Abstract
Background
Increased orthodontic treatment duration is associated with iatrogenic risks such as root resorption, white spot lesions etc. Recent research using pharmacological agents to accelerate tooth movement has mostly been conducted on animals and there is no reported research conducted on humans comparing the effects of Vitamin D3 and Platelet Rich Plasma (PRP) in the same subjects using a split mouth technique.
Objectives
To determine and compare effects of local injection of PRP and Vitamin D3 on the rate of tooth movement. Also, to assess association of external apical root resorption with the use of PRP, Vitamin D3 and compare it to a control group.
Materials & methods
11 subjects who diagnosed with Class I bi-maxillary malocclusion and who gave informed consent were recruited in the study. The patients were randomly divided using split mouth design and each quadrant served as either experimental or control group one. At the beginning of retraction phase, Vitamin D3 and PRP were injected to the randomly assigned quadrants while the contralateral side served as a control. The amount of space closure in 4 months was measured from distal surface of canine to mesial surface of 2nd premolar. Root resorption was assessed using CBCT taken at the beginning and at the closure of retraction phase.
Results
Mean rate of tooth movement was higher in PRP and Vitamin D3 groups compared to their controls. In the PRP group, the increased rate of tooth movement was observed throughout the study interval, but in the Vitamin D3 group it was only seen in first two months. Also, the PRP group demonstrated a higher rate of tooth movement compared to Vitamin D3 group. Root resorption was lesser in both experimental groups. Among the teeth assessed, lateral incisor showed maximum root resorption and canine the least.
Conclusions
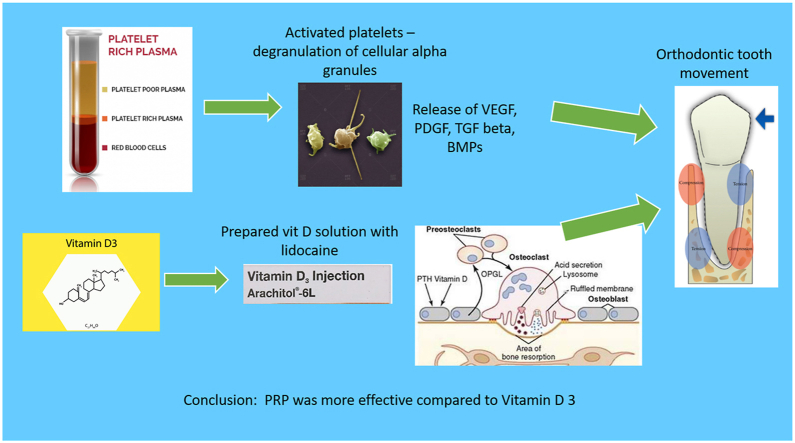
PRP is a more efficient pharmacological agent compared to Vitamin D3 for accelerating tooth movement.
Keywords: Accelerated orthodontics, Vitamin D3, PRP, Space closure, Orthodontic root resorption
Graphical abstract
1. Introduction
Orthodontic tooth movement is initiated by the application of mechanical forces and the force induced strain alters the periodontal ligament's vascularity. This results in the local synthesis and release of various neurotransmitters like cytokines, growth factors, colony-stimulating factors and arachidonic acid metabolites that are responsible to bring about bone deposition and resorption.1
The number of adults seeking orthodontic treatment has increased in the recent years. Bone metabolism which relates to the rate of tooth movement is innately slower in adults compared to adolescents which results in increased treatment duration. Adults are also more influenced by social standards and work pressures and hence they prefer to wear braces for shorter duration.2 Prolonged treatment duration also increases the susceptibility to iatrogenic issues such as root resorption, plaque induced conditions like caries and demineralization and periodontal problems.3 Hence accelerating the tooth movement offers advantages to both the orthodontist and the patients.
Attempts to accelerate tooth movement can be dated back to 1893.4 The surgically assisted approach requires multiple interventions and also has a high risk for root resorption. The patient's level of acceptance is also low for these surgical procedures. Although few studies have shown that there is a statistically significant increase in rate of tooth movement, the level of evidence is low. Further studies are required to show their effectiveness in accelerating orthodontic tooth movement.5
Local injection of biological agents could be used as a substitute to surgery.6, 7, 8, 9, 10 These biological agents are clinically more efficient, less invasive, more controlled and are target specific as they act on biological mediators. These are locally administered to avoid any systemic effects.11 Platelet Rich Plasma (PRP) contains inactive growth factors stored in alpha granules. These secretory granules contain numerous proteins and chemokines responsible for hemostasis and wound healing. Since PRP is a concentrate of platelets, it essentially simulates the natural clotting mechanism. Also, the concentrate does not promote bacterial proliferation. The PRP derived growth factors are trans-membranous, they are not mutagenic and hence only stimulate the natural healing process and have no role in tumour formation.12
Vitamin D3 is another biochemical agent that has shown to be effective in accelerating orthodontic tooth movement. Decrease in calcium levels stimulate the active form of 1,25, Dihydroxycholecalciferol which helps in maintenance of calcium hemostasis. According to Collins et al. and Kale et al. the local administration of Vitamin D3 results in a good balance between deposition and resorption of alveolar bone and well-modulated bone turnover compared to other pharmacological agents like prostaglandin and parathyroid hormone.7,8 The active form of vitamin D3 has shown to induce osteoclastic activity as well as osteoblastic activity in a dose-dependent fashion.7
The effect of PRP on bone and orthodontic tooth movement has been studied in literature.11, 12, 13, 14, 15, 16 But there are very few human studies to evaluate the effects of PRP and Vit D3 on acceleratory orthodontics and there is no study done till date where both these were used in the same subjects. This study hence is designed to evaluate the effects of both these factors when used in the same subjects. Also, this study considered en mass retraction of anteriors in contrast to separate canine retraction as in previous studies.6,7,11
Root resorption is one of the side effects of acceleratory orthodontics. Majority of the studies found in literature regarding iatrogenic root resorption associated with either PRP or Vit D3 are animal-based studies.13, 14, 15, 16 Only Tehranchi A et al. conducted a human study and found that Vit D doesn't have any effects on root resorption.17
Hence this study aimed to compare the effect of PRP and Vitamin D3 on accelerating orthodontic tooth movement and also to assess the amount of root resorption associated with it.
2. Materials and methods
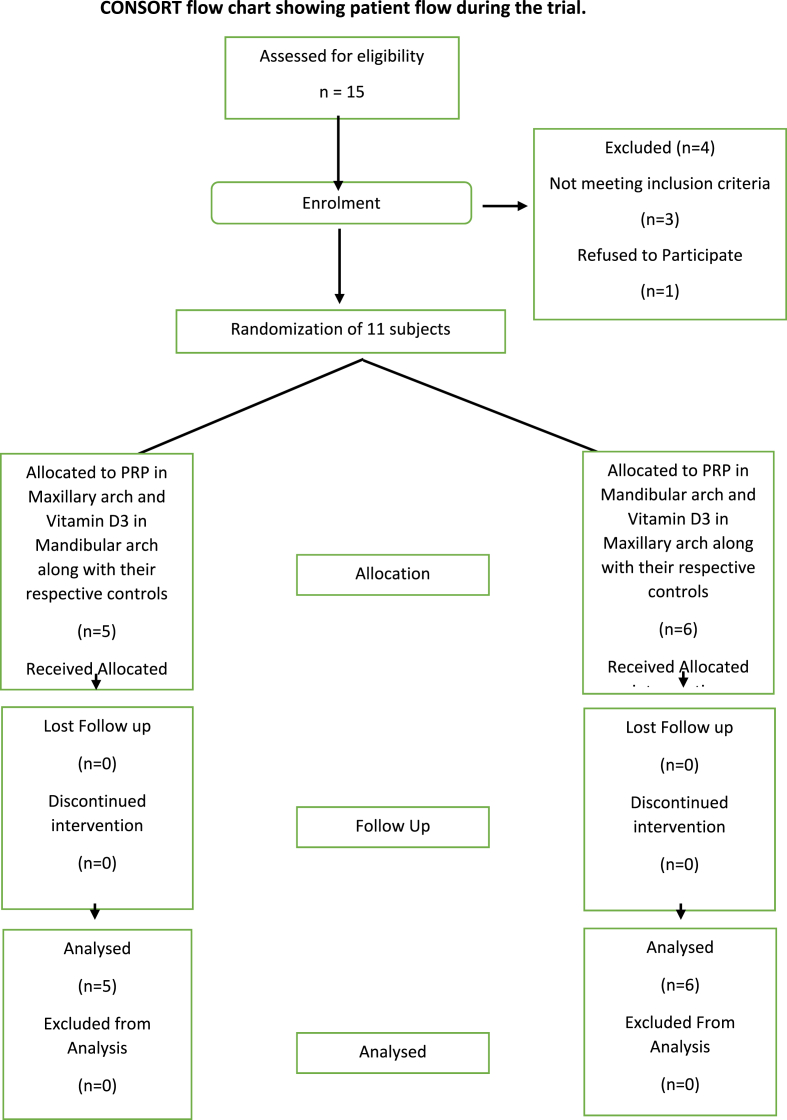
This study was a split-mouth, double blinded, prospective, single centre randomized control clinical trial conducted for a period of 4 months after obtaining approval from the Institutional Ethics Committee board. 11 healthy patients willing to undergo orthodontic treatment at the department of orthodontics, were recruited as study participants based on the inclusion criteria. The inclusion criteria were as follows: Patients with Class I bimaxillary protrusion malocclusion requiring all 4 first premolar extraction and in the age group of 18–30 years. The exclusion criteria included: Patients under any medication that affects tooth movement, with any craniofacial anomalies, patients with periodontal disease, patients with any kind of systemic disease that affects tooth movement, patients with a habit of smoking/alcohol consumption.
The statistically minimum sample size required was calculated based on the formula,
Substituting the values in the formula, sample size obtained was 11 with 95% power and the rate of extraction space closure was considered as the primary factor in calculating the sample size.
The study design is presented in Fig. 1. The study was carried out from September 2020 to April 2021. After obtaining a signed informed consent and reassessing of the cases by the recruiter, the maxillary and mandibular quadrants were randomised into experimental and control group, (diagonally opposite quadrants into experimental/control to take care of unequal masticatory forces) by the recruiter as per the allotment concealment sequence explained in Table 1. Hence each subject had two experimental and two control quadrants (Table 1).
Fig. 1.
Consort flow chart of the study protocol.
Table 1.
Randomization sequence.
| PATIENT | 1ST QUADRANT | 2ND QUADRANT | 3RD QUADRANT | 4TH QUADRANT |
|---|---|---|---|---|
| SUBJECT 1 | PRP | PRP CONTROL | VITAMIN D3 | VITAMIN D3 CONTROL |
| SUBJECT 2 | PRP CONTROL | PRP | VITAMIN D3 CONTROL | VTAMIN D3 |
| SUBJECT 3 | VITAMIN D3 | VITAMIN D3 CONTROL | PRP | PRP CONTROL |
| SUBJECT 4 | VITAMIN D3 CONTROL | VITAMIN D3 | PRP CONTROL | PRP |
| SUBJECT 5 | PRP CONTROL | PRP | VITAMIN D3 | VITAMIN D3 CONTROL |
| SUBJECT 6 | PRP | PRP CONTROL | VITAMIN D3 CONTROL | VITAMIN D3 |
| SUBJECT 7 | VITAMIN D3 CONTROL | VITAMIN D3 | PRP | PRP CONTROL |
| SUBJECT 8 | VITAMIN D3 | VITAMIN D3 CONTROL | PRP CONTROL | PRP |
| SUBJECT 9 | PRP | PRP CONTROL | VITAMIN D3 | VITAMIN D3 CONTROL |
| SUBJECT 10 | PRP CONTROL | PRP | VITAMIN D3 CONTROL | VITAMIN D3 |
| SUBJECT 11 | VITAMIN D3 | VITAMIN D3 CONTROL | PRP | PRP CONTROL |
| TOTAL | PRP = 3 | PRP = 3 | PRP = 3 | PRP = 2 |
| VITAMIN D3 = 3 | VITAMIN D3 = 2 | VITAMIN D3 = 3 | VITAMIN D3 = 3 | |
| VITAMIN D3 CONTROL = 2 | VITAMIN D3 CONTROL = 3 | VITAMIN D3 CONTROL = 3 | VITAMIN D3 CONTROL = 3 | |
| PRP CONTROL = 3 | PRP CONTROL = 3 | PRP CONTROL = 2 | PRP CONTROL = 3 |
PRP-Plasma Rich Protein.
2.1. Allocation
Allocation concealment was achieved with sequentially numbered and sealed envelopes. The operators and study participants were blinded about experimental and control sides. The 2nd author was involved in preparation of the PRP and vitamin D3 solutions and they were labelled as experiment 1 and 2. The injections were performed by the 1st author to the quadrants assigned by the recruiter. The amount of root resorption was calculated on CBCT by 1st author.
2.2. Patient preparation
The first premolars were extracted in all 4 quadrants as per the treatment plan after analysing the records. Pre-adjusted edgewise appliance with MBT prescription of 0.022-inch slot were bonded with transpalatal arch/lingual arch for anchorage reinforcement. After alignment and levelling, a final working wire of 19 × 25-inch ss was placed with power arms placed distal to lateral incisor. En-mass retraction was initiated after 21 days after the engagement of the wire. The intervention was carried out by the 1st author just before starting the retraction. PRP, Vit D3 and Saline (placebo for control side) were injected in the respective quadrants. A force of 150 g per side was applied on all sides. A CBCT was taken just before starting the retraction.
2.3. Platelet rich plasma preparation and administration
The autologous PRP was prepared under aseptic conditions by drawing 10 ml of venous blood form the medial cubital vein. This was transferred into a PRP tube containing 10% sodium citrate solution which acts as an anticoagulant. Heparin is not recommended due to its systemic side effects and bone-resorbing action. This was subjected to double centrifugation to obtain a leukocyte rich platelet rich plasma. First it was centrifuged at 1000 rpm for 12min at room temperature. The blood separates into 3 components with RBCs at the bottom, buffy coat in middle that is rich in platelets (PRP) and top layer of platelet poor plasma (PPP). The RBCs are discarded and remaining buffy coat and platelet poor plasma are centrifuged again at 3000 rpm for 8min. After the second centrifugation the PPP is removed until 4 ml remains at the bottom. The bottom 4 ml is the PRP containing anticoagulant, high concentration platelets and WBCs, and this has to be injected shortly after preparation.6 In this study 0.2 ml of PRP was injected through intra-ligamentous route distal to the central incisor, lateral incisor and canine, only once at the beginning of retraction.
2.4. Vit D3 preparation and administration
According to literature, 25 pg/ml of Vitamin D3 is considered to be the effective dose to accelerate orthodontic tooth movement.18 Arachitol injection contains 15 mg of Vitamin D3/ml of solution. Diluting 1 ml of Arachitol in 800 ml of 2% Xylocain, using the step solving method, a 240 pg/ml solution was prepared which was close to the recommended concentration by Noor A Hasani et al.18 0.1 ml of this solution which contains 24 pg was injected by intra-ligamentous route distal to the canine, lateral incisor and central incisor using 30-gauge needle on the 0, 7th, 21st and 47th days. These days were chosen based on the histological events of tooth movement.19 In the intra-ligamentous method the needle is inserted through the gingival sulcus into the PDL space between tooth and alveolar bone crest.20 The injected solution diffuses apically through marrow spaces into the inter septal bone. The traditional injection technique requires 1.8 ml of solution to be effective, whereas with intra-ligamentous technique only 0.2 ml of the solution was sufficient to demonstrate the effect. This not only reduces pain, but is also target area specific.
2.5. Measurement of tooth movement
The measurement of tooth movement was done using Vernier caliper intra-orally from the disto-incisal surface of the canine to the mesio-incisal surface of the 2nd premolar. The start of the study is considered to be T0, end of 1st month was T1, end of 2nd month was T2, end of 3rd month was T3 and end of 4th month was T4.
2.6. Root resorption
Root resorption was measured using CBCT (CS 3D Imaging v3.5.7 Carestream Health Inc.). The tooth length was measured from the mid-incisal point of the crown to the root apex. Apical root resorption was assessed as the difference between the length of the root at T1 and the length of the root at T2 in millimetres. Although very minimal craters were observed along the root surface area, they were difficult to measure due to beam hardening and cupping artefacts.
3. Results
Table 2 shows that the rate of tooth movement was higher in the PRP group when compared to the control group at the end of each month and at the end of 4 months and this was found to be statistically significant. Table 3 shows that the mean rate of tooth movement was significantly higher in the Vit D3 group when compared to its respective control group for first, second month as well as for the overall study duration. Table 4 shows the comparison of rate of tooth movement between Vitamin D3 and PRP groups. Table 5 shows that the mean rate of tooth movement was higher in the maxilla when compared to mandible.
Table 2.
Comparison of rate of tooth movement between PRP and control.
| N | MEAN | SD | Mean diff. | T | P | ||
|---|---|---|---|---|---|---|---|
| T0-T1 | PRP | 11 | 1.79 | 0.21 | 0.94 | 11.3 | <.0001* |
| CONTROL | 11 | 0.85 | 0.17 | ||||
| T1-T2 | PRP | 11 | 1.71 | 0.21 | 0.93 | 11.1 | <.0001* |
| CONTROL | 11 | 0.77 | 0.18 | ||||
| T2-T3 | PRP | 11 | 1.66 | 0.22 | 0.87 | 10.1 | <.0001* |
| CONTROL | 11 | 0.78 | 0.15 | ||||
| T3-T4 | PRP | 11 | 0.93 | 0.22 | 0.19 | 2.3 | 0.01* |
| CONTROL | 11 | 0.74 | 0.15 | ||||
| T0-T4 | PRP | 11 | 6.10 | 0.85 | 2.94 | 9.1 | <.0001* |
| CONTROL | 11 | 3.16 | 0.65 | ||||
PRP-Plasma Rich Protein.
Table 3.
Comparison of rate of tooth movement between Vitamin D3 and control.
| N | MEAN | SD | Mean diff. | T | P | ||
|---|---|---|---|---|---|---|---|
| T0-T1 | VIT-D3 | 11 | 1.19 | 0.16 | 0.33 | 5.1 | <.0001* |
| CONTROL | 11 | 0.86 | 0.14 | ||||
| T1-T2 | VIT-D3 | 11 | 1.15 | 0.16 | 0.31 | 5.9 | <.0001* |
| CONTROL | 11 | 0.83 | 0.14 | ||||
| T2-T3 | VIT-D3 | 11 | 0.90 | 0.14 | 0.08 | 1.3 | 0.1 |
| CONTROL | 11 | 0.81 | 0.11 | ||||
| T3-T4 | VIT-D3 | 11 | 0.86 | 0.10 | 0.10 | 1.9 | 0.07 |
| CONTROL | 11 | 0.76 | 0.12 | ||||
| T0-T4 | VIT-D3 | 11 | 4.11 | 0.54 | 0.82 | 3.7 | <0.05* |
| CONTROL | 11 | 3.29 | 0.50 | ||||
Table 4.
Comparison of rate of tooth movement between Vitamin D3 and PRP.
| N | MEAN | SD | Mean diff. | T | P | ||
|---|---|---|---|---|---|---|---|
| T0-T1 | PRP | 11 | 1.79 | 0.21 | 0.59 | 7.3 | <.0001* |
| VIT-D3 | 11 | 1.19 | 0.16 | ||||
| T1-T2 | PRP | 11 | 1.71 | 0.21 | 0.56 | 7.1 | <.0001* |
| VIT-D3 | 11 | 1.15 | 0.16 | ||||
| T2-T3 | PRP | 11 | 1.60 | 0.22 | 0.76 | 9.5 | <.0001* |
| VIT-D3 | 11 | 0.90 | 0.14 | ||||
| T3-T4 | PRP | 11 | 0.93 | 0.22 | 0.07 | 0.98 | 0.1 |
| VIT-D3 | 11 | 0.86 | 0.10 | ||||
| T0-T4 | PRP | 11 | 6.10 | 0.85 | 1.99 | 6.57 | <.0001* |
| VIT-D3 | 11 | 4.11 | 0.54 | ||||
PRP-Plasma Rich Protein.
Table 5.
Comparison of rate of tooth movement between Maxilla and mandible by the end of 4 months.
| N | MEAN | SD | Mean diff. | T | P | ||
|---|---|---|---|---|---|---|---|
| PRP | MAXILLA | 5 | 6.98 | 0.27 | 1.50 | 12.5 | <.0001* |
| MANDIBLE | 6 | 5.48 | 0.11 | ||||
| PRP CONTROL | MAXILLA | 5 | 3.82 | 0.13 | 1.21 | 12.9 | <.0001* |
| MANDIBLE | 6 | 2.60 | 0.17 | ||||
| VITAMIN D3 | MAXILLA | 6 | 4.56 | 0.20 | 0.98 | 9.1 | <.0001* |
| MANDIBLE | 5 | 3.57 | 0.14 | ||||
| VITAMIN D3 CONTROL | MAXILLA | 6 | 3.72 | 0.06 | 0.95 | 27.3 | <.0001* |
| MANDIBLE | 5 | 2.77 | 0.05 | ||||
PRP-Plasma Rich Protein.
Table 6 shows the comparison of amount of root resorption for each tooth in the 4-month interval. The mean root resorption was lesser in the PRP group when compared to its control in all the teeth assessed. The mean root resorption was lesser even in the Vit D3 group when compared to its control group in all the teeth assessed and the mean difference was found to be statistically significant only for the canine. The mean root resorption was higher in the PRP group when compared to the Vit D3 group for the central incisor and canine, where as it was found to be higher in Vit D3 group for the lateral incisor and second premolar. These mean differences were clinically as well as statistically insignificant.
Table 6.
Comparison of amount of root resorption for each tooth in 4 month interval.
| N | MEAN | SD | Mean diff. | T | P | |
|---|---|---|---|---|---|---|
| CENTRAL INCISOR | ||||||
| PRP | 11 | 0.60 | 0.10 | −0.05 | −0.7 | 0.2 |
| CONTROL | 11 | 0.65 | 0.18 | |||
| VITAMIN D3 | 11 | 0.56 | 0.17 | −0.06 | −0.7 | 0.2 |
| CONTROL | 11 | 0.62 | 0.21 | |||
| PRP | 11 | 0.60 | 0.10 | 0.04 | 0.7 | 0.2 |
| VITAMIN D3 | 11 | 0.56 | 0.17 | |||
| LATERAL INCISOR | ||||||
| PRP | 11 | 0.97 | 0.16 | −0.07 | −0.8 | 0.1 |
| CONTROL | 11 | 1.03 | 0.19 | |||
| VITAMIN D3 | 11 | 0.98 | 0.15 | −0.07 | −1.02 | 0.1 |
| CONTROL | 11 | 1.05 | 0.19 | |||
| PRP | 11 | 0.97 | 0.16 | −0.01 | −0.14 | 0.4 |
| VITAMIN D3 | 11 | 0.98 | 0.15 | |||
| CANINE | ||||||
| PRP | 11 | 0.41 | 0.12 | −0.09 | −1.27 | 0.2 |
| CONTROL | 11 | 0.50 | 0.18 | |||
| VITAMIN D3 | 11 | 0.40 | 0.08 | −0.12 | −2.38 | 0.02* |
| CONTROL | 11 | 0.52 | 0.14 | |||
| PRP | 11 | 0.41 | 0.12 | 0.01 | 0.2 | 0.4 |
| VITAMIN D3 | 11 | 0.40 | 0.08 | |||
| SECOND PRE-MOLAR | ||||||
| PRP | 0.52 | 0.21 | −0.05 | −0.6 | 0.2 | |
| CONTROL | 0.57 | 0.13 | ||||
| VITAMIN D3 | 0.49 | 0.15 | −0.01 | −1.37 | 0.09 | |
| CONTROL | 0.58 | 0.16 | ||||
| PRP | 0.52 | 0.21 | −0.05 | −0.6 | 0.2 | |
| VITAMIN D3 | 0.58 | 0.16 | ||||
PRP-Plasma Rich Protein.
4. Discussion
This study found a significant increase in rate of tooth movement induced by PRP compared to the control group and this was similar to the results obtained by Al Timamy et al.11 This can be attributed to the biological potential of PRP. Whole blood contains approximately 2,00,000 ± 75,000 platelets/μl whereas therapeutic PRP contains 400% higher platelet content.21 This increased platelet count is effective in accelerating bone healing and hence increasing the rate of tooth movement.22,23 The double spin technique used in our study was effective in achieving high platelet concentration.21 The effectiveness of therapeutic PRP also increased due to the use of intra-ligamentous route. The needle was inserted through the gingival sulcus into the PDL space on the distal aspect of the tooth so that the solution would diffuse into the intra-septal bone.6 Here PRP was injected only once at the beginning of study on the distal intra-ligamentous space of central incisor, lateral incisor and canine, since en-mass retraction was used. This differs from other protocols such as in the study by Al Timamy et al. where PRP was injected in 5 areas around the canine and separate canine retraction was employed.11
Liou E suggested not to use CaCl2 while preparing PRP as it can led to a burst release of the growth factors. Hence repeated injections would be needed in order to maintain a continuous effect.6 In another study, even with repeated injections on 0, 21st and 42nd day, they could achieve only a 15% faster rate of canine retraction on PRP side in the 1st month, 5% in 2 nd month and in the 3rd month there was a decreased rate of tooth movement on PRP side by 40% compared to control group.11
In the present study there was a 52.5% increased rate of anterior teeth retraction in 1st month, 59% in 2 nd month, 52.4% in 3rd month and 20% in 4th month in the PRP group compared to control group which was statistically significant. Even with a reduced number of sites of injection and frequency of injection, PRP proved to be effective.
Vitamin D3 also is effective in accelerating the bone turn-over and this is dependent on the concentration of vitamin D3 in the prepared solution. Noor Al Hasani et al., in 2011 compared effect of local administration of various concentrations of vitamin D3 on orthodontic tooth movement in human subjects and they concluded that 25 pg of Vitamin D3 showed 50% increase in the rate of canine retraction compared to the control group.18 Based on this, a solution was prepared with the concentration of 25 pg/ml. Since this required a very large quantity of solvent, step wise dissolution of vitamin D3 in 800 ml of 2% lidocain was done and a near value i.e. 24 pg of vitamin D3 solution was achieved. 0.1 ml solution of this was injected by intra-ligamentous route at 3 sites - distal to central incisor, lateral incisor and canine since en-mass retraction was done unlike previous studies where they injected only on the distal aspect of canine. The injections were carried out on the 0, 7, 21 and 47th day based on understanding of histological events of tooth movement and as suggested by Shetty A et al.19
This study showed that in the vitamin D3 group there was a 27.7% increase in the rate of anterior teeth retraction in the 1st month, 26.9% in the 2 nd month, 0.08% in the 3rd month and 11% in the 4th month as compared to the control group. There was an obvious decreasing trend in the rate of tooth movement as the study progressed. This showed that there is a need for multiple injections throughout the retraction phase for it to effectively accelerate the rate of space closure. Overall there was nearly a 20% increase in rate of anterior retraction in this study as compared to 51% in a study done Noor Al Hasani et al., 56% by Varughese S.T et al. and 70% by Ciur M.D.I et al. study.18,24,25 In this study lidocaine was used which is a polar solvent to dissolve vitamin D3 which is a nonpolar solute.19 The solubility of vitamin D3 in lidocaine is not as good as with dimethyl sulfoxide (DMSO) which was used in the previous studies done by Noor Al Hasani and Ciur M.D.I.18,25 This was probably the reason for the reduced rate of anterior teeth retraction in this study as well as the study by Shetty A et al.19 Dimethylsulfoxide is a better solvent due to its ability to penetrate body membranes and its ability to transport other pharmacological agents.25,26 But there is insufficient evidence regarding the safety of the intraoral use of dimethyl sulfoxide and its possible side effects.27 Since lidocaine is used in everyday dental practice that was chosen as the solvent.
This study was the first to compare the effect of PRP and vitamin D3 on the rate of anterior teeth retraction using split mouth technique. The results have shown that both PRP and Vitamin D3 show a significant increase in rate of anterior teeth retraction compared to their respective control groups. PRP group showed a 40% increase in the rate of tooth movement compared to vitamin D3 in the first month, 32% increase in 2 nd month, 47% increase in 3rd month and 7.5% in 4th month which is statistically significant. The rate of tooth movement was found to be faster in the maxilla as compared to the mandible in all groups. This could be related to the higher bone density of the mandible and the difference in remodelling rate.28
The mean root resorption was lesser in PRP and Vit D3 groups in all the teeth when compared to their controls. The mean difference was not found to be statistically significant except in the case of the canine for Vit D3 vs control group. The PRP group demonstrated more root resorption in the Central incisor and Canine whereas Vit D3 group showed more resorption in Lateral incisor and second premolar and these differences were not statistically significant.
This difference was very minor and not statically significant. Yet, the reason for this variation could be that the biological response to these two agents is different and there is a large variability in the response of individual teeth to the biological effects of these agents in relation to root resorption.
Among the teeth assessed, lateral incisor showed maximum root resorption and canine the least. The reason can be attributed to the morphology i.e slenderer root (Lateral incisor > Second premolar > Central incisor > Canine).29, 30, 31 The lateral incisor is most likely to exhibit pippet/spindle/trigonal shaped roots which leads to stress accumulation at the root apex making it highly susceptible to root resorption.
Study done by Abdel Haffiez et al. on rabbits, concluded that the PRP can significantly reduce the amount of orthodontic relapse following removal of the orthodontic force.32 There have been no long term investigations regarding the stability of the effects of local injection of PRP and Vitamin D3 on the rate of tooth movement in humans in literature. Most of the previous studies have been on conducted on animals and were of a shorter duration.8,9,13,14,16,19,21
5. Conclusion
The mean rate of tooth movement was higher in the PRP and Vit D3 groups compared to their respective controls, whereas the rate of tooth movement was significantly higher in the PRP group compared to Vit D3 group. Interestingly in the PRP group, the increased rate of tooth movement was observed throughout the study period, but in Vit D3 group it was only for the first two months. Root resorption was lesser in both PRP & Vit D3 groups compared to their controls. Among the teeth assessed, the lateral incisor showed maximum root resorption and canine the least.
6. Limitations of this study
Most of the subjects in this study were female, and equal gender distribution would have been more appropriate. The exact platelet concentration in the PRP used and Vitamin D3 concentration in Vitamin D3 prepared solution was not measured. The solvent used for vitamin D3 was a polar solvent in which vitamin D3 was not 100% dissolved so the concentration obtained could have been variable.
Another shortcoming of this study was that although aseptic conditions were followed for preparation of autologous PRP, there were no microbial evaluations done for the prepared PRP before its injection.
This study does not evaluate the long-term effects of local injection of PRP and Vitamin D3 on the rate of tooth movement. Hence this can be an area of future research.
Contributor Information
S. Navya, Email: navyachinnu26@gmail.com.
G.S. Prashantha, Email: pacchi77@yahoo.com.
S. Sabrish, Email: drsharanyaortho@gmail.com.
M.S. Roshan, Email: dentoroshan@gmail.com.
S. Mathew, Email: drsmathew@gmail.com.
References
- 1.Krishnan V., Davidovitch Z.E. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129(4) doi: 10.1016/j.ajodo.2005.10.007. 469.e1-469.e32. [DOI] [PubMed] [Google Scholar]
- 2.Sayers M.S., Newton J.T. Patients' expectations of orthodontic treatment: Part 2—findings from a questionnaire survey. J Orthod. 2007;34(1):25–35. doi: 10.1179/146531207225021888. [DOI] [PubMed] [Google Scholar]
- 3.Talic N.F. Adverse effects of orthodontic treatment: a clinical perspective. Saudi Dent J. 2011;23(2):55–59. doi: 10.1016/j.sdentj.2011.01.003. 10.1016/, j.sdentj.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzpatrick B.N. Corticotomy. Australian dental journal. 1980;25(5):255–258. doi: 10.1111/j.1834-7819.1980.tb05196.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalemaj Z., Cesare L.D., Jacopo B. Efficacy of surgical and non-surgical interventions on accelerating orthodontic tooth movement. A systematic review. Eur J Oral Implantol Spring. 2015;8(1):9–24. PMID: 25738176. [PubMed] [Google Scholar]
- 6.Liou E. The development of submucosal injection of platelet rich plasma for accelerating orthodontic tooth movement and preserving pressure side alveolar bone. APOS trends in orthodontics. 2016;6(1):5. [Google Scholar]
- 7.Collins M.K., Sinclair P.M. The local use of vitamin D to increase the rate of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1988;94(4):278–284. doi: 10.1016/0889-5406(88)90052-2. [DOI] [PubMed] [Google Scholar]
- 8.Kale S., Kocadereli I., Atilla P., Aşan E. Comparison of the effects of 1, 25 dihydroxycholecalciferol and prostaglandin E2 on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2004;125(5):607–614. doi: 10.1016/j.ajodo.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki K., Shibata Y., Fukuhara T. The effect of prostaglandins on experimental tooth movement in monkeys (Macaca fuscata) J Dent Res. 1982;61(12):1444–1446. doi: 10.1177/00220345820610121401. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki K., Miura F., Suda T. Prostaglandin as a mediator of bone resorption induced by experimental tooth movement in rats. J Dent Res. 1980;59(10):1635–1642. doi: 10.1177/00220345800590101301. [DOI] [PubMed] [Google Scholar]
- 11.El-Timamy A., El Sharaby F., Eid F., El Dakroury A., Mostafa Y., Shaker O. Effect of platelet-rich plasma on the rate of orthodontic tooth movement: a split-mouth randomized trial. Angle Orthod. 2020;90(3):354–361. doi: 10.2319/072119-483.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Sharkawy H., Kantarci A., Deady J., et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78(4):661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 13.Rashid A., El Sharaby F., Nassef E., Mehanni S., Mostafa Y. Effect of platelet-rich plasma on orthodontic tooth movement in dogs. Orthod Craniofac Res. 2017;20:102–110. doi: 10.1111/ocr.12146. [DOI] [PubMed] [Google Scholar]
- 14.Jeon Y.R., Jung B.K., Roh T.S., et al. Comparing the effect of nonactivated platelet-rich plasma, activated platelet-rich plasma, and bone morphogenetic protein-2 on calvarial bone regeneration. J Craniofac Surg. 2016;27(2):317–321. doi: 10.1097/SCS.0000000000002349. [DOI] [PubMed] [Google Scholar]
- 15.Akbulut S., Yagci A., Yay A.H., Yalcin B. Experimental investigation of effects of platelet-rich plasma on early phases of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2019;155(1):71–79. doi: 10.1016/j.ajodo.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Booij-Vrieling H.E., Ferbus D., Tryfonidou M.A., et al. Increased Vitamin D-driven signalling and expression of the Vitamin D receptor, MSX2, and RANKL in tooth resorption in cats. Eur J Oral Sci. 2010;118:39–46. doi: 10.1111/j.1600-0722.2009.00707.x. [DOI] [PubMed] [Google Scholar]
- 17.Tehranchi A., Sadighnia A., Younessian F., Abdi A.H., Shirvani A. Correlation of Vitamin D status and orthodontic-induced external apical root resorption. Dent Res J. 2017;14(6):403–411. doi: 10.4103/1735-3327.218565. PMID: 29238379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hasani N.R., Al-Bustani A.I., Ghareeb M.M., Hussain S.A. Clinical efficacy of locally injected calcitriol in orthodontic tooth movement. Int J Pharm Pharmaceut Sci. 2011;3(5):139–143. [Google Scholar]
- 19.Shetty A., Patil A.K., Ameet R., Sandhu P.K. Local infiltration of Vitamin D3 does not accelerate orthodontic tooth movement in humans: a preliminary study. Angle Orthod. 2015 doi: 10.2319/122214-935.1. [DOI] [Google Scholar]
- 20.Meechan J.G. Intraligamentary anaesthesia. J Dent. 1992;20(6):325–332. doi: 10.1016/0300-5712(92)90018-8. [DOI] [PubMed] [Google Scholar]
- 21.Nagata M.J.H., et al. Effectiveness of two methods for preparation of autologous platelet-rich plasma: an experimental study in rabbits. Eur J Dermatol. 2010:395–402. doi: 10.1055/s-0039-1697859. 04(04) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weibrich G., Hansen T., Kleis W., Buch R., Hitzler W. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34(4):665–671. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Graziani F., Ivanovski S., Cei S., Ducci F., Tonetti M., Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006 Apr;17(2):212–219. doi: 10.1111/j.1600-0501.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 24.Varughese S.T., et al. Effect of vitamin D on canine distalization and alveolar bone density using multi-slice spiral ct: a randomized controlled trial. J Contemp Dent Pract. 2019;20(12):1430–1435. doi: 10.5005/jp-journals-10024-2698. [DOI] [PubMed] [Google Scholar]
- 25.Ciur M.D.I., Zetu I.N., Danisia H., Viennot S., Bourgeois D., Andrian S. Evaluation of the influence of local administration of vitamin d on the rate of orthodontic tooth movement. Rev Med-Chir Soc Med Nat Iasi. 2016;120(3):694–699. [PubMed] [Google Scholar]
- 26.Wong L.K., Reinertson E.L. Clinical considerations of dimethyl sulfoxide. ISU Veterinarian. 1984;46(2):89–95. [Google Scholar]
- 27.Madsen B.K., Hilscher M., Zetner D., Rosenberg J. Adverse reactions of dimethyl sulfoxide in humans: a systematic review. F1000Res. 2018;7:1746. doi: 10.12688/f1000research.16642.2. Version 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deguchi T., Takano-Yamamoto T., Yabuuchi T., Ando R., Roberts W.E., Garetto L.P. Histomorphometric evaluation of alveolar bone turnover between the maxilla and the mandible during experimental tooth movement in dogs. Am J Orthod Dentofacial Orthop. 2008;133(6):889–897. doi: 10.1016/j.ajodo.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan V. Root resorption with orthodontic mechanics: pertinent areas revisited. Aust Dent J. 2017;62(Suppl 1):71–77. doi: 10.1111/adj.12483. [DOI] [PubMed] [Google Scholar]
- 30.Kjær I. Morphological characteristics of dentitions developing excessive root resorption during orthodontic treatment. Eur J Orthod. 1995;17(1):25–34. doi: 10.1093/ejo/17.1.25. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson O. Clinical significance of root resorption. Am J Orthod. 1952;38:687–696. doi: 10.1016/0002-9416(52)90206-6. [DOI] [Google Scholar]
- 32.Abdel-Haffiez Sherief H., Ismail Hanan, Elharouni Nadia, Ali Hanaa. The effect of platelet rich plasma injection on relapse of orthodontically moved teeth in rabbits. Egyptian Orthodontic Journal. June 2017;51(6):41–57. doi: 10.21608/eos.2017.78359. [DOI] [Google Scholar]