Abstract
Purpose
To investigate, in patients with gastric carcinoma undergoing laparoscopic radical gastrectomy, the effects of ultrasound-guided quadratus lumborum block (UG-QLB) combined with general anaesthesia (GA) on the postoperative recovery compared with GA alone.
Patients and Methods
The retrospective study enrolled 231 patients with gastric carcinoma undergoing laparoscopic radical gastrectomy, including 119 patients who received UG-QLB combined with GA (Group QG), and 112 patients undergoing GA alone (Group GA). The primary endpoint was the postoperative 3-year recurrence-free survival (RFS). The secondary endpoints were the average visual analogue scale (VAS) scores within 48 h after surgery, the first time of postoperative ambulation, the first time of flatus, postoperative hospitalization, perioperative opioid requirement and adverse effects after surgery.
Results
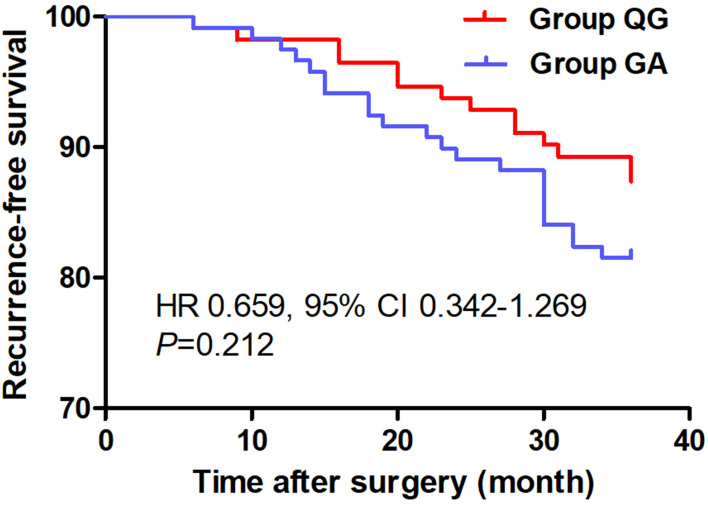
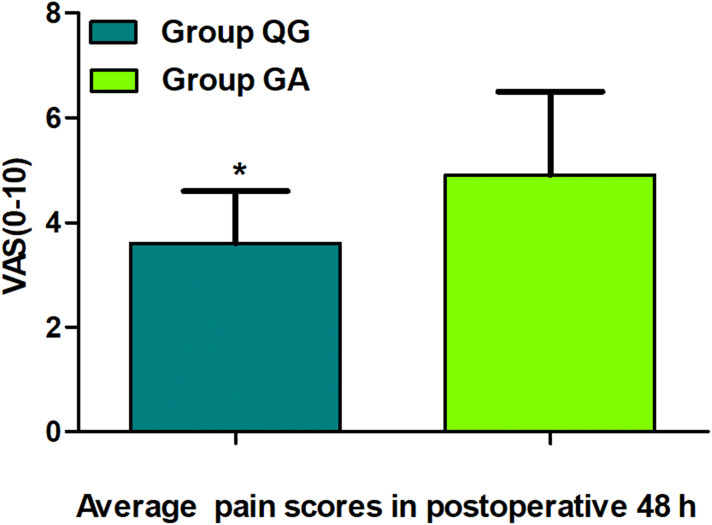
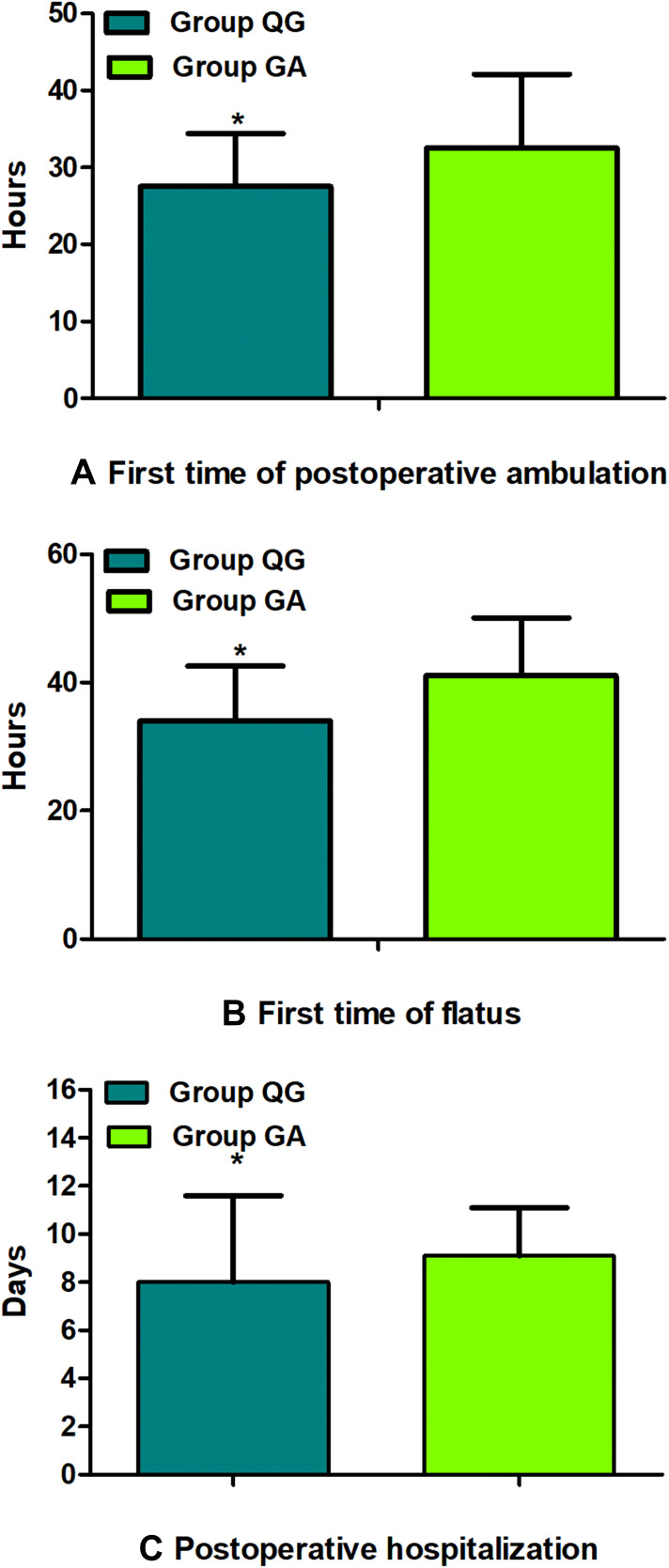
UG-QLB combined with GA did not affect the 3-year RFS in patients undergoing laparoscopic radical gastrectomy (HR 0.659, 95% CI 0.342–1.269, P=0.212). However, the VAS ranking analysis implicated that it could significantly alleviate the postoperative pain in laparoscopic radical gastrectomy patients (P<0.01). In addition, it dramatically facilitated the early recovery of postoperative ambulation and flatus, while shortening the duration of postoperative hospitalization (P<0.01). The most important was it could remarkably reduce the opioid consumption (P<0.01), which in the meanwhile, reduced the incidence of postoperative nausea and vomiting (PONV) (P=0.01).
Conclusion
Although UG-QLB combined with GA did not improve the 3-year RFS for patients with gastric carcinoma undergoing laparoscopic radical gastrectomy, it could provide satisfactory postoperative pain relief, reduce opioid consumption and adverse effects, which subsequently facilitates postoperative early rehabilitation.
Keywords: gastric adenocarcinoma, prognosis, postoperative early recovery, laparoscopic radical gastrectomy, quadratus lumborum block, ultrasound guidance
Introduction
Gastric cancer, as the sixth most common cancer, is the third leading cause of malignant tumour-related death around the world, and adenocarcinoma has been a common pathological type.1,2 At present, laparoscopic radical gastrectomy has been currently the best option for cure because of its minimal invasion, reduced pain, lower postoperative complications, early recovery, and improved quality of life.2–4 However, various aspects of surgery (eg suppressing antitumour cell-mediated immunity, interfering in the microenvironment of the tumour, directly affecting malignant tissue) generally promote the danger for the development of preexisting micro-metastases and, as a result, the survival time of patients reduces.5–7 Furthermore, perioperative other factors, such as surgical stress response and anaesthesia method, probably inhibit the body immune function and also contribute to the viability of minimal residual disease with consequential local recurrence / metastatic disease, and hence the above factors are an acknowledged risk of relevant promotion factors to prognosis.7–9 Consequently, it is essential to improve the prognosis of patients with malignant tumour for suppressing stress response and protecting immune function in the perioperative period.
So far as we know, a lot of latest literature has demonstrated that apart from surgery, more and more attention had been paid to the influence of anaesthesia technique on the oncologic long-term prognosis.10,11 Previous investigations indicated that the appropriate anaesthesia technique could suppress stress response and maintain immune system, which contributed to the postoperative rehabilitation.12–14 Although general anaesthesia (GA) alone is the most common technique in surgery, it made inflammation and immunosuppression stronger than local anaesthesia.9,15 Previous studies illuminated that regional analgesia had effective analgesic effect, moderate the neuroendocrine response to operative stress, and also relieve immunosuppression.9,15,16 Besides, it might potentially delay the risk of postoperative tumour recurrence and metastases.8,17–23 Interestingly, researchers have now demonstrated that amide local anesthetics probably had direct effects on cancer cell.24–26 Along with the progress of ultrasound technology, quadratus lumborum block (QLB) is being increasingly used in abdominal surgery for analgesia, which is a relatively recent technique first described by Blanco in 2007.27 Several variations of QLB have been developed since then, all of which involves the injection of local anesthetic into the fascial plane around the quadratus lumborum muscle. Up to date, there are 6 approaches including lateral QLB, posterior QLB, transmuscular QLB, intramuscular QLB, subcostal QLB and anterior QLB at the lateral supra-arcuate ligament. Moreover, it has lots of benefits such as effective analgesia, minimising opioid requirements, reduction side effect, suppressing inflammatory response and facilitating postoperative recovery.28–32
However, to our knowledge, there is still lack of study comparing the surgical outcomes between ultrasound-guided QLB (UG-QLB) combined with GA and GA alone in laparoscopic radical gastrectomy for gastric adenocarcinoma. Therefore, in the monocentric retrospective research, we tested the primary hypothesis that UG-QLB combined with GA could improve recurrence-free survival (RFS) compared with GA alone in patients with gastric carcinoma undergoing laparoscopic radical gastrectomy. In addition, we tested the secondary hypotheses that UG-QLB combined with GA might promote the postoperative early rehabilitation.
Materials and Methods
Ethical Statement for Collecting Clinical Information
The monocentric retrospective study was approved by the Research Ethics Committee of The Second Hospital of Shandong University (No: KYLL-2021LW-066) and was adhered to the Declaration of Helsinki, before the trial registration at the Chinese Clinical Trail Registry (ChiCTR2100050656). The data was anonymous and there was no damage or conflicts of interest to the patients, so that the requirement for obtaining informed consent was waived.
Study Design and Patients Selection
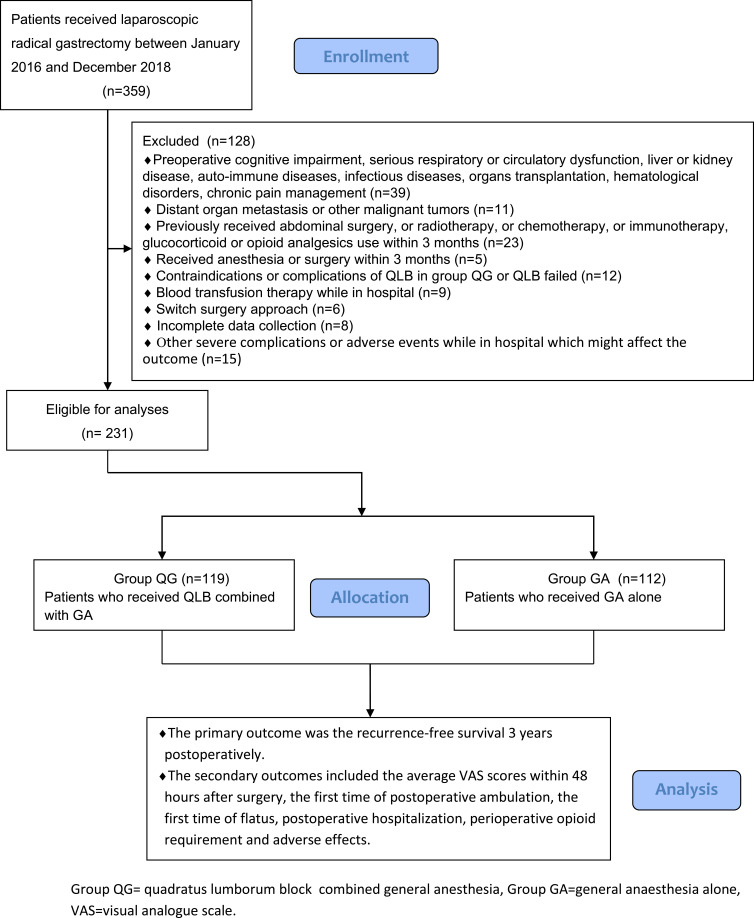
In all, 231 patients with gastric carcinoma undergoing laparoscopic radical gastrectomy in the second hospital of Shandong University between January 2016 and December 2018 were included in the research. The data were collected from the electronic medical records of patients, the anesthesiology database and patients. To minimize selection bias, data collection was performed by research workers who were blinded to the research objective. All the patients were split into two groups according to anaesthesia methods: QLB combined GA group (QG group, n=119) and GA alone group (GA group, n=112). Inclusion criteria were: (1) ages ranged from 20 to 80 years; American Society of Anesthesiologists (ASA) grade I~III; (2) histology confirmed adenocarcinoma and negative resection margin; (3) all patients underwent the same surgical technique (all patients had underwent 5-port laparoscopic gastrectomy, following the principles of gastric cancer treatment guidelines. A radical surgery with therapeutic purpose was performed adherence with operative standards in the treatment of gastric cancer in the Japanese.33 Total or subtotal gastrectomy was performed according to the tumour stage, tumour location and status of the regional lymph nodes.); (4) all patients received GA combined with QLB or GA alone; (5) all anaesthesia, surgeries management and nursing team were performed by the same team; (6) all the post-operative pain management methods were identical in the groups. The patient-controlled intravenous analgesia (PCIA) for 48-h postoperatively was signed, and the sufentanil was acted as ease pain if by any chance it was not satisfactory. Exclusion criteria were: (1) preoperative cognitive impairment, serious respiratory or circulatory dysfunction, liver or kidney disease (Child-Pugh grade C and serum creatinine >442 mol/L), auto-immune diseases, infectious diseases, organs transplantation, hematological disorders, chronic pain management; (2) distant organ metastasis or other malignant tumours (including malignant tumour history); (3) previously received abdominal surgery, or radiotherapy, or chemotherapy, or immunotherapy, use of glucocorticoid or opioid analgesics within 3 months; (4) received anaesthesia or surgery within 3 months; (5) contraindications or complications in response to QLB in group QG (eg local infection, local anaesthetic toxicity), failure to complete QLB or QLB failed; (6) blood transfusion therapy while in hospital; (7) switch surgery approach; (8) incomplete data collection; (9) other severe complications or adverse events while in hospital which might affect the outcome (including severe cardiopulmonary disease, abdominal infection, incision split or infection, urinary tract infection or retention, subphrenic infection, pneumonia, gastroparesis, intestinal obstruction, pancreatic leak, anastomotic leak, postoperative hemorrhage, dumping syndrome, reoperation for other reasons.).
Anaesthesia Methods
All participants were regularly monitored by electrocardiography (ECG), invasive blood pressure (IBP), saturation of peripheral oxygen (SPO2), end-tidal carbon dioxide (ETCO2), bispectral index (BIS) values and body temperature monitoring. For patients assigned to QG group, bilateral QLB (30 mL of 0.25% ropivacaine per side) was once carried out before induction of GA and the success of QLB was also confirmed through the pinprick test. All participants received GA induction with propofol, sufentanil, and cisatracurium, which maintained with combined propofol and sevoflurane. Additional sufentanil and cisatracurium were given as necessary. Postoperatively, the 48-h PCIA pump was performed for all patients. The formula was: sufentanil 2 μg/kg + tropisetron 5 mg, diluted to 100 mL with saline. Moreover, the load was 5 mL, the background infusion was 2 mL/h, the lock time was 15 minutes, and the additional volume 0.5 mL. In addition, a full-time anaesthesia nurse who was blinded to the anaesthesia method was usually arranged to follow up and evaluate the pain visual analogue scale (VAS, where 0 is no pain and 10 is the worst imaginable pain) scores at 24 h and 48 h post-surgery.
Outcomes
The primary endpoint was the postoperative 3-year recurrence-free survival (RFS), and it was described as the time from completion of surgery to the earliest date of recurrence, metastasis or gastric cancer-cause death, whichever happen first. (2) The secondary endpoints were as below: I. The average VAS scores within 48 h postoperatively. II. The recovery of the patients, including the first time of postoperative ambulation, the first time of flatus and postoperative hospitalization. III. Sufentanil consumption during and 48 h post-surgery. IV. The adverse effects within 48 h after surgery, including drowsiness, postoperative nausea and vomiting (PONV), pruritus, respiratory depression, urinary retention, hypotension and bradycardia.
Statistical Analysis
SPSS 23.0 (SPSS Inc. Armonk, NY, USA) was used for statistical analysis. Data were given as mean ± SD or number (percentage), as appropriate. The characteristics were compared by independent samples t-test/Mann–Whitney U for continuous data and chi-squared test/ Fisher’s exact test for categorical data. Postoperative 3-year RFS was analysed using a Kaplan–Meier estimator with differences between groups assessed by Log rank test. A Cox proportional hazard model was used to analyse the pTNM. Effect size was expressed as HR and 95% CI. All comparisons were two-sided and Probability (P) values < 0.05 represented statistically significant (P<0.05).
Results
Ultimately, from January 02, 2016 to December 30, 2018, 231 patients with gastric carcinoma undergoing laparoscopic radical gastrectomy were included, which consisted of 119 patients in Group QG and 112 patients in Group GA who met the standards for the research (Figure 1). Patients characteristics at baseline were well balanced between the groups (P>0.05, Table 1). UG-QLB combined with GA did not affect the 3-year RFS in patients undergoing laparoscopic radical gastrectomy (HR 0.659, 95% CI 0.342–1.269, P=0.212, Figure 2). We also conducted the Cox regression analyses for the study to determine clinically significant risk factors (pTNM stage) for gastric adenocarcinoma recurrence. As a result, the pTNM stage was found to be associated with higher risks of 3-year RFS (P=0.004). In Group QG and Group GA, pTNM stage was also closely related to 3-year RFS (P=0.040 and P=0.049) (Table 2). However, the VAS ranking analysis implicated UG-QLB could significantly alleviate the postoperative pain in laparoscopic radical gastrectomy patients (P<0.01, Figure 3). Furthermore, UG-QLB combined with GA dramatically facilitated the early recovery of postoperative ambulation and flatus, while shortening the duration of postoperative hospitalization (P<0.01, Figure 4). The most important was UG-QLB combined with GA could remarkably reduce the opioid consumption (P<0.01, Figure 5), which in the meanwhile, reduced the incidence of PONV (P=0.01, Table 3).
Figure 1.
Flow diagram of the study.
Table 1.
Patients Characteristics and Pathologic Outcomes in the Groups
| Characteristics | Group QG (n=119) | Group GA (n=112) | P value |
|---|---|---|---|
| Age, (year) | 64.3±8.6 | 62.3±9.2 | 0.10 |
| Male sex, n (%) | 72 (60.5) | 63 (56.3) | 0.51 |
| Weight (kg) | 67.2±11.1 | 64.6±10.3 | 0.06 |
| BMI (kg/m2) | 22.0±2.3 | 21.7±1.8 | 0.27 |
| ASA grade, n (%) | |||
| I | 11 (9.2) | 9 (8.0) | 0.19 |
| II | 83 (69.8) | 89 (79.5) | |
| III | 25 (21.0) | 14 (12.5) | |
| Hemoglobin (g/L) | 124.3±20.5 | 125.4±19.2 | 0.66 |
| Sevoflurane (mL) | 101.5±15.8 | 105.0±13.2 | 0.07 |
| Propofol (mg) | 1100.8±852.5 | 994.6±251.1 | 0.21 |
| Duration of anesthesia (min) | 208.0 ± 28.9 | 206.2 ± 27.5 | 0.63 |
| Duration of surgery (min) | 180.5 ± 28.5 | 178.3 ± 29.6 | 0.58 |
| Intraoperative blood loss (mL) | 89.9 ±33.7 | 98.1 ±35.7 | 0.08 |
| Urine volume (mL) | 135.5±34.5 | 128.8±39.8 | 0.17 |
| Tumor size, cm, n (%) | 119 | 112 | |
| ≤5 | 78 (65.5) | 78 (69.6) | 0.51 |
| >5 | 41 (34.5) | 34 (30.4) | |
| Tumor location, n (%) | |||
| Proximal stomach | 19 (16.0) | 20 (17.9) | 0.55 |
| Middle stomach | 32 (26.9) | 36 (32.1) | |
| Distal stomach | 68 (57.1) | 56 (50.0) | |
| Operative procedure, n (%) | |||
| Total gastrectomy | 72 (60.5) | 61 (54.5) | 0.38 |
| Distal gastrectomy | 46 (38.7) | 51 (45.5) | |
| Esophagogastric resection | 1 (0.8) | 0 | |
| c T stage, n (%) | |||
| cT1 | 29 (24.4) | 33 (29.5) | 0.51 |
| cT2 | 39 (32.8) | 40 (35.7) | |
| cT3 | 46 (38.6) | 37 (33.0) | |
| cT4 | 5 (4.2) | 2 (1.8) | |
| Lymphadenectomy, n (%) | |||
| D1 | 43 (36.1) | 45 (40.2) | 0.53 |
| D2 | 76 (63.9) | 67 (59.8) | |
| pTNM stage n (%) | |||
| I | 23 (19.3) | 22 (19.6) | 0.36 |
| I | 53 (44.5) | 59 (52.7) | |
| III | 43 (36.1) | 31 (27.7) | |
| Treatment regime, n (%) | |||
| Surgery only | 33 (27.7) | 35 (31.3) | 0.72 |
| Surgery &chemotherapy | 81 (68.1) | 74 (66.0) | |
| Other treatment | 5 (4.2) | 3 (2.7) |
Notes: The data are given as mean ± SD or n (%). Quantitative data were compared by independent-sample t test; Categorical data were compared by chi-squared test.
Figure 2.
Comparison of Kaplan-Meier survival curves for the 3-year postoperative RFS between groups. There were no statistical differences in the 3-year postoperative RFS between groups (HR 0.659, 95% CI 0.342-1.269, P=0.212). Group QG, quadratus lumborum block combined with general anaesthesia, Group GA, general anaesthesia alone.
Table 2.
Univariate Analyses of pTNM Stage Associated with 3-Year RFS in Entire Group, Group QG and Group GA
| Entire Group (n=231) | Group QG (n=119) | Group GA (n=112) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| pTNM stage | |||||||||
| I | 1.000 | 1.000 | 1.000 | ||||||
| II | 1.369 | 0.377–4.976 | 0.633 | 1.333 | 0.269–6.605 | 0.725 | 1.529 | 0.171–13.685 | 0.704 |
| III | 5.875 | 1.763–19.579 | 0.004 | 4.728 | 1.074–20.820 | 0.040 | 7.956 | 1.007–62.875 | 0.049 |
Figure 3.
Comparison of the average pain scores in 48 h postoperatively between groups. The data are given as mean ± SD, compared with Group GA, *P<0.01. Data were compared by independent-sample t-test. Group QG, quadratus lumborum block combined with general anaesthesia, Group GA, general anaesthesia alone, VAS, visual analogue scale.
Figure 4.
Comparison of postoperative early recovery between groups. First time of postoperative ambulation (A), First time of flatus (B), Postoperative hospitalization (C). The data are given as mean ± SD, compared with Group GA, *P<0.01. Data were compared by independent-sample t-test. Group QG, quadratus lumborum block combined with general anaesthesia, Group GA, general anaesthesia alone.
Figure 5.
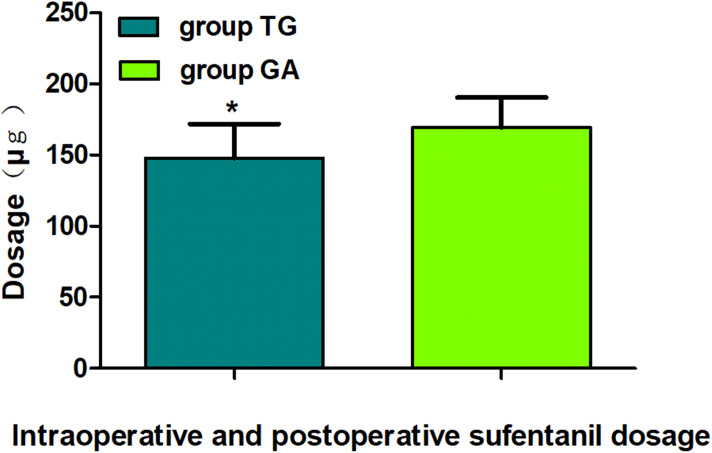
Comparison of intraoperative and postoperative sufentanil dosage between groups. The data are given as mean ± SD, compared with Group GA, *P<0.01. Data were compared by independent-sample t-test. Group QG, quadratus lumborum block combined with general anaesthesia, Group GA, general anaesthesia alone.
Table 3.
Adverse Reactions in the Groups (n (%))
| Group QG (n=119) | Group GA (n=112) | P value | |
|---|---|---|---|
| Drowsiness | 7 (5.9%) | 12 (10.7%) | 0.21 |
| PONV | 10 (8.4%) | 26 (23.2%) | 0.01 |
| Pruritus | 8 (6.7%) | 9 (8.0%) | 0.79 |
| Respiratory depression | 5 (4.2%) | 11 (9.8%) | 0.10 |
| Urinary retention | 0 (0.0) | 6 (5.4%) | 0.06 |
| Hypotension | 0 (0.0) | 4 (3.6%) | 0.12 |
| Bradycardia | 0 (0.0) | 1 (0.9%) | 1.00 |
Notes: The data are given as n (%). Categorical data were compared by chi-squared test.
Discussion
The most principal findings of the retrospective study were that the UG-QLB combined with GA for patients with gastric carcinoma undergoing laparoscopic radical gastrectomy did not prolong the postoperative 3-year RFS, but it significantly improved the analgesia effect postoperatively, decreased opioid requirement, lowered adverse effects. Additionally, it shortened the first time of postoperative ambulation, reduced the first time of flatus, and cut down the postoperative hospitalization, which facilitates the postoperative early rehabilitation.
Currently, surgical resection is the principal measure to treat gastric cancer, yet surgical trauma can result in a range of systemic metabolic, inflammatory, and neuroendocrine responses. Therefore, the immune surveillance function of patients is inhibited, which maybe facilitate tumour metastasis postoperatively.10,23 According to related reports, except surgical trauma, all kinds of perioperative factors could impact the anti-tumour immune function and were in connection with neoplasm metastasis. However, anaesthesia methods have received more and more attentions among above factors.34 In the light of the plenty of latest literature, regional anaesthesia can influence tumour progression, generally by the three main mechanisms: elimination of the adrenergic-inflammatory caused by the operation, systemic effects of local anaesthetic agents, and reducing opioid requirements. Nevertheless, it remains a subject for debate.8,35,36 Despite some observational researches in tumour patients shown benefits of regional anaesthesia, most others were the opposite.19–21,37 In a propensity score matching analysis, Pei et al38 reported that epidural combined with general anaesthesia (EGA) had a better survival rate than patients with GA alone in gastric cancer surgery. A population-based retrospective study by Cummings et al39 shown that there was no obvious difference between epidural analgesia and no epidural analgesia regarding treated recurrence or survival of gastric cancer. Furthermore, Shin et al40 suggested that postoperative applying of epidural analgesia was found to be no relevance to decreased recurrence or mortality compared to intravenous analgesia after curative gastric surgery in a retrospective analysis. Nevertheless, a retrospective study by Wang et al41 demonstrated that epidural anaesthesia was related to long-term survival among younger gastric cancer patients, but not among older patients. In another retrospective study, Wang et al14 demonstrated that the improved survival rate of gastric cancer was significantly association with EGA. To date, there are few studies on the effect of QLB on gastric adenocarcinoma. But then our outcome firstly shown that UG-QLB combined with GA had no effect on the 3-year RFS of patients for laparoscopic radical gastrectomy. The possible main factors are as below: reduced stress response, less opioids and relatively stable immune function during all the surgery, which were to a large extent related to UG-QLB.
Although laparoscope is a minimally invasive method that has helped to reduce the degree of pain, the patients still experience moderate to severe pain at the cut as well as internal organs after surgery, leading to physical stringent state, impaired immune function and promoting tumour recurrence or metastasis. Hence, we need to block visceral and somatic nerve fibres in order to control pain more effectively and improve the clinical effects.3,42 It has been shown that local anaesthesia combined with GA had better analgesia by completing the somatosensorial and sympathetic block and had an opioid sparing effect.10 The findings of this study suggest that the average VAS score in Group QG was dramatically reduced than that in Group GA (P<0.01), which was identical to the findings of Zhu et al28 who demonstrated that the VAS score was evidently lower for aged patients with laparoscopic radical gastrectomy during 48 h postoperatively in Group QLB in comparison with Control group in a randomized controlled trial. Additional, our results resemble a study by Nie et al28 who indicated that QLB provided analgesia which was superior to transversus abdominis plane block within 48 h after laparoscopic radical gastrectomy surgery in a randomized study. Furthermore, Wang et al31 revealed that QLB alleviated postoperative pain in patients with laparoscopic colon cancer.
Early postoperative recovery is affected by many factors, for example, degree of surgical trauma, post-surgical pain, postoperative activities, the first time of flatus and the postoperative hospitalization.30,36 Our findings demonstrated that these indicators (The first time of postoperative ambulation, the first time of flatus and postoperative hospitalization) were evidently shorter in Group QG by comparison to those in Group GA (P<0.01). Our findings were similar to those of Wang et al31 study which reported that QLB shortened the time of the first time of ambulation and anal flatus by comparison with Control group in laparoscopic radical gastrectomy surgery. A randomized trial by Nie et al28 reported that QLB could markedly shorten the first time of postoperative movement and flatus, as well as postoperative hospital stay for laparoscopic radical gastrectomy in contrast to GA. Another randomized study by Wang et al30 shown that QLB could significantly shortened the first time of movement, exhaust, taking semi-liquid food, and postoperative length of stay in open radical colon cancer surgery. More intriguingly, a latest randomized controlled trial by Zhu et al29 revealed that QLB reduced the incidence of postoperative cognitive function in the aged patients underwent laparoscopic gastrectomy, which might be associated with the suppression of the inflammatory response postoperatively.
QLB could accelerate recovery, maintain independent cellular immune. That is to say, it is in consistent with the concept of enhanced recovery after surgery (ERAS) to preserve organ function, decrease perioperative stress response, and facilitates the postoperative early rehabilitation.
The sufentanil consumption during operation and after operation of Group QG was remarkably less than in Group GA (P<0.01). This also indicated that QLB combined with GA could significantly decrease perioperative pain, which was in line with the findings of Nie et al29 and Zhu et al30 who found that QLB distinctively lowered perioperative opioid consumption. In addition, several randomized controlled trial also reported that QLB appeared to decrease the need for opioid requirement for laparoscopic hepatectomy, laparoscopic colorectal surgery and laparoscopic nephrectomy.43–45
Operative factors, residual narcotics and postoperative pains may cause side effect, for example, drowsiness, PONV, itching, urinary retention, bradycardia and hypotension. A recent meta-analysis by Korgvee et al46 concluded that QLB lessened the incidence of PONV, which seemed to be a better alternative for postoperative pain after abdominal and hip operation. The findings demonstrated that the incidence of PONV in Group QG was notably reduced than those in Group GA (P=0.01), which was in line with previous trials in laparoscopic operations.29,47 The possible reasons include the multimodal analgesia from QLB, the relieved pain, lower opioid usage, and rapid restoration of gastrointestinal function.48,49
There were some limitations in this research. Firstly, this was a single-centre retrospective study. The incomplete medical record and the bias of recalling history increased the complexity of the study, which may have been prone to selection bias, and some multicenter prospective trials should be carried out for further to test and verify. Secondly, both the groups were not randomly assigned, and the baseline information might be unbalanced and biased, which was also one of the defects of our retrospective study. Thirdly, although we tried to minimize confounders in this study to the greatest extent, there may be unmeasured variable and residual confounders on outcomes. Fourthly, the differentiation grade and chemotherapy regimens were not statistically analyzed, which may affect the clinical results. Fifthly, the follow-up cycle of postoperative pain efficacy were only 48 h, and a longer follow-up visit need to validate the results. Lastly, we did not consider the type of UG-QLB (which was divided into six approaches recently), which probably affect the accuracy of the outcomes.
Conclusions
This retrospective study suggested that UG-QLB combined with GA did not improve the 3-year RFS for patients with gastric carcinoma undergoing laparoscopic radical gastrectomy patients, yet it could provide satisfactory postoperative pain relief, reduce opioid consumption and adverse effects, which subsequently facilitates the postoperative early recovery. UG-QLB could be a safe and effective supplementary anesthesia for laparoscopic radical gastrectomy.
Acknowledgments
We would like to thank the participants who enrolled in this study, and the study team for essential contributions. Additionally, we thank Professor Liyuan Liu for her help in statistics.
Funding Statement
This work was supported by the scientific research fund of Shandong Medical Association under Grant No. YXH2020ZX021. This study was also supported by clinical medicine science and technology innovation plan of Jinan Science and Technology Bureau, Shandong Province, China (No. 202019127). Additionally, this study was supported by National health commission of China Key R&D Program(No. GWJJ2022100301-2.
Abbreviations
GA, general anesthesia; QLB, quadratus lumborum block; UG-QLB, ultrasound-guided quadratus lumborum block; BMI, body mass index; ASA, American Society of Anesthesiologists; HR, hazard ratio; CI, confidence interval; SD, standard deviation; P, probability; RFS, recurrence-free survival; pTNM, pathological tumor-node-metastasis; PCIA, patient-controlled intravenous analgesia; ECG, electrocardiography; IBP, invasive blood pressure; SPO2, saturation of peripheral oxygen; ETCO2, end-tidal carbon dioxide; BIS, bispectral index; EGA, epidural combined with general anesthesia.
Data Sharing Statement
The datasets of our study can be obtained from the corresponding author through reasonable requirements.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no conflicts of interest for this work and no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
References
- 1.Yu J, Huang C, Sun Y., et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the class-01 randomized clinical trial. JAMA. 2019;321(20):1983–1992. doi: 10.1001/jama.2019.5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Veen A, Brenkman HJF, Seesing MFJ, et al. Laparoscopic versus open gastrectomy for gastric cancer (LOGICA): a multicenter randomized linical trial. J Clin Oncol. 2021;39(9):978–989. doi: 10.1200/JCO.20.01540 [DOI] [PubMed] [Google Scholar]
- 3.Feng M, Feng Q, Chen Y, et al. Effect of dezocine on the ratio of Th1/Th2 cytokines in patients receiving postoperative analgesia following laparoscopic radical gastrectomy: a prospective randomised study. Drug Des Devel Ther. 2021;15:2289–2297. doi: 10.2147/DDDT.S306120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DJ, Seo SH, Kim KH, et al. Comparisons of clinicopathologic factors and survival rates between laparoscopic and open gastrectomy in gastric cancer. Int J Surg. 2016;34:161–168. doi: 10.1016/j.ijsu.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 5.Ben-Eliyahu S. Tumor excision as a metastatic Russian roulette: perioperative interventions to improve long-term survival of cancer patients. Trends Cancer. 2020;6(11):951–959. doi: 10.1016/j.trecan.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Missair A, Cata JP, Votta-Velis G, et al. Impact of perioperative pain management on cancer recurrence: an ASRA/ESRA special article. Reg Anesth Pain Med. 2019;44(1):13–28. doi: 10.1136/rapm-2018-000001 [DOI] [PubMed] [Google Scholar]
- 7.Wall T, Sherwin A, Ma D, Buggy DJ. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123(2):135–150. doi: 10.1016/j.bja.2019.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubowitz J, Hiller J, Riedel B. Anesthetic technique and cancer surgery outcomes. Curr Opin Anaesthesiol. 2021;34(3):317–325. doi: 10.1097/ACO.0000000000001002 [DOI] [PubMed] [Google Scholar]
- 9.Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can J Anaesth. 2016;63(2):184–192. doi: 10.1007/s12630-015-0523-8 [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Guo K, Sun X, et al. Impact of anesthesia methods on perioperative systemic inflammation and long-term outcomes in patients undergoing surgery for hepatocellular carcinoma: a propensity score-matched analysis. Ann Transl Med. 2021;9(1):49. doi: 10.21037/atm-20-3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105(2):106–115. doi: 10.1093/bja/aeq164 [DOI] [PubMed] [Google Scholar]
- 12.Plücker J, Wirsik NM, Ritter AS, et al. Anaesthesia as an influence in tumour progression. Langenbecks Arch Surg. 2021;406(5):1283–1294. doi: 10.1007/s00423-021-02078-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Sun Y, Chen H, et al. Effects of two different anesthetic methods on cellular immunity of patients after liver cancer resection. J Biol Regul Homeost Agents. 2016;30(4):1099–1106. [PubMed] [Google Scholar]
- 14.Xu Y, Sun Y, Chen H, et al. The effects of intra- and post-operative anaesthesia and analgesia choice on outcome after gastric cancer resection: a retrospective study. Oncotarget. 2017;8(37):62658–62665. doi: 10.18632/oncotarget.16724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez-González O, Cuéllar-Guzmán LF, Soliz J, et al. Impact of regional anesthesia on recurrence, metastasis, and immune response in breast cancer surgery: a systematic review of the literature. Reg Anesth Pain Med. 2017;42(6):751–756. doi: 10.1097/AAP.0000000000000662 [DOI] [PubMed] [Google Scholar]
- 16.Cui X, Zhu C, Chen P, et al. Effect of pectoral nerve block type II under general anesthesia on the immune function of patients with breast cancer. Am J Surg. 2020;220(4):938–944. doi: 10.1016/j.amjsurg.2020.03.008 [DOI] [PubMed] [Google Scholar]
- 17.Sessler DI, Pei L, Huang Y, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet. 2019;394(10211):1807–1815. doi: 10.1016/S0140-6736(19)32313-X [DOI] [PubMed] [Google Scholar]
- 18.Wahal C, Kumar A, Pyati S. Advances in regional anaesthesia: a review of current practice, newer techniques and outcomes. Indian J Anaesth. 2018;62(2):94–102. doi: 10.4103/ija.IJA_433_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev. 2017;36(1):159–177. doi: 10.1007/s10555-016-9647-8 [DOI] [PubMed] [Google Scholar]
- 20.Novak-Jankovič V, Markovič-Božič J. Regional anaesthesia in thoracic and abdominal surgery. Acta Clin Croat. 2019;58(Suppl 1):96–100. doi: 10.20471/acc.2019.58.s1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tedore T. Regional anaesthesia and analgesia: relationship to cancer recurrence and survival. Br J Anaesth. 2015;115(Suppl 2):ii34–45. doi: 10.1093/bja/aev375 [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Chang C, Lu C, et al. Paravertebral block in regional anesthesia with propofol sedation reduces locoregional recurrence in patients with breast cancer receiving breast conservative surgery compared with volatile inhalational without propofol in general anesthesia. Biomed Pharmacother. 2021;142:111991. doi: 10.1016/j.biopha.2021.111991 [DOI] [PubMed] [Google Scholar]
- 23.Hayden JM, Oras J, Block L, et al. Intraperitoneal ropivacaine reduces time interval to initiation of chemotherapy after surgery for advanced ovarian cancer: randomised controlled double-blind pilot study. Br J Anaesth. 2020;124(5):562–570. doi: 10.1016/j.bja.2020.01.026 [DOI] [PubMed] [Google Scholar]
- 24.Le Gac G, Angenard G, Clément B, et al. Local anesthetics inhibit the growth of human hepatocellular carcinoma cells. Anesth Analg. 2017;125(5):1600–1609. doi: 10.1213/ANE.0000000000002429 [DOI] [PubMed] [Google Scholar]
- 25.Dan J, Gong X, Li D, et al. Inhibition of gastric cancer by local anesthetic bupivacaine through multiple mechanisms independent of sodium channel blockade. Biomed Pharmacother. 2018;103:823–828. doi: 10.1016/j.biopha.2018.04.106 [DOI] [PubMed] [Google Scholar]
- 26.Baptista-Hon DT, Robertson FM, Robertson GB, et al. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1.5 channel function. Br J Anaesth. 2014;113(Suppl 1):i39–i48. doi: 10.1093/bja/aeu104 [DOI] [PubMed] [Google Scholar]
- 27.Blanco R. Tap block under ultrasound guidance: the description of a ‘no pops’ technique: 271 [abstract]. Region Anesth Pain Med. 2007;32:S1–130. [Google Scholar]
- 28.Nie B, Niu L, Yang E, et al. Effect of subcostal anterior quadratus lumborum block vs. oblique subcostal transversus abdominis plane block after laparoscopic radical gastrectomy. Curr Med Sci. 2021;41(5):974–980. doi: 10.1007/s11596-021-2429-8 [DOI] [PubMed] [Google Scholar]
- 29.Zhu M, Qi Y, He H, et al. Effect of quadratus lumborum block on postoperative cognitive function in elderly patients undergoing laparoscopic radical gastrectomy: a randomized controlled trial. BMC Geriatr. 2021;21(1):238. doi: 10.1186/s12877-021-02179-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Hu H, Feng C, et al. Effect of ultrasound-guided quadratus lumborum block preemptive analgesia on postoperative recovery of patients with open radical colon cancer surgery: a retrospective study. Cancer Manag Res. 2021;13:6859–6867. doi: 10.2147/CMAR.S322678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, He Y, Chen X, et al. Ultrasound guided lateral quadratus lumborum block enhanced recovery in patients undergoing laparoscopic colorectal surgery. Adv Med Sci. 2021;66(1):41–45. doi: 10.1016/j.advms.2020.12.002 [DOI] [PubMed] [Google Scholar]
- 32.Liu Q, Xie J, Dai Y, et al. Quadratus lumborum block spares postoperative opioid usage but does not appear to prevent the development of chronic pain after gastrointestinal surgery. Pain Physician. 2021;24(8):E1191–E1198. [PubMed] [Google Scholar]
- 33.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018. Gastric Cancer. 2021;24(1):1–21. doi: 10.1007/s10120-020-01042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu Y, Wu J, Huang Q, et al. Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: a noninferiority randomised trial. Eur J Anaesthesiol. 2021;38(Suppl2):S97–S105. doi: 10.1097/EJA.0000000000001450 [DOI] [PubMed] [Google Scholar]
- 35.Cata JP, Gottumukkala V, Thakar D, et al. Effects of postoperative epidural analgesia on recurrence-free and overall survival in patients with nonsmall cell lung cancer. J Clin Anesth. 2014;26(1):3–17. doi: 10.1016/j.jclinane.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 36.Feng M, Wang L, Sun J, et al. Thoracic paravertebral block combined with general anaesthesia or general anaesthesia alone for thoracoscopic lung adenocarcinoma surgery: a retrospective study. Cancer Manag Res. 2022;14:953–965. doi: 10.2147/CMAR.S346285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cata JP. Outcomes of regional anesthesia in cancer patients. Curr Opin Anaesthesiol. 2018;31(5):593–600. doi: 10.1097/ACO.0000000000000636 [DOI] [PubMed] [Google Scholar]
- 38.Pei J, Zhang C, Liang Y, et al. Effects of epidural combined with general anesthesia versus general anesthesia alone in gastric cancer surgery: a propensity score matching analysis. Ann Transl Med. 2020;8(7):473. doi: 10.21037/atm.2020.03.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummings KC, Patel M, Htoo PT, et al. A comparison of the effects of epidural analgesia versus traditional pain management on outcomes after gastric cancer resection: a population-based study. Reg Anesth Pain Med. 2014;39(3):200–207. doi: 10.1097/AAP.0000000000000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin S, Kim HI, Kim NY, et al. Effect of postoperative analgesia technique on the prognosis of gastric cancer: a retrospective analysis. Oncotarget. 2017;8(61):104594–104604. doi: 10.18632/oncotarget.21979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Guo W, Wu Q, et al. Impact of combination epidural and general anesthesia on the long-term survival of gastric cancer patients: a retrospective study. Med Sci Monit. 2016;22:2379–2385. doi: 10.12659/msm.899543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang W, Tai Y, Lin S, et al. An investigation of the relationships between postoperative pain trajectories and outcomes after surgery for colorectal cancer. J Chin Med Assoc. 2019;82(11):865–871. doi: 10.1097/JCMA.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 43.Pang M, Sun G, Yao W, et al. Ultrasound-guided transmuscular quadratus lumborum block reduced postoperative opioids consumptions in patients after laparoscopic hepatectomy: a three-arm randomized controlled trial. BMC Anesthesiol. 2021;21(1):45. doi: 10.1186/s12871-021-01255-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang D, Song L, Li Y, et al. Posteromedial quadratus lumborum block versus transversus abdominal plane block for postoperative analgesia following laparoscopic colorectal surgery: a randomized controlled trial. J Clin Anesth. 2020;62:109716. doi: 10.1016/j.jclinane.2020.109716 [DOI] [PubMed] [Google Scholar]
- 45.Li H, Shi R, Shi D, et al. Anterior quadratus lumborum block at the lateral supra-arcuate ligament versus transmuscular quadratus lumborum block for postoperative analgesia in patients undergoing laparoscopic nephrectomy: a randomized controlled trial. J Clin Anesth. 2021;75:110561. doi: 10.1016/j.jclinane.2021.110561 [DOI] [PubMed] [Google Scholar]
- 46.Korgvee A, Junttila E, Koskinen H, et al. Ultrasound-guided quadratus lumborum block for postoperative analgesia: a systematic review and meta-analysis. Eur J Anaesthesiol. 2021;38(2):115–129. doi: 10.1097/EJA.0000000000001368 [DOI] [PubMed] [Google Scholar]
- 47.Vamnes JS, Sørenstua M, Solbakk KI, et al. Anterior quadratus lumborum block for ambulatory laparoscopic cholecystectomy: a randomized controlled trial. Croat Med J. 2021;62(2):137–145. doi: 10.3325/cmj.2021.62.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koksal E, Aygun H, Genç C, et al. Comparison of the analgesic effects of two quadratus lumborum blocks (QLBs), QLB type II vs QLB type III, in caesarean delivery: a randomised study. Int J Clin Pract. 2021;12:e14513. doi: 10.1111/ijcp.14513 [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Liang S, Chen H, et al. The effects of epidural anaesthesia and analgesia on T lymphocytes differentiation markers and cytokines in patients after gastric cancer resection. BMC Anesthesiol. 2019;19(1):102. doi: 10.1186/s12871-019-0778-7 [DOI] [PMC free article] [PubMed] [Google Scholar]