Alzheimer’s disease (AD) is the most common cause of dementia, accounting for 60% to 80% of cases worldwide.1 The pathologic hallmark of the disease is the accumulation of extracellular beta amyloid plaques and intracellular tau filaments, ultimately leading to neuronal death. The current approved treatments slow down the progression of the disease, but do not stop the neurodegeneration caused by it. The past 2 decades have witnessed repeated failures of clinical trials using disease-modifying therapies targeting amyloid.2 These failures have prompted a major rethinking of the approach, with the general consensus being that potential treatments would work better if started at an earlier, possibly preclinical, stage of the disease.3
With the redoubled efforts to diagnose AD at earlier stages that could be amenable to preventive therapy, the field has been in search of biomarkers, clues that aid in diagnosing the disease early and help differentiate AD from other causes of dementia. These biomarkers would be most useful if they were noninvasive, widely available, reliable, sensitive, and specific to AD. Furthermore, the ideal biomarker would allow clinicians to make the diagnosis during the prodromal stages of AD, before the onset of debilitating dementia, as it is these earlier stages that are now the focus of therapeutic efforts. However, diagnosing patients at these stages of the disease remains difficult and costly. Thus, if retinal imaging can be validated as a biomarker to confirm the clinical suspicion of AD, this would be a highly attractive potential tool.
In this population-based, cross-sectional study in the Saku region of Japan (see page 107), persons 65 to 86 years of age underwent retinal OCT imaging and neuropsychological testing to explore the association between cognitive impairment and retinal thickness.4 The study revealed an inverse association between macular ganglion cell complex thickness and the presence dementia after adjusting for potential confounders including age and refractive error. Additionally, in the macular inferior sectors, several OCT measures were associated with the presence of dementia, defined as having a score of less than 23 on the Mini-Mental State Exam. The authors included psychiatric assessment to exclude participants with depression and also attempted to exclude participants with vascular dementia based on their history of stroke. Importantly, they did not report the functional impact of cognitive status on participants’ activities of daily living. In clinical practice, a score of 23 on the Mini-Mental State Exam alone does not establish the diagnosis of dementia because the same score can be obtained by an individual with normal activities of daily living who then would belong in the diagnostic category of mild cognitive impairment (MCI), not dementia. This subtle difference also highlights why the field could benefit greatly from additional noninvasive and quantitative biomarkers when evaluating patients with cognitive impairment.
Macular pathologic involvement in AD was described first in histopathologic specimens of human donors by Blanks et al5 in 1989. These authors showed that in the macula, the ganglion cells were affected in the temporal parafoveal region.6 The ganglion cell loss extended to the entire retina, particularly superior and inferior mid-peripheral regions.7 These studies also showed significant histopathologic evidence of loss and degeneration of the ganglion cell axons in the optic nerve in the AD eyes.5 With that in mind, the field of retinal imaging has been in search of the best tools to reveal these pathologic changes. Studies have explored retinal blood flow and OCT of the nerve and macula with varying success in different populations.
A lack of finding of significant changes in the peripapillary retinal nerve fiber layer (RNFL), representing the axons of the ganglion cells, in the current study population is intriguing on many levels. Recently, using the United Kingdom Biobank database, which is a prospective multi-center, community-based study of United Kingdom residents 40 to 69 years of age at enrollment, Ko et al8 found that thinner peripapillary RNFL was associated with worse cognitive performance at baseline. Further, in a smaller subset of the population that underwent repeat cognitive testing (1251 participants), the authors found that those with the thinnest quintile of RNFL thickness were more likely to perform worse on repeat cognitive testing, suggesting a correlation between baseline RNFL and future cognitive decline, even after controlling for potential confounders. The discrepancy between the current study and others that found a correlation between AD and RNFL thickness may be explained by several factors. For example, the correlation between baseline cognitive function and RNFL data in the United Kingdom Biobank dataset demonstrated a nonlinear, but rather curvilinear, correlation with the lowest quintiles of RNFL (<45 μm and 45–50 μm) showing much stronger correlation than the higher quintiles. This finding could explain in part the lack of association in the current study, in which the authors considered the entire range of RNFL, rather than examining the different quartiles of thickness. Another possibility is the different inclusion criteria, especially glaucoma, which was a definite exclusion in the United Kingdom Biobank study design, but not in the current study. Another difference relates to the different cognitive tests used in these studies. The Mini-Mental State Exam used to categorize participants in the current study has been used widely, but suffers from a lack of discrimination for the milder range of cognitive impairment and may not be sufficient to distinguish control participants from those with MCI.9 In contrast, the United Kingdom Biobank study used a set of psychological tests that were specifically more sensitive to these milder stages of impairments. Another difference relates to the age of the populations, with the Asian/Japanese study having a distinctly older population, as well as a clear difference in the racial background of the 2 populations, with the United Kingdom Biobank study being overwhelmingly white, whereas the population in the current was entirely Asian/Japanese. It is a possibility that different populations can differ in their retinal manifestations of AD, although we are not aware of any research to support this.
Another salient negative finding in the current study is the lack of association of any of the OCT metrics with the MCI category, an earlier stage of cognitive dysfunction considered to be a precursor to dementia resulting from AD and representing an important population for studies aiming for earlier treatment to prevent the onset of dementia. As the authors stated, MCI encompasses a loosely defined clinical spectrum, with a range of cognitive deficits and a wide range of susceptibility to progression to AD. Although it would be highly desirable to find an ocular parameter that correlates with this earlier, prodromal state, the current study was not designed with that goal in mind. Within the MCI clinical spectrum, amnestic MCI is a subtype that is most likely to be caused by AD. Thus, 50% of these individuals progress to dementia resulting from AD within 30 months.10 A recent meta-analysis showed the potential of the peripapillary RNFL to be affected significantly in people with MCI when considering the total available evidence in 6 rigorously performed studies with a total of 161 MCI patients from 5 different populations.11 More recently, OCT angiography has revealed that macular capillary density is decreased significantly in patients with amnestic MCI and early AD, providing further evidence that the retina may be affected in well-defined populations with prodromal stages of AD.12
Confirming the diagnosis of AD relies on clinical suspicion, coupled with confirmatory imaging and fluid biomarker evidence, with AD diagnosis definitively confirmed only at brain autopsy. Cerebrospinal fluid biomarkers and amyloid positron emission tomography scans remain the most widely studied and validated, although they suffer from being relatively invasive and expensive, respectively, which has restricted their wide clinical implementation.13,14 Compared with these approaches, retinal OCT imaging presents a far less invasive, less costly, and higher resolution method that is more widely accessible globally. Therefore, an important need exists to investigate whether retinal OCT imaging parameters can be informative as potential biomarkers of AD in the clinical arena. The AD field is in dire need of noninvasive biomarkers that would aid in confirming or disproving the diagnosis of AD, when it is clinically suspected and before the onset of dementia. Future large-scale, multi-center, and multi-ethnic studies need to correlate the retinal (macular and optic nerve) parameters with other, more pathologically relevant, brain biomarkers such as cerebrospinal fluid amyloid and tau load, amyloid load, or both on positron emission tomography scans. Another important area of investigation would be whether these retinal findings progressively worsen as an individual patient’s cognitive impairment and brain neuronal loss advance, which would be critical validation of the potential for the retina to serve as a surrogate marker for AD pathologic progression, supplementing information from other biomarkers.
Overall, this is an exciting time for the field of retinal imaging in the arena of cognitive disorders of aging. Accumulating data show that the retina could provide valuable insights into the diagnosis of AD and cognitive impairment. Pending the next level of rigorous multi-center studies, it is fair to say that retinal imaging one day could serve as a potential biomarker in AD studies.
Pictures & Perspectives
Pembrolizumab-Related Enophthalmos.
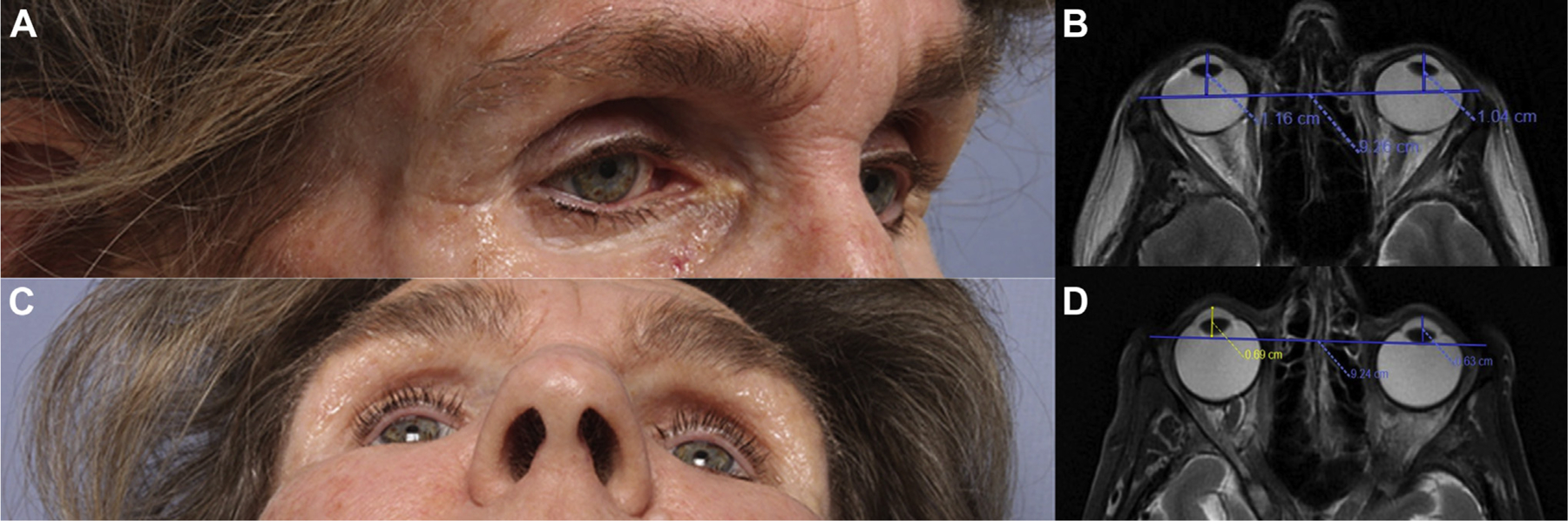
Pembrolizumab is a highly selective immune checkpoint inhibitor that targets the programmed cell death protein (PD-1) receptor on lymphocytes and has been approved for the treatment of malignant melanoma. A 59-year-old woman had been treated for 1 year with pembrolizumab for melanoma metastatic to lung, spleen, and central nervous system. She noted changes of her facial appearance with temporal wasting and sunken eyes while denying any decrease in body weight. Exophthalmometry was 11 mm for the right eye (OD) and 10 mm for the left eye (OS). (Fig A, B) Axial magnetic resonance imaging (MRI) scans performed 6 months apart (Fig C, D) demonstrated a significant change in globe position with 4.7 mm recession on the right and 4.1 mm on the left. There were no signs of orbital metastasis or inflammation. She had no other medical or iatrogenic factors to explain loss of orbital volume and enophthalmos. (Magnified version of Fig A-D is available online at www.aaojournal.org).
Footnotes
Financial Disclosure(s): The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Contributor Information
Amani A. Fawzi, Chicago, Illinois.
Sandra Weintraub, Chicago, Illinois.
Waleed Fawzi, London, United Kingdom.
References
- 1.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 2018;14(3):367–429. [Google Scholar]
- 2.Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 2014;6(4):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan TK. An algorithm for preclinical diagnosis of Alzheimer’s disease. Front Neurosci 2018;12:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito Y, Sasaki M, Takahashi H, et al. Quantitative assessment of the retina using OCT and associations with cognitive function. Ophthalmology 2019;127:107–118. [DOI] [PubMed] [Google Scholar]
- 5.Blanks JC, Hinton DR, Sadun AA, Miller CA. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res 1989;501(2):364–372. [DOI] [PubMed] [Google Scholar]
- 6.Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging 1996;17(3):377–384. [DOI] [PubMed] [Google Scholar]
- 7.Blanks JC, Schmidt SY, Torigoe Y, et al. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging 1996;17(3):385–395. [DOI] [PubMed] [Google Scholar]
- 8.Ko F, Muthy ZA, Gallacher J, et al. Association of retinal nerve fiber layer thinning with current and future cognitive decline: a study using optical coherence tomography. JAMA Neurol 2018;75(10):1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 2009;43(4):411–431. [DOI] [PubMed] [Google Scholar]
- 10.Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 2007;68(4):288–291. [DOI] [PubMed] [Google Scholar]
- 11.Knoll B, Simonett J, Volpe NJ, et al. Retinal nerve fiber layer thickness in amnestic mild cognitive impairment: case-control study and meta-analysis. Alzheimers Dement (Amst) 2016;4: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YS, Zhou N, Knoll BM, et al. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer’s disease on optical coherence tomography angiography. PLoS One 2019;14(4):e0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 2016;15(7): 673–684. [DOI] [PubMed] [Google Scholar]
- 14.Henriques AD, Benedet AL, Camargos EF, et al. Fluid and imaging biomarkers for Alzheimer’s disease: where we stand and where to head to. Exp Gerontol 2018;107:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]


