Abstract
Intracellular calcium (Ca2+) concentration ([Ca2+]i) is implicated in proliferation, invasion, and metastasis in cancerous tissues. A variety of oncologic therapies and some candidate drugs induce their antitumor effects (in part or in whole) through the modulation of [Ca2+]i. Cervical cancer is one of most common cancers among women worldwide. Recently, major research advances relating to the Ca2+ signals in cervical cancer are emerging. In this review, we comprehensively describe the current progress concerning the roles of Ca2+ signals in the occurrence, development, and prognosis of cervical cancer. It will enhance our understanding of the causative mechanism of Ca2+ signals in cervical cancer and thus provide new sights for identifying potential therapeutic targets for drug discovery.
Keywords: cervical cancer, Ca2+ signals, occurrence, development, prognosis
1. Introduction
Intracellular calcium (Ca2+) is an important messenger that exists in almost all cells, and exerts biological effects through various signal pathways [1,2,3]. Increasing intracellular free Ca2+([Ca2+]i) is a universal mechanism of signal transduction, which controls a variety of cellular processes, including proliferation, metabolism, and gene transcription [1,2]. However, under certain conditions, the increase in [Ca2+]i concentration is cytotoxic. For example, intracellular Ca2+ overloading promotes apoptosis of myoblasts, and increasing intracellular Ca2+ can cause an escalation of reactive oxidative species [4,5]. The concentration of [Ca2+]i depends on various calcium channels, calcium pumps, and interacting proteins and ligands in the body to ensure its level within an appropriate range and give full play to its biological effects. Therefore, as a messenger, the concentration of [Ca2+]i is kept within a strict range [1].
Cervical cancer is the fourth most common cancer in women worldwide, the fourth highest cancer-related mortality rate in the world, and second only to breast cancer among cancers of the female reproductive system [6]. Among women, cervical cancer diagnoses accounted for 6.6% of all cancer types diagnosed during the same period, with a mortality rate of 7.5% [6]. Human papillomavirus (HPV, most notably HPV16 and HPV18) has been clearly defined as a human carcinogen, and its persistent infection in the cervix has been identified as an important cause of cervical cancer [7]. However, only a small part of persistent infection may develop into cervical cancer, which indicates that in addition to HPV, there are many other factors in the body that also contribute to the development of cervical cancer. Among them, the Ca2+ signaling pathway is closely related to the occurrence, development, and prognosis of cervical cancer [1,8]. Cellular Ca2+ homeostasis and signaling are maintained by a special set of proteins, such as Ca2+ channels, pumps, exchangers, sensors, and Ca2+ activation effectors [9]. When the expression, structure, and/or function of these proteins change, it may cause corresponding adverse effects. This review summarizes the latest findings regarding the pathogenic roles of Ca2+ channels, pumps and sensors, and Ca2+ activation effectors in cervical cancer, with the aim of providing new ideas and corresponding therapeutic targets for cervical cancer (Table 1).
Table 1.
Ca2+ channels/pumps in cervical cancer.
| Ca2+ Channels/Pumps | Expression | Effects on Cervical Cancer | References |
|---|---|---|---|
| TRPV1 | Increased | Increased hazard ratio for overall survival, cell viability, and colony formation | [10,11] |
| TRPV6 | Decreased | Lead to poor prognosis | [12] |
| TRPM4 | Increased | Promote cancer cell proliferation | [13,14,15] |
| TRPM7 | Increased | Promote cancer cell proliferation and invasion | [16,17,18] |
| Orai1 and STIM1 | Increased | Cancer cell proliferation, migration, and angiogenesis increase; correlates with prognosis | [19,20] |
| IP3R3/ITPKC | / | Genetic polymorphism is associated with an increased risk of cervical squamous cell carcinoma | [21,22] |
| SERCA2 | Increased | Positive correlation with clinical stage | [23] |
| S100A7, S100A9, S100A11, S100A14 | Increased | Correlation with tumor grade and lymph node metastasis; promotes cancer cell proliferation, migration, and invasion | [24,25] |
| IKCa1 | Increased | Positively correlated with malignancy, promoting dedifferentiation and cancer cell proliferation | [26] |
2. Ca2+ Channels, Pumps, and Interacting Proteins and Ligands in Cervical Cancer
2.1. Transient Receptor Potential (TRP) Channels
TRP channels are a ubiquitous superfamily of ion channels in the body, responsible for a wide range of cellular functions, many of which regulate [Ca2+]i homeostasis and transduction [27,28,29]. TRP channels are gated by multiple stimuli, which differ between different family members. Some of them are triggered by mechanical stimuli and drive intracellular signaling pathways through spatiotemporally controlled Ca2+ influx [30]. Mechanosensitive Ca2+ channels play a key role in the rapid transmission of physical signals into biocompatible messages to influence key processes during development, morphogenesis, and regeneration [30]. TRP channels are divided into seven mammalian subfamilies according to their structural homology, including TRPV (vanilloid), TRPM (melastatin), TRPC (canonical), TRPP (polycystin), TRPML (mucolipin), TRPA (ankyrin), and TRPN (NOMPC-like) [27]. Each family has different activation modes [27,29,31]. As one of the channels for the influx of extracellular Ca2+, TRP channels play an important role in regulating [Ca2+]i concentrations [27]. Activation of TRP channels results in changes in [Ca2+]i concentrations, which are also required for the function of intracellular organelles, such as endosomes and lysosomes.
TRPV1 could be gated by multiple stimuli, such as proinflammatory substances, heat, endocannabinoids, resiniferatoxin, lipoxygenase products, and peptide toxins [32]. TRPV1 mediated apoptosis mainly through protein signaling; in addition, this channel also stimulated proliferation through ATP release, purinoceptor 2 (P2Y2) activation, and epidermal growth factor receptor (EGFR) transactivation in [33]. Therefore, exogenous and endogenous TRPV1 agonists combined with P2Y2 and EGFR antagonists may constitute a potential form of anticancer therapy [33]. Some studies have found that the expression of TRPV1 in cervical cancer tissue is significantly higher than that in cervical intraepithelial neoplasia and normal epithelial tissue [10,11]. In cervical cancer tissue, the expression of TRPV1 was negatively correlated with the expression of PTEN (a suppressor gene) in [10]. High expression of TRPV1 was an independent prognostic factor for overall survival in cervical cancer in [10]. In particular, high TRPV1/low PTEN expression showed the highest hazard ratio for overall survival. In addition to this, the in vitro results of this study showed that overexpression of TRPV1 was associated with increased cell viability and colony formation, making it a potential candidate biomarker to predict the responses of cervical cancer cells to chemoradiation [10]. In addition, a study by Lucido CT et al. found that TRPV1 expression was significantly increased with the development of cervical cancer [11]. However, no relevant studies have confirmed whether the increased TRPV1 expression is associated with cervical cancer progression through pathways such as ATP release, P2Y2 activation, and EGFR transactivation. Based on existing research on the combined application of TRPV1 agonists and corresponding antagonists in cervical cancer and lung cancer cell [34,35], it is a very meaningful work to carry out TRPV1-related therapeutic targets for the treatment of cervical cancer.
TRPV6 is uniquely highly selective for Ca2+ and plays an important role in the extracellular Ca2+ influx pathway and the maintenance of Ca2+ homeostasis in organisms [30]. Ca2+ influx through this channel depends on intracellular and extracellular Ca2+ balance [36]. In addition to this, the activity of this channel can be regulated by hormones, such as estrogen and progesterone, tamoxifen, and vitamin D, leading to changes in cell proliferation and survival [37]. The relationship between TRPV6 and malignant transformation of various tissues has been extensively studied, and its expression level has been found to be elevated in many cancers, including breast cancer and pancreatic cancer [38,39]. The increased TRPV6 expression can stimulate cancer cell metastasis and generate chemoresistance [39,40]. In addition to this, high expression of TRPV6 is associated with cell proliferation and invasion through Ca2+-dependent pathways, and its elevated levels are thought to correlate with breast and prostate cancer prognosis [37,41,42]. However, in a study by Sun et al., it was found that both TRPV6 mRNA and protein levels were significantly reduced in early cervical squamous cell carcinoma tissues and cell lines [12]. The expression of TRPV6 in early-stage cervical cancer was significantly correlated with the tumor stage, tumor growth type, tumor size, differentiation grade, and poor prognosis [12]. Moreover, the early-stage cervical cancer patients with a low TRPV6 expression had a short progress-free survival and overall survival duration [12]. Univariate and multivariate analyses identified TRPV6 as an independent prognostic factor for early cervical cancer patients’ survival, indicating that TRPV6 may be used as a novel prognostic marker for early cervical cancer [12]. The expression of TRPV6 in cervical cancer is different from other cancers, and additional research will be needed to validate this difference, as well as to uncover the underlying mechanisms and develop novel therapeutic targets based on TRPV6.
TRPM4 belongs to the TRPM channel subfamily. Unlike other TRP families, it does not directly transduce Ca2+, but is directly activated by intracellular Ca2+, which then directs Na+ influx. The influx of Na+ depolarizes the plasma membrane, reducing the driving force of store-operated Ca2+ entry (SOCE) and other Ca2+ entry pathways, thereby regulating [Ca2+]i concentrations [43,44]. Although TRPM4 is impermeable to Ca2+, Na+ influx through TRPM4 reduces membrane potential and leads to a reduction in Ca2+ signaling in many different cells, including cancer cells [31]. Thus far, TRPM4 has been investigated in various cancers. In several types of cancer, including colorectal tumor and prostate cancer, TRPM4 is overexpressed and contributes to cancer hallmark functions, such as proliferation, migration, invasion, and the epithelial-to-mesenchymal transition [45,46]. Hence, TRPM4 is considered a potential diagnostic marker for cancer progression and a promising anticancer drug target candidate [47]. A gene expression study of cervical cancer cases reported that TRPM4 was overexpressed in cervical cancer specimens compared with normal cervical epithelium [13]. Hong et al. reported that prostate cancer cells with reduced TRPM4 expression were found to have changes in cell distribution in each cell cycle [14]. Consistent with the study on prostate cancer cells, knockdown of TRPM4 in the cervical cancer-derived cell line HeLa also caused corresponding cell cycle changes, thereby reducing the proliferation of HeLa cells via β-catenin degradation [15]. This study of the cell cycle distribution of shRNA-mediated downregulation of TRMP4 levels found a significant reduction in the S-phase cell population, but a significant increase in the G1 phase cell population [15]. Moreover, after TRPM4 gene knockout, the expression levels of cell-cycle-related proteins changed, such as the decreased expression level of cyclin D1. Cyclin D1 promotes the transition of cells from G1 to S phase, which may explain the decreased cell proliferation observed after TRPM4 knockout [14]. TRPM4 is a potential prognostic cancer marker; further studies using TRPM4-KO cells or a specific TRPM4 inhibitor (9-phenanthrol, flufenamic acid, NBA (2-(1-naphthyloxyacetamido)-4-chloro -benzoic acid), and LBA (4-chloro-2-(2-(4-chloro-2-methylphenoxy) propanamidei) benzoic acid)), CBA ((4-chloro-2-(2-chlorophenoxy) acetamido) benzoic acid, also called compound 5)) treatments could give a better overview of the role of TRPM4 in the development, growth, and metastasis of cervical cancer [31,48]. Furthermore, based on the fact that TRPM4 is a channel located on the cell membrane, it will be an interesting target on inhibiting proliferation of cervical cancer cells [31].
TRPM7 preferentially allows the flow of Mg2+ and Ca2+, and in some cell types, the influx of Mg2+ through TRPM7 channels can lead to changes in intracellular Ca2+ levels [49,50]. Accumulating evidence has shown that TRPM7 is aberrantly expressed and/or activated in human cancers. TRPM7 plays a variety of functional roles in the hallmarks of cancer, including survival, cell cycle progression, proliferation, migration, and invasion [51,52]. More and more data also show that TRPM7 has potential value as a molecular biomarker and therapeutic target for human malignant tumors [52]. TRPM7 is also found to be aberrantly overexpressed and/or activated in cervical cancer, and has been discovered as the direct therapeutic target for cervical cancer [16,17,18]. For instance, Liu et al. demonstrated that TRPM7 is a target of miR-543 (a class of miRNAs that play an important role in the occurrence and development of various human carcinogenesis) in cervical cancer [16]. Restoration of TRPM7 expression partially reversed the tumor suppressive role of miR-543 on cervical cancer progression by promoting cell proliferation and invasion and attenuating cell apoptosis [53]. Dong et al. reported that miR-192-5p (a miRNA with broad anticancer effects) performs an inhibitory role in cervical cancer proliferation and invasion by targeting TRPM7 [17]. In addition, TRPM7 channel activity has proven to play an important role in the death of necrotic volume increase and necrotic cell in the acid poisoning of HeLa cells. Progesterone can inhibit the expression and activity of TRPM7, thereby transforming acid poisoning cells from necrotic to apoptosis [54].
TRPC is a classic transient receptor potential channel in the body. It generally comprises of seven isoforms, labeled TRPC1-7. These channels can regulate Ca2+ balance and promote cell cycle regulation and the expression/activation of Ca2+ related factors, thereby playing a key role in cancer cell proliferation [55,56]. Studies have found that the expression of TRPC in breast cancer tissues has increased significantly [57]. TRPC can be used as a potential drug target for cancer diagnosis and treatment [57,58]. However, there is no related research on the relationship between this channel and cervical cancer.
Apart from these, there are many TRP channels that are associate with intracellular Ca2+ concentration in cancer. However, there are no relevant studies to confirm their relationship with cervical cancer. Follow-up related research can be carried out, and then find out the target of cervical cancer treatment related to these TRP channels.
2.2. Ca2+ Release-Activated Ca2+ (CRAC) Channel
Store-operated calcium entry (SOCE) through the CRAC channel is a central mechanism by which cells generate Ca2+ signals and mediate Ca2+-dependent gene expression. The CRAC channel, composed of Ca2+ release-activated Ca2+ channel protein 1 (Orai1) and stromal interaction molecule (STIM), represents a prototypical example of SOCE to mediate Ca2+ entry between the endoplasmic reticulum and the plasma membrane in most nonexcitable cells [59,60]. There is accumulating evidence to indicate that the CRAC channel can influence various processes associated with tumorigenesis [61,62]. Abnormal expression of CRAC channel proteins has been observed in several types of cancer cells, indicating that CRAC-channel-activated Ca2+ influx will be a potential therapeutic target for cancer [61,62]. STIM recognizes a signal of reduced [Ca2+] in the ER and transmits this signal to Orai1 channels in the cell surface membrane to promote Ca2+ influx. In cancer cells, SOCE plays an important role in the cell cycle process, proliferation, migration, metastasis, and evasion of apoptosis [63]. The changes in the expression of a key element of reshaping SOCE and Ca2+ steady state play an important role in the transformation of the phenotypes observed in the transformation cell [64].
In cervical cancer, a recent study found that histone deacetylase 6 (HDAC6) is required for STIM1 translocation on microtubules and thus activates Orai1-mediated SOCE [19]. The levels of Orai1, STIM1, and HDAC6 were upregulated in cervical cancer cells [19]. Inactivation of HDAC6 with drug inhibitors or molecular knockdowns leads to low acetylation of tubulin and SOCE abolition. Interestingly, the expression of STIM1 and Orai1 was increased in most cervical cancer specimens, while the acetylation of tubulin was decreased [19,20], suggesting that specific targeting of HDAC6 in cervical cancer can inhibit STIM1-Orai1-mediated cervical neogenesis. STIM1 is very important for cervical cancer cell proliferation, migration, and vascular generation [19]. Increasing STIM1 expression is related to increased metastasis and decreased survival. STIM1 silencing in cervical cancer cells can significantly inhibit cancer cell proliferation. The excessive expression of STIM1 enhances the invasion of cervical cancer cells, while the knockout of STIM1 weakens this migration [19,20]. In addition, STIM1 can regulate the secretion of VEGF-A by cancer cells [20]. STIM1′s expression in tumors is also closely related to the clinical prognosis of early cervical cancer [20]. Related studies have found that SOCE inhibitors have the effect of blocking tumor blood supply in cervical cancer, prostate cancer, and breast cancer [20,65,66]. The SOCE channel and its related protein-mediated Ca2+ signal transformation is of great significance for the biological effects of the cell. Therefore, deeper research on SOCE will help to better understand the occurrence, development, and prognosis of cervical cancer, contributing to the development of novel methods and targets for the treatment of cervical cancer.
2.3. Inositol 1,4,5-Triphosphate Receptor (IP3R) and Ryanodine Receptors (RyR) Channels
Intracellular Ca2+ release channels in ER consist of a subset: mainly including IP3R channels and RyR channels. IP3R channels are activated by IP3 binding, and RyR channels are activated by elevated [Ca2+]i or protein signaling and ER releases Ca2+ through these channels [67,68].
The IP3R/Ca2+ signaling pathway is an extremely important part of maintaining body homeostasis. The signaling pathway has direct and indirect effects on the action of cells, including control of cell metabolism, secretion, fertilization, proliferation, and smooth muscle contraction [69,70]. Changes in IP3R/Ca2+ signaling are important factors in the development of a large number of human diseases [71]. Increasing evidence has shown that changes in the IP3R/Ca2+ signaling system are responsible for altered Ca2+ signaling in many cancer cells, such as lung cancer and cholangiocarcinoma clear cell renal cell carcinoma, and are closely related to the occurrence, development, and prognosis of cancer [72,73,74]. There are three IP3R isoforms—IP3R1, IP3R2, and IP3R3—expressed in mammals in different amounts. Yang et, al. reported that IP3R3 genetic polymorphisms are associated with the risk of cervical cancer [21]. The ITPKC gene encodes inositol 1,4,5-trisphosphate 3-kinase C, which inactivates IP3R3. ITPKC can inhibit the IP3 pathway by phosphorylating the active ligand of IP3Rs, inositol-1,4,5-trisphosphate (IP3), to a less active/inactive form (inositol-1,3,4,5-tetrakisphosphate), thereby weakening the Ca2+ signaling pathway, and its genetic polymorphism is associated with an increased risk of cervical squamous cell carcinoma [22,75].
RyR channels are the largest known ion channels that are located in the membrane of the ER and are expressed in a restricted subset of cell subtypes, such as cardiac and skeletal muscle cells [76]. One of its important roles is the release of Ca2+ from intracellular stores during excitation–contraction coupling in cardiac and skeletal muscle [77]. RyRs have three isoforms, RyR1, RyR2, and RyR3. They are embedded in the ER, mediating intracellular Ca2+ release, thus leading to the generation of a quick, transient increase in cytosolic Ca2+ levels [78]. RyRs are known to play key roles in the control of some major biological processes, such as metabolism, cell proliferation, and apoptosis [78]. RyRs can also be involved in the occurrence and development of cancers, including ovarian cancer, head and neck cancer, and prostate cancer [79,80,81]. Schmitt et al. indicated that impaired RyR2 function by either somatic mutation or epigenetic silencing is a common event in head and neck squamous cell carcinoma pathogenesis. Detection of RyR2 expression may be useful in assessing the risk of malignant transformation in dysplastic lesions [79]. Abdul et al. indicated that RyRs expression correlates with tumor grade in breast cancer; RyRs could serve as a prognostic indicator and/or as a target for breast cancer treatment [82]. Law et al. revealed the cytotoxic mechanism of neferine-induced autophagy through RyRs activation in resistant cancers, providing insights into the exploitation of novel interventions based on RyRs [83]. However, there are few studies on the relationship between the occurrence, development, and prognosis of cervical cancer and RyRs/Ca2+ signaling. Such studies cannot conclusively provide ideas for obtaining corresponding therapeutic targets.
2.4. SERCA Pump
Calcium is actively accumulated in the ER by sarcoendoplasmic reticulum (SR) calcium transport ATPase (SERCA enzymes). Unlike the above-mentioned channels that regulate ER Ca2+ homeostasis by releasing Ca2+, SERCA-dependent Ca2+ transport is the only Ca2+ uptake mechanism in this organelle [9]. Therefore, regulation of SERCA function is a key mechanism for regulating ER Ca2+ homeostasis according to cell type and its differentiation state. Regulation of SERCA activity can affect cell differentiation and survival [9]. Three SERCA genes are known (SERCA1, SERCA2, and SERCA3) by alternative splicing. The expression level of SERCA changes significantly during cell differentiation or tumorigenesis, resulting in altered ER calcium storage [9,84]. Studies have shown that elevated SERCA2 expression was detected in malignant cervical cancer, and this change was positively correlated with the clinical stage of malignant cervical cancer [23]. SBF-1, a synthetic steroidal glycoside, binds directly to SERCA2 to inhibit its function [85]. In human cervical cancer cells, SBF-1 represses SERCA2 function both in vitro and in vivo, thereby disrupting ER Ca2+ homeostasis and inducing ER stress-mediated cancer cell death. The study also found that SBF-1 inhibited the growth and migration of HeLa cells depending on the activity and level of SERCA2 [23]. In addition to this, they also indicated that SERCA2 is a potential therapeutic target for human cervical cancer. The concept of using SBF-1 as a chemotherapeutic drug in the treatment of cervical cancer is interesting. However, the application of SBF-1 is very limited due to its immunosuppressive activity on T lymphocytes and its possible adverse cardiovascular effects by affecting cell-wide SERCA2 channels [86].
2.5. Mitochondrial Calcium Uniporter (MCU) Channel
MCU is the pore-forming subunit of the MCU complex, which consists of MCU, the scaffold protein EMRE (Escherichia coli efflux-multidrug resistance E), and the Ca2+ sensitive inhibitory regulatory subunits MICU1 and MICU2 [87]. The MCU channel and its associated regulators transport Ca2+ across the inner mitochondrial membrane to the mitochondrial matrix. Due to this central role and ability to influence cell behavior and fate, several research groups are investigating the role of the MCU complex in different cancers and cancer-related diseases [87,88,89]. Currently, there have been very limited data on the changes of MCU expression or activity in cervical cancer. In one study, it was found that siRNA silencing of MCU in HeLa cervical cancer cells significantly reduced mitochondrial Ca2+ uptake. Furthermore, in HeLa cervical cancer cells, overexpression of MCU or knockdown of MICU1 resulted in constitutive mitochondrial Ca2+ influx [90].
2.6. Others
There are other molecules related to Ca2+ signaling in the body, such as S100 calcium-binding protein. When S100 calcium-binding protein binds to Ca2+ and can change its structure and function, thereby acting as a Ca2+ sensor, translating fluctuations in intracellular Ca2+ levels into cellular responses [91,92]. There are many subtypes of S100 calcium-binding protein, and several studies have found that S100A7, S100A9, S100A11, and S100A14 are closely related to cervical cancer [24,25,93,94]. Studies have shown that the expression of S100A11 in cervical cancer tissue is significantly higher than that in paracancerous tissue and normal cervical tissue. The overexpression of S100A11 can promote the proliferation and metastasis of cervical cancer cells [24]. Wang et al. found that the expression of S100A14 was increased in cervical cancer tissues, and this increase in expression was closely related to whether cervical cancer had metastasis [25]. They also found that in cervical cancer cells, S100A14 overexpression increased the ratio of the G2/M phase, which in turn promoted cell proliferation, migration, and invasion. The S100A14 gene knockout can eliminate the above changes [25]. Similar to S100A14, the expression of S100A7 was also significantly upregulated in cervical cancer tissues compared with normal cervical tissues. There is a correlation between S100A7 expression and tumor grade and lymph node metastasis [93]. In addition, S100A9 is overexpressed in cervical cancer [94]. The above S100 proteins are all related to the proliferation, metastasis, and invasion of cervical cancer. However, whether these pathological processes are realized through the Ca2+ pathway has not been confirmed yet.
The intermediate-conductance calcium-activated potassium channels (IKCa1) functions are strictly dependent on Ca2+. IKCa1 are expressed in many tissues and play a variety of physiological roles, including regulation of intracellular Ca2+ homeostasis [95,96,97]. In normal cells, IKCa1 regulate K+ efflux and hyperpolarization after channel activation by releasing intracellular Ca2+ stores [98]. In addition to this, IKCa1 are involved in cell proliferation. Several studies have shown that the functions of IKCa1 are required for Ca2+-sensing steps in cell cycle progression. Cells in a hyperpolarized state exhibit enhanced Ca2+ entry and Ca2+ homeostasis through K+ channel activation, which are critical for controlling cells through the G0/G1 or G1/S phase transition [99,100]. Related studies have found that drug blockade or gene reduction of IKCa1 in vitro reduced pancreatic and hepatocellular carcinoma cell proliferation and inhibited cell growth [101,102,103]. Ling L. et al. found that IKCa1 are highly expressed in cervical cancer tissue; the higher the malignancy of cervical cancer tissue has, the higher the IKCa1 expression is [26]. The findings also suggest that IKCa1 are involved in promoting the dedifferentiation and proliferation of malignant cervical cancer cells [26]. When using clotrimazole (an IKCa1 blocker), or knocking down IKCa1 using siRNA, the decrease in IKCa1 currents reduces the ability of Ca2+ influx, which reduces intracellular Ca2+ signaling that regulates cell cycle progression [26]. It further leads to the increased apoptosis of cancer cells, thereby inhibiting the growth and proliferation of cervical cancer HeLa cells [26].
3. Summary and Outlook
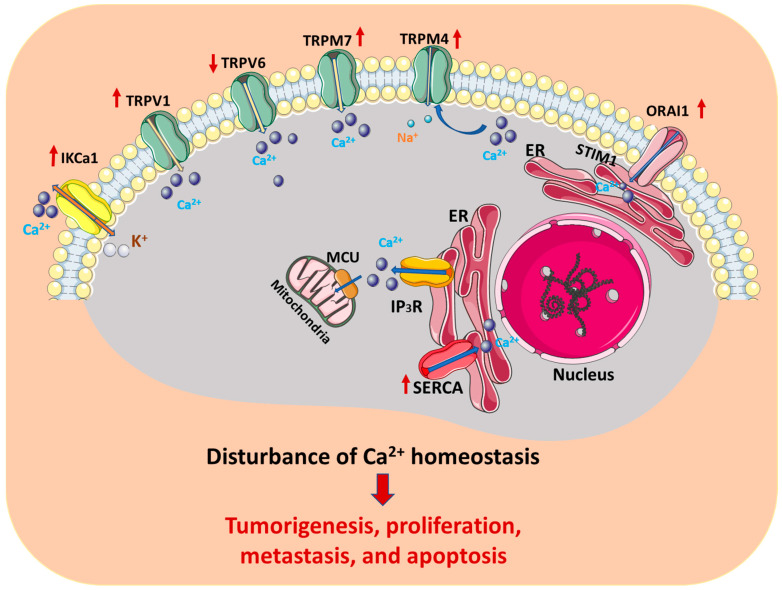
Through previous studies on the relationship between Ca2+ signaling and cervical cancer, we can easily find that Ca2+ signaling plays an important role in cervical cancer growth, proliferation, and metastasis. Changes in various Ca2+ channels, pumps, exchangers, sensors, and Ca2+ activation effectors related to the regulation of Ca2+ homeostasis can affect the concentration of Ca2+ inside and outside the cell, thereby affecting cervical cell metabolism, proliferation, and apoptosis, and eventually leading to the development of cervical cancer (Figure 1).
Figure 1.
Ca2+ channels/pumps/exchangers in cervical cancer. Ca2+ channels/pumps/exchangers play an important role in affecting the concentration of Ca2+ inside and outside the cell: (1) plasm membrane Ca2+ channels or transporters, including IKCa1, TRPs, and CRACs; (2) intracellular calcium release channels in ER mainly include IP3R channels; (3) SERCA-dependent Ca2+ transport is a Ca2+ uptake mechanism in ER. Changes in Ca2+ channels/pumps/exchangers related to the regulation of Ca2+ homeostasis could affect cervical cell metabolism, proliferation, and apoptosis, and lead to the development of cervical cancer. IKCa1: intermediate-conductance calcium-activated potassium channels; ER: endoplasmic reticulum; TRPs: transient receptor potential channels; MCU: mitochondrial calcium uniporter; IP3R: inositol 1,4,5-triphosphate receptor; SERCA: sarcoendoplasmic reticulum calcium transport ATPase; STIM1: stromal interaction molecule 1.
In addition to the Ca2+ channels, pumps, and interacting proteins and ligands mentioned above, there are still many Ca2+-regulatory factors that regulate Ca2+ homeostasis and signal transduction related to the occurrence of cancer in vivo. For example, studies have shown that Orai3 is involved in various processes of breast cancer, such as proliferation and survival and resistance to chemotherapy, and the expressions of secretory pathway Ca2+-ATPases (SPCA1) and SPCA2 genes are significantly increased in breast cancer [104,105]. The plasma membrane Ca2+-ATPase (PMCA) is ubiquitously expressed, which is critical for maintaining low resting cytosolic Ca2+ in cells. Studies spanning many years have revealed that some cancers are associated with a remodeling of PMCA expression [106]. The sodium/calcium exchanger (NCX) and the therapeutic potential of its inhibitors have been also studied in cancer [107]. Piezo1, a mechanically activated ion channel, can regulate Ca2+-dependent signaling cascades associated with tumor cell migration by promoting local Ca2+ influx [108]. Piezo1 is involved in colon cancer cell metastasis; its expression is significantly elevated in prostate cancer cell lines and human prostate cancer tissues, and downregulation of Piezo1 significantly inhibits prostate cancer cell survival, proliferation, and migration [108,109]. In addition, some studies have also found that the multidrug resistance of cancer cells can be overcome by regulating the T-type Ca2+ channel [110].
However, there is no relevant research to show whether these channels or protein- regulated Ca2+ homeostasis and signal transduction are related to the occurrence, development, and prognosis of cervical cancer. Research findings in other tumors may provide new ideas for cervical cancer research. Existing studies on Ca2+ signaling related to cervical cancer treatment are still few, and the current research has not included Ca2+ signaling targets as the focus of cervical cancer treatment. Increasing expression or silencing of some Ca2+ channel proteins related to tumor cell apoptosis has been confirmed in the treatment of other tumors, which provides a new idea for the targeted therapy of cervical cancer. Admittedly, little is known about the effect of Ca2+ signaling in cervical cancer; there is, thus, a need for additional research in this field.
Author Contributions
Writing—original draft preparation: Q.G. and J.L.; writing—review and editing: Q.G., J.Q., F.D. and M.L.; supervision: Q.G., J.L., B.J., H.D. and T.X.; project administration: Q.G., L.F., M.L., M.F. and J.Q.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Supported partly by the Ministry of Science and Technology (2019YFA0802600), National Nature and Science Foundation of China (82271724, 81873841, 81741024, and 81401244), Suzhou City “Wei Sheng Ren Cai (GSWS2019029)” program, Medical and Industrial Integration Collaborative Innovation Research Project (SLJ202012), and Clinical Trial Institution Capacity Enhancement Project (SLT202003). Jiangsu provincial key research and development plan of science and technology department (BE2018669), General Programs of Jiangsu Commission of Health (M2021087), and Nature and Science Foundation of Jiangsu (BK20221243).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patergnani S., Danese A., Bouhamida E., Aguiari G., Previati M., Pinton P., Giorgi C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020;21:8323. doi: 10.3390/ijms21218323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monteith G.R., Prevarskaya N., Roberts-Thomson S.J. The calcium-cancer signalling nexus. Nat. Rev. Cancer. 2017;17:367–380. doi: 10.1038/nrc.2017.18. [DOI] [PubMed] [Google Scholar]
- 3.Islam M.S. Calcium Signaling: From Basic to Bedside. Adv. Exp. Med. Biol. 2020;1131:1–6. doi: 10.1007/978-3-030-12457-1_1. [DOI] [PubMed] [Google Scholar]
- 4.Ren D., Liu R., Yan X., Zhang Q., Zeng X., Yuan X. Intensive stretch-activated CRT-PMCA1 feedback loop promoted apoptosis of myoblasts through Ca2+ overloading. Apoptosis. 2022 doi: 10.1007/s10495-022-01759-4. [DOI] [PubMed] [Google Scholar]
- 5.Fani G., La Torre C.E., Cascella R., Cecchi C., Vendruscolo M., Chiti F. Misfolded protein oligomers induce an increase of intracellular Ca2+ causing an escalation of reactive oxidative species. Cell Mol. Life Sci. 2022;79:500. doi: 10.1007/s00018-022-04513-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 7.Bouvard V., Baan R., Straif K., Grosse Y., Secretan B., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/S1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 8.Marchi S., Giorgi C., Galluzzi L., Pinton P. Ca2+ Fluxes and Cancer. Mol. Cell. 2020;78:1055–1069. doi: 10.1016/j.molcel.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Arbabian A., Brouland J.P., Gelebart P., Kovacs T., Bobe R., Enouf J., Papp B. Endoplasmic reticulum calcium pumps and cancer. Biofactors. 2011;37:139–149. doi: 10.1002/biof.142. [DOI] [PubMed] [Google Scholar]
- 10.Han G.H., Chay D.B., Nam S., Cho H., Chung J.Y., Kim J.H. The Combination of Transient Receptor Potential Vanilloid Type 1 (TRPV1) and Phosphatase and Tension Homolog (PTEN) is an Effective Prognostic Biomarker in Cervical Cancer. Int. J. Gynecol. Pathol. 2021;40:214–223. doi: 10.1097/PGP.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 11.Lucido C.T., Wynja E., Madeo M., Williamson C.S., Schwartz L.E., Imblum B.A., Drapkin R., Vermeer P.D. Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecol. Oncol. 2019;154:228–235. doi: 10.1016/j.ygyno.2019.04.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun F., Xiao L., Jang X.X., Xiong Y., Li Q., Yue X.J., Wei Y.J., Wei Y.X., Ma Y.L., Yu Y.H. TRPV6 is a prognostic marker in early-stage cervical squamous cell carcinoma. Tumour Biol. 2016;37:15743–15751. doi: 10.1007/s13277-016-5368-4. [DOI] [PubMed] [Google Scholar]
- 13.Narayan G., Bourdon V., Chaganti S., Arias-Pulido H., Nandula S.V., Rao P.H., Gissmann L., Durst M., Schneider A., Pothuri B., et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: Identification of candidate amplified and overexpressed genes. Genes Chromosom. Cancer. 2007;46:373–384. doi: 10.1002/gcc.20418. [DOI] [PubMed] [Google Scholar]
- 14.Hong X., Yu J.J. MicroRNA-150 suppresses epithelial-mesenchymal transition, invasion, and metastasis in prostate cancer through the TRPM4-mediated beta-catenin signaling pathway. Am. J. Physiol. Cell. Physiol. 2019;316:C463–C480. doi: 10.1152/ajpcell.00142.2018. [DOI] [PubMed] [Google Scholar]
- 15.Armisen R., Marcelain K., Simon F., Tapia J.C., Toro J., Quest A.F., Stutzin A. TRPM4 enhances cell proliferation through up-regulation of the beta-catenin signaling pathway. J. Cell. Physiol. 2011;226:103–109. doi: 10.1002/jcp.22310. [DOI] [PubMed] [Google Scholar]
- 16.Liu X., Gan L., Zhang J. miR-543 inhibites cervical cancer growth and metastasis by targeting TRPM7. Chem. Biol. Interact. 2019;302:83–92. doi: 10.1016/j.cbi.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Dong R.F., Zhuang Y.J., Wang Y., Zhang Z.Y., Xu X.Z., Mao Y.R., Yu J.J. Tumor suppressor miR-192-5p targets TRPM7 and inhibits proliferation and invasion in cervical cancer. Kaohsiung J. Med. Sci. 2021;37:699–708. doi: 10.1002/kjm2.12398. [DOI] [PubMed] [Google Scholar]
- 18.Qi H., Lu L., Wang L. Long Noncoding RNA ST7-AS1 Upregulates TRPM7 Expression by Sponging microRNA-543 to Promote Cervical Cancer Progression. Onco Targets Ther. 2020;13:7257–7269. doi: 10.2147/OTT.S253868. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Chen Y.T., Chen Y.F., Chiu W.T., Liu K.Y., Liu Y.L., Chang J.Y., Chang H.C., Shen M.R. Microtubule-associated histone deacetylase 6 supports the calcium store sensor STIM1 in mediating malignant cell behaviors. Cancer Res. 2013;73:4500–4509. doi: 10.1158/0008-5472.CAN-12-4127. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y.F., Chiu W.T., Chen Y.T., Lin P.Y., Huang H.J., Chou C.Y., Chang H.C., Tang M.J., Shen M.R. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc. Natl. Acad. Sci. USA. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y.C., Chang T.Y., Chen T.C., Lin W.S., Chang S.C., Lee Y.J. ITPR3 gene haplotype is associated with cervical squamous cell carcinoma risk in Taiwanese women. Oncotarget. 2017;8:10085–10090. doi: 10.18632/oncotarget.14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y.C., Chang T.Y., Chen T.C., Chang S.C., Chen W.F., Chan H.W., Lin W.S., Wu F.T., Lee Y.J. Genetic polymorphisms in the ITPKC gene and cervical squamous cell carcinoma risk. Cancer Immunol. Immunother. 2012;61:2153–2159. doi: 10.1007/s00262-012-1280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Ouyang Z., Zhang Q., Wang L., Shen Y., Wu X., Gu Y., Shu Y., Yu B., Wu X., et al. SBF-1 exerts strong anticervical cancer effect through inducing endoplasmic reticulum stress-associated cell death via targeting sarco/endoplasmic reticulum Ca(2+)-ATPase 2. Cell Death Dis. 2014;5:e1581. doi: 10.1038/cddis.2014.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng M., Sang L., Wang X. S100 Calcium Binding Protein A11 (S100A11) Promotes The Proliferation, Migration And Invasion Of Cervical Cancer Cells, And Activates Wnt/beta-Catenin Signaling. Onco Targets Ther. 2019;12:8675–8685. doi: 10.2147/OTT.S225248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Yang J., Qian J., Liu Z., Chen H., Cui Z. S100A14, a mediator of epithelial-mesenchymal transition, regulates proliferation, migration and invasion of human cervical cancer cells. Am. J. Cancer Res. 2015;5:1484–1495. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L., Zhan P., Nie D., Fan L., Lin H., Gao L., Mao X. Intermediate-Conductance-Ca2-Activated K Channel IKCa1 Is Upregulated and Promotes Cell Proliferation in Cervical Cancer. Med. Sci. Monit. Basic Res. 2017;23:45–57. doi: 10.12659/MSMBR.901462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilius B., Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vangeel L., Voets T. Transient Receptor Potential Channels and Calcium Signaling. Cold Spring Harb. Perspect Biol. 2019;11:a035048. doi: 10.1101/cshperspect.a035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H. TRP Channel Classification. Adv. Exp. Med. Biol. 2017;976:1–8. doi: 10.1007/978-94-024-1088-4_1. [DOI] [PubMed] [Google Scholar]
- 30.Karki T., Tojkander S. TRPV Protein Family-From Mechanosensing to Cancer Invasion. Biomolecules. 2021;11:1019. doi: 10.3390/biom11071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borgstrom A., Peinelt C., Stoklosa P. TRPM4 in Cancer—A New Potential Drug Target. Biomolecules. 2021;11:229. doi: 10.3390/biom11020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aghazadeh Tabrizi M., Baraldi P.G., Baraldi S., Gessi S., Merighi S., Borea P.A. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med. Res. Rev. 2017;37:936–983. doi: 10.1002/med.21427. [DOI] [PubMed] [Google Scholar]
- 33.Zhai K., Liskova A., Kubatka P., Busselberg D. Calcium Entry through TRPV1: A Potential Target for the Regulation of Proliferation and Apoptosis in Cancerous and Healthy Cells. Int. J. Mol. Sci. 2020;21:4177. doi: 10.3390/ijms21114177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De La Chapa J., Valdez M., Ruiz F., 3rd, Gonzales K., Mitchell W., McHardy S.F., Hart M., Polusani S.R., Gonzales C.B. Synthesis and SAR of novel capsazepine analogs with significant anti-cancer effects in multiple cancer types. Bioorg. Med. Chem. 2019;27:208–215. doi: 10.1016/j.bmc.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 35.Ramer R., Merkord J., Rohde H., Hinz B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010;79:955–966. doi: 10.1016/j.bcp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Yelshanskaya M.V., Nadezhdin K.D., Kurnikova M.G., Sobolevsky A.I. Structure and function of the calcium-selective TRP channel TRPV6. J. Physiol. 2021;599:2673–2697. doi: 10.1113/JP279024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolanz K.A., Hediger M.A., Landowski C.P. The role of TRPV6 in breast carcinogenesis. Mol. Cancer Ther. 2008;7:271–279. doi: 10.1158/1535-7163.MCT-07-0478. [DOI] [PubMed] [Google Scholar]
- 38.Xu X., Li N., Wang Y., Yu J., Mi J. Calcium channel TRPV6 promotes breast cancer metastasis by NFATC2IP. Cancer Lett. 2021;519:150–160. doi: 10.1016/j.canlet.2021.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Song H., Dong M., Zhou J., Sheng W., Li X., Gao W. Expression and prognostic significance of TRPV6 in the development and progression of pancreatic cancer. Oncol. Rep. 2018;39:1432–1440. doi: 10.3892/or.2018.6216. [DOI] [PubMed] [Google Scholar]
- 40.Fan H., Shen Y.X., Yuan Y.F. Expression and prognostic roles of TRPV5 and TRPV6 in non-small cell lung cancer after curative resection. Asian Pac. J. Cancer Prev. 2014;15:2559–2563. doi: 10.7314/APJCP.2014.15.6.2559. [DOI] [PubMed] [Google Scholar]
- 41.So C.L., Milevskiy M.J.G., Monteith G.R. Transient receptor potential cation channel subfamily V and breast cancer. Lab. Investig. 2020;100:199–206. doi: 10.1038/s41374-019-0348-0. [DOI] [PubMed] [Google Scholar]
- 42.Fixemer T., Wissenbach U., Flockerzi V., Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: A novel prognostic marker for tumor progression. Oncogene. 2003;22:7858–7861. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- 43.Mathar I., Jacobs G., Kecskes M., Menigoz A., Philippaert K., Vennekens R. Trpm4. Handb. Exp. Pharmacol. 2014;222:461–487. doi: 10.1007/978-3-642-54215-2_18. [DOI] [PubMed] [Google Scholar]
- 44.Fleig A., Penner R. The TRPM ion channel subfamily: Molecular, biophysical and functional features. Trends Pharmacol. Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Kappel S., Stoklosa P., Hauert B., Ross-Kaschitza D., Borgstrom A., Baur R., Galvan J.A., Zlobec I., Peinelt C. TRPM4 is highly expressed in human colorectal tumor buds and contributes to proliferation, cell cycle, and invasion of colorectal cancer cells. Mol. Oncol. 2019;13:2393–2405. doi: 10.1002/1878-0261.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sagredo A.I., Sagredo E.A., Pola V., Echeverria C., Andaur R., Michea L., Stutzin A., Simon F., Marcelain K., Armisen R. TRPM4 channel is involved in regulating epithelial to mesenchymal transition, migration, and invasion of prostate cancer cell lines. J. Cell. Physiol. 2019;234:2037–2050. doi: 10.1002/jcp.27371. [DOI] [PubMed] [Google Scholar]
- 47.Rivas J., Diaz N., Silva I., Morales D., Lavanderos B., Alvarez A., Saldias M.P., Pulgar E., Cruz P., Maureira D., et al. KCTD5, a novel TRPM4-regulatory protein required for cell migration as a new predictor for breast cancer prognosis. FASEB J. 2020;34:7847–7865. doi: 10.1096/fj.201901195RRR. [DOI] [PubMed] [Google Scholar]
- 48.Guinamard R., Hof T., Del Negro C.A. The TRPM4 channel inhibitor 9-phenanthrol. Br. J. Pharmacol. 2014;171:1600–1613. doi: 10.1111/bph.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou Z.G., Rios F.J., Montezano A.C., Touyz R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019;20:1877. doi: 10.3390/ijms20081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chubanov V., Gudermann T. Mapping TRPM7 Function by NS8593. Int. J. Mol. Sci. 2020;21:7017. doi: 10.3390/ijms21197017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pugliese D., Armuzzi A., Castri F., Benvenuto R., Mangoni A., Guidi L., Gasbarrini A., Rapaccini G.L., Wolf F.I., Trapani V. TRPM7 is overexpressed in human IBD-related and sporadic colorectal cancer and correlates with tumor grade. Dig. Liver Dis. 2020;52:1188–1194. doi: 10.1016/j.dld.2020.05.027. [DOI] [PubMed] [Google Scholar]
- 52.Yee N.S. Role of TRPM7 in Cancer: Potential as Molecular Biomarker and Therapeutic Target. Pharmaceuticals. 2017;10:39. doi: 10.3390/ph10020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou C., Zhao X., Duan S. The role of miR-543 in human cancerous and noncancerous diseases. J. Cell. Physiol. 2021;236:15–26. doi: 10.1002/jcp.29860. [DOI] [PubMed] [Google Scholar]
- 54.Numata T., Sato-Numata K., Okada Y. TRPM7 is involved in acid-induced necrotic cell death in a manner sensitive to progesterone in human cervical cancer cells. Physiol. Rep. 2019;7:e14157. doi: 10.14814/phy2.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaunt H.J., Vasudev N.S., Beech D.J. Transient receptor potential canonical 4 and 5 proteins as targets in cancer therapeutics. Eur. Biophys. J. 2016;45:611–620. doi: 10.1007/s00249-016-1142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Y., Ye C., Tian W., Ye W., Gao Y.Y., Feng Y.D., Zhang H.N., Ma G.Y., Wang S.J., Cao W., et al. TRPC1 promotes the genesis and progression of colorectal cancer via activating CaM-mediated PI3K/AKT signaling axis. Oncogenesis. 2021;10:67. doi: 10.1038/s41389-021-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhennin-Duthille I., Gautier M., Faouzi M., Guilbert A., Brevet M., Vaudry D., Ahidouch A., Sevestre H., Ouadid-Ahidouch H. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: Correlation with pathological parameters. Cell Physiol. Biochem. 2011;28:813–822. doi: 10.1159/000335795. [DOI] [PubMed] [Google Scholar]
- 58.Asghar M.Y., Tornquist K. Transient Receptor Potential Canonical (TRPC) Channels as Modulators of Migration and Invasion. Int. J. Mol. Sci. 2020;21:1739. doi: 10.3390/ijms21051739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zomot E., Achildiev Cohen H., Dagan I., Militsin R., Palty R. Bidirectional regulation of calcium release-activated calcium (CRAC) channel by SARAF. J. Cell. Biol. 2021;220:e202104007. doi: 10.1083/jcb.202104007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parekh A.B. Store-operated CRAC channels: Function in health and disease. Nat. Rev. Drug Discov. 2010;9:399–410. doi: 10.1038/nrd3136. [DOI] [PubMed] [Google Scholar]
- 61.Khan H.Y., Mazahir I., Reddy S., Fazili F., Azmi A. Roles of CRAC channel in cancer: Implications for therapeutic development. Expert Rev. Precis. Med. Drug Dev. 2020;5:371–382. doi: 10.1080/23808993.2020.1803062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chalmers S.B., Monteith G.R. ORAI channels and cancer. Cell Calcium. 2018;74:160–167. doi: 10.1016/j.ceca.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Hammad A.S., Machaca K. Store Operated Calcium Entry in Cell Migration and Cancer Metastasis. Cells. 2021;10:1246. doi: 10.3390/cells10051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jardin I., Rosado J.A. STIM and calcium channel complexes in cancer. Biochim. Biophys. Acta. 2016;1863:1418–1426. doi: 10.1016/j.bbamcr.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Huang X., Jan L.Y. Targeting potassium channels in cancer. J. Cell. Biol. 2014;206:151–162. doi: 10.1083/jcb.201404136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang S., Zhang J.J., Huang X.Y. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 67.Santulli G., Lewis D., des Georges A., Marks A.R., Frank J. Ryanodine Receptor Structure and Function in Health and Disease. Subcell Biochem. 2018;87:329–352. doi: 10.1007/978-981-10-7757-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seo M.D., Velamakanni S., Ishiyama N., Stathopulos P.B., Rossi A.M., Khan S.A., Dale P., Li C., Ames J.B., Ikura M., et al. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483:108–112. doi: 10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jia X., Yang Q., Gao C., Chen X., Li Y., Su H., Zheng Y., Zhang S., Wang Z., Wang H., et al. Stimulation of vascular smooth muscle cell proliferation by stiff matrix via the IKCa channel-dependent Ca2+ signaling. J. Cell. Physiol. 2021;236:6897–6906. doi: 10.1002/jcp.30349. [DOI] [PubMed] [Google Scholar]
- 70.Berridge M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Berridge M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016;96:1261–1296. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- 72.Schroder D., Todter K., Gonzalez B., Franco-Echevarria E., Rohaly G., Blecher C., Lin H.Y., Mayr G.W., Windhorst S. The new InsP3Kinase inhibitor BIP-4 is competitive to InsP3 and blocks proliferation and adhesion of lung cancer cells. Biochem. Pharmacol. 2015;96:143–150. doi: 10.1016/j.bcp.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Ueasilamongkol P., Khamphaya T., Guerra M.T., Rodrigues M.A., Gomes D.A., Kong Y., Wei W., Jain D., Trampert D.C., Ananthanarayanan M., et al. Type 3 Inositol 1,4,5-Trisphosphate Receptor Is Increased and Enhances Malignant Properties in Cholangiocarcinoma. Hepatology. 2020;71:583–599. doi: 10.1002/hep.30839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rezuchova I., Hudecova S., Soltysova A., Matuskova M., Durinikova E., Chovancova B., Zuzcak M., Cihova M., Burikova M., Penesova A., et al. Type 3 inositol 1,4,5-trisphosphate receptor has antiapoptotic and proliferative role in cancer cells. Cell Death Dis. 2019;10:186. doi: 10.1038/s41419-019-1433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imboden J.B., Pattison G. Regulation of inositol 1,4,5-trisphosphate kinase activity after stimulation of human T cell antigen receptor. J. Clin. Investig. 1987;79:1538–1541. doi: 10.1172/JCI112986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zalk R., Marks A.R. Ca2+ Release Channels Join the ‘Resolution Revolution’. Trends Biochem. Sci. 2017;42:543–555. doi: 10.1016/j.tibs.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lanner J.T., Georgiou D.K., Joshi A.D., Hamilton S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giannini G., Clementi E., Ceci R., Marziali G., Sorrentino V. Expression of a ryanodine receptor-Ca2+ channel that is regulated by TGF-beta. Science. 1992;257:91–94. doi: 10.1126/science.1320290. [DOI] [PubMed] [Google Scholar]
- 79.Schmitt K., Molfenter B., Laureano N.K., Tawk B., Bieg M., Hostench X.P., Weichenhan D., Ullrich N.D., Shang V., Richter D., et al. Somatic mutations and promotor methylation of the ryanodine receptor 2 is a common event in the pathogenesis of head and neck cancer. Int. J. Cancer. 2019;145:3299–3310. doi: 10.1002/ijc.32481. [DOI] [PubMed] [Google Scholar]
- 80.Mariot P., Prevarskaya N., Roudbaraki M.M., Le Bourhis X., Van Coppenolle F., Vanoverberghe K., Skryma R. Evidence of functional ryanodine receptor involved in apoptosis of prostate cancer (LNCaP) cells. Prostate. 2000;43:205–214. doi: 10.1002/(SICI)1097-0045(20000515)43:3<205::AID-PROS6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L., Liu Y., Song F., Zheng H., Hu L., Lu H., Liu P., Hao X., Zhang W., Chen K. Functional SNP in the microRNA-367 binding site in the 3’UTR of the calcium channel ryanodine receptor gene 3 (RYR3) affects breast cancer risk and calcification. Proc. Natl. Acad. Sci. USA. 2011;108:13653–13658. doi: 10.1073/pnas.1103360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdul M., Ramlal S., Hoosein N. Ryanodine receptor expression correlates with tumor grade in breast cancer. Pathol. Oncol. Res. 2008;14:157–160. doi: 10.1007/s12253-008-9045-9. [DOI] [PubMed] [Google Scholar]
- 83.Law B.Y.K., Michelangeli F., Qu Y.Q., Xu S.W., Han Y., Mok S.W.F., Dias I., Javed M.U., Chan W.K., Xue W.W., et al. Neferine induces autophagy-dependent cell death in apoptosis-resistant cancers via ryanodine receptor and Ca2+-dependent mechanism. Sci. Rep. 2019;9:20034. doi: 10.1038/s41598-019-56675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Christodoulou P., Yiallouris A., Michail A., Christodoulou M.I., Politis P.K., Patrikios I. Altered SERCA Expression in Breast Cancer. Medicina. 2021;57:1074. doi: 10.3390/medicina57101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li W., Song R., Fang X., Wang L., Chen W., Tang P., Yu B., Sun Y., Xu Q. SBF-1, a synthetic steroidal glycoside, inhibits melanoma growth and metastasis through blocking interaction between PDK1 and AKT3. Biochem. Pharmacol. 2012;84:172–181. doi: 10.1016/j.bcp.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 86.Chen W., Fang X., Gao Y., Shi K., Sun L., Yu B., Luo Q., Xu Q. SBF-1 inhibits contact hypersensitivity in mice through down-regulation of T-cell-mediated responses. BMC Pharmacol. Toxicol. 2019;20:86. doi: 10.1186/s40360-019-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X., Li Y., Li Z., Lin S., Wang H., Sun J., Lan C., Wu L., Sun D., Huang C., et al. Mitochondrial Calcium Uniporter Drives Metastasis and Confers a Targetable Cystine Dependency in Pancreatic Cancer. Cancer Res. 2022;82:2254–2268. doi: 10.1158/0008-5472.CAN-21-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miao Y., Wang X., Lai Y., Lin W., Huang Y., Yin H., Hou R., Zhang F. Mitochondrial calcium uniporter promotes cell proliferation and migration in esophageal cancer. Oncol. Lett. 2021;22:686. doi: 10.3892/ol.2021.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vultur A., Gibhardt C.S., Stanisz H., Bogeski I. The role of the mitochondrial calcium uniporter (MCU) complex in cancer. Pflugers Arch. 2018;470:1149–1163. doi: 10.1007/s00424-018-2162-8. [DOI] [PubMed] [Google Scholar]
- 90.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bresnick A.R., Weber D.J., Zimmer D.B. S100 proteins in cancer. Nat. Rev. Cancer. 2015;15:96–109. doi: 10.1038/nrc3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmer D.B., Weber D.J. The Calcium-Dependent Interaction of S100B with Its Protein Targets. Cardiovasc. Psychiatry Neurol. 2010;2010:728052. doi: 10.1155/2010/728052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tian T., Li X., Hua Z., Ma J., Wu X., Liu Z., Chen H., Cui Z. S100A7 promotes the migration, invasion and metastasis of human cervical cancer cells through epithelial-mesenchymal transition. Oncotarget. 2017;8:24964–24977. doi: 10.18632/oncotarget.15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Q., He Y., Wang X.L., Zhang Y.X., Wu Y.M. Differentially expressed proteins among normal cervix, cervical intraepithelial neoplasia and cervical squamous cell carcinoma. Clin. Transl. Oncol. 2015;17:620–631. doi: 10.1007/s12094-015-1287-x. [DOI] [PubMed] [Google Scholar]
- 95.Xia X.M., Fakler B., Rivard A., Wayman G., Johnson-Pais T., Keen J.E., Ishii T., Hirschberg B., Bond C.T., Lutsenko S., et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 96.Toyama K., Wulff H., Chandy K.G., Azam P., Raman G., Saito T., Fujiwara Y., Mattson D.L., Das S., Melvin J.E., et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J. Clin. Investig. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pedarzani P., Stocker M. Molecular and cellular basis of small--and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol. Life Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hua X., Deuse T., Chen Y.J., Wulff H., Stubbendorff M., Kohler R., Miura H., Langer F., Reichenspurner H., Robbins R.C., et al. The potassium channel KCa3.1 as new therapeutic target for the prevention of obliterative airway disease. Transplantation. 2013;95:285–292. doi: 10.1097/TP.0b013e318275a2f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kiefer H., Blume A.J., Kaback H.R. Membrane potential changes during mitogenic stimulation of mouse spleen lymphocytes. Proc. Natl. Acad. Sci. USA. 1980;77:2200–2204. doi: 10.1073/pnas.77.4.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strobl J.S., Wonderlin W.F., Flynn D.C. Mitogenic signal transduction in human breast cancer cells. Gen. Pharmacol. 1995;26:1643–1649. doi: 10.1016/0306-3623(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 101.Jager H., Dreker T., Buck A., Giehl K., Gress T., Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol. Pharmacol. 2004;65:630–638. doi: 10.1124/mol.65.3.630. [DOI] [PubMed] [Google Scholar]
- 102.Yang X.W., Liu J.W., Zhang R.C., Yin Q., Shen W.Z., Yi J.L. Inhibitory effects of blockage of intermediate conductance Ca2+-activated K+ channels on proliferation of hepatocellular carcinoma cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013;33:86–89. doi: 10.1007/s11596-013-1076-0. [DOI] [PubMed] [Google Scholar]
- 103.Wang Z.H., Shen B., Yao H.L., Jia Y.C., Ren J., Feng Y.J., Wang Y.Z. Blockage of intermediate-conductance-Ca2+-activated K+ channels inhibits progression of human endometrial cancer. Oncogene. 2007;26:5107–5114. doi: 10.1038/sj.onc.1210308. [DOI] [PubMed] [Google Scholar]
- 104.Chamlali M., Kouba S., Rodat-Despoix L., Todesca L.M., Petho Z., Schwab A., Ouadid-Ahidouch H. Orai3 Calcium Channel Regulates Breast Cancer Cell Migration through Calcium-Dependent and -Independent Mechanisms. Cells. 2021;10:3487. doi: 10.3390/cells10123487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dang D., Prasad H., Rao R. Secretory pathway Ca2+-ATPases promote in vitro microcalcifications in breast cancer cells. Mol. Carcinog. 2017;56:2474–2485. doi: 10.1002/mc.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Curry M.C., Roberts-Thomson S.J., Monteith G.R. Plasma membrane calcium ATPases and cancer. Biofactors. 2011;37:132–138. doi: 10.1002/biof.146. [DOI] [PubMed] [Google Scholar]
- 107.Rodrigues T., Estevez G.N.N., Tersariol I. Na+/Ca2+ exchangers: Unexploited opportunities for cancer therapy? Biochem. Pharmacol. 2019;163:357–361. doi: 10.1016/j.bcp.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 108.Sun Y., Li M., Liu G., Zhang X., Zhi L., Zhao J., Wang G. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. J. Cancer Res. Clin. Oncol. 2020;146:1139–1152. doi: 10.1007/s00432-020-03179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han Y., Liu C., Zhang D., Men H., Huo L., Geng Q., Wang S., Gao Y., Zhang W., Zhang Y., et al. Mechanosensitive ion channel Piezo1 promotes prostate cancer development through the activation of the Akt/mTOR pathway and acceleration of cell cycle. Int. J. Oncol. 2019;55:629–644. doi: 10.3892/ijo.2019.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao Z., Ji M., Wang Q., He N., Li Y. Ca2+ signaling modulation using cancer cell membrane coated chitosan nanoparticles to combat multidrug resistance of cancer. Carbohydr. Polym. 2020;238:116073. doi: 10.1016/j.carbpol.2020.116073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.