Abstract
Background
This study aims to develop PADjadjaran Mortality in Acute coronary syndrome (PADMA) Score to predict in-hospital mortality in acute coronary syndrome (ACS) patients based on clinical examination only. Additionally, we also compared the predictive value of the PADMA Score with the Global Registry of Acute Coronary Events (GRACE), Canada Acute Coronary Syndrome (C-ACS), and The Portuguese Registry of Acute Coronary Syndromes (ProACS) risk scores.
Methods
This retrospective cohort study included all ACS patients aged≥18 years who were admitted to Dr. Hasan Sadikin Central General Hospital from January 2018 to January 2022. Patients’ demographic, comorbidities and clinical presentation data were collected and analysed using multivariate logistic regression to create two models of scoring system (probability and cut-off model) to predict in-hospital all-cause mortality. The area under the curve (AUC) among PADMA, GRACE, C-ACS and ProACS risk scores was compared using the fisher Z test.
Results
Multivariate regression analysis of 1359 patients showed that older age, history of cerebrovascular disease, tachycardia, high Shock Index and Killip class III and IV were independent mortality predictors and included in the PADMA Score. PADMA Score ranged from 0 to 20, with a score≥5 that can predict all-cause mortality with 82.78% sensitivity and 72.35% specificity. The difference in AUC between PADMA and GRACE scores was insignificant (p=0.126). Moreover, the AUC of the PADMA Score was significantly higher compared with the C-ACS (p=0.002) and ProACS risk scores (p<0.001).
Conclusion
PADMA Score is a simple scoring system to predict in-hospital mortality in ACS patients. PADMA Score≥5 showed an accurate discriminative capability to predict in-hospital mortality, comparable with the GRACE Score and superior to C-ACS and ProACS scores.
Keywords: carotid artery diseases, acute coronary syndrome, myocardial infarction
WHAT IS ALREADY KNOWN ON THIS TOPIC
Although the Global Registry of Acute Coronary Events (GRACE) risk Score is the most widely used scoring system for predicting the risk of mortality in acute coronary syndrome (ACS) patients, this scoring system is time-consuming and cannot be performed in healthcare centres with limited facilities.
WHAT THIS STUDY ADDS
This study develops a novel scoring system to predict the risk of in-hospital mortality in ACS patients based only on clinical examination, namely PADjadjaran Mortality in Acute coronary syndrome (PADMA) risk Score.
The predictive value of the PADMA Score is proven to be comparable to the GRACE risk Score and superior to Canada Acute Coronary Syndrome and Portuguese Registry of Acute Coronary Syndromes scores.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Since the PADMA Score is simple and easy to calculate, clinicians can rapidly predict the risk of in-hospital mortality of ACS patients in the first medical contact.
Introduction
Cardiovascular diseases (CVD) are a global health burden and are responsible for one-third of mortality worldwide. In 2019, approximately 17.9 million people died from CVD. Its prevalence is expected to increase.1 Compared with other CVD, acute coronary syndrome (ACS) is a worldwide health issue with the highest mortality rate, with over three-quarters of CVD deaths in low-middle-income countries.1 2 Therefore, the risk stratification tool to predict mortality risk in this population is mandatory to assess the early prognosis and help clinicians decide the best therapeutical options for the patients.
Until now, several prognostic tools (eg, Global Registry of Acute Coronary Events [GRACE], Thrombolysis in Myocardial Infarction [TIMI], Zwolle De Luca, Primary Angioplasty in Myocardial Infarction [PAMI] Addala and Cadillac Halkin) have been discovered to predict the risk of mortality in ACS patients.3 Nonetheless, GRACE Score is still the most widely used scoring system and also the validated instruments that had the highest sensitivity and specificity in predicting mortality compared with other prognostic tools.4 5 However, this scoring system relies heavily on electrocardiography (ECG) and laboratory findings to complete the calculation; hence, it is time-consuming and cannot be performed in rural healthcare centres with limited facilities.
Several cohort studies evaluate the usefulness of the CHA2DS2VASc (Congestive heart failure, Hypertension, Aged ≥ 75 years, Diabetes mellitus, Stroke, Vascular disease, Aged 65-74 years, Sex category [female]) score as a clinical scoring system in which the calculation is based only on the history of a patient’s comorbidities.6–8 These cohort studies showed that a high CHA2DS2VASc Score significantly increased the risk of major adverse cardiac events in ACS patients regardless of atrial fibrillation status.6–8 Nevertheless, although this scoring system is convenient compared with the GRACE Score, it also has limitations as it does not include the patient’s clinical presentation into the calculation; therefore, it can underestimate the mortality risk in ACS patients with poor haemodynamic status. Thus, this study aims to develop a novel risk scoring system named PADjadjaran Mortality in Acute coronary syndrome (PADMA) Score to predict the risk of in-hospital mortality in ACS patients, which in the calculation is based on the patient’s history and clinical presentation at admission. This scoring is simple and can be completed in the first medical contact in all healthcare facilities. Additionally, we also compared the prognostic value of the PADMA Score with other scoring systems, including GRACE, Canada Acute Coronary Syndrome (C-ACS) and The Portuguese Registry of Acute Coronary Syndromes (ProACS) risk scores.
Methods
Study design and patient selection
This retrospective and single-centre cohort study included all ACS patients aged≥18 years hospitalised in Dr. Hasan Sadikin Central General Hospital from January 2018 to January 2022. Incomplete or missing data from medical records were excluded from the study.
Definition of variables and outcome
Variables that were considered in the model were as follows; demography (female sex, age, body mass index (BMI)), clinical presentation (initial systolic blood pressure (SBP), initial heart rate (HR), Shock Index (SI), which is defined as HR divided by SBP, Killip class and ST-segment deviation in ECG), history and cardiovascular risk factors (smoking, diabetes, hypertension, family history of coronary artery disease, history of cerebrovascular disease, history of angina and history of revascularisation, including primary percutaneous intervention and coronary artery bypass surgery). All these variables were collected from the patient’s medical records. The primary endpoint was in-hospital mortality, defined as all-cause death that occurred during the index hospitalisation of the ACS event.
Statistical analyses
All statistical analyses were performed using SPSS V.25.0. All categorical data were presented in numbers and percentages. In contrast, numerical data were presented in mean and SD if the distribution was normal or median and IQR if the distribution was not normal. Chi-square and Fisher’s tests were performed to analyse the association between predicting factors and in-hospital mortality. The logistic regression analysis was performed to find the significant p value (p<0.05) from the adjusted OR. All the significant parameters were included in logistic regression with a stepwise backward method. The scoring system was developed by numbering each independent variable based on the regression coefficient (B) from multivariate logistic regression analysis. Receiver operating characteristics (ROC) analysis was used to produce the area under the curve (AUC) for predicting the outcome of interest. Moreover, the DeLong method was used to generate the PADMA Score’s sensitivity and specificity and determine the ideal cut-off. The Granger model was performed to obtain the estimated probability of mortality for in-hospital death. The scoring system validated the previous sample to find diagnostic value. Finally, to compare the prognostic value of PADMA, GRACE, C-ACS and ProACS risk scores, we compared the AUC of these scoring systems using Fisher Z test.
Results
The baseline characteristics of the studies are summarised in table 1. Of 1359 subjects who enrolled in this study, most of the patients were male (76.6%) and less than 65 years (72.9%). Most patients were non-obese (6.8%), more than half of the subjects were smokers (59.7%) and had hypertension (62.8%). The initial presentation showed that most of the patients had no congestion (71%) with stable haemodynamics with HR less than 100 bpm (86%), blood pressure more than 100 mm Hg (76.5%) and SI less than 0.7 (59.6%). More than half of the ECG presented ST elevation (58.9%).
Table 1.
Baseline characteristic of the study participants
| Variable | Total n=1359 | Non-survivor n=151 | Survivor n=1208 | P value |
| Gender, n (%) | ||||
| Female | 318 (23.4) | 50 (33.1) | 268 (22.2) | 0.003* |
| Male | 1041 (76.6) | 101 (66.9) | 940 (77.8) | |
| Age (years), mean±SD | 58±11 | 64±12 | 57±11 | <0.001* |
| Age (years) classifications, n (%) | ||||
| <65 | 991 (72.9) | 74 (49) | 917 (75.9) | <0.001* |
| 65–75 | 263 (19.4) | 45 (29.8) | 218 (18) | |
| >75 | 105 (7.7) | 32 (21.2) | 73 (6) | |
| BMI (kg/m2), mean±SD | 24.3±3.5 | 23.9±3.6 | 24.4±3.5 | 0.209* |
| Obesity (BMI≥30 kg/m2) | 92 (6.8) | 9 (4) | 86 (7.1) | 0.147 |
| Smoking, n (%) | 812 (59.7) | 75 (49.7) | 737 (61.0) | 0.007* |
| Diabetes melitus, n (%) | 293 (21.6) | 41 (27.2) | 252 (20.9) | 0.076 |
| Hypertension, n (%) | 854 (62.8) | 100 (66.2) | 754 (62.4) | 0.361 |
| Family history of CAD, n (%) | 126 (9.3) | 12 (7.9) | 114 (9.4) | 0.552 |
| Cerebrovascular disease, n (%) | 96 (7.1) | 21 (13.9) | 75 (6.2) | <0.001* |
| History of angina, n (%) | 451 (33.2) | 57 (37.7) | 394 (32.6) | 0.207 |
| History of revascularisation, n (%) | 181 (13.3) | 17 (11.3) | 164 (13.6) | 0.429 |
| SBP (mm Hg), mean±SD | 121±25 | 108±30 | 123±23 | <0.001* |
| SBP (mm Hg) classifications, n (%) | ||||
| ≤100 | 319 (23.5) | 69 (45.7) | 250 (20.7) | <0.001* |
| >100 | 1040 (76.5) | 82 (54.3) | 958 (79.3) | |
| HR (bpm), mean±SD | 82±19 | 93±25 | 81±18 | <0.001* |
| HR (bpm) classifications, n (%) | ||||
| ≤100 | 1169 (86.0) | 93 (61.6) | 1076 (89.1) | <0.001* |
| >100 | 190 (14.0) | 58 (38.4) | 132 (10.9) | |
| Shock Index†, mean±SD | 0.71±0.32 | 0.93±0.36 | 0.68±0.30 | <0.001* |
| Shock Index classifications, n (%) | ||||
| ≤0.70 | 810 (59.6) | 41 (27.2) | 769 (63.7) | <0.001* |
| 0.71–1.00 | 431 (31.7) | 57 (37.7) | 374 (31) | |
| >1.00 | 118 (8.7) | 53 (35.1) | 65 (5.4) | |
| ACS classifications, n (%) | ||||
| STEMI | 801 (58.9) | 103 (68.2) | 698 (57.8) | 0.014* |
| NSTEMI/UAP | 558 (41.1) | 48 (31.8) | 510 (42.2) | |
| Killip class, n (%) | ||||
| I | 965 (71.0) | 52 (34.4) | 913 (75.6) | <0.001* |
| II | 218 (16.0) | 24 (15.9) | 194 (16.1) | |
| III | 38 (2.8) | 13 (8.6) | 25 (2.1) | |
| IV | 138 (10.2) | 62 (41.1) | 76 (6.3) | |
| Revascularisation procedures, n (%) | ||||
| PCI | 733 (53.9) | 62 (41.1) | 671 (55.5) | <0.001* |
| Fibrinolytic | 42 (3.1) | 4 (2.6) | 38 (3.1) | <0.001* |
| Pharmacological treatments, n (%) | ||||
| DAPT | 1338 (98.5) | 144 (95.8) | 1194 (98.8) | 0.006* |
| Anticoagulant | 1321 (97.2) | 142 (94.0) | 1179 (97.6) | 0.030* |
| ACE-I/ARB | 833 (61.3) | 31 (20.5) | 802 (66.4) | <0.001* |
| BB | 986 (72.6) | 34 (22.5) | 952 (78.8) | <0.001* |
| Statin | 1321 (97.2) | 129 (85.4) | 1192 (98.7) | <0.001* |
All categorical data are presented in n (%).
All numerical data are presented in mean±SD.
*Significant p value
*p<0.05, **p<0.01, ***p<0.001.
†Shock Index is calculated by heart rate divided by systolic blood pressure.
ACE-I, ACE inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BB, beta-blocker; BMI, body mass index; bpm, beat per minute; CAD, coronary artery disease; DAPT, dual antiplatelet; HR, heart rate; PCI, primary percutaneous intervention; SBP, systolic blood pressure.
In-hospital mortality occurred in 151 patients (11.1%). Female gender, older age, smoking and patients who had a history of cerebrovascular disease, low SBP, increased HR, high SI, ST-segment elevation myocardial infarction (STEMI) and high Killip class (III and IV) were significantly associated with higher mortality risk (p<0.05). Furthermore, the survivors had a higher number of revascularisation procedures and medical treatments in opposition to the non-survivors (p<0.05).
Table 2 showed the bivariate and multivariate regression analyses. Independence predictor in mortality were age 65–75 years (AOR=2.437 (95% CI=1.530 to 3.884); p<0.001), age>75 years (AOR=4.042 (95% CI=2.225 to 7.344); p<0.001), history of cerebrovascular disease (AOR=1.954 (95% CI=1.032 to 3.698); p=0.04), HR>100 bpm (AOR=1.859 (95% CI=1.080 to 3.202); p=0.025), SI 0.71–1.00 (AOR=2.189 (95% CI=1.341 to 3.572); p=0.002), SI>1.00 (OR=4.033 (95% CI=1.844 to 8.820); p<0.001), Killip III (OR=4.768 (95% CI=2.07 to 10.98); p<0.001) and Killip IV (OR=6.859 (95% CI=3.933 to 11.963); p<0.001).
Table 2.
Logistic regression analysis of in-hospital mortality risk
| Variable | Crude OR (95% CI) | P value | AOR (95% CI) | P value |
| Gender | ||||
| Female | 1.736 (1.205 to 2.501) | 0.003* | 1.389 (0.84 to 2.295) | 0.200 |
| Male | 1 (ref) | 1 (ref) | ||
| Age (years) | ||||
| <65 | 1 (ref) | 1 (ref) | ||
| 65–75 | 2.558 (1.717 to 3.812) | <0.001* | 2.437 (1.53 to 3.884) | <0001* |
| >75 | 5.432 (3.367 to 8.764) | <0.001* | 4.042 (2.225 to 7.344) | <0001* |
| Obesity (BMI>30 kg/m2) | 0.540 (0.232 to 1.257) | 0.153 | 0.705 (0.275 to 1.809) | 0.468 |
| Smoking | 0.631 (0.449 to 0.885) | 0.007* | 0.945 (0.587 to 1.52) | 0.814 |
| Diabetes melitus | 1.414 (0.963 to 2.077) | 0.076 | 1.139 (0.719 to 1.804) | 0.580 |
| Hypertension | 1.181 (0.826 to 1.687) | 0.361 | 1.321 (0.85 to 2.053) | 0.216 |
| Family history of CAD | 0.828 (0.445 to 1.541) | 0.552 | 1.03 (0.494 to 2.147) | 0.936 |
| History of cerebrovascular disease | 2.440 (1.455 to 4.092) | <0.001* | 1.954 (1.032 to 3.698) | 0.040* |
| History of angina | 1.253 (0.883 to 1.778) | 0.207 | 1.551 (0.972 to 2.475) | 0.066 |
| History of revascularisation | 0.808 (0.475 to 1.373) | 0.429 | 0.791 (0.398 to 1.573) | 0.505 |
| SBP (mm Hg) | ||||
| ≤100 | 3.224 (2.274 to 4.572) | <0.001* | 0.963 (0.558 to 1.661) | 0.892 |
| >100 | 1 (ref) | 1 (ref) | ||
| HR (beat/min) | ||||
| ≤100 | 1 (ref) | 1 (ref) | ||
| >100 | 5.084 (3.496 to 7.393) | <0.001* | 1.859 (1.08 to 3.202) | 0.025* |
| Shock Index† | ||||
| ≤0.70 | 1 (ref) | 1 (ref) | ||
| 0.71–1.00 | 2.859 (1.878 to 4.350) | <0.001* | 2.189 (1.341 to 3.572) | 0.002* |
| >1.00 | 15.293 (9.465 to 24.712) | <0.001* | 4.033 (1.844 to 8.82) | <0.001* |
| ACS | ||||
| STEMI | 1.568 (1.093 to 2.250) | 0.015* | 1.367 (0.879 to 2.125) | 0.166 |
| NSTEMI/UAP | 1 (ref) | 1 (ref) | ||
| Killip class | ||||
| I | 1 (ref) | 1 (ref) | ||
| II | 2.172 (1.307 to 3.610) | 0.003* | 1.244 (0.711 to 2.176) | 0.445 |
| III | 9.130 (4.417 to 18.872) | <0.001* | 4.768 (2.07 to 10.98) | <0.001* |
| IV | 14.323 (9.257 to 22.164) | <0.001* | 6.859 (3.933 to 11.963) | <0.001* |
*Significant p value
**p<0.05, **p<0.01, ***p<0.001.
†Shock Index is calculated by heart rate divided by systolic blood pressure.
AOR, adjusted OR; BMI, body mass index; CAD, coronary artery disease; HR, heart rate; NSTEMI, non-ST segment elevation myocardial infarction; SBP, systolic blood pressure; STEMI, ST-elevation myocardial infarction; UAP, unstable angina pectoris.
Several independent predictors that were calculated as a new scoring system were elaborated in table 3. The scoring result is based on regression coefficient (B) from multivariate logistic regression with a minimum score of 0 and the maximum score of +20. It showed that the lowest score is Killip II with 1 point, history of cerebrovascular disease and HR got 2 points, SI 0.7–1.0 got 3 points, age 65–75, SI>1.00 and Killip III got 4 points, age>75 years got 5 points and the highest score is Killip IV with 7 points.
Table 3.
Assessment of the score value from each independent predictor factor
| Variable | B | SE | B/SE | Score | |
| Age (years) | |||||
| <65 (ref) | |||||
| 65–75 | 0.923 | 0.232 | 3.98 | 3.92 | 4 |
| >75 | 1.458 | 0.290 | 5.03 | 4.96 | 5 |
| History of cerebrovascular disease | 0.703 | 0.321 | 2.19 | 2.16 | 2 |
| Heart rate (beat per minute) | |||||
| ≤100 (ref) | |||||
| >100 | 0.623 | 0.266 | 2.34 | 2.31 | 2 |
| Shock Index* | |||||
| ≤0.70 (ref) | |||||
| 0.71–1.00 | 0.767 | 0.238 | 3.22 | 3.18 | 3 |
| >1.00 | 1.392 | 0.345 | 4.03 | 3.98 | 4 |
| Killip class | |||||
| I (ref) | |||||
| II | 0.284 | 0.280 | 1.01 | 1.00 | 1 |
| III | 1.718 | 0.415 | 4.14 | 4.08 | 4 |
| IV | 1.913 | 0.265 | 7.22 | 7.12 | 7 |
*Shock Index is calculated by heart rate divided by systolic blood pressure.
Table 4 shows the scoring model based on the probability of mortality events—the greater the score, the higher chance of mortality, which indicates a dose–response related. ROC analysis revealed that with a cut-off of 5, the AUC, sensitivity and specificity of this scoring system in predicting in-hospital mortality were 0.842, 82.78% and 72.35%, respectively, (table 5 and figure 1).
Table 4.
Scoring system based on patient’s probability on mortality event
| Scoring | Probability (%) |
| 0 | 2.2 |
| 1 | 3.0 |
| 2 | 3.9 |
| 3 | 5.2 |
| 4 | 6.8 |
| 5 | 9.0 |
| 6 | 11.7 |
| 7 | 15.1 |
| 8 | 19.2 |
| 9 | 24.2 |
| 10 | 30.0 |
| 11 | 36.5 |
| 12 | 43.6 |
| 13 | 50.9 |
| 14 | 58.2 |
| 15 | 65.1 |
| 16 | 71.5 |
| 17 | 77.1 |
| 18 | 81.8 |
| 19 | 85.8 |
| 20 | 89.0 |
Table 5.
Receiver operating characteristics (ROC) analysis as mortality scoring system model
| Variable | AUC (95% CI) | P value | Cut-off value | Diagnostic value |
| Score | 0.842 (0.821 to 0.861) | <0.001 | ≥5 | Sensitivity: 82.78% |
| Specificity: 72.35% | ||||
| PPV: 27.2% | ||||
| NPV: 97.1% | ||||
| LR+: 2.99 | ||||
| LR−: 0.24 |
NPV, negative predictive value; AUC, area under curve; LR, likelihood ratio; PPV, positive predictive value.
Figure 1.
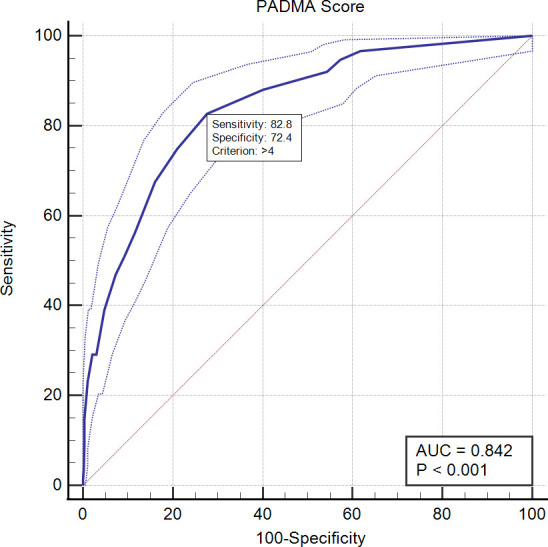
Receiver operating characteristics curve score to predict mortality. AUC, area under the curve; PADMA, PADjadjaran Mortality in Acute coronary syndrome.
Furthermore, we performed the Granger model to predict the incidence of mortality events based on three risk categories of PADMA Score (low risk: 0, moderate risk: 1–4 and high risk: 5–20). This model showed that low, intermediate and high risk yielded a probability of death of<3.0%, 3.0–6.8% and>6.8%, respectively, and an accumulated patient of 457 (33.6%), 463 (34%) and 440 (32.4%), respectively, (table 6).
Table 6.
PADMA scoring system based on patient’s probability on mortality event
| Risk category (tertiles) | PADMA Score | Probability of death |
| Low | 0 | <3.0 |
| Intermediate | 1–4 | 3.0–6.8 |
| High | 5–20 | >6.8 |
PADMA, PADjadjaran Mortality in Acute coronary syndrome.
We compared the predictive value of the PADMA Score with the GRACE, C-ACS and ProACS scores using the Fisher Z test. The AUC value between PADMA and GRACE risk scores was not statistically significant for predicting in-hospital mortality (p=0.126). Furthermore, it showed that the AUC value of the PADMA Score was significantly higher in comparison to C-ACS (p=0.002) and ProACS risk scores (p<0.001) (table 7 and figure 2).
Table 7.
ROC analysis result between GRACE and PADMA scoring system based on mortality
| Variable | AUC (95% CI) | AUC difference (95% CI) | P value |
| PADMA Score | 0.842 (0.821 to 0.861) | Ref | |
| GRACE Score | 0.863 (0.843 to 0.881) | 0.021 (−0.006 to 0.048) | 0.126 |
| C-ACS | 0.798 (0.775 to 0.819) | 0.042 (0.017 to 0.071) | 0.002* |
| ProACS | 0.760 (0.737 to 0.783) | 0.082 (0.049 to 0.115) | <0.001* |
*Significant p value
AUC, area under curve; C-ACS, Canada Acute Coronary Syndrome; GRACE, Global Registry of Acute Coronary Events; PADMA, PADjadjaran Mortality in Acute coronary syndrome; ProACS, The Portuguese Registry of Acute Coronary Syndromes; ROC, receiver operating characteristics.
Figure 2.
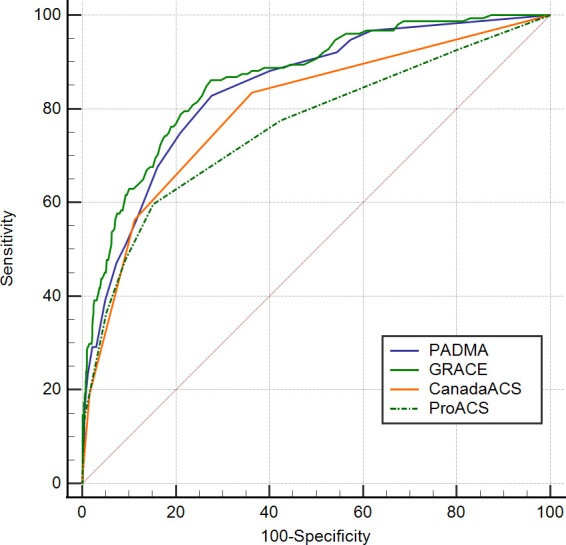
Comparison of AUC of PADMA Score with GRACE, C-ACS and ProACS risk scores to predict in-hospital mortality. AUC, area under curve; C-ACS, Canada Acute Coronary Syndrome; GRACE, Global Registry of Acute Coronary Events; PADMA, PADjadjaran Mortality in Acute coronary syndrome; ProACS, The Portuguese Registry of Acute Coronary Syndromes.
Discussion
The major findings of this cohort study are as follows. First, the components of the PADMA Score were age, history of cerebrovascular disease, HR, SI and Killip class. Second, the association between PADMA scores and in-hospital mortality in ACS patients showed that every increment of one score presented a higher mortality risk. PADMA Score was classified into three risk stratification: low, intermediate and high, with the probability of in-hospital mortality being <3%, 3%–6.8% and>6.8%, respectively. Third, using the cut-off of 5, a high PADMA Score substantially increased the risk of in-hospital mortality with AUC, sensitivity and specificity were 0.842, 82.78%, and 72.35%, respectively. Fourth, the PADMA Score’s predictive value was comparable to the GRACE Score and superior to the C-ACS and ProACS scores in predicting the risk of in-hospital mortality in ACS patients. Thus, we recommended using the PADMA Score as a simple prognosticator of in-hospital mortality in ACS patients.
There are two similar scoring systems to ours that only require a clinical examination to complete the calculation: C-ACS and ProACS risk scores. The C-ACS and ProACS were initiated in Canada and Portuguese, respectively.9 10 In this study, the PADMA Score was superior in predicting in-hospital mortality compared with C-ACS and ProACS scores based on the AUC values. According to the C-ACS and ProACS development cohorts, these two scoring systems had significantly lower AUC values than the GRACE risk Score.9 10 Otherwise, the predictive value of the PADMA Score was shown to be comparable with the GRACE Score, indicating that the PADMA Score can be useful as an alternative scoring system to the GRACE risk Score. However, there are several differences in baseline characteristics between this study and other cohorts. In contrast to GRACE, C-ACS and ProACS cohorts, patients in our study were younger, had fewer females and incidences of diabetes mellitus and history of angina, but had higher smokers, STEMI, and Killip class IV presentation.11–13 In addition, our patients had lower SBP and BMI levels than other cohorts. Moreover, as opposed to the ProACS development cohort, the revascularisation procedures in this study were higher.10 The in-hospital mortality rate in GRACE, C-ACS and ProACS cohorts were 4%, 5.2% and 5.4%, respectively, in which these numbers are approximately half of the mortality rate in our study.9 10 13 The possible explanation for this phenomenon is that compared with other cohorts, our study had a higher number of STEMI patients and Killip class IV presentation, thereby significantly increasing the risk of in-hospital mortality.
Generally, the PADMA Score encompasses two main elements, including demographic and haemodynamic status at admission. Demographic status is represented by age and history of cerebrovascular disease. Whereas HR, SI and Killip class reflect haemodynamic status. Every component of this scoring system was statistically significant and independent in increasing mortality in ACS patients and relatively reasonable to be included, given that every component had its mechanism related to mortality.
According to the current study, older age was one of the independent predictors of mortality, with age 65–75 and >75 years had 2.5 times and 4 times, respectively, higher mortality risk compared with those with age<65 years. Consistently, previous two cohort studies stated that old patients (>65 years and >75 years) significantly increased the risk of mortality in ACS patients.14 15 Furthermore, there are several explanations for why old ACS patients tend to present with high mortality risk. First, older patients are more likely to have comorbidities such as kidney disorders and hypertension.16 Second, mostly old patients who were finally diagnosed with ACS were presented with atypical symptoms at admission, often getting delayed diagnosis and treatment. Third, since old patients have a higher risk of bleeding, several old patients are not tolerable to undergo revascularisation procedures according to guidelines.17
This study showed that a history of cerebrovascular disease had a significant effect on mortality in ACS patients, which this variable was not used in GRACE or TIMI scores.18 19 Consistent with our findings, the association between cerebrovascular disease in the ACS population and mortality has been revealed by Mukherjee et al.20 Furthermore, patients with the cerebrovascular disease were less likely to be treated invasively or had limited medical drugs.21 Additionally, when coronary angiography was performed, patients with prior cerebrovascular disease often had multivessel disease, which increased the risk of death.22
Another novel and interesting finding in our study, we include the SI as a parameter in this study. The SI comprises two main components: HR and SBP. Originally, this index was used in patients who experienced hypovolemic and haemorrhagic shock.23 Nonetheless, several studies proved that high SI was significantly correlated with higher mortality risk in ACS populations.24–29 In this study, we divided SI into three categories, including <0.70, 0.71–1.00 and>1.00. This study showed that those with moderate (0.71–1.00) and high SI (>1.00) significantly and independently increased the risk of mortality by two times and four times higher as opposed to patients with low SI (<0.7). This finding is aligned with several cohort studies results, which revealed that SI higher than 0.7 and 1 significantly elevated the risk of mortality in ACS patients.24–29
In this study, high HR (>100) at admission was associated with increased mortality by approximately two times higher in contrast to those with low HR (<100). Consistently, previous studies supporting this result revealed that high admission HR was independently linked to mortality in ACS patients.9 15 30–32 It is widely known that sympathetic hyperactivity caused by a catecholamine surge in ACS patients can increase the HR. A study by Petterson et al found that high catecholamine levels in ACS patients were correlated with low left ventricular ejection fraction (LVEF).33 Moreover, sympathetic stimulation in ACS patients can cause ischemia-induced ventricular fibrillation and lead to sudden cardiac death.34 In addition, Yuksek et al stated that ACS patients who presented with elevated HR had higher serum troponin, glucose levels, lower LVEF and were more likely to have acute heart failure.35
An observational study conducted by Shlomai et al stated that admission SBP in ACS patients had a predictive value on mortality, as those with low SBP had a significant mortality risk compared with those with normal SBP.36 On the contrary, our study demonstrated that blood pressure less than 100 mm Hg was not significantly increased the risk of mortality in multivariate analysis. However, when SBP is included in the calculation of SI, the significance was numerically obtained. Theoretically, low SBP reflects low mean arterial pressure, as patients with low SBP, can lead to end-organ hypoperfusion and death.37 38 Thus, it concludes that ACS patients with high HR and low SBP indicated by high SI were associated with reduced LV function, increased arrhythmia risk and induced end-organ damage, leading to elevated mortality risk.
Our last PADMA Score component is the Killip class which is the standard classification to predict mortality in myocardial infarction. The higher Killip class was linked with a higher risk of in-hospital mortality.39 Persistently, the current study revealed that Killip class III and IV were independently associated with higher mortality risk. Basically, the Killip class is determined by pre-existing heart failure conditions (acute or chronic) and cardiogenic shock status. Heart failure markedly increased mortality risk in ACS patients, as it reduced the cardiac output, diminished the oxygen perfusion into the lung circulatory and induced renin–angiotensin–aldosterone system activation.40 On the other hand, cardiogenic shock, as an indicator of end-organ hypoperfusion due to cardiac dysfunction, significantly increased the risk of mortality in ACS patients.41 42
This study has several limitations. First, this study had a relatively small sample size and was only conducted in a single centre. Second, according to its retrospective design, it can lead to recall and selection bias. Third, due to the high amount of loss to follow-up, this scoring is limited to predicting only in-hospital mortality. Last, to better evaluate the association between PADMA Score and mortality in ACS patients, this scoring system still needs further validation by a multicentre cohort study with numerous participants and a longer duration of follow-up.
Conclusion
In conclusion, this study proudly presented the new scoring system to predict in-hospital mortality in ACS patients, PADMA Score, with several components including age, history of cerebrovascular disease, HR, SI and Killip class. This study revealed that the association between PADMA Score and in-hospital mortality was dose–response related. Moreover, this scoring system showed a good predictive value for predicting in-hospital mortality, which was comparable to the GRACE Score and superior to C-ACS and ProACS risk scores. Therefore, we highly suggest using the PADMA Score as a convenient scoring system for all ACS patients in an emergency setting to predict the risk of in-hospital mortality.
Footnotes
MP: conceptualisation, methodology, investigation, writing—original draft, writing—review & editing. TLB: conceptualisation, methodology, data curation, formal analysis, writing—original draft. AFY: methodology, data curation, formal analysis, writing—original draft. ICSP: methodology, formal analysis, investigation, writing—original draft. MP accepts full responsibility for the work, conduct of the study, had access to the data, and controlled the decision to publish. All co-authors contributed to the production of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. This study was approved by the medical research ethics committee of Dr. Hasan Sadikin Central General Hospital, West Java, Indonesia. (ID: 25/UN6.C.5.14/PT/2022). Participants gave informed consent to participate in the study before taking part.
References
- 1.World Health Organization . Cardiovascular diseases (CVDs) fact sheet, 2021. Available: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 2.Sanchis-Gomar F, Perez-Quilis C, Leischik R, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016;4:256. 10.21037/atm.2016.06.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Littnerova S, Kala P, Jarkovsky J, et al. Grace score among six risk scoring systems (CADILLAC, PAMI, TIMI, dynamic TIMI, Zwolle) demonstrated the best predictive value for prediction of long-term mortality in patients with ST-elevation myocardial infarction. PLoS One 2015;10:e0123215. 10.1371/journal.pone.0123215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collet J-P, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–367. 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 5.D'Ascenzo F, Biondi-Zoccai G, Moretti C, et al. Timi, grace and alternative risk scores in acute coronary syndromes: a meta-analysis of 40 derivation studies on 216,552 patients and of 42 validation studies on 31,625 patients. Contemp Clin Trials 2012;33:507–14. 10.1016/j.cct.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 6.Li C-Y, Chang C-J, Chung W-J, et al. Assessment of CHA2DS2-VASc score for predicting cardiovascular and cerebrovascular outcomes in acute myocardial infarction patients. Medicine 2018;97:e11230. 10.1097/MD.0000000000011230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng H, Sun Z, Chen H, et al. Usefulness of the CHA2DS2-VASc Score to Predict Adverse Outcomes in Acute Coronary Syndrome Patients Without Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. Am J Cardiol 2019;124:476–84. 10.1016/j.amjcard.2019.05.036 [DOI] [PubMed] [Google Scholar]
- 8.Kim KH, Kim W, Hwang SH, et al. The CHA2DS2VASc score can be used to stratify the prognosis of acute myocardial infarction patients irrespective of presence of atrial fibrillation. J Cardiol 2015;65:121–7. 10.1016/j.jjcc.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 9.Huynh T, Kouz S, Yan AT, et al. Canada acute coronary syndrome risk score: a new risk score for early prognostication in acute coronary syndromes. Am Heart J 2013;166:58–63. 10.1016/j.ahj.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 10.Timóteo AT, Aguiar Rosa S, Afonso Nogueira M, et al. ProACS risk score: an early and simple score for risk stratification of patients with acute coronary syndromes. Rev Port Cardiol 2017;36:77–83. 10.1016/j.repc.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 11.Fox KAA, Fitzgerald G, Puymirat E, et al. Should patients with acute coronary disease be stratified for management according to their risk? derivation, external validation and outcomes using the updated grace risk score. BMJ Open 2014;4:e004425. 10.1136/bmjopen-2013-004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elbarouni B, Goodman SG, Yan RT, et al. Validation of the global registry of acute coronary event (grace) risk score for in-hospital mortality in patients with acute coronary syndrome in Canada. Am Heart J 2009;158:392–9. 10.1016/j.ahj.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 13.Fox KAA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (grace). BMJ 2006;333:1091. 10.1136/bmj.38985.646481.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang HY, Ahn MJ, Jeong MH, et al. Predictors of in-hospital mortality in Korean patients with acute myocardial infarction. Chonnam Med J 2019;55:40–6. 10.4068/cmj.2019.55.1.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pogorevici A, Citu IM, Bordejevic DA, et al. Canada acute coronary syndrome score was a stronger baseline predictor than age ≥75 years of in-hospital mortality in acute coronary syndrome patients in Western Romania. Clin Interv Aging 2016;11:481. 10.2147/CIA.S104943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fillenbaum GG, Pieper CF, Cohen HJ, et al. Comorbidity of five chronic health conditions in elderly community residents: determinants and impact on mortality. J Gerontol A Biol Sci Med Sci 2000;55:84–9. 10.1093/gerona/55.2.m84 [DOI] [PubMed] [Google Scholar]
- 17.Avezum A, Makdisse M, Spencer F, et al. Impact of age on management and outcome of acute coronary syndrome: observations from the global registry of acute coronary events (grace). Am Heart J 2005;149:67–73. 10.1016/j.ahj.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 18.Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003;163:2345–53. 10.1001/archinte.163.19.2345 [DOI] [PubMed] [Google Scholar]
- 19.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284:835–42. 10.1001/jama.284.7.835 [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee D, Eagle KA, Kline-Rogers E, et al. Impact of prior peripheral arterial disease and stroke on outcomes of acute coronary syndromes and effect of evidence-based therapies (from the global registry of acute coronary events). Am J Cardiol 2007;100:1–6. 10.1016/j.amjcard.2007.02.046 [DOI] [PubMed] [Google Scholar]
- 21.Vagnarelli F, Corsini A, Lorenzini M, et al. Long-Term prognostic role of cerebrovascular disease and peripheral arterial disease across the spectrum of acute coronary syndromes. Atherosclerosis 2016;245:43–9. 10.1016/j.atherosclerosis.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 22.Cotter G, Cannon CP, McCabe CH, et al. Prior peripheral arterial disease and cerebrovascular disease are independent predictors of adverse outcome in patients with acute coronary syndromes: are we doing enough? results from the Orbofiban in patients with unstable coronary Syndromes-Thrombolysis in myocardial infarction (OPUS-TIMI) 16 study. Am Heart J 2003;145:622–7. 10.1067/mhj.2003.6 [DOI] [PubMed] [Google Scholar]
- 23.Mutschler M, Nienaber U, Münzberg M, et al. The Shock Index revisited - a fast guide to transfusion requirement? A retrospective analysis on 21,853 patients derived from the TraumaRegister DGU. Crit Care 2013;17:R172. 10.1186/cc12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chunawala ZS, Hall ME, Arora S, et al. Prognostic value of shock index in patients admitted with non-ST-segment elevation myocardial infarction: the ARIC study community surveillance. Eur Heart J Acute Cardiovasc Care 2021;10:869–77. 10.1093/ehjacc/zuab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemradj VV, Ottervanger JP, de Boer MJ, et al. Shock index more sensitive than cardiogenic shock in ST-elevation myocardial infarction treated by primary percutaneous coronary intervention. Circ J 2017;81:199–205. 10.1253/circj.CJ-16-0616 [DOI] [PubMed] [Google Scholar]
- 26.Huang B, Yang Y, Zhu J, et al. Usefulness of the admission shock index for predicting short-term outcomes in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2014;114:1315–21. 10.1016/j.amjcard.2014.07.062 [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi A, Misumida N, Luger D, et al. Shock index as a predictor for in-hospital mortality in patients with non-ST-segment elevation myocardial infarction. Cardiovasc Revasc Med 2016;17:225–8. 10.1016/j.carrev.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 28.Shangguan Q, Xu J-song, Su H, et al. Modified shock index is a predictor for 7-day outcomes in patients with STEMI. Am J Emerg Med 2015;33:1072–5. 10.1016/j.ajem.2015.04.066 [DOI] [PubMed] [Google Scholar]
- 29.Spyridopoulos I, Noman A, Ahmed JM, et al. Shock-index as a novel predictor of long-term outcome following primary percutaneous coronary intervention. Eur Heart J 2015;4:270–7. 10.1177/2048872614561480 [DOI] [PubMed] [Google Scholar]
- 30.Jensen MT, Pereira M, Araujo C, et al. Heart rate at admission is a predictor of in-hospital mortality in patients with acute coronary syndromes: results from 58 European hospitals: the European Hospital benchmarking by outcomes in acute coronary syndrome processes study. Eur Heart J Acute Cardiovasc Care 2018;7:149–57. 10.1177/2048872616672077 [DOI] [PubMed] [Google Scholar]
- 31.Honda T, Kanazawa H, Koga H, et al. Heart rate on admission is an independent risk factor for poor cardiac function and in-hospital death after acute myocardial infarction. J Cardiol 2010;56:197–203. 10.1016/j.jjcc.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 32.Bangalore S, Messerli FH, Ou F-S, et al. The association of admission heart rate and in-hospital cardiovascular events in patients with non-ST-segment elevation acute coronary syndromes: results from 135 164 patients in the crusade quality improvement initiative. Eur Heart J 2010;31:552–60. 10.1093/eurheartj/ehp397 [DOI] [PubMed] [Google Scholar]
- 33.Petersen CL, Nielsen JR, Petersen BL, et al. Catecholaminergic activation in acute myocardial infarction: time course and relation to left ventricular performance. Cardiology 2003;100:23–8. 10.1159/000072388 [DOI] [PubMed] [Google Scholar]
- 34.Kolettis TM. Coronary artery disease and ventricular tachyarrhythmia: pathophysiology and treatment. Curr Opin Pharmacol 2013;13:210–7. 10.1016/j.coph.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 35.Yuksek U, Cerit L, Yaman B, et al. Increased discharge heart rate might be associated with increased short-term mortality after acute coronary syndrome. Acta Cardiol 2021;7:1–7. 10.1080/00015385.2021.1979785 [DOI] [PubMed] [Google Scholar]
- 36.Shlomai G, Kopel E, Goldenberg I, et al. The association between elevated admission systolic blood pressure in patients with acute coronary syndrome and favorable early and late outcomes. J Am Soc Hypertens 2015;9:97–103. 10.1016/j.jash.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 37.DeMers DWD. Physiology, Mean Arterial Pressure. In: StatPearls. StatPearls publishing, 2022. http://www.ncbi.nlm.nih.gov/books/NBK538226/ [PubMed] [Google Scholar]
- 38.Vahdatpour C, Collins D, Goldberg S. Cardiogenic shock. J Am Heart Assoc 2019;8:e011991. 10.1161/JAHA.119.011991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol 1967;20:457–64. 10.1016/0002-9149(67)90023-9 [DOI] [PubMed] [Google Scholar]
- 40.Harjola V-P, Parissis J, Bauersachs J, et al. Acute coronary syndromes and acute heart failure: a diagnostic dilemma and high-risk combination. A statement from the acute heart failure Committee of the heart failure association of the European Society of cardiology. Eur J Heart Fail 2020;22:1298–314. 10.1002/ejhf.1831 [DOI] [PubMed] [Google Scholar]
- 41.Supeł K, Kacprzak M, Zielińska M. Shock index and TIMI risk index as valuable prognostic tools in patients with acute coronary syndrome complicated by cardiogenic shock. PLoS One 2020;15:e0227374. 10.1371/journal.pone.0227374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kosaraju A, Pendela VS, Hai O. Cardiogenic Shock. In: StatPearls [Internet]. StatPearls Publishing, 2020. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.


