mental disorders group using OHIP Database diagnostic codes. The following codes are outlined in online supplemental appendix A.
bmjopen-2022-060857supp001.pdf (192.1KB, pdf)
UDS frequency
UDS billing information, including the following OHIP fee codes: G040, G041, G042, G043, was extracted from the OHIP Database. Patients were assigned to one of four groups: less than once in 30 days, biweekly (>1–≤3 in 30 days), weekly (>3–≤5 in 30 days) and more than weekly (>5 in 30 days). The classification of groups was decided based on the distribution of the means of the UDS in 30 days.
One-year treatment retention
One-year treatment retention is a common measure used in several studies as a positive treatment outcome.15 17 33–37 After their first treatment episode, all patients were followed to a maximum follow-up date of 31 December 2016. Continuous OAT (1-year treatment retention) was assessed based on prescription refill data (from the ODB Database). The 30-day cut-off was chosen based on this interval that has been well established in this field of research.15 33 36 The database used for medication dispensing in this study might not capture doses administered in a hospital or provincial correctional setting. However, in Ontario, patients will typically continue to receive methadone or buprenorphine in these settings. Since most hospital admissions or provincial incarcerations are less than 30 days, this approach allows the analysis to be conducted without misinterpreting such events as treatment interruption.
Statistical analysis
Descriptive statistics were calculated for all UDS groups and used standardised differences (d) where d less than 10% indicated a clinically relevant difference. Standardised differences are not affected by sample size. Therefore, standard differences can be used to compare the balance in measured variables between exposure groups in the study.38
A logistic regression model was then used to calculate ORs for the association between UDS frequency and 1-year treatment retention. We adjusted for baseline covariates in the models, including sex, age, location of residence, income quintile, mental disorders, HIV status and deep tissue infections. All data were analysed using SAS V.9.4.39
Results
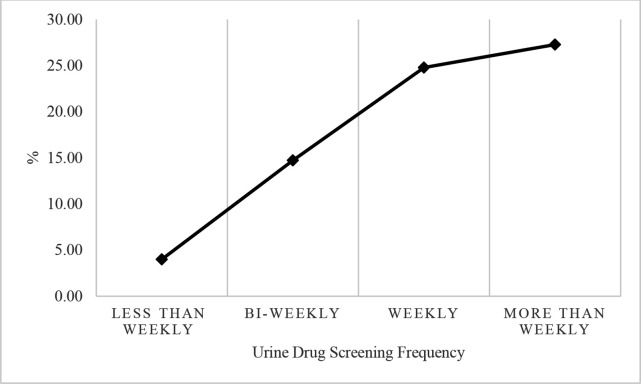
Figure 2 shows that between January 2011 and December 2015, a total of 55 921 individuals were included in the study. Of these, 6252 (11.20%) had UDS monthly or less, 9495 (16.98%) had biweekly UDS, 24 948 (44.61%) had weekly UDS and 15 226 (27.23%) had UDS six or more times in 30 days.
Figure 2.
Proportion of individuals retained for 1 year by urine drug screening frequency groups.
There were significant differences between the UDS frequency groups. Notably, we observed that the proportion of younger patients (aged 15–34 years) increased and that the proportion of older patients (55–65+ years) decreased with increased UDS frequency. Similarly, the proportion of northern rural patients increased, and the proportion of southern rural patients decreased with higher UDS frequency. Other demographic characteristics at OAT initiation are shown in table 1.
Table 1.
Summary statistics of individuals with OUD by UDS frequency group
| Urine drug test frequency | ||||||||
| Monthly or less | Biweekly | Weekly | More than weekly | |||||
| n=6252 (11.20) | d | n=9495 (16.98) | d | n=24 948 (44.61) | d | n=15 226 (27.23) | d | |
| Sex | 0.02 | 0.03 | 0.09 | 0.12* | ||||
| Female | 2268 (36.28) | 3217 (33.88) | 8217 (32.94) | 5992 (39.35) | ||||
| Male | 3984 (63.72) | 6278 (66.12) | 16 731 (67.06) | 9234 (60.65) | ||||
| Age | 0.64* | 0.36* | 0.24* | 0.40* | ||||
| 15–24 | 720 (11.20) | 1064 (11.21) | 4716 (18.90) | 3741 (24.57) | ||||
| 25–34 | 1358 (21.72) | 2656 (27.97) | 9086 (36.42) | 5906 (38.79) | ||||
| 35–44 | 1186 (18.967) | 2249 (23.69) | 5573 (22.34) | 3087 (20.27) | ||||
| 45–54 | 1415 (22.63) | 2277 (23.98) | 4161 (16.68) | 1947 (12.79) | ||||
| 55–64 | 887 (14.19) | 1004 (10.57) | 1289 (5.17) | 505 (3.32) | ||||
| 65+ | 686 (10.97) | 245 (2.58) | 123 (0.49) | 40 (0.26) | ||||
| Geography | 0.11* | 0.29* | 0.22* | 0.45* | ||||
| Northern rural | 366 (5.85) | 239 (2.52) | 828 (3.32) | 1400 (9.19) | ||||
| Northern urban | 445 (7.12) | 441 (4.64) | 1753 (7.03) | 2655 (17.44) | ||||
| Southern rural | 457 (7.31) | 672 (7.08) | 2107 (8.45) | 1462 (9.60) | ||||
| Southern urban | 4984 (79.72) | 8143 (85.76) | 20 260 (81.21) | 9709 (63.77) | ||||
| Income | 0.10 | 0.11* | 0.05 | 0.15* | ||||
| 1 (lowest) | 1999 (31.97) | 2847 (29.98) | 8293 (33.24) | 5953 (39.10) | ||||
| 2 | 1348 (21.56) | 2206 (23.23) | 5644 (22.62) | 3301 (21.68) | ||||
| 3 | 1089 (17.42) | 1777 (18.72) | 4586 (18.38) | 2520 (16.55) | ||||
| 4 | 956 (15.29) | 1497 (15.77) | 3755 (15.05) | 1884 (12.37) | ||||
| 5 | 860 (13.76) | 1168 (12.30) | 2670 (10.70) | 1568 (10.30) | ||||
| Mental health | 5544 (88.68) | 0.06 | 8426 (88.74) | 0.06 | 21 472 (86.07) | 0.05 | 13 234 (86.92) | 0.01 |
| HIV positive | 59 (0.94) | 0.03 | 111 (1.17) | 0.06 | 158 (0.63) | 0.02 | 83 (0.55) | 0.03 |
| Deep tissue infection | 344 (5.50) | 0.14* | 420 (4.42) | 0.09 | 591 (2.37) | 0.07 | 321 (2.11) | 0.08 |
*Statistically significant.
d, standardised difference; OUD, opioid use disorder; UDS, urine drug screening.
As shown in table 2, a logistic regression model was conducted to determine the association between UDS frequency and 1-year treatment retention. A total of 250 (4.00%) patients who were retained for 1 year had less than one UDS in 30 days, 1398 (14.72%) had biweekly UDS, 6185 (24.79%) had weekly UDS and 4153 (27.28%) had more than weekly UDS. UDS frequency was positively associated with 1-year treatment retention within our cohort. Compared with patients who had less than monthly UDS, biweekly UDS was associated with an increase in 1-year treatment retention (adjusted OR (aOR)=3.20, 95% CI 2.75 to 3.75); weekly UDS was associated with an increase in 1-year treatment retention (aOR=6.86, 95% CI 5.88 to 8.00) and more than weekly UDS was associated with an increase in 1-year treatment retention (aOR=8.03, 95% CI 6.87 to 9.38).
Table 2.
Urine drug screening (UDS) frequency and 1-year treatment retention
| UDS frequency per month | Patients (N) | One-year retention, N (%) | Unadjusted OR | Unadjusted 95% CI | Adjusted OR | Adjusted 95% CI |
| Less than monthly* | 6252 | 250 (4.0) | ||||
| Biweekly | 9495 | 1398 (14.72) | 3.18 | 2.71 to 3.72 | 3.20 | 2.75 to 3.75 |
| Weekly | 24 948 | 6185 (24.79) | 6.07 | 5.22 to 7.05 | 6.86 | 5.88 to 8.00 |
| More than weekly | 15 226 | 4153 (27.28) | 6.90 | 5.93 to 8.03 | 8.03 | 6.87 to 9.38 |
*Reference group.
Discussion
The study sought to evaluate the relationship between the frequency of UDS tests and 1-year retention in OAT. Drawing on longitudinal data from publicly funded health administrative data in Ontario, Canada, it was observed that more frequent UDS tests are associated with a significantly increased likelihood of 1-year treatment retention in OAT.
We found a certain degree of heterogeneity in the UDS frequency groups. UDS frequency can vary based on patient drug use, treatment compliance, time in treatment and some physician discretion. Since UDS is part of contingency management in Ontario, the lowest frequency of urine testing would typically be seen in two groups of patients. First, less frequent testing is done for those patients who are chronically unstable (most often due to sustained use of other drugs, homelessness, or ineffectively treated mental health problems or a combination of these) and thus have the frequency or urine testing reduced as they are not engaged in demonstrating increasing levels of stability. Second, those patients who have demonstrated sustained periods of stability, including cessation of problematic use of other substances, will have observed dosing and urine testing less frequently and sufficient only to monitor for continued stability.
In our data, we found that younger patients and those living in northern rural areas had more frequent UDS tests. This observation is likely reflective of physician and patient factors which may account for the higher frequency of urine testing in the northern Ontario patient group. The physicians practising in this geographical area may place more emphasis on adherence to the contingency management schedule in determining frequency of both UDS and take-home doses. Alternatively, given the longer distances between patients and providers,35 the patients in this area may be more motivated to engage in the process of increased UDS in the short term in order to obtain less frequent testing and higher frequency take-home doses in the long term. It is worth noting that our repeated observation in earlier papers34 35 40 of higher treatment retention in the northern Ontario geographical area and the higher frequency of testing in this geographical area demonstrated in this paper is consistent with the overall relationship between UDS frequency and retention reported here.
In this study, when evaluating 1-year treatment retention as the primary outcome, we accounted for variations in UDS frequency by adjusting for baseline patient characteristics. Compared with monthly UDS, increased frequency of urine screening was associated with a higher likelihood of 1-year treatment retention in OAT. Importantly, we observed that the more frequent the UDS, the stronger the association was with 1-year treatment retention. Research has shown that 1-year treatment is correlated with various positive health outcomes for OAT patients, including reduced rates of drug use, hospitalisation, criminal activity and mortality.15 33 Therefore, it is often used as a marker for a positive treatment outcome.
In our review of the literature, we found that only one other study has examined the impact of UDS frequency on OAT patient outcomes. Our search was consistent with a recent critical review of the literature by McEachern et al,10 which only identified one full-text report that met their search criteria studies focusing on individuals with substance use disorders and comparing UDS frequency to evaluate health outcomes. The other study evaluating UDS frequency was a three-arm randomised open-label trial (N=53) by Chutuape et al. The main intervention was random weekly or monthly testing, which was associated with higher retention rates over time, compared with no urine testing or contingency management.41 Although there is minimal research on UDS frequency and OAT outcomes, our study and the other study by Chutuape et al were consistent in demonstrating the positive effect of more frequent UDS on retention. Additional research is required to continue to add to this evidence base to provide clinicians with clearer, consistent guidelines on UDS frequency across Canada.
Some limitations in the current study require consideration. First, we acknowledge that this study cannot determine whether the requirement for UDS is a barrier to potential patients ever engaging in care. However, the high level of treatment engagement in Ontario compared with other jurisdictions (for example, the USA where the large majority of those with OUD have never been prescribed OAT)42 weighs against this being a substantial factor from a public health perspective. Second, there is the possibility of data entry and reporting errors associated with using administrative-level data. Third, the data are collected for physician remuneration and funding; therefore, its initial intention is not for research. Fourth, although we considered various factors associated with treatment retention, there is potential for unmeasured confounding, including confounding related to other substance use,36 43 44 social and interpersonal factors,45–48 the lack of patient descriptors that assess addiction severity and clinical characteristics49 50 due to our study only having access to routinely collected data. Fifth, in this study, methadone and buprenorphine/naloxone patients were grouped due to low frequency of buprenorphine/naloxone prescriptions during our study period. Research has shown that OAT medication type can impact retention. Therefore, further study is needed to compare UDS frequency between methadone and buprenorphine/naloxone patients. Finally, some expert opinions have suggested that routine use of urine toxicology testing reinforces a power dynamic and invites shame, stigma and judgement. We were not able to account for such factors in our analysis.51
Conclusion
In summary, our study identified a significant association between the frequency of UDS and 1-year treatment retention in OAT. There is an active discussion within Canada about the utility of UDS with some practitioners arguing that they should not be collected at all or very rarely, while others collect them frequently and tie them to increased take-home doses under as part of a contingency management strategy. The lack of evidence for the impact of UDS on retention has left it open to some to argue they simply provide a barrier to patient engagement. Therefore, it is timely for this study to demonstrate that more frequent urine testing is not associated with a reduction in treatment retention. The results can be generalised to any other locations with similar OAT regulations. This study adds to previous research showing the association between UDS frequency and positive OAT treatment outcomes, and more research is needed to strengthen the evidence base for UDS frequency in OAT.
Supplementary Material
Acknowledgments
We thank ICES Data Analytics Services, more specifically Ryan Ng, for his assistance with data extraction and database set-up. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed herein are those of the author, and not necessarily those of CIHI. We thank IQVIA Solutions Canada Inc. for use of their Drug Information Database.
Footnotes
Twitter: @dmarshnosm
Contributors: KAM participated in the conceptualisation, design, data analysis, writing and preparation of the article in question. JRD participated in the conceptualisation, data analysis and final revision of the article in question. FV participated in database management, cleaning and organisation, data analysis and final revision of the article in question. DM is the corresponding author. He played a leadership role in planning of this study as part of a larger research project. He also has contributed to the interpretation of results and final review of the article in question. DM is the acting guarantor for this study
Funding: This research was partially supported by the Northern Ontario Academic Medicine Association Clinical Innovation Grant AFP Innovation Fund (Project #A-21-10).
Competing interests: DM maintains the following roles: chief medical director at CATC (Canadian Addiction Treatment Center) and opioid agonist therapy provider. DM has no ownership stake in the CATC as a stipendiary employee. We do not foresee any conflict of interest as data will be made freely available to the public and the CATC, and the universities have no ability to prevent publication and dissemination of knowledge. The authors have no conflicts declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The dataset from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre- specified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analyticcode are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification. ICES is an independent, non-profit research institute funded by an annual grant from the Ontario Ministry of Health (MOH). As a prescribed entity under Ontario’s privacy legislation, ICES is authorized to collect and use health care data for the purposes of health system analysis, evaluation and decision support. Secure access to these data is governed by policies and procedures that are approved by the Information and Privacy Commissioner of Ontario.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The Laurentian University Research Ethics Board provided ethical approval for this study under project number 6009752.
References
- 1. Ontario PH, Tool IO. Opioid-related mortality and morbidity in Ontario Toronto, 2021. Available: https://www.publichealthontario.ca/en/data-and-analysis/substance-use/interactive-opioid-tool#/trends
- 2. Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ 2020;368:m772. 10.1136/bmj.m772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public agency of Canada. apparent opioid and stimulant toxicity deaths. Ottawa, Canada: 2021. Surveillance of Opioid- and Stimulant-Related Harms in Canada. [Google Scholar]
- 4. Special Advisory Committee on the Epidemic of Opioid Overdoses . Opioid and Stimulant-related harms in Canada, 2020.
- 5. Government of Canada . Opioid-related harms in Canada, 2020. Available: https://health-infobase.canada.ca/substance-related-harms/opioids/
- 6. Ball CRA. The effectiveness of methadone maintenance treatment. New York: Springer-Verlag, 1991. [Google Scholar]
- 7. Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry 2019;24:1868–83. 10.1038/s41380-018-0094-5 [DOI] [PubMed] [Google Scholar]
- 8. Krebs E, Enns B, Evans E, et al. Cost-Effectiveness of publicly funded treatment of opioid use disorder in California. Ann Intern Med 2018;168:10–19. 10.7326/M17-0611 [DOI] [PubMed] [Google Scholar]
- 9. Ching JH, Owens DK, Trafton JA, et al. Impact of treatment duration on mortality among veterans with opioid use disorder in the United States Veterans health administration. Addiction 2021;116:3494–503. 10.1111/add.15574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McEachern J, Adye-White L, Priest KC, et al. Lacking evidence for the association between frequent urine drug screening and health outcomes of persons on opioid agonist therapy. Int J Drug Policy 2019;64:30–3. 10.1016/j.drugpo.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction 1999;94:961–72. 10.1046/j.1360-0443.1999.9479612.x [DOI] [PubMed] [Google Scholar]
- 12. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017;357:j1550. 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cousins G, Teljeur C, Motterlini N, et al. Risk of drug-related mortality during periods of transition in methadone maintenance treatment: a cohort study. J Subst Abuse Treat 2011;41:252–60. 10.1016/j.jsat.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 14. Buster MCA, van Brussel GHA, van den Brink W. An increase in overdose mortality during the first 2 weeks after entering or re-entering methadone treatment in Amsterdam. Addiction 2002;97:993–1001. 10.1046/j.1360-0443.2002.00179.x [DOI] [PubMed] [Google Scholar]
- 15. Peles E, Linzy S, Kreek M, et al. One-Year and cumulative retention as predictors of success in methadone maintenance treatment: a comparison of two clinics in the United States and Israel. J Addict Dis 2008;27:11–25. 10.1080/10550880802324382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stone AC, Carroll JJ, Rich JD, et al. One year of methadone maintenance treatment in a fentanyl endemic area: safety, repeated exposure, retention, and remission. J Subst Abuse Treat 2020;115:108031. 10.1016/j.jsat.2020.108031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weisner C, Matzger H, Kaskutas LA. How important is treatment? one-year outcomes of treated and untreated alcohol-dependent individuals. Addiction 2003;98:901–11. 10.1046/j.1360-0443.2003.00438.x [DOI] [PubMed] [Google Scholar]
- 18. Barthwell AG. Clinical and public health considerations in urine drug testing to identify and treat substance use. Subst Use Misuse 2016;51:700–10. 10.3109/10826084.2015.1135953 [DOI] [PubMed] [Google Scholar]
- 19. British Columbia Centre on Substance Use & CIHR Canadian Research Initiative on Substance Misuse . A guideline for the management of opioid use disorder, 2017.
- 20. Ministry of Health and Long Term Care . Opioid agonist maintenance program (OAMP) monthly management fee and point of care drug testing health services branch, 2012. Available: http://www.health.gov.on.ca/en/pro/programs/ohip/bulletins/4000/bul4548.pdf
- 21. Centre for Addiction and Mental Health . Opioid agonist therapy: a synthesis of Canadian guidelines for treating opioid use disorder; 2021.
- 22. Moss E, McEachern J, Adye-White L, et al. Large variation in provincial guidelines for urine drug screening during opioid agonist treatment in Canada. Can J Addict 2018;9:6–9. 10.1097/CXA.0000000000000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ICES Data Dictionary Toronto Ontario , 2016. Available: https://datadictionary.ices.on.ca/Applications/DataDictionary/Default.aspx
- 24. Mamdani M, Rochon P, Juurlink DN, et al. Effect of selective cyclooxygenase 2 inhibitors and naproxen on short-term risk of acute myocardial infarction in the elderly. Arch Intern Med 2003;163:481–6. 10.1001/archinte.163.4.481 [DOI] [PubMed] [Google Scholar]
- 25. Juurlink DN. Proton pump inhibitors and clopidogrel: putting the interaction in perspective. Circulation 2009;120:2310–2. 10.1161/CIRCULATIONAHA.109.907295 [DOI] [PubMed] [Google Scholar]
- 26. Juurlink DN, Gomes T, Lipscombe LL, et al. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ 2009;339:b2942. 10.1136/bmj.b2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Elm E, Altman DG, Egger M, et al. [The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies]. Rev Esp Salud Publica 2008;82:344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 28. Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction 2006;101:212–22. 10.1111/j.1360-0443.2006.01310.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bell J, Burrell T, Indig D, et al. Cycling in and out of treatment; participation in methadone treatment in NSW, 1990-2002. Drug Alcohol Depend 2006;81:55–61. 10.1016/j.drugalcdep.2005.05.010 [DOI] [PubMed] [Google Scholar]
- 30. Termorshuizen F, Krol A, Prins M, et al. Prediction of relapse to frequent heroin use and the role of methadone prescription: an analysis of the Amsterdam cohort study among drug users. Drug Alcohol Depend 2005;79:231–40. 10.1016/j.drugalcdep.2005.01.013 [DOI] [PubMed] [Google Scholar]
- 31. Volkow ND. America’s Addiction to Opioids: Heroin and Prescription Drug Abuse. Available: https://www.nih.gov/sites/default/files/institutes/olpa/20140514-senate-testimony-volkow.pdf
- 32. Nosyk B, MacNab YC, Sun H, et al. Proportional hazards frailty models for recurrent methadone maintenance treatment. Am J Epidemiol 2009;170:783–92. 10.1093/aje/kwp186 [DOI] [PubMed] [Google Scholar]
- 33. Nosyk B, Marsh DC, Sun H, et al. Trends in methadone maintenance treatment participation, retention, and compliance to dosing guidelines in British Columbia, Canada: 1996-2006. J Subst Abuse Treat 2010;39:22–31. 10.1016/j.jsat.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 34. Eibl JK, Gauthier G, Pellegrini D, et al. The effectiveness of telemedicine-delivered opioid agonist therapy in a supervised clinical setting. Drug Alcohol Depend 2017;176:133–8. 10.1016/j.drugalcdep.2017.01.048 [DOI] [PubMed] [Google Scholar]
- 35. Eibl JK, Gomes T, Martins D, et al. Evaluating the effectiveness of first-time methadone maintenance therapy across Northern, rural, and urban regions of Ontario, Canada. J Addict Med 2015;9:440–6. 10.1097/ADM.0000000000000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franklyn AM, Eibl JK, Gauthier G, et al. The impact of benzodiazepine use in patients enrolled in opioid agonist therapy in northern and rural Ontario. Harm Reduct J 2017;14:6. 10.1186/s12954-017-0134-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA 2000;283:1303–10. 10.1001/jama.283.10.1303 [DOI] [PubMed] [Google Scholar]
- 38. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228–34. 10.1080/03610910902859574 [DOI] [Google Scholar]
- 39. Sas version 9.4 Cary, North Carolina, 2020. Available: https://www.sas.com/en_us/software/sas9.html
- 40. Franklyn MAE, N.E JKLightfoot, Marsh DC. Journal of Addiction Medicine and Therapy. In: Geography, treatment modality, and substance use: evaluating factors that impact opioid agonist therapy in northern Ontario. Canada, 2016. [Google Scholar]
- 41. Chutuape MA, Silverman K, Stitzer ML. Effects of urine testing frequency on outcome in a methadone take-home contingency program. Drug Alcohol Depend 2001;62:69–76. 10.1016/S0376-8716(00)00160-5 [DOI] [PubMed] [Google Scholar]
- 42. Priest KC, Gorfinkel L, Klimas J, et al. Comparing Canadian and United States opioid agonist therapy policies. Int J Drug Policy 2019;74:257–65. 10.1016/j.drugpo.2019.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brands B, Blake J, Marsh DC, et al. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J Addict Dis 2008;27:37–48. 10.1080/10550880802122620 [DOI] [PubMed] [Google Scholar]
- 44. Franklyn AM, Eibl JK, Gauthier GJ, et al. The impact of cannabis use on patients enrolled in opioid agonist therapy in Ontario, Canada. PLoS One 2017;12:e0187633. 10.1371/journal.pone.0187633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stein DJ, van Honk J, Ipser J, et al. Opioids: from physical pain to the pain of social isolation. CNS Spectr 2007;12:669–74. 10.1017/S1092852900021490 [DOI] [PubMed] [Google Scholar]
- 46. Mattoo SK, Chakrabarti S, Anjaiah M. Psychosocial factors associated with relapse in men with alcohol or opioid dependence. Indian J Med Res 2009;130:702–8. [PubMed] [Google Scholar]
- 47. Krakowski M, Smart RG. Social and psychological characteristics of heroin addicts dropping out of methadone treatment. Can Psychiatr Assoc J 1974;19:41–7. 10.1177/070674377401900108 [DOI] [PubMed] [Google Scholar]
- 48. Stein MD, Conti MT, Kenney S, et al. Adverse childhood experience effects on opioid use initiation, injection drug use, and overdose among persons with opioid use disorder. Drug Alcohol Depend 2017;179:325–9. 10.1016/j.drugalcdep.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Strain EC, Stitzer ML, Liebson IA, et al. Methadone dose and treatment outcome. Drug Alcohol Depend 1993;33:105–17. 10.1016/0376-8716(93)90052-R [DOI] [PubMed] [Google Scholar]
- 50. Strain EC, Bigelow GE, Liebson IA, et al. Moderate- vs high-dose methadone in the treatment of opioid dependence. JAMA 1999;281:1000–5. 10.1001/jama.281.11.1000 [DOI] [PubMed] [Google Scholar]
- 51. Incze MA. Reassessing the role of routine urine drug screening in opioid use disorder treatment. JAMA Intern Med 2021;181:1282–3. 10.1001/jamainternmed.2021.4109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-060857supp001.pdf (192.1KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The dataset from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre- specified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analyticcode are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification. ICES is an independent, non-profit research institute funded by an annual grant from the Ontario Ministry of Health (MOH). As a prescribed entity under Ontario’s privacy legislation, ICES is authorized to collect and use health care data for the purposes of health system analysis, evaluation and decision support. Secure access to these data is governed by policies and procedures that are approved by the Information and Privacy Commissioner of Ontario.