Abstract
The fiber-optic biosensor, originally developed to detect hazardous biological agents such as protein toxins or bacterial cells, has been utilized to quantify the concentration of serum antiplague antibodies. This biosensor has been used to detect and quantify the plague fraction 1 antigen in serum, plasma, and whole-blood samples, but its ability to quantify serum antibodies has not been demonstrated. By using a competitive assay, the concentration of serum antiplague antibodies was ascertained in the range of 2 to 15 μg/ml. By making simple dilutions, concentrations for 11 serum samples whose antiplague antibody concentrations were unknown were determined and were found to be in good agreement with enzyme-linked immunosorbent assay results. The competitive assay method could be used to effectively determine the exposure to plague of animals or humans or could be applied to other diseases, such as hepatitis or AIDS, where the presence of antibodies is used to diagnose infection.
Yersinia pestis, the etiologic agent of bubonic plague, continues to be endemic in many parts of the world. Although it occurs most frequently as an infection in wild rodents, plague can be transmitted to domesticated cats or dogs either by the bites of fleas or through the consumption of plague-infected carrion (8, 10). Subsequently, these animals may become vectors for transmission to humans. Serologic surveys of wild and domesticated animals in high-risk areas would permit an early warning of the spreading disease (18). The availability of a simple test performable in the field would allow the rapid screening of animals and people to monitor for exposure or immunization effectiveness (15).
Since plague-infected animals produce a strong humoral response to the fraction 1 (F1) antigen, the detection of F1 antibodies is the basis for standard serological tests in plague surveillance and diagnosis (20). The F1 antigen is a protein-polysaccharide complex that forms a major component of the outer membrane capsule of Y. pestis (5). Capsule production occurs at 37°C upon transmission from fleas to warmer mammalian hosts. The F1 antigen is thought to confer resistance to phagocytosis (5). The F1 antigen is also thought to be the primary immunogen in the whole-cell vaccines with protection-inducing properties (19).
The fiber-optic biosensor is being developed to conduct fluoroimmunoassays in a rapid, user-friendly form (11). The assays that have been developed have primarily been for hazardous biological substances. For example, sandwich immunoassays have been developed for plague F1 antigen (6), staphylococcal enterotoxin B (17), and ricin (13). Another use for the sensor has been the detection of small molecules. A competitive assay has been used to quantify trinitrotoluene contamination in groundwater (16). The principal advantage of this biosensor is that it permits samples to be tested in the field. While the standard enzyme-linked immunosorbent assay (ELISA) takes a skilled technician in a laboratory several hours to complete, the biosensor is capable of producing an answer within 10 to 20 min. In addition, the biosensor has recently been miniaturized (11), and an automated version which will further facilitate sample analysis is in development.
The sandwich fluoroimmunoassay has been the method of choice to detect biological molecules with the fiber-optic biosensor. In this assay, antibodies directed towards an antigen of interest are immobilized on the probe. When the probes are exposed to an antigen-containing sample, the antigen is bound by the antibody on the probe surface. The amount bound is determined by the application of a high concentration of fluorescently labeled antibody, which forms a fluorescent complex at the probe surface. The amount of fluorescent complex is quantified by the optoelectronics, which launches excitation light into the proximal end of the probe and measures the generated fluorescence returning back up the probe (8). Though this method has worked well for toxins and for proteins such as the F1 antigen, a different assay method was required to quantify serum antiplague antibodies.
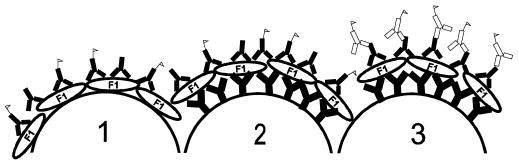
Several different methods were explored in order to obtain the most effective protocol for quantifying antiplague antibodies (Fig. 1). The first method tested was a competitive assay, in which probes were prepared by directly immobilizing the F1 antigen onto the probe surface. When these probes were exposed to serum containing anti-F1 antigen antibodies, a portion of the F1 antigen on the probes was bound. This resulted in a decrease of signal generated by the subsequent incubation with a standard quantity of fluorescently labeled antiplague antibody. The inhibition of signal compared to that of unexposed probes was indicative of the amount of antiplague antibodies in the serum.
FIG. 1.
Schematic of immunoassay methods. (1) Competitive assay with probes coated with F1 antigen. The fluorescently labeled antibodies are indicated by flags. (2) Competitive assay with probes with antiplague antibody that was immobilized and then coated with F1 antigen. (3) Sandwich immunoassay with fluorescent anti-human antibody to generate the signal.
A modified competitive assay was also investigated. In this protocol, antiplague immunoglobulin G (IgG)-coated probes were first exposed to a limited amount of F1 antigen. Next, they were exposed to the serum sample and finally to the fluorescently labeled antiplague antibody. Again, the degrees of signal inhibition between probes which had and had not been exposed to serum were compared.
The final method examined was a sandwich immunoassay. Fiber probes with immobilized antiplague IgG were coated with F1 antigen and then incubated with serum samples. The quantity of antiplague serum antibodies which bound to the probe surface was then determined with fluorescent rabbit anti-human IgG.
MATERIALS AND METHODS
Reagents.
The F1 antigen (3), sera from immunized personnel, rabbit antiplague IgG purified with protein G, and ascites fluid containing the monoclonal antibody YPF1-6H3-1-1-IgG, henceforth referred to as 6H3-IgG, were provided by the U.S. Army Medical Research Institute of Infectious Disease (USAMRIID). The 6H3-IgG monoclonal antibody was developed at USAMRIID by injecting F1 antigen (lot 4, produced by J. E. Williams, Walter Reed Army Institute of Research, Washington, D.C.) into BALB/c mice. The F1 antigen preparation used in this study was the same as that utilized in the hybridoma screening process. 6H3-IgG was affinity purified on a 3-ml Avidchrom column (Unisyn Technologies, San Diego, Calif.), by eluting the bound antibody with 0.1 M sodium acetate (pH 3.0) plus 20% glycerol. The purified antibody was then dialyzed three times against phosphate-buffered saline plus 0.01% sodium azide (PBS).
The anti-F1 antigen titers of the serum samples from immunized personnel used in this study were estimated by using twofold dilutions in a capture ELISA. Negative control serum was obtained from Gibco (Gaithersburg, Md.).
Rhodamine-conjugated rabbit anti-human IgG was purchased from Accurate Chemical (Westbury, N.Y.). Human IgG containing antiplague antibody was purified from a sample from an immunized volunteer on an Avidchrom column as described above. The quantity of antiplague IgG was determined by the modified competitive method described below. Other fluorescently labeled antibodies were prepared by dialysis of 1 mg of each antibody (1 mg/ml) against 50 mM borate (pH 9.3) plus 50 mM NaCl, followed by dialysis overnight in the same buffer containing 0.01 mg of tetramethylrhodamine-5-isothiocyanate, isomer G (TRITC) (Molecular Probes, Eugene, Oreg.), per ml. Free dye was removed by gel filtration on Bio-Gel P-10 (Bio-Rad, Hercules, Calif.) equilibrated with PBS. The dye-to-protein molar ratio for each antibody preparation was determined to be between 1.0 and 1.5 by the method of Amante et al. (1). Bovine serum albumin (2 mg/ml) was added to the labeled antibody prior to storage at 4°C.
Fiber preparation.
Optical probes were prepared from plastic-clad optical fiber with a 200-μm-diameter silica core (Quartz Products, Tuckerton, Del.). The sensing region was formed by removing 12.5 cm of cladding at the fiber’s distal end to expose the silica core. Residual cladding from the probe area was removed by immersion in concentrated hydrofluoric acid for 1 min. The fibers were subjected to computer-controlled immersion in hydrofluoric acid to form tapered probes (2).
Rabbit antiplague IgG or F1 antigen was immobilized onto the tapered core according to the procedure of Bhatia et al. (4, 9). Briefly, clean probes were incubated for 30 min in 4% thiol-terminal silane in toluene (3-mercaptopropyl trimethoxysilane; Fluka, Hauppauge, N.Y.) and then for 1 h with the heterobifunctional cross-linker N-succinimidyl 4-maleimidobutyrate (Fluka) (2 mM) in ethanol. Finally, the probes were incubated with the capture antibody or F1 antigen at 0.05 mg/ml in PBS for 2 h. This procedure immobilized the antibody on the fiber surface at approximately 2 ng/mm2 (4). The fiber probes were placed in storage in PBS at 4°C. Prior to testing, the probes were placed into flow chambers as previously described (2). All testing was performed with the laboratory breadboard biosensor, which has been previously described (12).
RESULTS
Competitive assay with F1 antigen-coated probes.
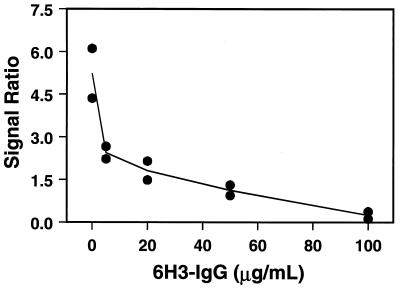
The first method used to quantify serum antiplague antibodies was a two-step competitive assay. First, a standard response curve for the probes coated with F1 antigen was constructed. The probe response was first standardized by incubation with 200 ng of TRITC-labeled 6H3-IgG (T-6H3-IgG)/ml for 5 min. Subsequently, various known amounts of unlabeled 6H3-IgG (0 to 100 μg/ml) were incubated on the probes for 10 min. Finally, to determine the degree of binding inhibition, the probes were incubated with 1,000 ng of T-6H3-IgG/ml. The additional fluorescent signal generated after 5 min of incubation with 1,000 ng of T-6H3-IgG/ml was divided by the signal generated by the initial exposure to 200 ng of T-6H3-IgG/ml. A ratio (R) can be described by the following equation: R = (signal 2 − signal 1)/(signal 1 − background). The plot of this ratio versus the concentration of unlabeled 6H3-IgG is shown in Fig. 2. As expected, the ratio dropped with increasing concentrations of unlabeled antibody. However, since very high levels of antibody were required to inhibit the response, additional methods were investigated.
FIG. 2.
Competitive assay standard curve for antiplague antibody with probes with immobilized F1 antigen. An initial signal (signal 1) was generated on the optical probes by the addition of T-6H3-IgG (200 ng/ml). The probes were then exposed to various amounts of unlabeled 6H3-IgG. The amount of binding was determined by the reapplication of T-6H3-IgG (1,000 ng/ml), yielding signal 2. The signal 2/signal 1 ratio versus 6H3-IgG concentration is shown. The ratios for two probes are shown at each concentration.
Competitive assay with rabbit antiplague IgG-coated probes.
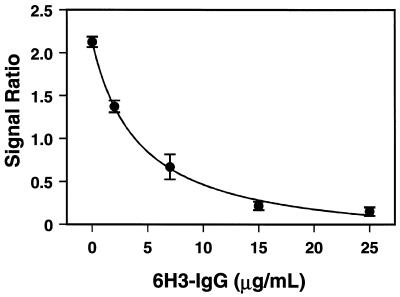
Probes coated with rabbit antiplague IgG, the same type used in the previously developed sandwich immunoassay to quantify F1 antigen (6), were used to develop an assay to quantify serum antiplague antibody. The antibody-coated probes were first incubated twice with T-6H3-IgG (5 μg/ml) for 5 min each time to determine nonspecific background signal due to adsorption of the labeled antibody. The probes were rinsed with PBS plus 0.1% Triton X-100 between each step. After the background signal had been determined, the probes were exposed to F1 antigen (200 ng/ml) for 5 min and then to T-6H3-IgG (5 μg/ml) for 5 min to standardize the probe response (signal 1). Following standardization, the probes were reprimed by exposure to 500 ng of F1 antigen/ml. Ultimately, either known amounts of 6H3-IgG in PBS (to generate a standard curve) or serum samples were incubated on the probes for 5 min. The amount of blocking that occurred was determined by a final incubation with T-6H3-IgG (5 μg/ml) for 5 min (signal 2). The signal ratio was determined as described above and plotted versus the concentration of 6H3-IgG (Fig. 3). The curve was fit by using the hyperbolic second-order-decay equation Y = (a + bc)/(c + X). The fit to this equation was used to calculate the concentrations of antibody in serum samples. This competitive method quantified the antibody concentration in the 2- to 15-μg/ml range.
FIG. 3.
Competitive assay standard curve for antiplague antibody with probes with immobilized rabbit antiplague IgG. To obtain the ratios, an initial signal (signal 1) was generated on the probes by addition of F1 antigen (200 ng/ml) for 5 min and then T-6H3-IgG (5 μg/ml) for 5 min. The second signal (signal 2) was obtained by repriming the probes with 500 ng of F1 antigen/ml and incubating them with various amounts of 6H3-IgG for 5 min and then with T-6H3-IgG for 5 min. The signal 2/signal 1 ratio versus 6H3-IgG concentration is shown. The mean ± standard error for three probes is shown at each concentration.
Sandwich immunoassay for antiplague antibodies.
The final method investigated was a standard sandwich immunoassay. Again, probes with rabbit antiplague IgG were prepared. The background signal generated by 5 μg of TRITC-labeled rabbit anti-human IgG/ml was determined by incubating probes twice with the fluorophore-labeled antibody for 5 min each time, as described above. These probes were then primed with F1 antigen (10 μg/ml) for 5 min, treated with various amounts of human antiplague IgG for 5 min, and washed, and then the amount bound was determined by incubation with 5 μg of TRITC-labeled rabbit anti-human IgG/ml for 5 min (signal 1). This response was calibrated by incubating the probes with a standard concentration of human antiplague IgG (10 μg/ml) for 5 min followed by a final incubation with TRITC-labeled rabbit anti-human IgG for 5 min (signal 2). The signal 1/signal 2 ratio was plotted versus the known amount of human antiplague IgG (Fig. 4). This method was not utilized to analyze any samples with unknown amounts of antiplague antibody due to problems associated with nonspecific adsorption of normal human IgG.
FIG. 4.
Sandwich immunoassay standard curve for serum antiplague antibody. Probes coated with rabbit antiplague IgG were primed with F1 antigen (10 μg/ml) and incubated with increasing amounts of purified human antiplague IgG. Finally, the amount of human antiplague IgG bound to the probe was delineated by the binding of TRITC-labeled rabbit anti-human IgG (5 μg/ml) for 5 min. The mean ± standard error for three probes is shown at each concentration.
Evaluation of samples with unknown amounts of antiplague antibody.
USAMRIID provided 11 numbered serum samples to be quantified. These samples were obtained from human volunteers who had been immunized against plague (Table 1). By using the modified competitive method with probes coated with rabbit antiplague IgG, the concentration of each sample was determined. After an initial screen, it was sometimes necessary to dilute the strongly positive samples with PBS in order to obtain a ratio on the sensitive portion of the standard curve. Samples that generated a signal ratio of 1.50 or above were considered to contain no detectable amount of antiplague antibody.
TABLE 1.
Analysis of serum samplesa
| Sample no. | Reciprocal ELISA titer | Signal ratio | Sample concn (μg/ml)
|
|
|---|---|---|---|---|
| Diluted (dilution) | Original | |||
| 9 | None (negative control serum) | 2.86 | 0 | 0 |
| 7 | <32 | 2.0 | 0 | 0 |
| 8 | <32 | 1.98 | 0 | 0 |
| 3 | 256 | 1.3 | 2.3 (1:4) | 9 |
| 1 | 4,096 | 0.38 | 11.7 (1:4) | 47 |
| 2 | 4,096 | 0.71 | 6.1 (1:9) | 55 |
| 4 | 4,096 | 0.52 | 8.6 (1:4) | 34 |
| 5 | 16,384 | 0.3 | 14.3 (1:29) | 415 |
| 6 | 16,384 | 0.22 | 18.1 (1:19) | 344 |
| 11 | 16,348 | 0.69 | 6.3 (1:29) | 183 |
| 10 | 32,768 | 0.51 | 8.7 (1:29) | 252 |
The serum samples prepared at USAMRIID were tested at the Naval Research Laboratory. The samples were tested neat by competitive immunoassay and diluted and retested if necessary to obtain a signal ratio on a responsive portion of the standard curve (Fig. 3). These values are comparable to the ELISA titers determined at USAMRIID.
DISCUSSION
A number of methods to quantify antiplague antibodies with the fiber-optic biosensor were examined. The competitive assay with probes coated with F1 antigen failed to provide adequate concentration discrimination. While a large signal decrease occurred after exposure to 5 μg of 6H3-IgG/ml, the F1 antigen-coated probes required very high concentrations of antibody (100 μg/ml) to block subsequent signal generation.
To circumvent this difficulty, a competitive assay that used rabbit antiplague probes was tried. In this assay, a small amount of F1 antigen was bound to these probes. This was done to calibrate the probes’ response. The probes were then coated with a larger amount of F1 antigen. This second amount could then be bound by antiplague IgG in the sample or by the signal-generating T-6H3-IgG. This method resulted in a response which could discriminate 0 to 15 μg/ml of antiplague IgG/ml.
A sandwich immunoassay with TRITC-labeled rabbit anti-human IgG as the signal-generating antibody was also investigated. Not being a competitive assay, this method was expected to be significantly more sensitive. In addition, the sandwich assay discriminates between immunoglobulin classes, while the competitive assays do not. The standard curve did show a limit of detection improved to 0.25 μg of human antiplague IgG/ml (Fig. 4), but this method was abandoned when it was observed that the control samples often generated signals due to the nonspecific adsorption of normal human IgG. Carvalho et al. (7), who have also investigated various assay formats for plague antibody detection, did not experience this difficulty, indicating that it may be a surface-specific phenomenon.
Of the various methods investigated, the competitive assay with probes coated with rabbit antiplague IgG was selected for an additional trial to quantify antiplague IgG in a series of samples supplied by USAMRIID (Table 1). The correlation between ELISA data and that obtained with the fiber-optic biosensor was excellent. The biosensor identified three negative serum samples while also correctly identifying positive samples with low (<10 μg/ml), medium (10 to 100 μg/ml), and high (>100 μg/ml) concentrations of antiplague IgG. To quantify a sample often required one or two dilutions to place the response on the sensitive portion of the standard curve, increasing total assay time. However, for rapid screening purposes, either no dilution or a 1:4 dilution, which would permit “yes” or “no” answers or rough concentration determinations, could be utilized.
These experiments demonstrate that this biosensor can successfully be utilized to determine serum antiplague-antibody levels. This method would simplify and decrease the cost of determining the effectiveness of immunizations. With further biosensor development, it may one day aid in the field screening of animals when testing for previous exposure to plague (8, 14, 18).
ACKNOWLEDGMENTS
This work was supported by the Office of Naval Research, the Naval Medical Research and Development Command, and the U.S. Army Medical Material Development Agency.
REFERENCES
- 1.Amante L A, Ancona A, Forni L. Conjugation of immunoglobulins with tetramethylrhodamine isothiocyanate. J Immunol Methods. 1972;1:289. doi: 10.1016/0022-1759(72)90006-3. [DOI] [PubMed] [Google Scholar]
- 2.Anderson G P, Golden J P, Ligler F S. Fiber optic biosensor: combination tapered fibers designed for improved signal acquisition. Biosens Bioelectron. 1993;8:249–256. [Google Scholar]
- 3.Baker E E, Sommer H, Foster L E, Meyer E, Meyer K F. Studies on immunization against plague. J Immunol. 1951;68:131–145. [PubMed] [Google Scholar]
- 4.Bhatia S K, Shriver-Lake L C, Prior K J, Georger J, Calvert J M, Bredehorst R, Ligler F S. Use of thiol-terminal silanes and heterobifunctional crosslinkers for immobilization of antibodies on silica surfaces. Anal Biochem. 1989;178:408–413. doi: 10.1016/0003-2697(89)90662-3. [DOI] [PubMed] [Google Scholar]
- 5.Butler T C. Plague and other yersinia infections. New York, N.Y: Plenum Medical Book Co.; 1983. [Google Scholar]
- 6.Cao L K, Anderson G P, Ligler F S, Ezzell J. Detection of Yersinia pestis fraction 1 antigen with a fiber optic biosensor. J Clin Microbiol. 1995;33:336–341. doi: 10.1128/jcm.33.2.336-341.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho L B, Araujo A M, Almeida A M P, Azevedo W M. The use of polyvinyl alcohol glutaraldehyde antigen coated discs for laser induced fluorescence detection of plague. Sens Actuators. 1996;B35-36:427–430. [Google Scholar]
- 8.Chomel B B, Jay M T, Smith C R, Kass P H, Ryan C P, Barrett L R. Serological surveillance of plague in dogs and cats, California, 1979–1991. Comp Immunol Microbiol Infect Dis. 1994;17:111–123. doi: 10.1016/0147-9571(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 9.Eigler, F. S., J. Georger, S. K. Bhatia, J. Calvert, L. C. Shriver-Lake, and R. Bredehorst. December 1991. Immobilization of active agents on substrates with a silane and heterobifunctional crosslinking agent. U.S. patent 5,077,210.
- 10.Gasper P W, Barnes A M, Quan T J, Benziger J P, Carter L G, Beard M L, Maupin G O. Plague (Yersinia pestis) in cats. Description of experimentally induced disease. J Med Entomol. 1993;30:20–26. doi: 10.1093/jmedent/30.1.20. [DOI] [PubMed] [Google Scholar]
- 11.Golden J P, Saaski E W, Shriver-Lake L C, Anderson G P, Ligler F S. Portable multichannel fiber optic biosensor for field detection. Optical Eng. 1997;36:1008–1013. [Google Scholar]
- 12.Golden J P, Shriver-Lake L C, Anderson G P, Thompson R B, Ligler F S. Fluorometer and tapered fiber optic probes for sensing in the evanescent wave. Optical Eng. 1992;31:1458–1462. [Google Scholar]
- 13.Narang U, Anderson G P, Ligler F S, Burans J. Fiber optic biosensor for ricin. Biosens Bioelectron. 1997;12:937–945. doi: 10.1016/s0956-5663(97)00027-4. [DOI] [PubMed] [Google Scholar]
- 14.Paulmurphy J, Work T, Hunter D, Mcfie E, Fjelline D. Serologic survey and serum biochemical reference ranges of the free-ranging mountain lion (Felis-concolor) in California. J Wildl Dis. 1994;30:205–215. doi: 10.7589/0090-3558-30.2.205. [DOI] [PubMed] [Google Scholar]
- 15.Rasoamanana B, Leroy F, Boisier P, Rasolomaharo M, Buchy P, Carniel E, Chanteau S. Field evaluation of an immunoglobulin G anti-F1 enzyme-linked immunosorbent assay for serodiagnosis of human plague in Madagascar. Clin Diagn Lab Immunol. 1997;4:587–591. doi: 10.1128/cdli.4.5.587-591.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shriver-Lake L C, Breslin K A, Charles P T, Conrad D W, Golden J P, Ligler F S. Detection of TNT in water using an evanescent wave fiber optic biosensor. Anal Chem. 1995;34:2431–2435. [Google Scholar]
- 17.Tempelman L A, King K D, Anderson G P, Ligler F S. Quantitating staphylococcal enterotoxin B in diverse media using a portable fiber-optic biosensor. Anal Biochem. 1996;233:50–57. doi: 10.1006/abio.1996.0006. [DOI] [PubMed] [Google Scholar]
- 18.Thomas C U, Hughes P E. Plague surveillance by serological testing of coyotes (Canis-latrans) in Los Angeles County, California. J Wildl Dis. 1992;28:610–613. doi: 10.7589/0090-3558-28.4.610. [DOI] [PubMed] [Google Scholar]
- 19.Williams J E, Altieri P L, Berman S, Lowenthal J P, Cavanaugh D C. Potency of killed plague vaccines prepared from avirulent Yersinia pestis. Bull W H O. 1980;58:753–756. [PMC free article] [PubMed] [Google Scholar]
- 20.Williams J E, Arntzen L, Tyndal G L, Isaacson M. Application of enzyme immunoassay for the confirmation of clinically suspect plague in Namibia. Bull W H O. 1986;64:745–752. [PMC free article] [PubMed] [Google Scholar]