Abstract
Advances in regenerative medicine manufacturing continue to be a priority for achieving the full commercial potential of important breakthrough therapies. Equally important will be the establishment of distribution chains that support the transport of live cells and engineered tissues and organs resulting from these advanced biomanufacturing processes. The importance of a well-managed distribution chain for products requiring specialized handling procedures was highlighted during the COVID-19 pandemic and serves as a reminder of the critical role of logistics and distribution in the success of breakthrough therapies. This perspective article will provide insight into current practices and future considerations for creating global distribution chains that facilitate the successful deployment of regenerative medicine therapies to the vast number of patients that would benefit from them worldwide.
Keywords: regenerative medicine, manufacturing, distribution, shipping and logistics
Graphical Abstract
Graphical Abstract.
The establishment of distribution chains that support the transport of live cells and engineered tissues and organs is necessary for the dissemination of tissue-engineered products to patients worldwide. This perspective article provides insight into current practices and future considerations for creating global distribution chains, including current best practices for maintaining cell/tissue viability and the FDA codes that regulate these processes.
Significance Statement.
Advances in regenerative medicine provide new therapies for diseased tissues and organs, and manufacturing of these therapies continues to be a priority for achieving their full commercial potential. Equally important will be the establishment of distribution chains that support the transport of live cells and engineered tissues and organs resulting from these advanced biomanufacturing processes. This perspective article reviews current and future considerations for creating an international supply chain for distribution of these therapies.
Recent Distribution Examples and Future Trends
The complexity of current and future distribution chains that support the transport of live cells and engineered tissues, organs, and other perishable medical supplies has been highlighted by the worldwide experience of the last 2 years. Coronavirus Disease 2019 (COVID-19), caused by SARS-CoV-2, has been the focus of the world since the beginning of 2020. The pandemic resulted in the US Food and Drug Administration (FDA) granting Emergency Use Authorization (EUA) for 3 mRNA vaccines in record time: Pfizer-BioNTech (December 2020),1 Moderna (December 2020),2 and Janssen (February 2021).3 Shipping and storage conditions (ultra-cold vs refrigerated) of these vaccines were the subject of significant discussion (reviewed in Table 1).4–7 Ultra-cold shipping and storage, lack of a well-managed distribution chain, and endpoint distribution proved problematic during the initial distribution of vaccines, with a significant number of doses lost due to incorrect shipping or handling.8,9 This is not a new problem as the World Health Organization (WHO) reported in 2005 that more than 50% of vaccines released globally each year end up being discarded due to supply-chain and distribution problems, such as not having enough freezer space or proper transportation.9,10 Along with increasing demand for mRNA vaccines, the number of gene-, cell-, and tissue-based therapies in development continues to show steady growth. The Alliance for Regenerative Medicine estimates more than 1100 developers of products worldwide in 2020, a 10% increase from 2019.11
Table 1.
An overview of shipping logistics for COVID-19 vaccines
| COVID-19 vaccine | Shipping condition | Storage condition(s) | Dose |
|---|---|---|---|
| Pfizer-BioNTech | Dry ice | Ultra-cold freezer: Before mixing, the vaccine may be stored in an ultra-cold freezer between −80°C and −60°C (−112°F and −76°F). Vaccine may be stored until expiration date. Freezer: Before mixing, the vaccine may be stored in the freezer between −25°C and −15°C (−13°F to 5°F) for up to 2 weeks. Refrigerator: Before mixing, the vaccine may be stored in the refrigerator between 2°C and 8°C (36°F and 46°F) for up to 1 month (31 days). Mixed vaccine: Once mixed, vaccine can be left at room temperature (2°C to 25°C [35°F to 77°F]) for up to 6 hours. |
Multiple—2 doses (0.3 mL) (3 weeks apart) |
| Moderna | Frozen between −50°C and −15°C (−58°F and 5°F) | Unpunctured vials may be stored in the refrigerator between 2°C and 8°C (36° to 46°F) for up to 30 days. Punctured vials may be stored between 2°F and 25°C (36°F and 77°F) for up to 12 hours The expiration date is not printed on the vaccine vial or carton. To determine the expiration date: Scan the QR code located on the outer carton or go to www.modernatx.com/covid19vaccine-eua/ |
Multiple—2 doses (0.5 mL) (1 month apart) |
| Janssen (J&J) | The Janssen COVID-19 vaccine is initially stored frozen by the manufacturer and then shipped at 2°C to 8°C (36°F to 46°F). If vaccine is still frozen upon receipt, thaw at 2°C to 8°C (36°F to 46°F). If needed immediately, thaw at room temperature (maximally 25°C/77°F). Do not refreeze once thawed. | Unpunctured vials: Store multi-dose vials of the Janssen COVID-19 vaccine at 2°C to 8°C (36°F to 46°F) and protect from light. Do not store frozen. Punctured vials of Janssen COVID-19 vaccine may be stored between 9°C and 25°C (47°F to 77°F) for up to 12 hours. Storage after first puncture: Hold the vial between 2° and 8°C (36° to 46°F) for up to 6 hours or at room temperature (maximally 25°C/77°F) for up to 2 hours. Discard the vial if vaccine is not used within these times. |
Single dose (0.5 mL) |
Current Best Practices
Cell and Tissue Products
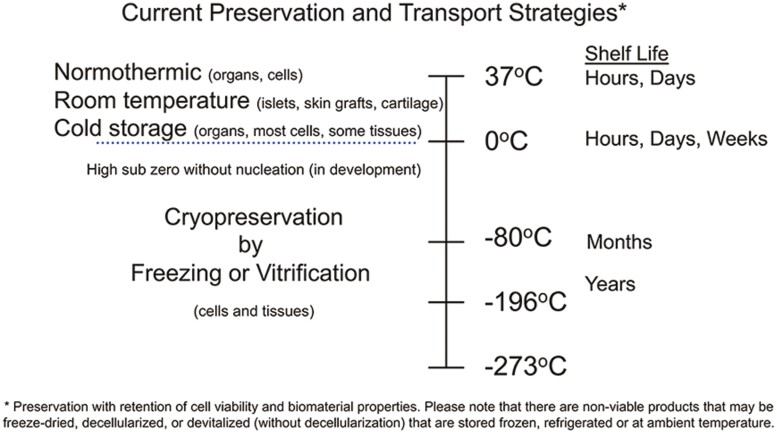
The major objective of storage and transport of cell- and tissue-engineered products is the preservation of cell/tissue viability and function. Cell therapies, such as hematopoietic stem cells, mesenchymal stem cells, and differentiated cell products can be stored and transported at 0-4°C for hours or a few days depending upon the storage solution used (Fig. 1).12 Longer-term storage requires cryopreservation by freezing in the presence of cryoprotectants such as DMSO either alone or in combination with high molecular weight substances such as hydroxyethyl starch.12–14 Gametes, such as sperm and ova for reproductive medicine and veterinary practices, most often use freezing or ice-free vitrification cryopreservation strategies with low and high cryoprotectant formulations, respectively. Cryopreserved cells are best maintained below the glass transition (Tg) of the cryoprotectant formulation used in vapor or liquid phase nitrogen. Stable storage is achieved at temperatures below −130°C where change should not occur for many years. Cryopreserved cell transport can be achieved using nitrogen dry shippers. In many cases, after verification, fresh dry ice at −78.5°C can be used.12 Currently, bioengineered tissue constructs are usually manufactured and delivered on wet ice “just-in-time.” The exceptions to this include the FDA-approved and commercially available tissue-engineered skin replacement products Dermagraft and Apligraf which are stored and delivered at −75°C and 20-23°C, respectively.15,16
Figure 1.
Current preservation and transport strategies. Certain cells, tissues, and organs can survive for hours to days when stored and transported at temperatures above freezing. Living cells and tissues must be kept at sub-zero temperatures in order to ensure long-term viability.
Tissue and Organ Transplantation
In 2020, approximately 39 000 organs17 and 1 million tissues18 were transplanted within the US. Despite increases in registered donors in recent years,19 there is still a considerable shortage of viable organs and tissues for transplant with estimates of nearly 7000 deaths annually in the US due to organs not reaching a recipient in time.20 Due to organ preservation being limited to hours of refrigerated or on ice storage (Fig. 1), organs are rushed to their locations and often not well matched to recipients due to the short geographical distance they may travel within their preservation limit.21 The lack of appropriate donor pool size is argued to be the greatest limiting factor for patients in need of organ transplantation,22 however, recent advances in organ storage, preservation, and transport have emerged as viable opportunities to extend organ preservation limit and/or transport distance.23 To meet the burgeoning demand of organ transplantation, the future will require a robust integration of donor matching services, advances in preservation, as well as supply-chain logistics associated with storage and transport.21
Unlike organs, cell viability is not an issue for most tissue allografts. Tissue allografts with retention of biomaterial, structural, properties can be stored on the order of weeks to years before use, albeit at the cost of increased processing to maintain safety and clinical efficacy, as well as complex storage requirements.24 Fresh tissues may be stored at low temperatures (4°C) using storage media or freeze-dried if ambient temperatures are necessary.24 The benefit of low temperature storage is the ability to maintain cell and tissue viability, when viability is needed, while also reducing the complexity of shipping and transport containers. While the increased viability and function of refrigerated fresh allografts presents an advantage to other options,25 current time constraints for tissue expiration can make their use logistically challenging. For instance, osteochondral allografts, the most common fresh tissue used in orthopedic surgery, are stored aseptically at 1-10°C with current expiration times around 28 days, including the quarantine period for bacterial, fungal, and viral tests.26 This presents a challenge for clinicians, patients, and hospitals to coordinate the use of these tissues within this short time frame. Combining effective recipient matching programs with advanced shipping and transport logistics could foreseeably reduce the discard of fresh allografts by increasing the likelihood of pairing these tissues to a patient prior to expiration.
In recent years, breakthroughs in cryopreservation of tissue allografts have emerged demonstrating enhanced preservation of cell viability and tissue function to months if not years.27 While these advances offer a clear advantage to conventionally stored fresh allograft tissues, they come at the cost of complex shipping and storage requirements as well as the added variable of thawing time for clinicians.24 Currently, cryopreserved, or frozen tissue specimens, are shipped in insulated containers with liquid nitrogen (so-called dry shipper) or dry ice to prevent thawing during transport. These containers often require rigorous validation with considerations in mode of transport, labeling, materials used, regional regulations, and environmental control.28 Improvements in packaging and real-time environmental control could result in sizeable cost-savings to tissue allograft manufacturers as well as hospitals and clinics where ultra-low temperature storage is not readily available.
Biological Considerations for Logistics within a Global Supply Chain
Regenerative Medicine Therapies
According to section 506(g)(8) of the US Food Drug & Cosmetic Act, regenerative medicine therapies (RMTs) include “cell therapies, therapeutic tissue engineering products, human cell and tissue products and combination products using any such therapies or products, except for those regulated solely under section 361 of the Public Health Service Act (42 U.S.C. 264) and Title 21 of the Code of Federal Regulations Part 1271 (21 CFR Part 1271).”29 The FDA further interprets section 506(g) so that RMTs may apply to human gene therapy products, which includes cells that have been genetically modified and will have a sustained influence on cells or tissues, as well as xenogeneic cell products. From the aforementioned definition, the scope of biological material that can potentially be transported as RMTs is diverse and it may not be possible to find a single solution that can be universally applied as a commercial logistics strategy.30
Whole blood transfusion, arguably the earliest form of cell therapy,31 can be seen as a success story for a well-managed biological supply chain. Annually, 6.8 million Americans donate blood, which results in the transfusion of approximately 21 million blood components in the same calendar year,32 The success is in part due to the 42-day shelf-life of red blood cells at 1-6°C that affords some flexibility in planning the collection, storage, and distribution of the fresh material.33 Other components of the whole blood, such as pooled platelets can survive for up to 5 days at 22°C and plasma is generally cryopreserved and stable for up to 36 months at −25°C.34,35 The apheresis (whole blood) for the isolation of peripheral blood mononuclear cells (PBMCs) destined as source material for immune therapies, are routinely shipped fresh at 4°C or carefully cryopreserved and shipped under cryogenic conditions (lower than −135°C for best viability).36 From the above examples, on structurally simple liquid-based samples, it already becomes apparent that the storage and subsequently transport temperature can vary considerably depending on the source material (Fig. 1). This is similarly reflected in the temperatures used for the storage and transport of hematopoietic stem cells, embryonic stem cells, and other primary cells that may serve as source material for RMTs or used in life science research. Final products in the RMT sector can be even more heterogenous, including, but not limited to 2D products such as iPSC-derived cells and co-cultures ranging to structurally complex 3D products such as organoids, tissue-engineered products, bioprinted products, or even lab-grown whole organs. The rate at which these novel therapies and research models are developing are rapidly outpacing the temperature-controlled logistics solutions that are currently available from −196° to +37°C, resulting in a new drive for innovative shipping technologies.37
It is therefore of utmost importance that RMT developers have the entire supply chain in mind at the beginning of product development. Within this context the risks of failing to bring the therapy to the market can be reduced, at least in the short term, by developing a commercially feasible and robust temperature-controlled logistics strategy, including qualified shippers, that balances manufacturing with the needs of each therapy and are compliant with all regulatory frameworks that apply.30 Because RMT products are considerably more valuable than traditional pharmaceutical products and may even be irreplaceable,38 it is essential to ensure they are shipped within the prescribed temperature range at the correct time for a successful delivery within a single country.39 Moreover, the dynamic and often deteriorating condition of the patient places even more pressure on ensuring the time-critical delivery window is met.39,40 Deliveries that cross international borders are particularly burdensome because regional regulations and regulatory jurisdictions are not consistent throughout the transport chain.39
RMT manufacturers can look to third-party logistics providers, who are experienced with providing a “white glove” service for high-value products, to aid in designing a tailor-made logistics solution. Not only can these providers help therapy manufacturers at an early stage to de-risk their supply chain, but they can also execute the strategy on the operational level.30 That being said, these organizations will manage the entire shipment in a GDP (Good Distribution Practice) compliant manner, track and trace all the required data for regulatory purposes and assist with all paperwork and labeling associated with shipping biological material across international borders.30,38 Excursions may not always be avoidable but having a partner that has access to restricted areas, such as the airport, to provide intervention services can mean the difference between a treated patient and a rejected shipment (https://www.time-matters.com).
Expanding the Definition of Global beyond the Earth
Regenerative medicine is an area of focus for research in microgravity on the International Space Station (ISS) that to date has included stem cell, organoid, tissue chip, and 3D bioprinting research. Changes in gene expression, cell signaling, aggregation, proliferation, differentiation, and cell-matrix interaction have been studied in a variety of cell types.41 Early work performed on the space shuttle reporting increased proliferation and delayed differentiation of stem cells has been a catalyst for continued research into potential future on-orbit manufacturing applications that could address current industry bottlenecks in production and advanced the understanding of stem cell biology.42 Since the space shuttle was retired in 2011, mission durations have extended from weeks to months, with the first long-duration stem cell culture performed on the ISS in 2016, which continues to confirm these early results.43 The logistics of launching biological payloads, time in transport to ISS, maintaining cell viability and health on-orbit, and return via splashdown into the Pacific (and now Atlantic) ocean require capabilities that address the unique considerations for transport in an extended supply chain beyond the planet.
Future Vision
Biopreservation
Bioengineered construct cryopreservation by freezing and vitrification strategies are in development.14 Drying methods based upon lyophilization (freeze-drying) and desiccation, although not currently reproducibly feasible, are also anticipated in the future.44,45 In the pharmaceutical and biopharmaceutical industries, drying and freeze-drying have been used increasingly for the manufacturing of small molecule drugs and biologically active molecules such as enzymes, hormones, antibiotics, and monoclonal antibodies (mAb), owing to their robust and reproducible procedures.46 Biological materials can be dried by either lyophilization or dehydration, also known as desiccation, to residual moisture levels not to exceed a limit linked to biomaterial quality. Presently, drying is applied primarily to products without living cells, such as bone and amniotic membrane-derived products.
In addition, a variety of novel approaches have been conceived to address the challenges of biopreservation method development for cells, tissues, and organs with retention of cell viability, biomaterial properties, and function. These approaches are inspired by mechanisms of freeze tolerance or freeze avoidance practiced by animals in nature47,48 and techniques using nanotechnology (ie, radiofrequency inductive heating of Fe nanoparticles to produce rapid warming rates), isochoric pressures, and non-Newtonian fluids to achieve cryopreservation.48 While still in development, other methods, such as intracellular trehalose, late embryogenesis abundant (LEA) protein expression, glass transition, and anti-plasticization, along with novel drying methods for removing water from mammalian cells continue to be explored.49–52 Cellphire Therapeutics, Inc. has developed 2 products currently in clinical trials that use long-term cryopreservation techniques, including Thrombosomes, a freeze-dried hemostatic derived from human platelets, and cryopreserved platelets (CPP) which are shelf-stable for up to 2 years (www.cellphire.com). Additionally, there have been a number of reports on lyophilized cell preservation, in addition to platelets, including red blood cells, mononuclear cells, and hematopoietic stem cells.53 This technology has potential applications across a wide range of medical applications, including point of care therapy, battlefield medicine, and regenerative medicine. Enabling the preservation of cell therapies in a dry state could improve supply-chain logistics, and bring both economic and practical benefits to patients, health care providers, and biopharmaceutical companies.54
Active and Passive Shipping Configurations
The sensitive nature of pharmaceuticals and biopharmaceuticals, along with the current shortcomings in maintaining the cold-chain throughout shipments, are leading to an upwards trend in the adoption of new passive and active configuration shippers.55 The trend appears to be skewed toward passive shippers, because hold-times of up to 120 hours are now becoming more common.55 Nonetheless, the need for active shippers is predicted to grow too, as electric or active shipper have a near unlimited running time when periodically recharged and are ultimately the safest solution for precious cargo.55
Innovation can also be seen in the rise of hybrid solutions or systems offering unique environmental control features. A noteworthy addition to the cold-chain is the VIA Capsule from Cytiva, a liquid nitrogen-free cryogenic shipper, that can be docked with a cooling engine for local storage when connected to an external power source.56 To complement the existing active configuration shippers, that are mostly aimed at the cold-chain, shippable CO2 incubators have recently been developed to transport living cell and tissue cultures under laboratory conditions in a so-called warm-chain (eg, 37°C). One such device, the Cellbox, is the only system with its own integrated CO2 source and an international flight allowance.57 Shippable CO2 incubators have demonstrated their value in providing a robust solution for shipping among others, “Multi-Organ-Chip” samples and bioprinted human full-thickness skin models under standard laboratory conditions.58
Regardless of the type of shipping container selected, it is critical to monitor the data relevant to the product integrity in a regulatory compliant manner throughout the shipment. Fortunately, there are many commercially available temperature loggers that can be placed inside the container along with the product to be monitored. When other parameters, such as humidity, gas concentrations, air pressure, and even vibration need to be monitored, this can be achieved by implementing multi-sensor temperature monitors instead.
In the simplest format, the loggers are offline devices that record measured values at regular intervals to an internal memory. Upon delivery of the package, the recipient is able to export the data from the device by means of a USB cable or similar physical connection in order to generate a shipment report. With many services migrating to a cloud infrastructure, hybrid approaches are also being offered by manufacturers. These devices will store the data locally and through access to a cellular network, Wi-Fi, or mobile access point, the information can be uploaded for secure storage in the cloud, providing the possibility of creating reports online without any physical access to the temperature logger.
Finally, after selecting the type of temperature monitor best suited for the application, companies that are FDA-regulated will need to ensure that they select a device that is also fully compliant with CFR21 part 11.29 This framework from the CFR published by the federal government of the US, provides detailed guidance on ensuring compliance and security when working with electronic data records. To simplify the entire process for the shipper, manufacturers of intelligent shipping containers, like the Cellbox, have integrated multi-sensor temperature monitors that can manage electronic data records in a CFR21 part 11 compliant manner. As the need to ship worldwide and even off world continues to increase, the ways in which data are collected, handled, and stored will need to adapt in order to provide regulators with a transparent audit trail for reviewing the safety and integrity of shipped products.
Space Industry Logistics Chain
Current capabilities that exist to accommodate continued regenerative medicine research in microgravity on ISS will continue to expand as potential future manufacturing applications are further explored and identified. These will include expanded capabilities for transport systems to ISS and return of future products, along with incorporation of current Good Manufacturing Practices (cGMP) into the logistics chain.
Conclusions
Regenerative medicine is poised to become a significant industry within the medical field. The important breakthrough products that this industry will produce require well-managed logistics and distribution chains that accommodate the successful transport of live cells and tissue from manufacturing sites to pharmacies and ultimately to patients. Failure in the transport chain is costly to manufacturers, the industry, and to the patients in need. Along with manufacturing initiatives, expanding existing capabilities and distribution chains to accommodate living cells and tissues is an equally critical component to achieving the full commercial potential of this industry.
Funding
None declared.
Conflict of Interest
K.B. declared employment, patent holder, and stock ownership with Tissue Testing Technologies LLC; advisory role with LifeNet Health; and research funding from Johnson and Johnson and Detraxi. The other authors declared no potential conflicts of interest.
Author Contributions
T.C.: Manuscript writing, final approval of manuscript. C.S., J.S., K.B., M.F., S.E.: Conception and design, collection of information, manuscript writing. J.H.: Final approval of manuscript, financial support.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. FDA authorizes Pfizer-BioNTech COVID-19 vaccine. Med Lett Drugs Ther. 2021;63(1615):1–2. [PubMed] [Google Scholar]
- 2. FDA authorizes Moderna COVID-19 vaccine. Med Lett Drugs Ther. 2021;63(1616):9–10. [PubMed] [Google Scholar]
- 3. U.S. Food and Drug Administration. J&J COVID-19 vaccine: emergency use authorization. Published Feb 27, 2021. Accessed July 18, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine
- 4. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine: storage and handling. Published 2021. Accessed July 18, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/downloads/storage-summary.pdf
- 5. Centers for Disease Control and Prevention. Moderna COVID-19 vaccine: storage and handling. Published 2021. Accessed July 18, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/downloads/storage-summary.pdf
- 6. Jouhnson J. Janssen storage, dosage, and administration guide . Published 2021. Accessed July 18, 2021. https://www.in.gov/health/immunization/files/Janssen-COVID-19-Vaccine-EUA-Launch-Storage,-Dosing,-and-Administration-Guide.pdf
- 7. Branswell H. Comparing the Covid-19 vaccines developed by Pfizer, Moderna, and Johnson & Johnson . Published 2021. Accessed July 18, 2021. https://www.statnews.com/2021/02/02/comparing-the-covid-19-vaccines-developed-by-pfizer-moderna-and-johnson-johnson/
- 8. Salcedo A. More than 16,000 vaccine does potentially spoiled in Maine and Michigan by temperature problems. The Washington Post. 2020. Accessed January 20, 2021. https://www.washingtonpost.com/nation/2021/01/20/moderna-vaccine-spoiled-maine-michigan [Google Scholar]
- 9. Siemaszko C. Thousands of COVID-19 vaccines wind up in the garbage because of fed, state guidelines . 2021. Accessed August 19, 2021. https://www.nbcnews.com/news/us-news/thousands-covid-19-vaccines-wind-garbage-because-fed-state-guidelines-n1254364
- 10. Setia S, Mainzer H, Washington ML, et al. Frequency and causes of vaccine wastage. Vaccine. 2002;20(7-8):1148–1156. 10.1016/s0264-410x(01)00433-9 [DOI] [PubMed] [Google Scholar]
- 11. Alliance for Regenerative Medicine. Growth & Resilience in Regenerative Medicine. Alliance for Regenerative Medicine; 2020. [Google Scholar]
- 12. Meneghel J, Kilbride P, Morris GJ.. Cryopreservation as a key element in the successful delivery of cell-based therapies—a review. Front Med (Lausanne). 2020;7:592242. 10.3389/fmed.2020.592242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baust JM, Campbell LH, Harbell JW.. Best practices for cryopreserving, thawing, recovering, and assessing cells. In Vitro Cell Dev Biol Anim. 2017;53(10):855–871. 10.1007/s11626-017-0201-y [DOI] [PubMed] [Google Scholar]
- 14. Bojic S, Murray A, Bentley BL, et al. Winter is coming: the future of cryopreservation. BMC Biol. 2021;19(1):56. 10.1186/s12915-021-00976-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Organogenesis. Dermagraft: Human Fibroblast-Derived Dermal Substitute. Organogenesis Inc.; 2021. [Google Scholar]
- 16. Organogenesis. Apligraf: Living Cellular Skin Substitute. Organogenesis Inc.; 2021. [Google Scholar]
- 17. Centers for Disease Control and Prevention. Transplant safety overview. Published 2021. Accessed August 25, 2021. https://www.cdc.gov/transplantsafety/overview/key-facts.html
- 18. Health Resources and Services Administration. Organ donation statistics. Published 2021. Accessed July 18, 2021. https://www.organdonor.gov/learn/organ-donation-statistics
- 19. U.S. Department of Health and Human Services. Annual Record Trend Continues for Deceased Organ Donation, Deceased Donor Transplants. U.S. Department of Health and Human Services; 2021. [Google Scholar]
- 20. Donate Life America. Organ, Eye and Tissue Donation Statistics. Published 2021. Accessed August 25, 2021. https://www.donatelife.net/statistics/ [Google Scholar]
- 21. Giwa S, Lewis JK, Alvarez L, et al. The promise of organ and tissue preservation to transform medicine. Nat Biotechnol. 2017;35(6):530–542. 10.1038/nbt.3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cameron AM, Barandiaran Cornejo JF.. Organ preservation review: history of organ preservation. Curr Opin Organ Transplant. 2015;20(2):146–151. 10.1097/MOT.0000000000000175 [DOI] [PubMed] [Google Scholar]
- 23. Jing L, Yao L, Zhao M, et al. Organ preservation: from the past to the future. Acta Pharmacol Sin. 2018;39(5):845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moore MA, Samsell B, McLean J.. Allograft tissue safety and technology. In: Wolfe K, Horigan J, eds. Biologics in Orthopaedic Surgery. 1st ed.Elsevier; 2019:49–62. [Google Scholar]
- 25. Demange M, Gomoll AH.. The use of osteochondral allografts in the management of cartilage defects. Curr Rev Musculoskelet Med. 2012;5(3):229–235. 10.1007/s12178-012-9132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torrie AM, Kesler WW, Elkin J, et al. Osteochondral allograft. Curr Rev Musculoskelet Med. 2015;8(4):413–422. 10.1007/s12178-015-9298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rorick CB, Mitchell JA, Bledsoe RH, et al. Cryopreserved, thin, laser-etched osteochondral allograft maintains the functional components of articular cartilage after 2 years of storage. J Orthop Surg Res. 2020;15(1):521. 10.1186/s13018-020-02049-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Association of Tissue Banks. Qualification of packaging and validation of shipping/transport procedures. Published 2017. Accessed July 18, 2021. https://www.aatb.org/sites/default/files/guidance-docs/AATB-Guidance-Doucment-No9-10-23-17.pdf
- 29. U.S. Food and Drug Administration. Resources related to regenerative medicine therapies. Published 2020. Accessed August 22, 2021. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/resources-related-regenerative-medicine-therapies
- 30. Ellison S, McCoy R, Bell M, Frend K, Ward S.. Logistics by design: framework for advanced therapy developers to create optimal Logistics Platforms [Expert Insight]. Cell Gene Ther Insights. 2018;4(10):1019–1040. [Google Scholar]
- 31. Li Z, Liu D.. Cell therapy must be regulated as medicine. Exp Hematol Oncol. 2015;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. American Red Cross. Importance of the blood supply. Published 2021. Accessed August 19, 2021. https://www.redcrossblood.org/donate-blood/how-to-donate/how-blood-donations-help/blood-needs-blood-supply.html
- 33. Cancelas JA, Dumont LJ, Maes LA, et al. Additive solution-7 reduces the red blood cell cold storage lesion. Transfusion. 2015;55(3):491–498. 10.1111/trf.12867 [DOI] [PubMed] [Google Scholar]
- 34. Aubron C, Flint AWJ, Ozier Y, et al. Platelet storage duration and its clinical and transfusion outcomes: a systematic review. Crit Care. 2018;22(1):185. 10.1186/s13054-018-2114-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Snyder EL, Stack G, Napychank P, et al. Storage of pooled platelet concentrates: in vitro and in vivo analysis. Transfusion. 1989;29(5):390–395. 10.1046/j.1537-2995.1989.29589284136.x [DOI] [PubMed] [Google Scholar]
- 36. Kofanova OA, Davis K, Glazer B, et al. Viable mononuclear cell stability study for implementation in a proficiency testing program: impact of shipment conditions. Biopreserv Biobank. 2014;12(3):206-216. 10.1089/bio.2013.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirchner VKA. Packaging for the Most Challenging Shipments. World Courier; 2017. Accessed July 18, 2021. https://www.worldcourier.com/insights/packaging-for-the-most-challenging-shipments [Google Scholar]
- 38. Cryoport Systems. The regenerative medicine supply chain: anticipate new compliance standard now or risk falling behind. Published 2019. Accessed August 19, 2021. https://www.cryoport.com/chain-of-compliance-white-paper-download?submissionGuid=311687c7-6f0e-4732-871e-dbd43aa05481
- 39. Stephens N, Morrison M, Martin P, Hogle L.. Spatiotemporal readiness is key to preparing regenerative medicine for the clinic [Commentary]. Regen Med. 2021;16(3):229-235. [DOI] [PubMed] [Google Scholar]
- 40. Wang K, Liu Y, Li J, et al. A multiscale simulation framework for the manufacturing facility and supply chain of autologous cell therapies. Cytotherapy. 2019;21(10):1081-1093. [DOI] [PubMed] [Google Scholar]
- 41. Mains R, Reynolds S, Baker T, Sato K.. A researchers guide to cell biology. ISS researcher’s guide series. 2015. Accessed August 19, 2021. https://www.nasa.gov/connect/ebooks/researchers_guide_cellular_biology_detail.html
- 42. Blaber EA, Finkelstein H, Dvorochkin N, et al. Microgravity reduces the differentiation and regenerative potential of embryonic stem cells. Stem Cells Dev. 2015;24(22):2605–2621. 10.1089/scd.2015.0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wnorowski A, Sharma A, Chen H, et al. Effects of spaceflight on human induced pluripotent stem cell-derived cardiomyocyte structure and function. Stem Cell Rep. 2019;13(6):960–969. 10.1016/j.stemcr.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Drake AC, Lee Y, Burgess EM, et al. Effect of water content on the glass transition temperature of mixtures of sugars, polymers, and penetrating cryoprotectants in physiological buffer. PLoS One. 2018;13(1):e0190713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang M, Oldenhof H, Sydykov B, et al. Freeze-drying of mammalian cells using trehalose: preservation of DNA integrity. Sci Rep. 2017;7(1):6198. 10.1038/s41598-017-06542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ward KR, Matejtschuk P.. The principles of freeze-drying and application of analytical technologies. Methods Mol Biol. 2021;2180:99–127. 10.1007/978-1-0716-0783-1_3 [DOI] [PubMed] [Google Scholar]
- 47. Brockbank KG, Campbell LH, Greene ED, et al. Lessons from nature for preservation of mammalian cells, tissues, and organs. In Vitro Cell Dev Biol Anim. 2011;47(3):210–217. 10.1007/s11626-010-9383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taylor MJ, Weegman BP, Baicu SC, et al. New approaches to cryopreservation of cells, tissues, and organs. Transfus Med Hemother. 2019;46(3):197-215. 10.1159/000499453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Solanki PK, Rabin Y.. Thermomechanical stress analysis of rabbit kidney and human kidney during cryopreservation by vitrification with the application of radiofrequency heating. Cryobiology. 2021;100:180–192. 10.1016/j.cryobiol.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kahvand F, Fasihi M.. Plasticizing and anti-plasticizing effects of polyvinyl alcohol in blend with thermoplastic starch. Int J Biol Macromol. 2019;140:775–781. 10.1016/j.ijbiomac.2019.08.185 [DOI] [PubMed] [Google Scholar]
- 51. Czernik M, Fidanza A, Luongo FP, et al. Late Embryogenesis Abundant (LEA) proteins confer water stress tolerance to mammalian somatic cells. Cryobiology. 2020;92:189–196. 10.1016/j.cryobiol.2020.01.009 [DOI] [PubMed] [Google Scholar]
- 52. Brogna R, Oldenhof H, Sieme H, et al. Increasing storage stability of freeze-dried plasma using trehalose. PLoS One. 2020;15(6):e0234502. 10.1371/journal.pone.0234502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Merivaara A, Zini J, Koivunotko E, et al. Preservation of biomaterials and cells by freeze-drying: change of paradigm. J Control Release. 2021;336:480-498. 10.1016/j.jconrel.2021.06.042 [DOI] [PubMed] [Google Scholar]
- 54. Weng L. Technologies and applications toward preservation of cells in a dry state for therapies. Biopreserv Biobank. 2021;19(4):332–341. 10.1089/bio.2020.0130 [DOI] [PubMed] [Google Scholar]
- 55. Jafferi N. Innovation is driving pharma air cargo growth [Industry Report]. STAT. 2019:1–17. [Google Scholar]
- 56. cytiva. VIA Capsule cryogenic shipment system. Published 2021. Accessed August 24, 2021. https://cdn.cytivalifesciences.com/dmm3bwsv3/AssetStream.aspx?mediaformatid=10061&destinationid=10016&assetid=34099
- 57. Cellbox Solutions. Published 2021. Accessed August 24, 2021. https://cellbox-solutions.com
- 58. Cellbox Solutions. A taste of Cellbox applications. Published 2021. Accessed August 24, 2021. https://cellbox-solutions.com/application
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.