Abstract
The potential roles of specific antibodies of the different immunoglobulin G (IgG) subclasses in the serological diagnosis of cystic echinococcosis (CE) and alveolar echinococcosis (AE) were investigated by an enzyme-linked immunosorbent assay based on hydatid fluid as antigen. Specific antibodies of subclass 1 were found to be of major importance. In sera collected at the time of diagnosis (i.e., before any therapeutic intervention was initiated) they could be demonstrated in 14 of 15 sera from patients with CE and in all 12 sera from patients with AE. The most discriminatory and the most specific antibodies found in this study belonged to IgG subclass 4. Only one false-positive reaction was observed with 253 sera from healthy volunteers, and no cross-reactions occurred in 80 sera from patients with different parasitic infections. Specific IgG4 antibodies could be demonstrated in 61.0 to 66.7% (CE) or 47.6 to 66.7% (AE) of the cases. Antibody levels of IgG subclass 2 were elevated only moderately, and subclass 3 antibodies were detected in a few cases only. In addition, nonspecific reactions in sera of healthy volunteers or patients with other parasitic infections could partially be attributed to antibodies of subclasses 2 and 3.
Echinococcosis is caused by metacestode stages of tapeworms of the genus Echinococcus (family Taeniidae). Within this genus, four species, Echinococcus granulosus, E. multilocularis, E. vogeli, and E. oligarthrus, are recognized which all may establish and develop in the human host. Among them, E. granulosus and E. multilocularis are the clinically most relevant species which are responsible for cystic echinococcosis (CE) and alveolar echinococcosis (AE) in humans, respectively.
The disease is usually diagnosed by clinical examinations using different imaging techniques (ultrasonography, computerized tomography, magnetic resonance imaging), which are supported by the demonstration of specific serum antibodies. The serological diagnosis in a routine laboratory depends mainly on the detection of immunoglobulin class G (IgG) antibodies directed against different antigens of E. granulosus or E. multilocularis. Sensitivity and specificity of the serological tests depend on the stage of the disease, the localization of the parasites, the antigens, and the techniques used (2, 4).
Cyst fluid (CF) of E. granulosus cysts of sheep or cattle origin is one of the most widely used antigens, and the enzyme-linked immunosorbent assay (ELISA) is one of the most commonly used techniques in serodiagnostic laboratories. In cases of CE of the liver, antibodies against CF antigens can be detected with a high diagnostic sensitivity by this method. In eight independent studies, CF-based ELISA systems detected 90% (83.2 to 100%) of the cases with CE (6). The overall specificities of the tests were reported to be very high (96.0 to 100%; average, 99.3%) in these studies, but considerable cross-reactivity due to other parasitic infections (1.7 to 48.7%; average, 17.6%) was recorded. Therefore, additional serological tests and/or clinical examinations are required for a reliable diagnosis. For cases of AE, similar detection rates have been reported in the literature (4) for this method. However, better-defined highly specific antigens are available for the serological diagnosis of AE, as reviewed by Gottstein (4).
A number of recent reports demonstrate the value of analyzing specific IgG subclass antibodies for the sensitive and specific serological diagnosis of echinococcosis or for follow-up studies after surgery or after initiation of chemotherapy (1, 5, 7–10).
The present study was designed to assess the value of the detection of specific IgG subclass antibodies for the serological diagnosis of CE and AE in a standard CF-based ELISA system.
MATERIALS AND METHODS
Sera.
Fifty-six sera from patients with clinically confirmed CE of the liver (group CE) and 54 sera from patients with hepatic AE (group AE) were used in this study. In 41 patients (73%) of group CE and in 42 (78%) of group AE, parasitic lesions had been surgically removed 1 to 5 years ago. Cutoff values were calculated on the basis of 240 sera from healthy adult individuals. An additional group of 253 healthy volunteers (group C) was used for the determination of the different specificities. A group of 80 sera from patients with various other parasitic infections (group P) (malaria, 4; leishmaniasis, 8; amebiasis, 8; toxoplasmosis, 4; filariasis, 8; strongyloidosis, 8; trichinellosis, 8; toxocariasis, 8; fasciolosis, 8; schistosomiasis, 8; cysticercosis, 8) was used for cross-reactivity studies.
ELISA.
All sera were diluted (1:200) in phosphate-buffered saline containing 0.3% Tween 20 and analyzed according to a standard ELISA procedure using CF collected from E. granulosus cysts of cattle. The preparation of the test plates and the immunoassays were performed as described elsewhere (3). Specific antibodies were detected with γ-chain-specific affinity-purified (polyclonal) goat anti-human IgG (Dako) and IgG1-, IgG2-, IgG3-, and IgG4-specific secondary antibodies (The Binding Site) conjugated to alkaline phosphatase. Optimal antigen concentration and dilutions of secondary antibodies were previously determined by checkerboard titrations. All experiments were performed at a final antigen concentration of 5 μg/ml, and final dilutions of secondary antibodies were 1:800 for anti-IgG; 1:1,000 for anti-IgG2, anti-IgG3, and anti-IgG4; and 1:2,000 for anti-IgG1 antibodies. Optical densities at 405 nm (OD405) were read after incubation periods of 15 min at 37°C. All experiments were repeated twice.
Discrimination coefficients.
In a first step, batches of 12 sera each from groups CE, AE, P, and C were selected to determine the power of specific IgG or IgG subclass antibodies to discriminate between positive and negative reactions. Discrimination coefficients were calculated by division of the mean OD405 values of the sera of groups CE and AE by the mean OD405 values of sera from groups P and C. In order to distinctly demonstrate any possible differences, sera which were known to give rather high background or high nonspecific reactions in a standard ELISA were specifically selected from groups P and C. No threshold values were determined for this part of the study.
Sensitivity, specificity, and cross-reactions.
In order to assess the value of the individual detection of specific IgG subclass antibodies for the serological diagnosis of echinococcosis in a diagnostic laboratory by a standard ELISA, the analysis of specific antibodies of the IgG subclasses 1 and 4 was compared to the analysis of specific IgG antibodies by comparing individual sensitivities, specificities, and cross-reactions of the different methods for the sera described above.
RESULTS
Discrimination coefficients.
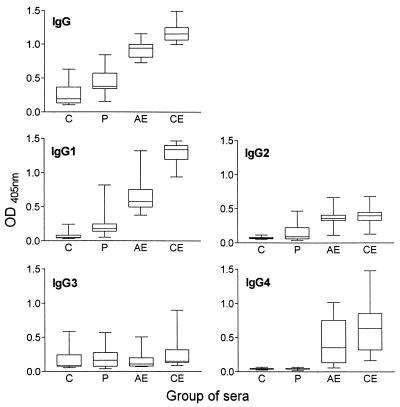
The OD405 values of the sera are given in Fig. 1 for all antibody detection systems, and the discrimination coefficients are listed in Table 1. For both groups, CE and AE, distinct levels of specific IgG1 antibodies were detected in all sera tested whereas specific IgG4 antibodies could be demonstrated in 50 to 60% of the cases only. The marked reactivities of many sera of groups C and P observed in the standard IgG ELISA were greatly reduced when antibodies of the IgG subclasses 1 and 4 were analyzed in separate tests. For IgG1, only one serum of group C showed a distinct reaction, whereas for IgG4 no nonspecific reactions were recorded.
FIG. 1.
IgG- and IgG subclass-specific OD405 values for 12 sera each of groups C, P, AE, and CE.
TABLE 1.
Group discrimination coefficients
| Discriminationa | Discrimination coefficient
|
||||
|---|---|---|---|---|---|
| IgG | IgG1 | IgG2 | IgG3 | IgG4 | |
| Group CE | |||||
| Group C | 4.7 | 17.6 | 5.4 | 1.5 | 14.7 |
| Group P | 2.6 | 5.7 | 2.6 | 1.3 | 14.4 |
| Group AE | |||||
| Group C | 3.7 | 9.2 | 4.9 | 1.0 | 9.5 |
| Group P | 2.0 | 3.0 | 2.4 | 0.9 | 9.3 |
Twelve sera from each group were tested.
In all sera of groups CE and AE, reactions of IgG subclass 2 antibodies were generally low and specific IgG3 antibodies were detected in a few cases only. On the other hand, pronounced nonspecific reactions were observed in a number of sera from groups P and C for IgG3 antibodies and in a few sera of group P for IgG2 antibodies.
The highest discrimination coefficients between sera of group CE and group C were calculated for IgG1 (17.6) and IgG4 (14.7). In these cases, the coefficients were enhanced more than three times compared to the analysis of specific IgG antibodies. A clear improvement (more than 2.5 times) in the corresponding coefficients was also found for the sera of group AE.
Antibodies of subclass 4 allowed the clearest discrimination between CE or AE sera and sera of patients with other parasitic infections (group P), with discrimination coefficients of 14.7 and 9.5, respectively. For IgG1, the corresponding values were also clearly enhanced compared to IgG. For IgG2, all discrimination coefficients were in the same range as for total IgG. Coefficients determined for IgG3 antibodies were in the range of 0.9 to 1.5 and allowed no discrimination of the different groups.
Sensitivity and specificity.
For the detection of specific IgG antibodies, the cutoff value was calculated as the mean OD405 of 240 sera from healthy individuals plus 3 standard deviations. Based on the enhanced discrimination coefficients found for IgG1 and IgG4 antibodies, the corresponding IgG1- and IgG4-specific cutoff values were arbitrarily set at 5 standard deviations above the mean OD405 of the same 240 sera.
In the CE group, between 93.0 and 97.6% positive reactions were recorded for specific IgG and IgG1 antibodies, whereas specific IgG4 antibodies could be detected in only 61.0 to 66.7% of the sera tested. No significant differences between sera from patients collected at the time of diagnosis (i.e., before any therapeutic intervention was initiated) and sera from patients who were surgically and/or chemotherapeutically treated were observed.
In sera from patients with AE collected at the time of diagnosis, specific IgG and IgG1 antibodies could be detected in all cases. Specific IgG4 antibodies were found in 66.7% of the sera. In sera collected from patients after surgical intervention and initiation of chemotherapy, specific IgG, IgG1, and IgG4 antibodies were present in 78.6, 76.2, and 47.6% of the sera, respectively (Table 2).
TABLE 2.
Sensitivity
| Group (n) | No. of positive reactions (%)
|
||
|---|---|---|---|
| IgG | IgG1 | IgG4 | |
| CE | |||
| Group Aa (15) | 14 (93.0) | 14 (93.0) | 10 (66.7) |
| Group Bb (41) | 40 (97.6) | 40 (97.6) | 25 (61.0) |
| AE | |||
| Group Aa (12) | 12 (100.0) | 12 (100.0) | 8 (66.7) |
| Group Bb (42) | 33 (78.6) | 32 (76.2) | 20 (47.6) |
Serum samples were taken before any therapeutic intervention (surgery and chemotherapy) was initiated.
Serum samples were taken 0.5 to 5 years after surgery (complete resection for CE, complete or partial resection for AE) and initiation of chemotherapy.
Only a few positive reactions were observed among 253 sera from healthy volunteers (Table 3). Six nonspecific reactions were recorded for IgG antibodies, three were recorded for IgG1, and one was recorded for IgG4. Therefore, specificity was highest for IgG4 (99.6%). Specific IgG4 antibodies could be demonstrated only in sera in which specific antibodies of subclass 1 were also present. All sera positive for specific IgG1 antibodies were also positive in the standard IgG ELISA.
TABLE 3.
Specificity
| Ig | No. of positive reactions (n = 253a) | % Specificity |
|---|---|---|
| IgG | 6 | 97.6 |
| IgG1 | 3 | 98.8 |
| IgG4 | 1 | 99.6 |
Sera from healthy blood donors.
Cross-reactions.
Eighteen cross-reactions were observed when sera from patients with different other parasitic infections were analyzed for antiechinococcus IgG antibodies (Table 4). The highest proportion was found in sera from patients with trematode infections (8 of 16) and in patients with cysticercosis (3 of 8). An additional six positive reactions were recorded among patients with nematode infections (n = 32). The number of nonspecific reactions was reduced to 12 when specific IgG1 antibodies were analyzed. However, 7 of 32 nonspecific reactions were found in nematode-infected patients, whereas in patients with trematode infections (n = 16) or cysticercosis (n = 8) only 2 false-positive reactions occurred. Only one false-positive reaction was noted for patients with protozoan infections (n = 24) for both IgG and IgG1. In this system, IgG4 antibodies proved to be highly specific. No positive reactions were recorded among the 80 sera from patients with parasitic infections.
TABLE 4.
Cross-reactions
| Group (no. of sera tested) | No. of positive reactions
|
||
|---|---|---|---|
| IgG | IgG1 | IgG4 | |
| Protozoan infections (24)a | 1 | 1 | 0 |
| Nematode infections (32)b | 6 | 7 | 0 |
| Trematode infections (16)c | 8 | 2 | 0 |
| Cysticercosis (8) | 3 | 2 | 0 |
| Total (80) | 18 | 12 | 0 |
| Cross-reactions (%) | 22.5 | 15.0 | |
Malaria, 4; leishmaniasis, 8; amebiasis, 8; toxoplasmosis, 4.
Filariasis, 8; strongyloidosis, 8; trichinellosis, 8; toxocariasis, 8.
Fasciolosis, 8; schistosomiasis, 8.
DISCUSSION
This study was designed to assess the value of the demonstration of single specific IgG subclass antibodies for the serological diagnosis of echinococcosis in a standard ELISA as it is used by many diagnostic laboratories. No attempts were made to compare the levels of specific subclass antibodies quantitatively, and all comparisons are valid only on a qualitative scale.
The results of our study demonstrate that specific antibodies of the IgG subclasses 1 and 4 are of major importance for the serological diagnosis of CE and AE in a CF-based ELISA system. The important role of antibodies of these two subclasses has also been demonstrated in earlier reports (1, 8, 9). In serum samples of patients with CE, parasite-specific antibodies were found mainly among subclasses 1 (63%) and 4 (30%). Furthermore, 18% of all IgG4 antibodies were found to be parasite specific (1). Specific IgG4 and IgG1 antibodies were found more frequently than specific IgG2 or IgG3 antibodies in sera from CE or AE patients with partially purified antigen B or a crude antigen prepared from protoscolices of E. multilocularis, respectively (9). In a follow-up study of AE patients after surgical treatment or chemotherapy, decreasing levels of specific IgG4 antibodies seemed to be a promising serological marker for a beneficial outcome (10).
These results are in contrast to an earlier report (5) where antibodies directed against antigen 5 were analyzed by means of an ELISA. Specific antibodies in serum samples from patients with CE were found among all subclasses, and specific IgG3 antibodies proved to be the most discriminatory. Furthermore, specific antibodies of all IgG subclasses were also found in patients with different responses to pharmacological treatment (7).
However, since all these studies analyzed IgG subclass antibodies directed against different antigens by means of different ELISA systems, the results cannot be directly compared.
In our system, based on crude CF as antigen, the detection of specific IgG and IgG1 antibodies was as sensitive with diagnostic serum samples from patients with CE as with those from patients with AE. Although not completely eliminated, nonspecific reactions in nonechinococcosis sera were considerably reduced for this subclass compared to the number of false-positive reactions observed for specific IgG antibodies. The higher specificity can be partially explained statistically. The enhanced discrimination between positive and negative sera (Fig. 1) allowed us to fix the threshold value at 5 standard deviations above the mean OD405 of the negative-control sera. Surprisingly, this had no negative effect on the sensitivity compared to the analysis of specific IgG antibodies.
Since IgG2 antibody levels were elevated in patients with CE and AE to a moderate extent only (Fig. 1), they seemed to contribute little to the discrimination between positive and negative sera. On the contrary, 3 of 12 sera of group P also gave distinct responses and might have been partially responsible for elevated reactions when specific IgG antibodies were analyzed.
Distinctly elevated IgG3 antibody levels were also found in some of the sera of groups C (3 of 12) and P (4 of 12), and no differences between the mean group OD405 discrimination coefficients of Echinococcus and non-Echinococcus sera could be found. The results suggest that nonspecific reactions are at least partially due to reactions of antibodies of the IgG subclasses 2 and 3 and that antibodies of these two subclasses contribute little to the discrimination between positive and negative sera in our system.
A single nonspecific reaction among 253 sera from healthy volunteers and no positive reaction at all in sera from patients with various parasitic infections were recorded for IgG4 antibodies, suggesting that the most specific antibodies produced during the course of the infection belong to this subclass. The finding that specific IgG4 antibodies could be demonstrated in only 66.7% of the sera (CE and AE) collected at the time of diagnosis makes the analyses of these antibodies unsuitable for screening purposes.
One of the major drawbacks of our study is the use of an undefined antigen. The humoral immune response observed in such systems is necessarily a mixed response characterized by various antibodies directed against a number of different antigens. Changes in the antibody response to one particular antigen during the course of the infection may well be overshadowed by changes in antibody responses against other antigens. Therefore, such systems are of little value for the follow-up of the development of the antibody response during the course of an infection, and no attempts were made to evaluate our system for this purpose. However, hydatid fluid collected from E. granulosus cysts is the most sensitive and probably the most widely used antigen for the serological diagnosis of CE. Owing to the fact that in cases of AE cross-reacting antibodies can also be detected, this antigen may also be used in screening systems for AE.
Based on our observations, the specificity of such standard test systems for the demonstration of anti-Echinococcus antibodies might be enhanced without loss of sensitivity by substituting anti-IgG1 antibodies for anti-IgG antibodies in the detection system. Furthermore, the demonstration of specific IgG4 antibodies in this system might serve as a confirmatory test with a high positive predictive value in a proportion of the cases. Also, for the characterization of specific immunodominant Echinococcus antigens, e.g., by a two-dimensional Western blot, IgG4 antibodies hold the best promise.
ACKNOWLEDGMENTS
We thank J. Eckert and R. C. A. Thompson for helpful discussions and for carefully reading the manuscript.
REFERENCES
- 1.Aceti A, Pennica A, Teggi A, Fondacaro L M, Caferro M, Leri O, Tacchi G, Celestino D, Quaranta G, DeRosa F, Sebastiani A. IgG subclasses in human hydatid disease: prominence of the IgG4 response. Int Arch Allergy Immunol. 1993;102:347–351. doi: 10.1159/000236582. [DOI] [PubMed] [Google Scholar]
- 2.Craig P S. Immunodiagnosis of Echinococcus granulosus. In: Anderson F L, et al., editors. Compendium on cystic echinococcosis. Provo, Utah: Brigham Young University; 1993. pp. 85–118. [Google Scholar]
- 3.Gottstein B, Eckert J, Frey H. Serological differentiation between Echinococcus granulosus and E. multilocularis infection in man. Parasitol Res. 1983;69:347–356. doi: 10.1007/BF00927876. [DOI] [PubMed] [Google Scholar]
- 4.Gottstein B. Molecular and immunological diagnosis of echinococcosis. Clin Microbiol Rev. 1992;5:248–261. doi: 10.1128/cmr.5.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hira P R, Bahr G M, Shweiki H M, Behbehani K. Diagnostic value of anti-arc 5 IgG antibody and analysis of the IgG subclasses in sera of patients with cystic hydatid disease. Serodiagn Immunother Infect Dis. 1990;4:285–293. [Google Scholar]
- 6.Llano R. M.D. thesis. Zurich, Switzerland: University of Zurich; 1997. [Google Scholar]
- 7.Rigano R, Profumo E, Ioppolo S, Notargiacomo S, Ortona E, Teggi A, Siracusano A. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol. 1995;102:281–285. doi: 10.1111/j.1365-2249.1995.tb03778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shambesh M K, Craig P S, Wen H, Rogan M T, Paolillo E. IgG1 and IgG4 serum antibody responses in asymptomatic and clinically expressed cystic echinococcosis patients. Acta Trop. 1997;64:53–63. doi: 10.1016/s0001-706x(96)00637-7. [DOI] [PubMed] [Google Scholar]
- 9.Wen H, Craig P S. Immunoglobulin G subclass responses in human cystic and alveolar echinococcosis. Am J Trop Med Hyg. 1994;51:741–748. doi: 10.4269/ajtmh.1994.51.741. [DOI] [PubMed] [Google Scholar]
- 10.Wen H, Bresson-Hadni S, Vuitton D A, Lenys D, Yang B M, Ding Z X, Craig P S. Analysis of immunoglobulin G subclass in the serum antibody response of alveolar echinococcosis patients after surgical treatment and chemotherapy as an aid to assessing the outcome. Trans R Soc Trop Med Hyg. 1995;89:692–697. doi: 10.1016/0035-9203(95)90449-2. [DOI] [PubMed] [Google Scholar]