Summary
Regulatory T cells (Tregs) can inhibit the occurrence of autoimmune diseases and increase the activation threshold of the immune response. Here, we present schemes for the isolation and culture of natural human regulatory T cells (nTregs) and in vitro-induced Tregs (iTregs). Appropriate concentrations of TGF-β, IL-2, retinoic acid (atRA), and rapamycin were used to promote proliferation to meet sample needs in basic research, especially in technologies such as sequencing.
For complete details on the use and execution of this protocol, please refer to Lu et al. (2014a) and Gu et al. (2022).
Subject areas: Cell biology, Cell culture, Cell isolation, Immunology
Graphical abstract
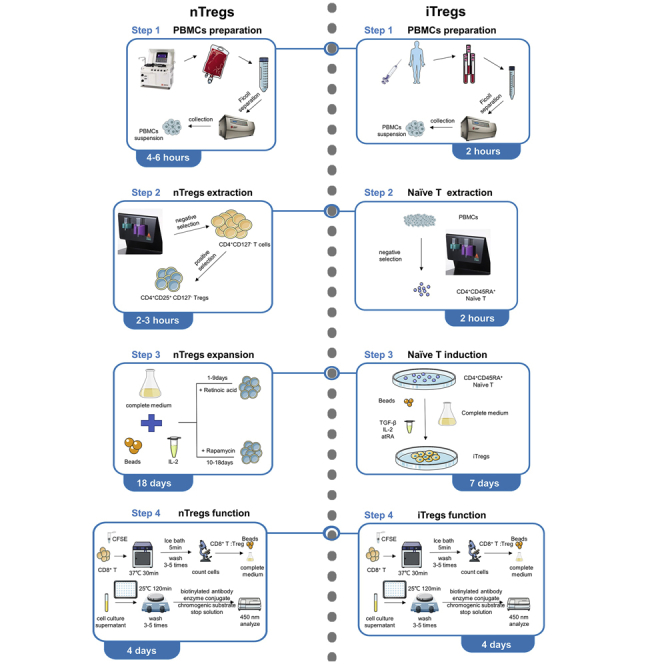
Highlights
-
•
Isolation and culture of human natural regulatory T cells (nTregs)
-
•
Isolation and culture of human in vitro-induced Tregs (iTregs)
-
•
Assays for immunosuppressive function of Tregs
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Regulatory T cells (Tregs) can inhibit the occurrence of autoimmune diseases and increase the activation threshold of the immune response. Here, we present schemes for the isolation and culture of natural human regulatory T cells (nTregs) and in vitro-induced Tregs (iTregs). Appropriate concentrations of TGF-β, IL-2, retinoic acid (atRA), and rapamycin were used to promote proliferation to meet sample needs in basic research, especially in technologies such as sequencing.
Before you begin
Tregs play important roles in autoreactive lymphocyte suppression and regulation of immune homeostasis. This protocol describes a procedure to extract nTreg and naive CD4+ T cells from the peripheral blood of volunteers and induce their expansion.
Due to the low content of nTreg in peripheral blood, we usually extract 100 mL peripheral blood mononuclear lymphocyte cells from the peripheral blood of a single volunteer and then further extract nTreg. To consider the experimental cost, we will extract 100 mL of peripheral blood from volunteers and extract naïve CD4+ T cells (Lu et al., 2010).
Selecting the appropriate culture environment and culture system, and adding the appropriate concentration of cytokines, such as IL-10, TGF-β, atRA, and rapamycin (Lu et al., 2014b), are essential for inducing and expanding Tregs (Lu et al., 2014a). Therefore, before starting, the reagents and equipment listed in the following and attached key resources table should be prepared.
All research work can only be carried out after obtaining the approval of the Institutional Review Board (IRB).
Institutional permissions
Peripheral blood mononuclear lymphocytes from normal volunteers were obtained from the First Affiliated Hospital of Nanjing Medical University under protocols approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (2021-SRFA-346).
Preparation of Tregs isolation and culture
Timing: 1 day
-
1.
Contact volunteers in advance with precautions and signed informed consent forms according to local ethics committee requirements.
-
2.
Check the equipment status and consumables expiration date.
-
3.
Dispense the following required reagents into the corresponding solutions and store them in aliquots.
Note: Transfer of volunteer blood to the laboratory requires a transfer box and ice packs.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Alexa Fluor® 647 anti-human FOXP3 Antibody (clone 206D) (Working dilution: 1:100) | BioLegend | Cat # 320114, RRID: AB_439753 |
| APC anti-human CD45RA Antibody (clone HI100) (Working dilution: 1:100) | BioLegend | Cat # 304112, RRID: AB_314415 |
| APC anti-human CD8a Antibody (clone RPA-T8) (Working dilution: 1:100) | BioLegend | Cat # 301014, RRID: AB_314132 |
| Brilliant Violet 510™ anti-human CD4 Antibody (clone OKT4) (Working dilution: 1:100) | BioLegend | Cat # 357420, RRID: AB_2561377 |
| FITC anti-human CD127 (IL-7Rα) Antibody (clone A019D5) (Working dilution: 1:100) | BioLegend | Cat # 351312, RRID: AB_10933247 |
| FITC anti-human CD4 Antibody (clone OKT4) (Working dilution: 1:100) | BioLegend | Cat # 357406, RRID: AB_571950 |
| PE anti-human CD25 Antibody (clone BC96) (Working dilution: 1:100) | BioLegend | Cat # 302606, RRID: AB_314275 |
| PerCP/Cyanine5.5 anti-human CD25 Antibody (clone M-A251) (Working dilution: 1:100) | BioLegend | Cat # 302626, RRID: AB_2561978 |
| PerCP/Cyanine5.5 anti-human CD4 Antibody (clone RPA-T4) (Working dilution: 1:100) | BioLegend | Cat # 357414, RRID: AB_893328 |
| Biological samples | ||
| Human blood | Healthy volunteers, male and female, aged 18–65 years | Approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (2021-SRFA-346) |
| Chemicals, peptides, and recombinant proteins | ||
| CellTrace™ CFSE Cell Proliferation Kit, for flow cytometry | Invitrogen | C34554 |
| Dimethyl Sulfoxide, 950 mL | Thermo Scientific | 20688 |
| Dynabeads™ Human T-Activator CD3/CD28 | Gibco | 11132D |
| eBioscience™ Foxp3 / Transcription Factor Staining PBS Set | Invitrogen | 00-5523-00 |
| Flow Cytometry Staining Buffer (FACS buffer) | Invitrogen | 00-4222-26 |
| LIVE/DEAD™ Fixable Near-IR Dead Cell Stain | Invitrogen | L34976 |
| Lymphocyte Serum-Free Medium | Corning | 88-581-CM |
| Lymphoprep™ | STEMCELL | Cat # 07851 |
| PBS (1×) | Gibco | 20012027 |
| Rapamycin | MCE | HY-10219 |
| Recombinant Human IL-2 | PeproTech | Cat # 200-02 |
| Recombinant Human TGF-beta 1 Protein | R&D | Cat # 240-B-002 |
| Retinoic acid | MCE | HY-14649 |
| Trypan Blue Stain (0.4%) | Gibco | 15250061 |
| Critical commercial assays | ||
| CD127 MicroBead Kit, human | Miltenyi Biotec | Cat # 130-094-945 |
| CD4+CD25+ Regulatory T Cell Isolation Kit, human | Miltenyi Biotec | Cat # 130-091-301 |
| CD8 MicroBeads, human | Miltenyi Biotec | Cat # 130-045-201 |
| Human interleukin-10 (IL-10) ELISA Kit | Jingmei | JM-03221H1 |
| Human transforming growth factorβ1TGF-β 1 ELISA KIT | Jingmei | JM-03245H1 |
| Mini&MidiMACSTM Starting Kits (MS, LS) | Miltenyi Biotec | Cat #130-042-501 |
| Naive CD4+ T Cell Isolation Kit II, human | Miltenyi Biotec | Cat # 130-094-131 |
| Software and algorithms | ||
| Excel | Microsoft | N/A |
| FlowJo 10.6.1 | BD | https://www.flowjo.com/solutions/flowjo/downloads |
| GraphPad Prism 9 | GraphPad Inc | https://www.graphpad.com/scientific-software/prism/ |
| Other | ||
| 10 mL serological pipette | Labsecet | SP-003-10 |
| 96 Well Cell Culture Cluster | Corning | Cat # 3599 |
| Anticoagulation Solution (Sodium citrate) | JIERUI | https://www.weigaoholding.com/product_info/164.html |
| BD FACS ARIA II SORP (Special Order Research Product) | BD | FACS Aria™ II |
| Beckman Allegra X-15R Benchtop Centrifuge | Beckman Counter | 8043-30-1206 |
| Blood Collection Tubes | BD | 367525 |
| Centrifuge tube, 15 mL | Labsecet | CT-002-15 |
| Centrifuge tube, 50 mL | Labsecet | CT-002-50 |
| Countess™ 3 Automated Cell Counter | Invitrogen | AMQAX2000 |
| Countess™ Cell Counting Chamber Slides | Invitrogen | C10228 |
| DynaMag™-15 | Invitrogen | 12301D |
| Finnpipette 9501 C1 Pipet Controller, 1–100 ML | Thermo Scientific | CN-22800-20 |
| Forma™ Direct Heat CO2 Incubator, 184 L | Thermo Scientific | 320 |
| MCS®+ 9000 Mobile Platelet Collection System | Haemonetics | https://persona.haemonetics.com/en/products/devices/blood-center-devices/mcs-9000 |
| Peripheral Blood Stem Cell Disposable Set | Haemonetics | Cat #0971E-00 |
| 25cm2 Rectangular Canted Neck Cell Culture Flask with Plug Seal Cap | Corning | Cat # 430168 |
| Corning 75cm2 Rectangular Canted Neck Cell Culture Flask with Plug Seal Cap | Corning | Cat # 430720 |
| Round Bottom Polystyrene Tubes | Falcon | 352054 |
Step-by-step method details
Isolation of natural human regulatory T cells (nTregs)
Timing: 3 h for extracting human peripheral blood mononuclear lymphocytes, 3 h for separating nTregs
In this step, human peripheral blood mononuclear lymphocytes were first extracted by MCS®+ 9000 Mobile Platelet Collection System, and then human nTregs were purified by magnetically activated cell sorting (MACS). Alternatively, you can get commercially available buffy coats and skip step 1.
-
1.Collection of human peripheral blood mononuclear lymphocytes (PBMCs).
-
a.Use MCS®+ 9000 Mobile Platelet Collection System to extract 100 mL PBMCs from the peripheral blood of volunteers.
-
b.Lymphoprep™: PBMCs:PBS = 1:1:1.
-
i.Fully mix PBS and PBMCs with the same volume in centrifuge tubes.
-
ii.Place the same volume of lymphoprep ™ below the mixing solution. Indeed the PBS: PBMC is layered ON TOP of the Lymphoprep solution and not vice versa.
 CRITICAL: Due to solution density issues, take care of the Lymphoprep™. Make sure it is not mixed with blood and PBS before centrifugation.
CRITICAL: Due to solution density issues, take care of the Lymphoprep™. Make sure it is not mixed with blood and PBS before centrifugation.
-
i.
-
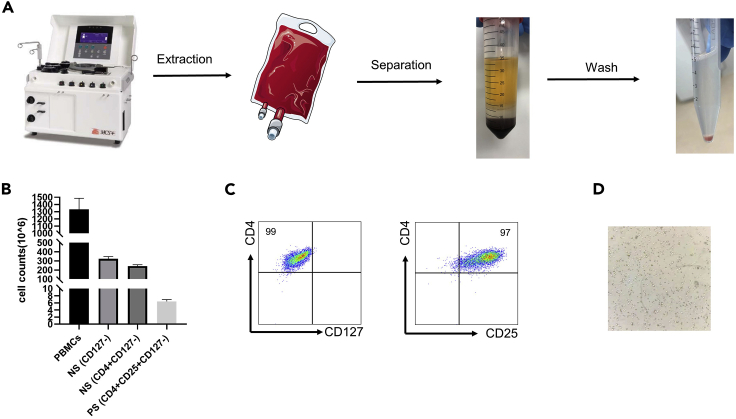
c.Take the middle white film layer after centrifugation (393 g, 23 min, acceleration 0, acceleration 0, 4°C) (Figure 1A).
-
d.Wash by adding one volume of PBS (167 g, 5 min, acceleration 9, deceleration 9, 4°C), and collect the cell pellet. (e.g., 1 mL leukocyte suspension with 1 mL PBS).
-
e.Add the same volume of PBS as the supernatant, and repeat step.1.d 3–5 times until the liquid was clear.
-
f.Collect all the cell pellets and count PBMCs.Note: All of the work with Lymphoprep needs to be at 25°C. Temperature fluctuations will result in poor separation. And all subsequent work (after PBMC isolation) is performed ‘ON ICE’.
-
a.
-
2.Isolation of CD4+CD25+CD127- nTregs.
-
a.Resuspend cell pellet in 30 μL of PBS per 10⁷ total cells.
-
b.Negative selection of CD127- T cells.
-
c.Add 10 μL of FcR Blocking Reagent and 10 μL of CD127 Biotin per 10⁷ total cells.
-
i.Mix well and incubate for 10 min in the refrigerator (2°C–8°C). Add 30 μL of PBS and 20 μL of Anti-Biotin MicroBeads per 10⁷ total cells.
-
ii.Mix well and incubate for 15 min in the refrigerator (2°C–8°C). Wash cells by adding 10–20 mL of PBS per 108 cells and resuspend up to 10⁸ cells in 500 μL of PBS.
-
i.
-
d.Proceed to magnetic separation and place it in the magnetic field of a suitable MACS Separator.
-
i.After rinsing the LS Column with 2 mL of PBS, apply cell suspension to the column.
-
ii.Take unlabeled cells that pass through the column and two 1 mL PBS washing steps are done on the same cell suspension.
-
i.
-
e.Centrifuge cell suspension, discard the supernatant, and count cells.
-
f.Negative selection of CD4+CD127- T cells.
-
g.For each 10⁷ total cells, suspend the cell pellet in 90 μL of PBS.
-
i.To this, add 10 μL of CD4+ T Cell Biotin-Antibody Cocktail and incubate for 5 min in the refrigerator (2°C–8°C).
-
ii.Mix well and incubate for another 10 min in the refrigerator (2°C–8°C) with 20 μL of Anti-Biotin MicroBeads per 107 cells.
-
i.
-
h.Repeat steps 2.d. and 2.e.
-
i.Positive selection of CD4+CD25+CD127- nTregs.
-
j.Centrifuge cell suspension and count cells.
-
k.Resuspend cell pellet in 90 μL of PBS.
-
i.Add 10 μL of CD25 MicroBeads, mix well and incubate for 15 min in the dark in the refrigerator (2°C–8°C).
-
ii.Wash cells by adding 1–2 mL of PBS, centrifuge, and resuspend up to 10⁸ cells in 500 μL of PBS.
-
i.
-
l.Place MS Column in the magnetic field of a suitable MACS Separator.
-
i.Rinse the column with 500 μL of PBS, apply cell suspension to the column, and wash the column with 500 μL of PBS.
-
ii.Remove the column from the separator, and place it on a suitable collection tube.
-
i.
-
m.Pipette 1 mL of PBS onto the column. Push the plunger firmly into the column to flush out the magnetically labeled cells immediately.
-
n.Centrifuge cell suspension and count cells (Figure 1B).
-
a.
-
3.Identification of nTregs by flow cytometry.
-
a.Save an aliquot after each separation step and check each aliquot for purity.
-
b.Take 0.3∗106 cells in 100μL FACS buffer.
-
i.For each tube, add staining antibodies, 0.5 μL of CD4-BV510, 0.5 μL of CD25-PE, 0.5 μL of CD127-FITC, and 0.1 μL of LIVE/DEAD™ and vortex.
-
ii.Incubate in the refrigerator (2°C–8°C) for 15 min in the dark.
-
i.
-
c.Wash cells with 500 μL FACS buffer, decant the supernatant and retain about 100 μL residual volume.Note: Wash the stained cell suspension before being analyzed by flow cytometry to remove unbound.
-
d.The stained cells were resuspended in 500 μL FACS buffer and analyzed by flow cytometry (Figure 1C).Note: About 0.3–1.0 million nTreg can be obtained from the starting cell number of 100 million.Alternatives: Most of the time we choose FACS buffer. If there is no FACS buffer temporarily, you can choose PBS instead.
-
a.
Figure 1.
Isolation of natural human regulatory T cells (nTregs)
(A) Human peripheral blood mononuclear lymphocytes (PBMCs) were sorted from samples extracted by MCS®+ 9000 Mobile Platelet Collection System.
(B) The number of cells obtained by magnetic column sorting at each step. On the first negative selection (NS) step, CD127- cells were sorted from PBMCs. On the second negative selection step, CD4+CD127- cells were got. On the third positive selection (PS) step, CD4+CD25+CD127- nTregs were finally received.
(C) The purity of CD4+ CD127- cells were identified after NS selection, and the purity of CD4+CD25+CD127- nTregs were identified after PS selection on day 0.
(D) State of nTregs after selection on day 0 under the microscope (10×).
Culture of natural human regulatory T cells (nTregs)
Timing: 18 days
This step describes the activation and culture of nTregs.
-
4.Wash Human T-Activator CD3/CD28 Beads.
-
a.106 nTregs need 25 μL Human T-Activator CD3/CD28 Beads.
-
b.Place the magnetic bead suspension in the 1.5 mL tube.
-
c.Place the tube in a magnetic stand and aspirate the liquid after the magnetic beads attach to the inner wall of the tube.
-
d.After magnetic beads were resuspended with PBS, repeat step 4.c.
-
e.Repeat 4.c and 4.d 3–5 times, and obtain magnetic bead precipitation.
-
a.
CRITICAL: The preservation solution of Human T-Activator CD3/CD28 Beads is cytotoxic, thus requiring washing the beads three times with PBS (Gu et al., 2017).
-
5.Culture for the first 9 days.
-
a.106 nTregs were cultured in 1 mL complete medium (10% Fetal Bovine Serum and 1% Penicillin-Streptomycin Solution in Lymphocyte Serum-Free Medium). Select a suitable culture container according to the volume of the liquid, and the culture liquid needs to cover the bottom of the container.Alternatives: Regular serum-based media can be used to culture Tregs.
-
b.Add 25 μL Human T-Activator CD3/CD28 Beads to 106 cells, 300 IU/mL IL-2, and 100 nM/mL retinoic acid (Figure 1D).
 CRITICAL: The relative quiescence of the whole culture system should be maintained within 48 h after adding Human T-Activator CD3/CD28 Beads.
CRITICAL: The relative quiescence of the whole culture system should be maintained within 48 h after adding Human T-Activator CD3/CD28 Beads. -
c.After 48 h of incubation (36.7 Temp°C, 5.1% CO2), one volume of complete medium was added. 300 IU/mL IL-2 and 50 nM/mL retinoic acid were added to the total volume. (e.g., 1 mL initial culture volume, addition of 1 mL incubation solution, 600 IU IL-2 and 100 nM retinoic acid).
-
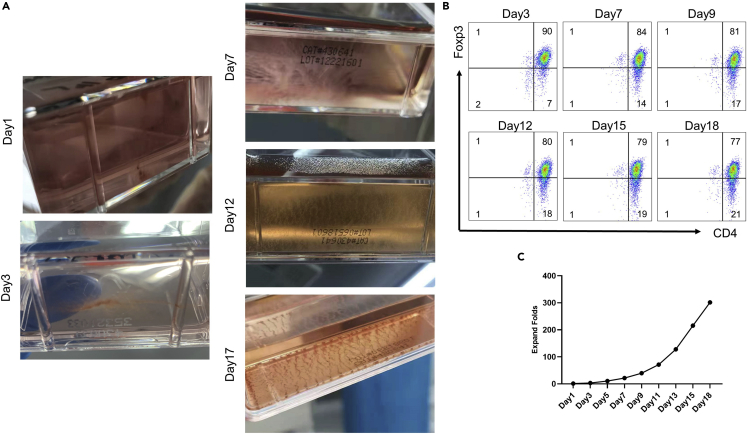
d.Observe the cultural solution daily. If the color turns yellow, add IL-2, retinoic acid, and complete medium as above.Note: Be careful to change flasks in time to ensure adequate growth space during the rapid cell proliferation phase (Figure 2A). If the total volume of the culture medium exceeds 2/3 of the bottle volume, the flask needs to be replaced.Note: The cell proliferation phase is rapid (day 8–15), nutrient consumption is rapid and metabolites are acidic, making the culture fluid yellow. (Figure 1A on day 12).
-
a.
-
6.Culture for the second 9 days.
-
a.Centrifuge cell suspension and count cells.
-
b.(Optional) Freeze a portion of nTregs.
-
c.nTregs were cultured at 106/mL with a complete medium. Add 25 μL Human T-Activator CD3/CD28 Beads to 106 cells, 300 IU/mL IL-2, and 100 nM/mL rapamycin.
-
d.After 48 h of incubation, one volume of complete medium was added. 300 IU/mL IL-2 and 50 nM/mL rapamycin were added to the total volume. (e.g., 1 mL initial culture volume, addition of 1 mL incubation solution, 600 IU IL-2 and 100 nM rapamycin).
-
e.Observe the cultural solution daily. If the color turns yellow, add IL-2, rapamycin, and complete the medium.
-
a.
Pause point: The cell cryopreservation solution is formulated from 90% FBS and 10% DMSO and is subjected to programmed freezing for final storage in liquid nitrogen. Cells are viable for 6 months.
-
7.Identification of nTregs by flow cytometry.
-
a.Take 0.3∗106 cells in 100 μLFACS buffer.
-
i.Add staining antibodies, 0.5 μL of CD4-FITC, 0.5 μL of CD25-PE, and 0.1 μL of LIVE/DEAD™ to each tube and vortex.
-
ii.Incubate in the refrigerator (2°C–8°C) for 15 min in the dark.
-
i.
-
b.Wash cells with 500 μL FACS buffer, abandon the supernatant and retain about 100 μL residual volume.
-
c.Add 500 μL 1× Fixation / Permeabilization Concentrate working solution to each tube and pulse vortex. Incubate for 30–60 min in the dark in the refrigerator (2°C–8°C).
-
d.Centrifuge cell suspension and abandon the supernatant. Add 500 μL of 1× Permeabilization PBS to each tube and discard the supernatant after centrifugation. Repeat once.
-
e.Resuspend pellet in remaining volume of 1× Permeabilization PBS, typically 500 μL.
-
f.Add staining antibodies, 0.5 μL of Foxp3-647 to each tube, pulse vortex and incubate for 30 min in the dark in the refrigerator (2°C–8°C).
-
g.Add 500 μL of 1× Permeabilization PBS to each tube and discard the supernatant after centrifugation.
-
h.The stained cells were resuspended in 500 μL FACS buffer and analyzed by flow cytometry (Figure 2B).
-
a.
Figure 2.
The cultural status of natural human regulatory T cells (nTregs) at different time points
(A) Representative plots of changes in cell status and cell culture medium in general on day 1, 3, 7, 12, and 17.
(B) Representative plots of CD4+Foxp3+ nTregs at different time points by flow cytometry.
(C) The expansion fold of nTregs (The first day set to 1).
Isolation and culture of human in vitro-induced Tregs (iTregs)
Timing: 3 h for separating iTregs, 7 days for active and culture iTregs
In this step, iTregs were first extracted by PBMCs through MACS, and then cultured with IL-2, TGF-β, and retinoic acid.
-
8.Isolation of CD4+CD45RA+T cells.
-
a.Determine the cell number of PBMCs, which can be obtained from peripheral blood and apheresis products.
-
b.For each 10⁷ total cells, suspend the cell pellet in 40 μL of PBS. To this, add 10 μL of Naive CD4+ T Cell Biotin-Antibody Cocktail II and incubate for 5 min in the refrigerator (2°C–8°C).
-
c.Mix well and incubate for another 10 min in the refrigerator (2°C–8°C) with 20 μL of Naive CD4+ T Cell MicroBeads Cocktail II and 30 μL of PBS per 107 cells.
-
d.Proceed to magnetic separation.
-
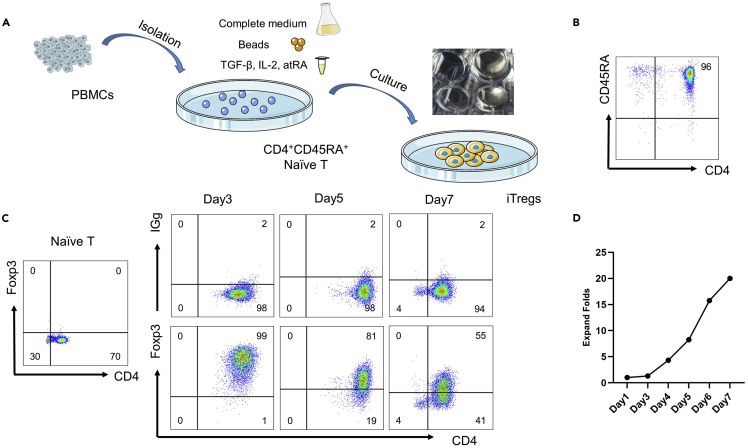
e.Place LS Column in the magnetic field of a suitable MACS Separator. After rinsing the LS Column with 3 mL of PBS, apply cell suspension to the column. Take unlabeled cells that pass through the column and wash it with 2×1 mL of PBS (Figure 3A).
-
f.Centrifuge cell suspension and count cells.
-
a.
-
9.Identification of CD4+CD45RA+T cells by flow cytometry.
-
a.Take 0.3∗106 cells in 100μL FACS buffer.
-
i.Add staining antibodies, 0.5 μL of CD4-BV510, 0.5 μL of CD45RA-PE, and 0.1 μL of LIVE/DEAD™ to each tube and vortex.
-
ii.Incubate in the refrigerator (2°C–8°C) for 15 min in the dark.
-
i.
-
b.Wash cells with 500 μL FACS buffer, abandon the supernatant and retain about 100μL residual volume.
-
c.The stained cells were resuspended in 500 μL FACS buffer and analyzed by flow cytometry (Figure 3B).
-
a.
-
10.Wash Human T-Activator CD3/CD28 Beads.
-
a.106 nTregs need 25 μL Human T-Activator CD3/CD28 Beads.
-
b.Place the magnetic bead suspension in the 1.5 mL tube.
-
c.Place the tube in a magnetic stand and aspirate the liquid after the magnetic beads attach to the inner wall of the tube.
-
d.After magnetic beads were resuspended with PBS, repeat step 10.c.
-
e.Repeat 10.c and 10.d 3–5 times, and obtain magnetic bead precipitation.
-
a.
-
11.Induction of naïve CD4+ T cells into iTregs.
-
a.Naïve CD4+ T cells were cultured at 5∗105/mL with a complete medium (10% Fetal Bovine Serum and 1% Penicillin-Streptomycin Solution in Lymphocyte Serum-Free Medium).
-
b.Add 25 μL Human T-Activator CD3/CD28 Beads to 106 cells, 100 IU/mL IL-2, 1 ng/mL TGF-β, and 100 nM/mL retinoic acid.
-
c.After 48 h of incubation, one volume of complete medium was added. 300 IU/mL IL-2 and 50 nM/mL rapamycin were added to the total volume. (e.g., 1 mL initial culture volume, addition of 1 mL incubation solution, 600 IU IL-2 and 100 nM retinoic acid).
-
d.Observe the cultural solution daily. If the color turns yellow, add IL-2, retinoic acid, and complete medium as above.
-
a.
Note: iTregs can maintain activity and function for up to 7 days.
Note: iTregs are less functional upon reactivation and the required experiments should be performed on days 3–5.
-
12.Identification of iTregs by flow cytometry.
-
a.Take 0.3∗106 cells in 100 μL FACS buffer.
-
i.Add staining antibodies, 0.5 μL of CD4-FITC, 0.5 μL of CD25-Percp/Cy5.5, and 0.1 μL of LIVE/DEAD™ to each tube and vortex.
-
ii.Incubate in the refrigerator (2°C–8°C) for 15 min in the dark.
-
i.
-
b.Wash cells with 500 μL FACS buffer, abandon the supernatant and retain about 100 μL residual volume.
-
c.Add 500 μL 1× Fixation / Permeabilization Concentrate working solution to each tube and pulse vortex. Incubate for 30–60 min in the dark in the refrigerator (2°C–8°C).
-
d.Centrifuge cell suspension and abandon the supernatant. Add 500 μL of 1× Permeabilization PBS to each tube and discard the supernatant after centrifugation. Repeat once.
-
e.Resuspend pellet in remaining volume of 1× Permeabilization PBS, typically 500 μL.
-
f.Add staining antibodies, 0.5 μL of Foxp3-647 to each tube, pulse vortex and incubate for 30 min in the dark in the refrigerator (2°C–8°C).
-
g.Add 500 μL of 1× Permeabilization PBS to each tube and discard the supernatant after centrifugation.
-
h.The stained cells were resuspended in 500 μL FACS buffer and analyzed by flow cytometry (Figure 3C).
-
a.
Note: About 6–10 million naïve CD4+ T cells can be obtained from the starting cell number of 100–120 million in 100 mL blood, and can be induced to 250–300 million iTregs in the next 7 days.
Figure 3.
Isolation and culture of human in vitro-induced Tregs (iTregs)
(A) Naïve CD4+ T cells were sorted from PBMCs by MACS, then cultured with TGF-B, IL-2, and atRA to generate iTregs.
(B) The purity of naïve CD4+ T cells was identified by flow cytometry after sorting.
(C) Representative plots of CD4+Foxp3+ iTregs by flow cytometry on day 0, 3, 5, 7.
(D) The expansion fold of iTregs (The first day set to 1).
Treg functional assays—ELISA and CFSE
Timing: 3 h for ELISA, 4 days for CFSE
In this step, ELISA and CFSE were used to detect Tregs function.
-
13.ELISA.
-
a.Bring all reagents to 25°C before use.
 CRITICAL: It is strongly recommended that all standards and samples be run in duplicate or triplicate. A standard curve is required for each ELISA assay.
CRITICAL: It is strongly recommended that all standards and samples be run in duplicate or triplicate. A standard curve is required for each ELISA assay. -
b.If not all microplate strips will be used, remove the excess strips by pressing them up from underneath each strip. Place excess strips back in the foil pouch with the included desiccant pack and reseal.
-
c.Wash plate 4 times with at least 300 μL Wash PBS per well and blot residual PBS by firmly tapping the plate upside down on absorbent paper. All subsequent washes should be performed similarly.
-
d.100 μL of standards and assay samples were added to the reaction wells and the plates were then incubated at 25°C with shaking for 120 min after blocking. Wash plate 4 times with Wash PBS.
-
e.Add 100 μL of biotinylated antibody working solution to the reaction wells and incubate with shaking for 60 min at 25°C after blocking the plate. Wash plate 4 times with Wash PBS.
-
f.100 μL of enzyme conjugate working solution was added to the reaction wells, and the plates were then incubated for 30 min at 25°C with shaking. Wash plate 5 times with Wash PBS.
-
g.100 μL chromogenic substrate was added to the reaction wells, and the color was developed at 25°C for 15 min in the dark after plate sealing.
-
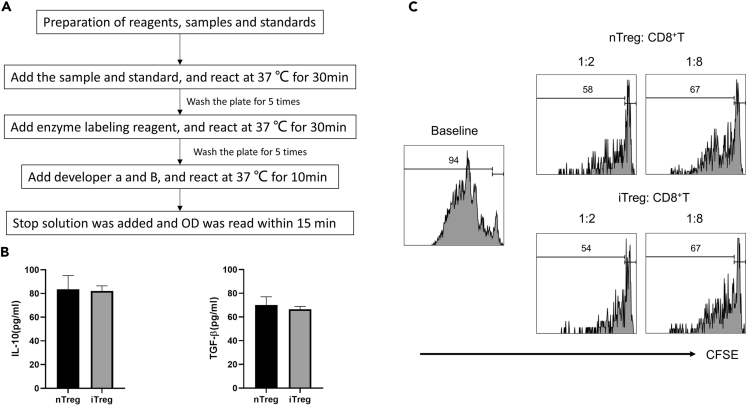
h.50 μL stop solution was added and OD was measured immediately (within 15 min) at 450 nm using a microplate reader (Figure 4A).
-
a.
-
14.Isolation of CD8+ T cells.
-
a.Determine the cell number of PBMCs.
-
b.Resuspend cell pellet in 80 μL of PBS per 107 total cells. Add 20 μL of CD8 MicroBeads per 107 total cells. Mix well and incubate for 15 min in the refrigerator (2°C–8°C).
-
c.Wash cells by adding 1 mL–2 mL of PBS per 107 cells, and resuspend up to 108 cells in 500 μL of PBS. Place MS Column in the magnetic field of a suitable MACS Separator.
-
d.Rinse the column with 500 μL of PBS, apply cell suspension to the column, and wash the column with 500 μL of PBS. Remove the column from the separator, and place it on a suitable collection tube.
-
e.Pipette 1 mL of PBS onto the column. Push the plunger firmly into the column to flush out the magnetically labeled cells immediately.
-
f.Centrifuge cell suspension and count cells.
-
a.
-
15.CFSE.
-
a.Resuspend 1–10∗106 CD8+ cells in 1 mL PBS, then add 1μL CFSE Cell Proliferation Kit and mix well.
-
b.Incubate cells for 30 min in a 37°C Incubator.
-
c.Add 5 mL of Lymphocyte Serum-Free Medium and ice bath for 5 min to stop staining. Wash three times with PBS.Note: Ice baths need to be adequate and complete to ensure that no CFSE fuel remains (Ni et al., 2018).
-
d.Tregs: CD8+T cells= 1:2, 1:8, and the total number of cells was consistent in each group (Figure 4B).
-
e.Cells were cultured at 106/mL with a complete medium, and add 5 μL of Human T-Activator CD3/CD28 Beads per 106 CD8+T cells.Note: Excessive addition of activated magnetic beads will lead to no obvious cell inhibition.
-
f.After 72–96 h, take 0.3∗106 cells in 100μL FACS buffer.
-
i.Add staining antibodies, 0.5 μL of CD4-FITC, 0.5 μL of CD25-Percp/Cy5.5, and 0.1 μL of LIVE/DEAD™ to each tube and vortex.
-
ii.Incubate in the refrigerator (2°C–8°C) for 15 min in the dark.
-
i.
-
g.Wash cells with 500 μL FACS buffer, abandon the supernatant, and retain about 100μL residual volume.
-
h.The stained cells were resuspended in 500 μL FACS buffer and analyzed by flow cytometry.
-
a.
Figure 4.
The immunosuppressive function of nTregs and iTregs
(A) Brief protocol for ELISA assay for the expression of IL-10 and TGF-β in Tregs.
(B) Histograms of IL-10 and TGF-β secretion in the cell culture supernatant of two Treg cell groups after three days of culture. The result is representative of three independent experiments.
(C) CFSE-labeled CD8+ T cells were co-cultured with nTregs and iTregs in the presence of Human T-Activator CD3/CD28 Beads for 4 days. The cells were stained with an anti-human CD8 antibody and assessed with flow cytometry.
Expected outcomes
This step-by-step protocol provides detailed information for the isolation and purification of human nTregs as well as culture. nTregs are expected to expand 200–300 fold within 12–14 days (Figure 2C) and do not lose the associated phenotype. And iTregs are expected to expand 25–30 fold within 7 days (Figure 3D). The cellular phenotype of Tregs during culture can be determined by flow cytometry.
Limitations
This culture method can be applied to human nTregs. However, we observe differences in nTregs cell activity and proliferation obtained from different volunteers. These may be due to individual variability (age, health status, lifestyle effects, etc.). In addition, human nTregs have substantially elevated rates of cell death after several rounds of passaging, possibly showing limited growth. Therefore, we suggest that when initially cultured for a week with optimal cell activity, nTregs can be partially cryopreserved and quality control measures instituted or changed to iTregs for experiments.
Troubleshooting
Problem 1
The needle of the Peripheral Blood Stem Cell Disposable Set is dislodged or blocked (related to step 1).
Potential solution
Select appropriate veins for puncture and standby indwelling needles.
Problem 2
Lymphoprep™ mixed with blood or PBS (related to step 1).
Potential solution
To avoid this, use the pipette carefully and pay attention to the liquid level at all times. If it has been mixed, there is no corresponding remedy.
Problem 3
Sort magnetic column clogging (related to step 2).
Potential solution
There is a maximum cell loading for the sorting magnetic column, and it is suggested that the maximum cell loading for LS is at 500 M cells and for MS is at 50 M cells. Also, you can filter the cells before adding them to the column.
Problem 4
Cells do not proliferate or die more (related to step 4).
Potential solution
Incorrect cell culture density. IL-2 varies from batch to batch, stored too long (no longer than 1 month at 4°C)or repeated freeze-thawing may decrease activity. Take care to wash the Human T-Activator CD3/CD28 Beads.
Problem 5
The inhibitory function was not apparent (related to step 15).
Potential solution
Cell viability and function declined during the third stimulation. And a single expansion of more than 12 days may also reduce the suppressive function.
Inadequately washing CFSE staining may decrease the proliferation of CD8+ T cells. Increasing ice bath time or the number of PBS washes may be useful.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Ling Lu, lvling@njmu.edu.cn.
Materials availability
This study did not generate unique reagents.
Acknowledgments
This study was supported by grants from the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-035). The graphical abstract was modified from Servier Medical Art (smart.servier.com/), licensed under a Creative Attribution 3.0 Generic License. (creativecommons.org/lic).
Author contributions
Conceptualization, L.L. and J.G.; methodology, J.G. and Q.S.; investigation, J.Z.; writing, J.G. and Q.S.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate datasets/codes.
References
- Gu J., Ni X., Pan X., Lu H., Lu Y., Zhao J., Guo Zheng S., Hippen K.L., Wang X., Lu L. Human CD39(hi) regulatory T cells present stronger stability and function under inflammatory conditions. Cell. Mol. Immunol. 2017;14:521–528. doi: 10.1038/cmi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Zhou J., Chen Q., Xu X., Gao J., Li X., Shao Q., Zhou B., Zhou H., Wei S., et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-beta signaling in regulatory T cells. Cell Rep. 2022;39:110986. doi: 10.1016/j.celrep.2022.110986. [DOI] [PubMed] [Google Scholar]
- Lu L., Lan Q., Li Z., Zhou X., Gu J., Li Q., Wang J., Chen M., Liu Y., Shen Y., et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc. Natl. Acad. Sci. USA. 2014;111:E3432–E3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Wang J., Zhang F., Chai Y., Brand D., Wang X., Horwitz D.A., Shi W., Zheng S.G. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J. Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Wang J., Gu J., Lu H., Li X., Qian X., Liu X., Wang X., Zhang F., Lu L. Rapamycin regulates iTreg function through CD39 and Runx1 pathways. J. Immunol. Res. 2014;2014:989434. doi: 10.1155/2014/989434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X., Tao J., Barbi J., Chen Q., Park B.V., Li Z., Zhang N., Lebid A., Ramaswamy A., Wei P., et al. YAP is essential for Treg-mediated suppression of antitumor immunity. Cancer Discov. 2018;8:1026–1043. doi: 10.1158/2159-8290.CD-17-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate datasets/codes.