Abstract
Background
P311, a highly conserved 8 kDa intracellular protein, has recently been reported to play an important role in aggravating hypertrophic scaring by promoting the differentiation and secretion of fibroblasts. Nevertheless, how P311 regulates the differentiation and function of fibroblasts to affect granulation tissue formation remains unclear. In this work, we studied the underlying mechanisms via which P311 affects fibroblasts and promotes acute skin wound repair.
Methods
To explore the role of P311, both in vitro and in vivo wound-healing models were used. Full-thickness skin excisional wounds were made in wild-type and P311−/− C57 adult mice. Wound healing rate, re-epithelialization, granulation tissue formation and collagen deposition were measured at days 3, 6 and 9 after skin injury. The biological phenotypes of fibroblasts, the expression of target proteins and relevant signaling pathways were examined both in vitro and in vivo.
Results
P311 could promote the proliferation and differentiation of fibroblasts, enhance the ability of myofibroblasts to secrete extracellular matrix and promote cell contraction, and then facilitate the formation of granulation tissue and eventually accelerate skin wound closure. Importantly, we discovered that P311 acts via up-regulating the expression of type II transforming growth factor-β receptor (TGF-βRII) in fibroblasts and promoting the activation of the TGF-βRII-Smad signaling pathway. Mechanistically, the mammalian target of rapamycin signaling pathway is closely implicated in the regulation of the TGF-βRII-Smad pathway in fibroblasts mediated by P311.
Conclusions
P311 plays a critical role in activation of the TGF-βRII-Smad pathway to promote fibroblast proliferation and differentiation as well as granulation tissue formation in the process of skin wound repair.
Keywords: P311, Wound healing, Fibroblasts, TGF-βRII-Smad pathway, Re-epithelialization, Granulation tissue formation, Collagen deposition
Highlights.
We demonstrated that P311 could promote the proliferation and differentiation of fibroblasts, enhance the ability of myofibroblasts to secrete extracellular matrix and promote cell contraction, and then facilitate the formation of granulation tissue and eventually accelerate skin wound closure.
P311 promotes TGFβRII-mediated fibroblast activation and granulation tissue formation in wound healing.
We verify that mammalian target of rapamycin plays a critical role in the regulation of P311-mediated TGFβRII expression in fibroblasts.
Background
In-depth understanding of the regulatory factors underlying the skin wound healing process is the basis for developing effective clinical treatments for wound healing and reducing the occurrence of refractory wounds [1]. As a key component of granulation tissue, the migration and proliferation of fibroblasts and subsequently their differentiation into myofibroblasts are important cellular biological processes for the formation and development of granulation tissue [2]. Under the stress conditions of wound, through secreting a large amount of extracellular matrix and their own strong contraction ability, myofibroblasts play a pivotal role in promoting the formation of granulation tissue and ultimately effective wound healing [3,4]. Mechanistically, the transforming growth factor-β–type II transforming growth factor-β receptor–Smad (TGF-β-TGF-βRII-Smad) signaling pathway plays critical roles in controlling fibroblast differentiation and function, such as contractile ability and collagen secretion [5].
Among the TGF-β superfamily, TGF-β1 is probably the most abundant isoform elevated in the context of tissue damage [6]. TGF-β1 initially binds to its receptor type II (RII), which assembles and then phosphorylates receptor type I (RI) to activate down-stream Smads [7]. Thus, TGF-βRII, as a key subunit of the TGF-βR complex, plays a crucial role in regulating the differentiation of fibroblasts into myofibroblasts [8].
P311, first reported by Studler et al [9], is a highly conserved 8 kDa intracellular protein. P311 contains a proline, glutamic acid, serine, and threonine-rich (PEST) domain that is responsible for its rapid degradation by the ubiquitin–proteasome system, as well as by an unknown metalloprotease, resulting in a half-life of ~5 min [9]. P311 plays a role in nerve and lung regeneration, blood pressure homeostasis and myofibroblast differentiation [10]. Mice with P311 deletion showed no remarkable phenotypes in mice sensory and motor function, while there were significant alterations in behavioral responses in learning and memory [11]. Our previous studies, using gene expression profiling and comparative proteomic analysis, showed that P311 expression is significantly elevated in human hypertrophic scars [12], suggesting that P311 is closely implicated in regulation of the differentiation and function of fibroblasts to affect skin wound healing. It is known that P311 requires a binding partner to acquire tertiary structure and function [13]. Previous studies have shown that eukaryotic translation initiation factor 3 subunit b (eIF3b), a subunit of the multiple translation initiation factor eIF3, is a direct binding partner of P311 [13]. P311 binds to eIF3b and the 5′ non-translational region of TGF-β1, TGF-β2 and TGF-β3 mRNAs concomitantly [14]. By directly promoting the enrichment of mRNAs in the translation machinery, P311 enhances TGF-β protein levels [15]. However, the underlying mechanisms by which P311 regulates the differentiation and function of fibroblasts and subsequently affects granulation tissue formation and skin wound healing remains largely unknown.
In this study, we demonstrated that P311 promotes the differentiation and function of fibroblasts, facilitates the formation of granulation tissue and eventually accelerates skin wound closure. Mechanistically, P311 is capable of elevating the expression of TGF-βRII in fibroblasts, resulting in activation of the TGF-βRII-Smad signaling pathway, a process that occurs via activation of mammalian target of rapamycin (mTOR).
Methods
Mice and wound-healing model
All experimental animal protocols were accepted by the Third Military Medical University’s Animal Experimental Ethics Committees and carried out according to the Third Military Medical University guidelines. P311 knockout mice (P311−/−) (a gift from Prof. Gregory, Department of Medicine, Duke University, USA) and C57 mice were raised by the Institute of Experimental Animal Research, Army Medical University. Three batches of P311−/− and C57 adult mice and newborn mice ≤3 days old were used for subsequent experiments. According to the previous program [16], full-thickness skin excisional wounds were incised dorsally with a sterile circular skin biopsy punch (6 mm in diameter). The size of wounds were recorded using IPP 6.0 software.
Immunohistochemistry, immunofluorescence and hematoxylin and eosin staining analysis
As in our previous research [17], measurement of expression of the target protein was conducted by immunohistochemistry (IHC) and immunofluorescence (IF). The primary antibodies were: anti-vimentin (1:200, abcam, ab92547)), anti-TGF-β1 (1:200, abcam, ab229856), anti-alpha smooth muscle actin (α-SMA) (1:200, abcam, ab7817), anti-TGF-βRII (1:200, abcam, ab186838), anti-collagen III (1:200, abcam, ab6320) and anti-collagen I (1:200, abcam, ab 138 492). The sections were stained with hematoxylin and eosin (H&E).
Photomicrographs
Fluorescent images were captured by a spinning disk confocal super-resolution microscope (Olympus SpinSR). The IHC and H&E images were scanned by a slide scanner (Olympus Slideview VS200). All images were analyzed with Olympus confocal software.
Masson, Victoria blue and reticular staining
According to the normal operating conditions and using procedures from previous research [16,18], Masson’s trichrome blue (Solarbio, G1340), Victoria blue (Solarbio, G1596) and modified Gomori (Solarbio, G3535) were used to stain tissue sections (Solarbio, G1340).
Isolation and identification of fibroblasts
Mice fibroblasts were isolated and identified from newborn (day 0–3) mice skin, as previously reported [19]. Identification of the fibroblasts was achieved by flow cytometry, stained with the Alexa Fluor® 488 anti-vimentin antibody (Abcam, Cambridge, UK). Adenovirus with P311 and tag (called fibroblastsad-P311) or control vehicle (named fibroblastsad-NC) was used to infect the spread fibroblasts. The efficiency of infection was calculated by microscope and FCAS image of the fluorescent signal. P311 expression was assessed by real-time polymerase chain reaction (rtPCR).
Collagen contraction assay
Collagen contraction by fibroblasts was measured by using a contraction test. Firstly, fibroblasts (1 × 104 cells/ml) were combined with 1.5 mg/ml neutralized rat tail collagen solution (2 mg/ml collagen solution and dulbecco’s modified eagle medium (DMEM) medium in a 3:1 ratio). In 12-well cell culture plates, 0.5 ml of fibroblast-containing collagen solution was added and polymerized for 30 min at 37°C. The gels were then treated with 0.5 ml of serum-free cell culture medium for 12 h. The medium was removed and reconstituted with DMEM cell culture medium with 10% serum or TGF-β1 (10 ng/ml). Following 3 days of exposure to these conditions, gel contraction was measured.
Analysis of hydroxyproline content of wound skin
A hydroxyproline assay kit was used to determine the hydroxyproline level of wound skin (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The procedures were carried out in accordance with the approved guidelines.
Fibroblast proliferation analysis
Following the manufacturer’s recommendations, cell proliferation was measured by using a CCK8 cell counting kit (Dojindo, Japan). 5-Ethynyl-2′ –deoxyuridine (EdU) [20] staining was performed with an EDU kit (Beyotime, China), In brief, the cells were fixed, permeabilized, blocked and stained with a DAPI solution containing an anti-fluorescence quencher (Beyotime, China). The fluorescence signal of fibroblasts was measured under the microscope.
Transwell and scratch wound migration assays in vitro
Transwell chambers (24-well insert, 8 μm, Corning, NY, USA) were used to test cell migration ability. In the upper compartment, 4 × 104 transfected cells were planted with 300 μl of serum-free medium. In the lower chamber, 600 μl of DMEM containing 10% FBS was added. After 24 h of incubation, the cells that had gone through the bottom membrane were stained with a 10% crystal violet solution (Sigma, NY, USA). Under the microscope, the migrating cells were counted. For scratch wound migration, an artificial wound was generated in monolayer cells using a 10 μl plastic pipette tip. At 0 and 18 h, the wound closure was photographed under a microscope. Every experiment was repeated at least three times.
RNA extraction and rtPCR for gene expression
As in the protocol used in our previous research [17], RNA was extracted with Trizol (Invitrogen) and rtPCR was carried out. cDNAs were synthesized following DNase digestion (Takara). The diluted cDNAs (1:100) were then used for rtPCR with SYBR green (Takara). The relative gene expression was calculated using 2−ΔCt = 2−(target Ct−reference Ct). The primer sequences are available in Supplementary Table S1, see online supplementary material.
Western blot analysis
Western blot analysis was carried out as described previously [21, 22]. The antibodies in this study were as follows: anti-vimentin (1:1000, abcam, ab92547), anti-α-SMA (1:1000, abcam, ab7817), anti-TGF-βRII (1:1000, abcam, ab186838), anti-collagen I (1:1000, abcam, 138 492), anti-collagen III (1:1000, abcam, ab6320), anti-smad2 (1:1000, CST, #5339), anti-smad3 (1:1000, CST, #9523), anti-smad (phosphor) (1:1000, CST, #18338,), anti-smad3 (phosphor) (1:1000, CST, #9520), anti-mTOR (1:1000, CST, #2983), anti-akt (1:1000,CST, #4691), anti-PI3K (1:1000, CST, #4249), anti-p70 S6K (1:1000, CST, #9208), anti-mTOR (phosphor) (1:1000, CST, #5536), anti-akt (phosphor) (1:1000, CST, #4060), anti-PI3K (phosphor) (1:1000,CST, # 17366), anti-p70 S6K (phosphor) (1:1000, CST, #9204, anti-GAPDH (1:1000, CST, #5174).
Enzyme-linked immunosorbent assay
According to the manufacturer’s instructions, a Quantikine enzyme-linked immunosorbent assay (ELISA) kit (MB100B; R&D systems) was used to detect the total amount of collagen I and collagen III in the cell culture medium by using the fibroblast culture supernatant.
Statistical analysis
All values are reported as mean ± standard deviation (SD) unless otherwise stated. One-way or two-way analysis of variance with Bonferroni correction was used to assess statistical difference. The graphs were created by GraphPad Prism 8.0 software. The results are based on this analysis. Statistically significance was deemed as p < 0.05.
Results
P311 deficiency results in delayed skin wound repair
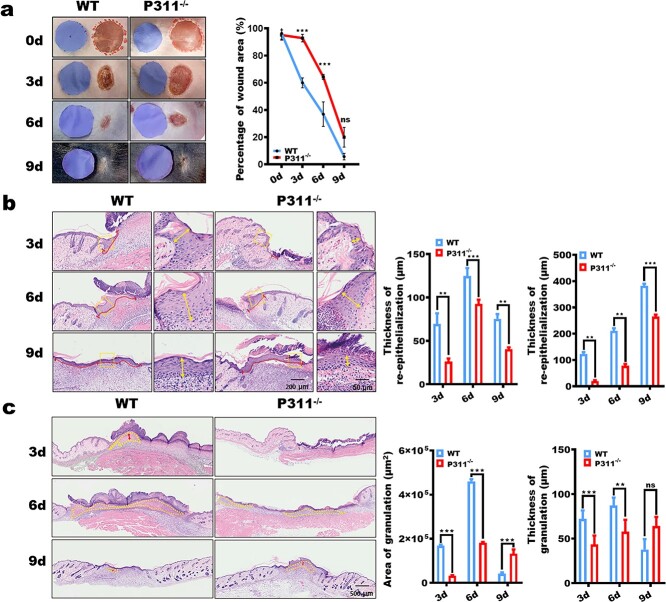
To precisely estimate the effects of P311 on wound healing, we utilized the P311 deletion mice and a murine full-thickness wound model. First, we found that P311−/− mice showed significantly delayed wound repair (Figure 1a) compared to the wild-type (WT) mice. Moreover, we demonstrated that P311 deficiency also affected the re-epithelialization process (Figure 1b) and granulation tissue formation (Figure 1c). Thus, our results demonstrated that retarded granulation tissue formation is one of the key mechanisms in delayed skin wound closure in P311−/−mice.
Figure 1.
P311 deficiency results in delayed skin wound repair. (a) Representative wound images (left panel) and quantification of wound area (right panel) in WT and P311−/− groups. (b) Wound re-epithelialization was examined utilizing hematoxylin and eosin (H&E) staining. Representative images (right panel) in WT and P311−/− groups and the length (red line) and thickness (yellow arrows) of the neo-epithelial tongue (left panel) are shown. Scale bar = 200, 50 μm. (c) Granulation tissue formation in wound tissue was examined utilizing H&E in WT and P311−/− groups staining. Representative images (right panel) and the area (yellow lines) and thickness (red arrows) of granulation tissues (right panel) are shown. Scale bar = 500 μm. Mean ± standard deviation, n = 3. (**p < 0.01; ***p < 0.001; ns not significant), WT wild type
P311 deficiency leads to reduced proliferation and motility of fibroblasts
Fibroblasts are the main components of granulation tissue. Here we first investigated the effects of P311 deficiency on the proliferation and motility of fibroblasts. As shown in Figure 2a, P311−/− mice had a 3-fold decrease in the number of vimentin-positive fibroblasts in granulation tissues when compared with the WT mice at day 6 post-injury, indicating that P311 deficiency might attenuate the recruitment and proliferation of fibroblasts. Then, we found that the protein and mRNA expression of vimentin in WT fibroblasts was significantly higher than in P311−/− fibroblasts. We also found that the protein and mRNA expression of vimentin was significantly higher in P311−/−ad-P311 fibroblasts compared to P311−/−ad-NC fibroblasts with TGF-β1 stimulation (Figure 2b–d).
Figure 2.

P311 deficiency leads to reduced proliferation and motility of fibroblasts. (a) Representative confocal image for vimentin-positive fibroblasts in wild type (WT) and P311−/− groups. The numbers of fibroblasts per view field were counted and analyzed, respectively. Scale bar = 50 μm. (b–c) Representative western blot (WB) and mRNA expression of vimentin in WT and P311−/− fibroblasts with or without transforming growth factor-β1 (TGF-β1) stimulation. (d–e) Representative WB and mRNA expression of vimentin in P311−/− fibroblasts with P311 encoding adenovirus transfection and P311−/− fibroblasts with empty adenovirus transfection and with or without TGF-β1 stimulation. (f, h) Cell proliferation of primary fibroblasts freshly isolated from WT and P311−/− mice skin was estimated by cell counting kit (CCK8) and 5-ethynyl-2′ -deoxyuridine (EdU) assays. (g, i) Cell motility of WT and P311−/− fibroblasts evaluated utilizing scratch and transwell assays. (j, l) Cell proliferation of P311−/−ad-NC (fibroblasts of P311−/− transfected with no-load adenovirus) and P311−/−ad-P311 fibroblasts (fibroblasts of P311−/− transfected with P311 adenovirus) was estimated by CCK8 and EdU assays. Scale bar = 200 µm. (k, m) Cell motility of P311−/−ad-NC and P311−/−ad-P311 fibroblasts was evaluated utilizing scratch and transwell assays. Scale bar = 200 µm. Mean ± standard deviation, n = 3. (*p < 0.05, **p < 0.01; ***p < 0.001; ns not significant)
Next, the proliferation rate of fibroblasts overall and at the single-cell level was examined using a cell-counting assay at optical density (OD) 450 nm and an EdU incorporation assay, respectively. As shown in Figure 2f, g, overall, P311−/− fibroblasts have ~2.7-fold significantly lower viability at 24 h compared with that of WT fibroblasts (Figure 2f). Similarly, at the single-cell level, 52% of WT fibroblasts displayed EdU-positive cells compared with ~37% of P311−/− fibroblasts (Figure 2g). Collectively, these results suggest that P311 deficiency can reduce fibroblast proliferation.
To further characterize the role of P311 in promoting cell mobility, we examined the effect of P311 on the mobility of fibroblasts by utilizing scratch migration and transwell invasion assays. Compared with WT fibroblasts, P311−/− fibroblasts displayed a 2-fold decrease in cell migration (Figure 2h). Likewise, the results of the transwell invasion assays also showed that P311−/− fibroblasts had a 3-fold reduction in cell invasion (Figure 2i).
Finally, we evaluated the proliferation and motility of P311−/− fibroblasts with or without P311 gene rescue. Supplementary Figure S1 (see online supplementary material) showed successful transfection of P311 adenovirus into mice fibroblasts. As expected, reconstitution of P311 in P311−/− fibroblasts let to significantly enhanced cell viability, proliferation, migration and invasion (Figure 2j–m, respectively) compared with P311−/− fibroblasts with empty adenovirus transfection. Taken together, these results suggest that P311 deficiency down-regulates the proliferation and motility of fibroblasts, which then contributes to retarded granulation tissue formation and wound healing.
P311 deficiency results in an impaired TGF-βRII-Smad signaling pathway in fibroblasts
The TGF-β1-TGF-βRII-Smad pathway is a core signaling transduction axis for regulating the differentiation of fibroblasts into myofibroblasts and the collagen secretion and contraction of myofibroblasts [5]. We found a marked reduction in TGF-β1 and TGF-βRII (Figure 3a and b, respectively) levels in wound granulation tissue of P311−/− mice as compared with that of WT animals at days 3 and 6 post-injury. Consistently, we observed a lower level of TGF-β-RII in P311−/− fibroblasts under basal and TGF-β1 stimulation conditions in comparison with the WT fibroblasts (Figure 3c, d). Moreover, the activation of Smad2/3 in P311−/− fibroblasts was significantly weakened (Figure 3e). These results thus suggest that P311 deficiency results in the downregulation of the TGF-βRII-Smad signaling pathway in fibroblasts.
Figure 3.
P311 deficiency leads to an impaired TGF-βRII-Smad signaling pathway in fibroblasts. (a, b) The expression of transforming growth factor-β1 (TGF-β1) and type II transforming growth factor-β receptor (TGF-βRII) in wound granulation tissues of wild type and P311−/− animals were examined by immunohistochemistry (IHC) assays at days 3 and 6 post-injury. Representative images of granulation tissues are shown and the optical density (OD) values were analyzed (below). Scale bar = 20 μm. (c, d) Representative western blot (WB) and confocal images of TGF-βRII in fibroblasts with or without TGF-β1 co-incubation for 24 h are shown in control and treatment groups and the OD/mean fluorescence intensity (MFI) value of TGF-βRII was statistically analyzed (below). Scale bar = 50 μm. (e) Activation of the Smad2/3signaling pathway in WT and P311−/− fibroblasts with or without TGF-β1 stimulation for 0, 15, 30 and 60 min was detected by WB. Representative WB images for the Smad signaling pathway are shown, and the OD ratio of phosphorylated and total proteins was statistically analyzed. Mean ± standard deviation, n = 3. (**p < 0.01; ***p < 0.001; ns not significant)
P311 deficiency impairs the differentiation of fibroblasts into myofibroblasts
Based on the well-established role of the TGFβ-RII-Smad signaling pathway in fibroblast differentiation [23], we further investigated the role of P311 in the differentiation of fibroblasts into myofibroblasts. We found that upon TGF-β1 stimulation, P311−/− fibroblasts failed to increase the expression of α-SMA, a myofibroblast marker, at both protein and mRNA levels compared with WT fibroblasts (Figure 4a, b). Importantly, considerably poorer differentiation of P311−/−fibroblasts into α-SMA-positive myofibroblasts (Figure 4c) resulted in significantly weaker contractility of P311−/− fibroblasts (Figure 4d). Consistently, we observed significantly reduced levels of α-SMA and the number of α-SMA-positive myofibroblasts in wound tissues of the P311−/− mice at day 6 post-wounding in comparison with the WT mice (Figure 4e–h). These data collectively suggest that dysfunction of P311−/− fibroblasts in differentiating into myofibroblasts might be an important mechanism for the reduced healing ability of the P311−/− mice.
Figure 4.
P311 deficiency brought about impaired differentiation of fibroblasts into myofibroblasts. (a, b) After 24 hours of co-incubation with or without transforming growth factor-1β (TGF-1β) in wild type (WT) and P311−/− fibroblasts, representative western blot (WB) images and real time polymerase chain reaction (rtPCR) analysis of α-smooth muscle actin (α-SMA) are shown and statistically analyzed. (c) A representative confocal image of vimentin+α-SMA+ myofibroblasts with or without TGF-β1 co-incubation is shown and was statistically analyzed. Scale bar = 50 μm. (d) Representative cell contraction images of WT and P311−/− fibroblasts with TGF-β1 stimulation at days 0 and 3 are shown and were analyzed. Scale bar = 50 μm. (e, f) Immunohistochemical and immunofluorescence staining for α-SMA (red arrow) at days 3 and 6 after skin injury in WT and P311−/− mice is shown and was statistically analyzed. Scale bar = 200 μm. (g) Representative fluorescence activated cell sorter (FACS) images of vimentin+α-SMA+ myofibroblasts in WT and P311−/− mice at days 3 and 6 are shown and were statistically analyzed. (h) Representative WB images of α-SMA in wound tissues of WT and P311−/− mice at day 6 post-wounding; the OD value of α-SMA was statistically analyzed. Mean ± standard deviation, n = 3. (*p < 0.05, **p < 0.01; ***p < 0.001; ns not significant)
P311 deficiency results in significantly decreased collagen secretion by myofibroblasts and collagen deposition in wound granulation tissues
Collagen deposition in skin wound healing is a key determinant of granulation tissue formation [22]. To clarify whether P311 deficiency could result in reduced collagen secretion from myofibroblasts, we found that the production of collagen I and collagen III was significantly reduced at both mRNA and protein levels in TGF-β1-induced P311−/− myofibroblasts in vitro in comparison to WT myofibroblasts (Figure 5a–d).
Figure 5.
P311 deficiency resulted in significantly decreased collagen secretion by myofibroblasts and collagen deposition in wound granulation tissues. With or without transforming growth factor-β1 (TGF-β1)-stimulation for 24 h, (a) the content of collagen I and collagen III were examined using enzyme-linked immunosorbent assay in WT and P311−/− groups (ELISA); (b) representative western blot (WB) images of collagen I and collagen III in wild type (WT) and P311−/− primary fibroblasts; the optical density value was statistically analyzed; (c) representative confocal images of collagen I and collagen III in WT and P311−/− primary fibroblasts; the mean fluorescence intensity (MFI) value was statistically analyzed; scale bar = 50 μm. (d) mRNA expression of collagen I and collagen III in WT and P311−/− primary fibroblasts was detected by rtPCR. The supernatant of wound tissue extract at day 6 post-injury was applied for identifying the content of hydroxyproline (e), collagen I (f) and collagen III (g) by ELISA in WT and P311−/− groups. The contents of collagen fibers (h), elastic fibers (i) and reticular fibers (j) in wound granulation tissues in WT and P311−/− mice were determined by Masson and Weigert Elastic Fiber Dyeing Solution, modified Gomori staining, and analyzed, respectively. Scale bar = 200 μm. Mean ± standard deviation, n = 3. (*p < 0.05, **p < 0.01; ***p < 0.001; ns not significant)
Moreover, we further evaluated the content of total collagen (represented as the content of hydroxyproline) and several different types of fiber deposition in wound tissues at days 3, 6 and 9 post-injury in vivo. The results showed a significantly decreased content of total collagen, collagen I, collagen III, collagen fibers and elastic fibers (Figure 5e–i, respectively) in P311−/− mice in comparison with WT mice. In contrast, we observed an opposite trend for the level of reticular fibers in wound tissues on day 3 after skin injury (Figure 5j). Collectively, these results suggest that P311 deficiency reduces the collagen secretion of myofibroblasts, which leads to disordered collagen deposition in wound tissue of P311−/− animals.
P311 reconstitution restores myofibroblast differentiation and collagen secretion mediated by the TGF-βRII-Smad signaling pathway in P311 −/− fibroblasts
So far, we have demonstrated that P311−/− fibroblasts exhibit a weakened activation of the TGF-β-RII-Smad signaling pathway, resulting in dysfunctional differentiation and collagen secretion of myofibroblasts. In order to confirm the function of P311, we reconstituted P311 gene expression in the P311−/− fibroblasts and re-examined the above-mentioned changes. The results showed that P311 reconstitution could significantly elevate the expression of TGF-β-RII (Figure 6a, b), enhance the activation of Smad2/3 signal (Figure 6c), increase the production of α-SMA (Figure 6d), the differentiation into myofibroblasts (Figure 6e), cell contraction (Figure 6f) and collagen secretion (Figure 6g, h) of P311−/− fibroblasts. Collectively, these results demonstrate that through down-regulating the TGF-βRII-Smad signaling pathway, P311 plays a critical role in myofibroblasts differentiation, cell contraction, collagen secretion and eventually wound healing.
Figure 6.
Forced P311 expression restores a weakened TGF-βRII-Smad–myofibroblast differentiation–collagen secretion axis of P311−/− fibroblasts. With or without transforming growth factor-β1 (TGF-β1) stimulation for 24 h, (a) representative western blot (WB), and (b) confocal images of type II transforming growth factor-β receptor (TGF-βRII) (left) and corresponding statistical analysis (right). Representative WB images of Smad2/3 and p-Smad2/3 in fibroblasts after stimulation for 15 min (c) and α-smooth muscle actin (α-SMA) after 24 h (d) in P311−/−ad-NC (fibroblasts of P311−/− transfected with no-load adenovirus) and P311−/−ad-P311 (fibroblasts of P311−/− transfected with P311 adenovirus), with quantification shown on the right. (e) Representative confocal image of vimentin+α -SMA+ myofibroblasts with or without TGF-β 1 co-incubation is shown in P311−/−ad-NC and P311−/−ad-P311 groups, and was statistically analyzed. (f) Representative cell contraction images of P311−/−ad-NC and P311−/−ad-P311 with TGF-β1 stimulation at days 0 and 3 are shown and were analyzed. (g) Representative enzyme-linked immunosorbent assay (ELISA) analysis and (h) WB images of collagen III and collagen I P311−/−ad-NC and P311−/−ad-P311 at 24 h after TGF-β1 stimulation; the OD values of collagen III and collagen I were statistically analyzed. Mean ± standard deviation, n = 3 independent biological replicates. (*p < 0.05, **p < 0.01; ***p < 0.001; ns not significant)
mTOR is involved in the regulatory role of P311 in the activation of the TGF-βRII-Smad signaling pathway
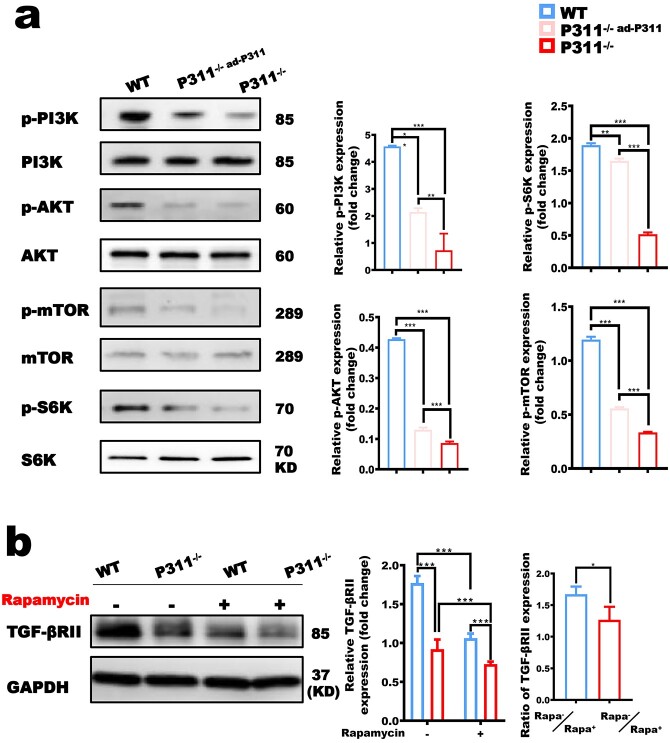
At present, the mechanisms underlying the decreased expression of TGF-βRII in P311−/− fibroblasts are still unknown. Since the mTOR signaling pathway plays a critical role in protein synthesis via controlling initiation of protein translation [24], it is reasonable to assume that the mTOR signaling pathway would be involved in the regulatory role of P311 in the expression of TGF-βRII. To test this hypothesis, we firstly evaluated the relationship between P311 and the activation of the mTOR signaling pathway in fibroblasts in vitro. As expected, the activation of the mTOR signaling pathway in P311−/− fibroblasts was significantly weakened as compared with that in WT fibroblasts, and P311 reconstitution partially restored the activation of the mTOR signaling pathway (Figure 7a). Next, the effect of the mTOR signaling pathway on P311-mediated activation of the TGF-βRII-Smad signaling pathway was evaluated by comparative analysis of fibroblasts with or without treatment with rapamycin, a specific mTOR inhibitor. As shown in Figure 7b, rapamycin fibroblasts. Importantly, the difference in TGF-βRII expression between WT and P311−/− fibroblasts was significantly reduced after rapamycin treatment (Figure 7b). Correspondingly, the activation of Smad2/3 in both WT and P311−/− fibroblasts upon TGF-β1 stimulation was significantly attenuated with rapamycin, and the difference in Smad2/3 activation between WT and P311−/− fibroblasts was eliminated after rapamycin treatment (Figure 7c). Thus, these results suggest that mTOR signaling is involved in regulating the P311-mediated activation of the TGF-βRII-Smad signaling pathway. Furthermore, we also found that inhibition of mTOR by rapamycin significantly decreased the expression of α-SMA and collagen I/III in both WT and P311−/− fibroblasts (Figure 7d). Consistently, the difference in α-SMA and collagen I/III expression between WT and P311−/− fibroblasts was significantly reduced after rapamycin treatment (Figure 7d). Together, these results reveal that the mTOR signaling pathway plays an important role in the regulation of TGF-βRII expression and downstream events including the TGF-βRII-Smad signaling pathway and myofibroblast differentiation and function.
Figure 7.

The mTOR pathway is significantly reduced the TGF-βRII expression level in both WT and P311−/− deeply involved in the regulation of P311 in the activation of the TGF-βRII-Smad signaling pathway. (a) Representative western blot (WB) images of mammalian target of rapamycin (mTOR) pathway expression from WT, P311−/−ad-P311 (fibroblasts of P311−/− transfected with P311 adenovirus) and P311−/− fibroblasts are shown (left), and the optical density value of protein was statistically analyzed. The expression of the (b) TGF-βRII, (c) TGF-β1-Smads (Smad2/3) signaling pathway and (d) α-SMA, collagen I, collagen III in fibroblasts with and without rapamycin (5 nM) treatment for 15 min in the presence of TGF-β1 stimulation was evaluated using WB (left); the corresponding analysis is shown. Mean ± standard deviation, n = 3 independent biological replicates. (**p < 0.01; ***p < 0.001). WT wild type, TGF-βRII type II transforming growth factor-β receptor mediated, α-SMA α-smooth muscle actin, TGF-β transforming growth factor-β
Discussion
The formation of granulation tissue is a critical process for skin wound healing. The activation, proliferation, differentiation and collagen secretion of fibroblasts are essential for the formation and development of granulation tissue in the process of skin wound repair [2]. P311, as we have previously reported, plays an important role in promoting wound healing and post-healing fibrosis [25]. It has been reported that P311 stimulates the translation of TGFβ1 mRNA and integrin β1 recycling in wound healing [26], since P311 interacts with filamin A and MYH9; while the former is an interconnecting protein between F-actin and integrin β1, the latter is responsible for integrin β1 recycling with F-actin, which is stimulated by the RAC1 pathway [27]. Nevertheless, the exact role of P311 in skin wound healing remains unknown. In this study, we demonstrate that P311 is capable of promoting activation, proliferation, differentiation and collagen secretion of fibroblasts and facilitates granulation tissue formation and wound closure by enhancing activation of the TGF-β1-TGF-βRII-Smad signaling axis.
It is commonly known that fibroblasts are the core effector cells leading to tissue fibrosis [25]. Moreover, P311 could promote fibroblasts to differentiate into myofibroblasts and aggravate hypertrophic scaring [28]. Meanwhile, P311 has been recently shown to play a positive role in accelerating skin wound repair by promoting epidermal stem cell migration and angiogenesis [4,25]. Nevertheless, whether and how P311 regulates fibroblasts to affect wound healing remains unclear. In this study, we systemically investigated the effects of P311 on fibroblast proliferation, differentiation and secretion of fibroblasts, as well as on the formation of granulation tissue in the context of skin wound closure. Here we found that P311−/− fibroblasts in vitro displayed markedly weakened migration and proliferation ability, impaired differentiation into myofibroblasts and dramatically reduced collagen secretion and cell contraction capacity, all of which could be restored by reconstitution of the P311 gene. Importantly, for the first time we demonstrated that the number of both fibroblasts and myofibroblasts and the content of collagen and fiber in wound tissue were dramatically decreased in P311−/− mice compared to WT animals, resulting in retarded formation of granulation tissues and wound healing. Our results thus suggest that a positive regulatory role of P311 in fibroblasts is beneficial to skin wound healing.
The TGF-β-TGF-βR-Smad signaling axis is known to play a pivotal role in regulating the activation, proliferation, differentiation and secretion of fibroblasts [23]. Previous studies have shown that P311 promotes the expression of TGF-β and the activation of Smad signaling to participate in the regulation of biological functions in a variety of cells [23]. For instance, forced P311 expression significantly elevated the TGF-β protein level, TGF-βR I/II mRNA level and activation of Smad signaling [17]. In this study, we found that P311−/− fibroblasts exhibited a markedly weakened activation of the TGF-βR-Smad pathway upon TGF-β1 stimulation in vitro, which was restored by reconstitution of the P311 gene. Thus, it is believed that P311 up-regulates TGF-βRII to strengthen the activation of the TGF-βRII-Smad pathway in fibroblasts and ultimately impacts a series of downstream events to facilitate wound healing.
mTOR is a conserved serine/threonine-protein kinase that is composed of two structurally and functionally distinct multiprotein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 [29]. mTORC1 is rapamycin-sensitive and mediates temporal control of cell growth by regulating several cellular processes, including translation, transcription, ribosome biogenesis, nutrient transport and autophagy [30]. Currently, there is increasing evidence indicating that the mTOR signaling pathway plays a crucial role in skin wound repair [31,32]. For example, the mTOR signaling pathway was up-regulated in wound healing, whereas pharmacological inhibition of mTOR with rapamycin delayed wound closure [33,34]. This pathway was also attenuated in diabetic patients with impaired wound healing, while activation of the PI3K-AKT signaling pathway upstream of mTOR improved the wound healing of diabetic animals [35]. Moreover, continuous activation of the mTOR signaling pathway has been considered to be an important cause of tissue fibrosis after injury, and the application of mTOR inhibitors can significantly relieve tissue fibrosis [36]. Given that mTORC1 phosphorylates and controls the translation of protein [30], it is reasonable to assume that activation of the mTORC1 signaling pathway may promote the expression of TGF-βRII protein in fibroblasts. To verify this hypothesis, we firstly tested the effect of P311 on the activation of the mTOR signaling pathway. P311−/− fibroblasts exhibited lower activation of the mTOR signaling pathway than WT fibroblasts, which could be restored by reconstitution of the P311 gene. Moreover, with mTOR inhibitor rapamycin treatment, the TGF-βRII expression difference between WT and P311−/− fibroblasts was almost eliminated. Overall, we demonstrated the critical role of mTOR in the regulation of P311 in TGF-βRII expression of fibroblasts. The whole process is summarized in Figure 8.
Figure 8.
Effect of P311 in promoting the proliferation and secretion of fibroblasts to facilitate granulation tissue formation and wound healing by activating the TGF-βRII-Smad pathway. TGF-βRII type II transforming growth factor-β receptor mediated, α-SMA α-smooth muscle actin, TGF-β Transforming growth factor-β, ECM extracellular matrix
P311 has recently been proved to promote angiogenesis [4], via which P311 may improve the speed and quality of granulation tissue formation to promote skin wound healing. Thus, targeting the P311-regualted angiogenesis process might provide a new opportunity in developing therapeutics for wound healing. Therefore, P311 might become a potential target for the development of therapeutic agents for the treatment of refractory wounds and hypertrophic scars. Here a key question remains: what is the underlying molecular mechanism regulating P311 expression? P311 is an easily degraded 8 kDa protein, which makes the detection of this protein technically difficult. However, our recently unpublished data demonstrated that P311, similar to hypoxia-inducible factor-1α (HIF-1α), can be produced in large quantities under hypoxic conditions (data not shown). This will greatly facilitate future study on the mechanism of regulation of P311 expression. Another important question is about the precise mechanisms underlying the regulatory effect of P311 on the activation of the mTOR pathway. Although our study has revealed that P311 can promote the mTOR signal, the specific target molecule via which P311 interacts to modulate the mTOR signal needs to be further clarified.
Conclusions
In conclusion, our present data demonstrate that P311 plays a critical role in the activation of the TGF-βRII-Smad pathway to promote fibroblast proliferation and differentiation, as well as to enhance granulation tissue formation in the process of skin wound repair. Mechanistically, the mTOR signaling pathway is closely implicated in the P311-mediated regulation of the TGF-βRII-Smad pathway in fibroblasts.
Supplementary Material
Acknowledgments
National Natural Sciences Foundation of China (No. 31872742 to W.F.H. and No. 81630055 to G.X.L.), Military Medical Science and Technology Youth Training Program of Army Military Medical University (Third Military Medical University) (No. 20QNPY024 to W.F.H.), the Special Project for Enhancing Science and Technology Innovation Ability (frontier exploration) of Army Military Medical University (Third Military Medical University) (No. 2019XQY12 to W.F.H.).
Contributor Information
Jue Wang, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Ruoyu Shang, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Jiacai Yang, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Zhihui Liu, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Yunxia Chen, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Cheng Chen, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Wenxia Zheng, Department of Technical Support, Chengdu Zhijing Technology Co., Ltd, Chengdu 610041, China.
Yuanyang Tang, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Academy of Biological Engineering, Chongqing University, Chongqing 400038, China.
Xiaorong Zhang, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Xiaohong Hu, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Yong Huang, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Han-Ming Shen, Faculty of Health Sciences, University of Macau, Macau, China.
Gaoxing Luo, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Weifeng He, State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing 400038, China; Chongqing Key Laboratory for Disease Proteomics, Chongqing 400038, China.
Authors’ contributions
J.W. and R.Y.S. performed the experiments and prepared the first draft of the manuscript. J.C.Y., Y.H. carried out partial animal experiments. Y.X.C., C.C., Z.H.L., Y.Y.T., X.R.Z., X.H.H. and performed most of the experiments and prepared the manuscript. W.X.Z. provided technical support for confocal microscopy and the full slide scanning microscope. H.M.S., W.F.H. and G.X.L. conceived and designed the research and edited the manuscript.
Abbreviations
- eIF3b: Eukaryotic translation initiation factor 3 subunit b; ELISA: enzyme-linked immunosorbent assay; mTOR: mammalian target of rapamycin; mTORC1: mTOR complex 1; rtPCR: real-time polymerase chain reaction; α-SMA: α-smooth muscle actin; TGF-β: Transforming growth factor-β; TGF-βRII: Type II transforming growth factor-β receptor; WT: wild type.
Ethics approval and consent to participate
In this study, all animal experiments were approved by the Committee on the Use and Care of Animals (The Army Medical University, Chongqing, China) and performed following institution guidelines.
Conflicts of interest
None declared.
References
- 1. Hutchings G, Kruszyna Ł, Nawrocki MJ, Strauss E, Bryl R, Spaczyńska J, et al. Molecular mechanisms associated with ROS-dependent angiogenesis in lower extremity artery disease. Antioxidants (Basel). 2021;10:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Werner S, Krieg T, Smola HJ. Keratinocyte–Fibroblast Interactions in Wound Healing. J Invest Dermatol. 2007;127:998–1008. [DOI] [PubMed] [Google Scholar]
- 3. Hinz B. The role of myofibroblasts in wound healing. Curr Res Transl Med. 2016;64:171–7. [DOI] [PubMed] [Google Scholar]
- 4. Wang S, Zhang X, Qian W, Zhou D, Yu X, Zhan R, et al. P311 deficiency leads to attenuated angiogenesis in cutaneous wound healing. Front Physiol. 2017;8:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Oliveira RC, Wilson SE. Fibrocytes, wound healing, and corneal fibrosis. Invest Ophthalmol Vis Sci. 2020;61:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gilbert RW, Vickaryous MK, Viloria-Petit AM. Signalling by transforming growth factor beta isoforms in wound healing and tissue regeneration. J Dev Biol. 2016;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–84. [DOI] [PubMed] [Google Scholar]
- 8. Heldin C-H, Miyazono K, Ten Dijke PJ. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–71. [DOI] [PubMed] [Google Scholar]
- 9. Studler JM, Glowinski J, Lévi-Strauss M. An abundant mRNA of the embryonic brain persists at a high level in cerebellum, hippocampus and olfactory bulb during adulthood. Eur J Neurosci. 1993;5:614–23. [DOI] [PubMed] [Google Scholar]
- 10. Badri KR, Yue M, Carretero OA, Aramgam SL, Cao J, Sharkady S, et al. Blood pressure homeostasis is maintained by a P311–TGF-β axis. J Clin Invest. 2013;123:4502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stradiot L, Mannaerts I, van Grunsven LA. P311, Friend, or Foe of Tissue Fibrosis? Front Pharmacol. 2018;9:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu J, Ma B, Yi S, Wang Z, He W, Luo G, et al. Gene expression of early hypertrophic scar tissue screened by means of cDNA microarrays. J Trauma. 2004;57:1276–86. [DOI] [PubMed] [Google Scholar]
- 13. Yao Z, Yang S, He W, Li L, Xu R, Zhang X, et al. P311 promotes renal fibrosis via TGFβ1/Smad signaling. Sci Rep. 2015;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. [DOI] [PubMed] [Google Scholar]
- 15. Yue MM, Lv K, Meredith SC, Martindale JL, Gorospe M, Schuger L. Novel RNA-binding protein P311 binds eukaryotic translation initiation factor 3 subunit b (eIF3b) to promote translation of transforming growth factor β1-3 (TGF-β1-3). J Biol Chem. 2014;289:33971–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu P, Wu Y, Zhou L, Yang Z, Zhang X, Hu X, et al. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burns Trauma. 2020;8:tkaa028. 10.1093/burnst/tkaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li H, Yao Z, He W, Gao H, Bai Y, Yang S, et al. P311 induces the transdifferentiation of epidermal stem cells to myofibroblast-like cells by stimulating transforming growth factor β1 expression. Stem Cell Res Ther. 2016;7:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen BE, Geronemus RG, Mcdaniel DH, Brauer JA. The role of elastic fibers in scar formation and treatment. Dermatol Surg. 2017;43:S19–24. [DOI] [PubMed] [Google Scholar]
- 19. Christen T, Verin V, Bochaton-Piallat M-L, Popowski Y, Ramaekers F, Debruyne P, et al. Mechanisms of neointima formation and remodeling in the porcine coronary artery. Circulation. 2001;103:882–8. [DOI] [PubMed] [Google Scholar]
- 20. Meduri GU, Belenchia JM, Estes RJ, Wunderink RG, Torky ME, LeeperKV, Jr. Fibroproliferative phase of ARDS: clinical findings and effects of corticosteroids. Chest. 1991;100:943–52. [DOI] [PubMed] [Google Scholar]
- 21. Joe A, Yi L, Natarajan A, le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Millanes-Romero A, Herranz N, Perrera V, Iturbide A, Loubat-Casanovas J, Gil J, et al. Regulation of heterochromatin transcription by Snail1/LOXL2 during epithelial-to-mesenchymal transition. Mol Cell. 2013;52:746–57. [DOI] [PubMed] [Google Scholar]
- 23. Hata A, Chen Y-G. TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8:a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12:379–92. [DOI] [PubMed] [Google Scholar]
- 25. Yao Z, Li H, He W, Yang S, Zhang X, Zhan R, et al. P311 Accelerates Skin Wound Reepithelialization by Promoting Epidermal Stem Cell Migration Through RhoA and Rac1 Activation. Stem Cells Dev. 2017;26:451–60. [DOI] [PubMed] [Google Scholar]
- 26. McDonough WS, Tran NL, Berens ME. Regulation of glioma cell migration by serine-phosphorylated P311. Neoplasia. 2005;7:862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chantaravisoot N, Wongkongkathep P, Loo JA, Mischel PS, Tamanoi F. Significance of filamin A in mTORC2 function in glioblastoma. Mol Cancer. 2015;14:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan J, Peng X, Luo G, Ma B, Cao C, He W, et al. Investigating the role of P311 in the hypertrophic scar. PLoS One. 2010;5:e9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18:744–57. [DOI] [PubMed] [Google Scholar]
- 30. Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda). 2006;21:362–9. [DOI] [PubMed] [Google Scholar]
- 31. Wei P, Zhong C, Yang X, Shu F, Xiao S, Gong T, et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burns Trauma. 2020;8:tkaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Castilho RM, Squarize CH, Gutkind JS. Exploiting PI3K/mTOR signaling to accelerate epithelial wound healing. Oral Dis. 2013;19:551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen C, Meng Z, Ren H, Zhao N, Shang R, He W, et al. The molecular mechanisms supporting the homeostasis and activation of dendritic epidermal T cell and its role in promoting wound healing. Burns Trauma. 2021;9:tkab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei F, Wang A, Wang Q, Han W, Rong R, Wang L, et al. Plasma endothelial cells-derived extracellular vesicles promote wound healing in diabetes through YAP and the PI3K/Akt/mTOR pathway. Aging (Albany NY). 2020;12:12002–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suto T, Karonitsch TJ. The immunobiology of mTOR in autoimmunity. J Autoimmun. 2020;110:102373. [DOI] [PubMed] [Google Scholar]
- 36. Gralinski LE, Ferris MT, Aylor DL, Whitmore AC, Green R, Frieman MB, et al. Genome wide identification of SARS-CoV susceptibility loci using the collaborative cross. PLoS Genet. 2015;11:e1005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dorn LE, Petrosino JM, Wright P, Accornero F. CTGF/CCN2 is an autocrine regulator of cardiac fibrosis. J Mol Cell Cardiol. 2018;121:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cabrera-Benítez NE, Parotto M, Post M, Han B, Spieth PM, Cheng WE, et al. Mechanical stress induces lung fibrosis by epithelial-mesenchymal transition (EMT). Crit Care Med. 2012;40:510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.